High-Pressure Homogenization and Biocontrol Agent as Innovative Approaches Increase Shelf Life and Functionality of Carrot Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carrot Juice
2.2. Selection of the Appropriate High-Pressure Homogenization Treatment (HPH)
2.3. Shelf-Life Assessment of Carrot Juice Considering the Combination of HPH Treatment and Biocontrol Agent
2.3.1. HPH Treatment
2.3.2. Fermentation Agent
2.4. Shelf-Life Assessment
2.5. Microbiological Analyses, pH, Nisin Concentration
2.6. Color Analysis
2.7. Volatile Molecule Profiles
2.8. Carotenoid Content
2.8.1. Extraction of Carotenoids from Carrot Juice
2.8.2. Determination of Carotenoids by High-Performance Liquid Chromatography (HPLC)
2.9. Statistical Analysis
3. Results
3.1. Selection of the Appropriate High-Pressure Homogenization Treatment (HPH)
3.2. Samples Stored at 4 and 10 °C
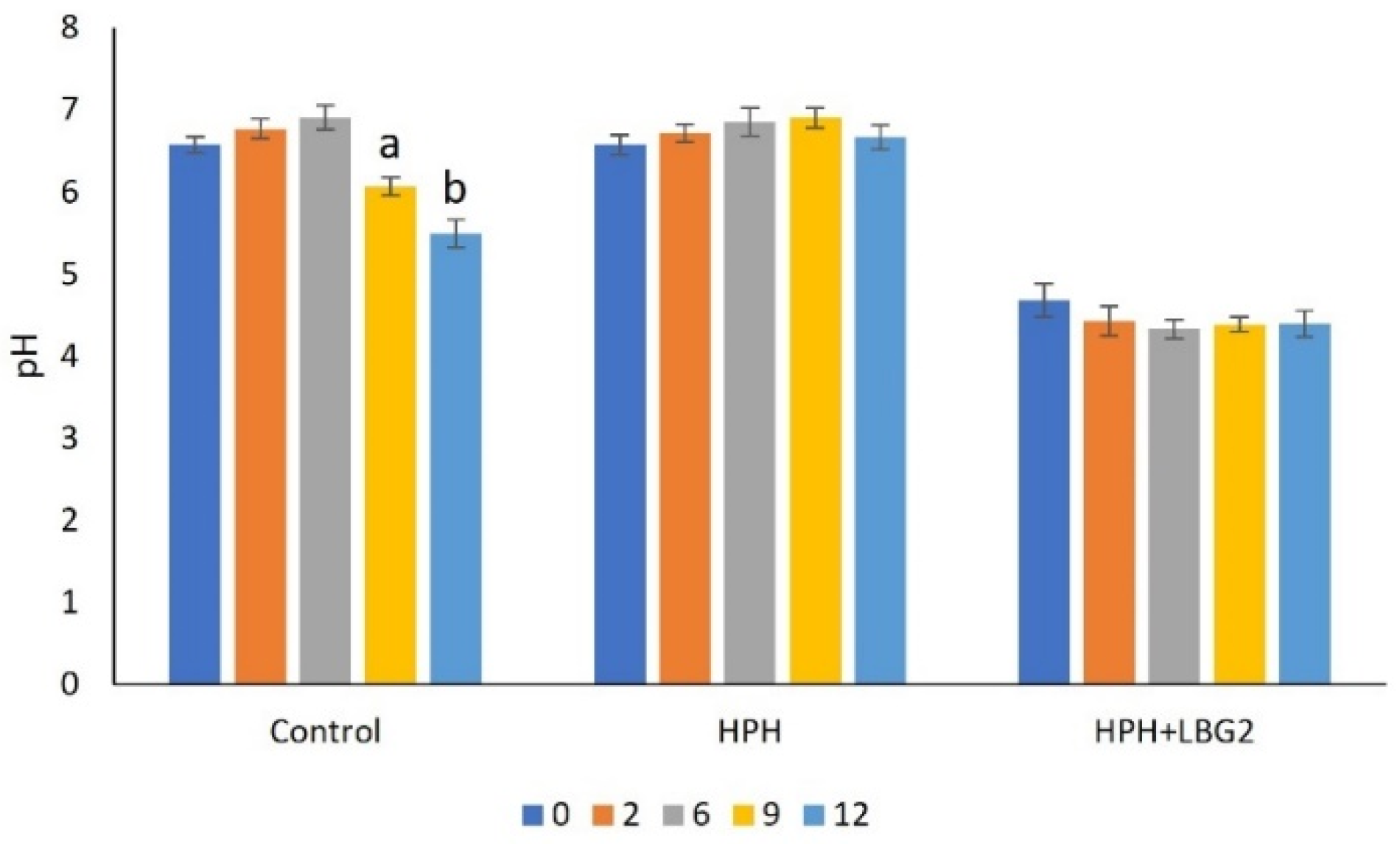
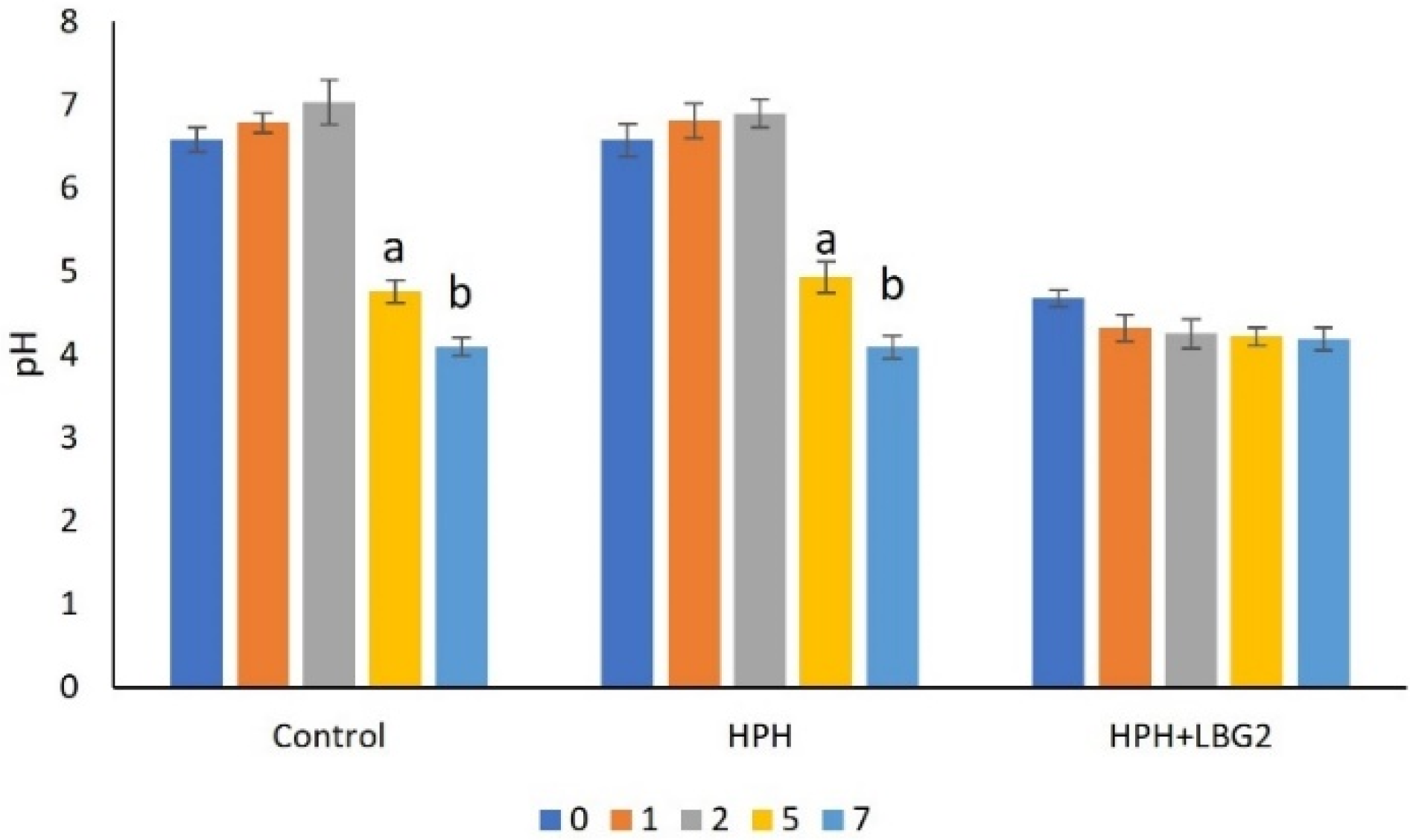
3.2.1. pH
3.2.2. Microbiological Analyses
3.2.3. Color Analyses
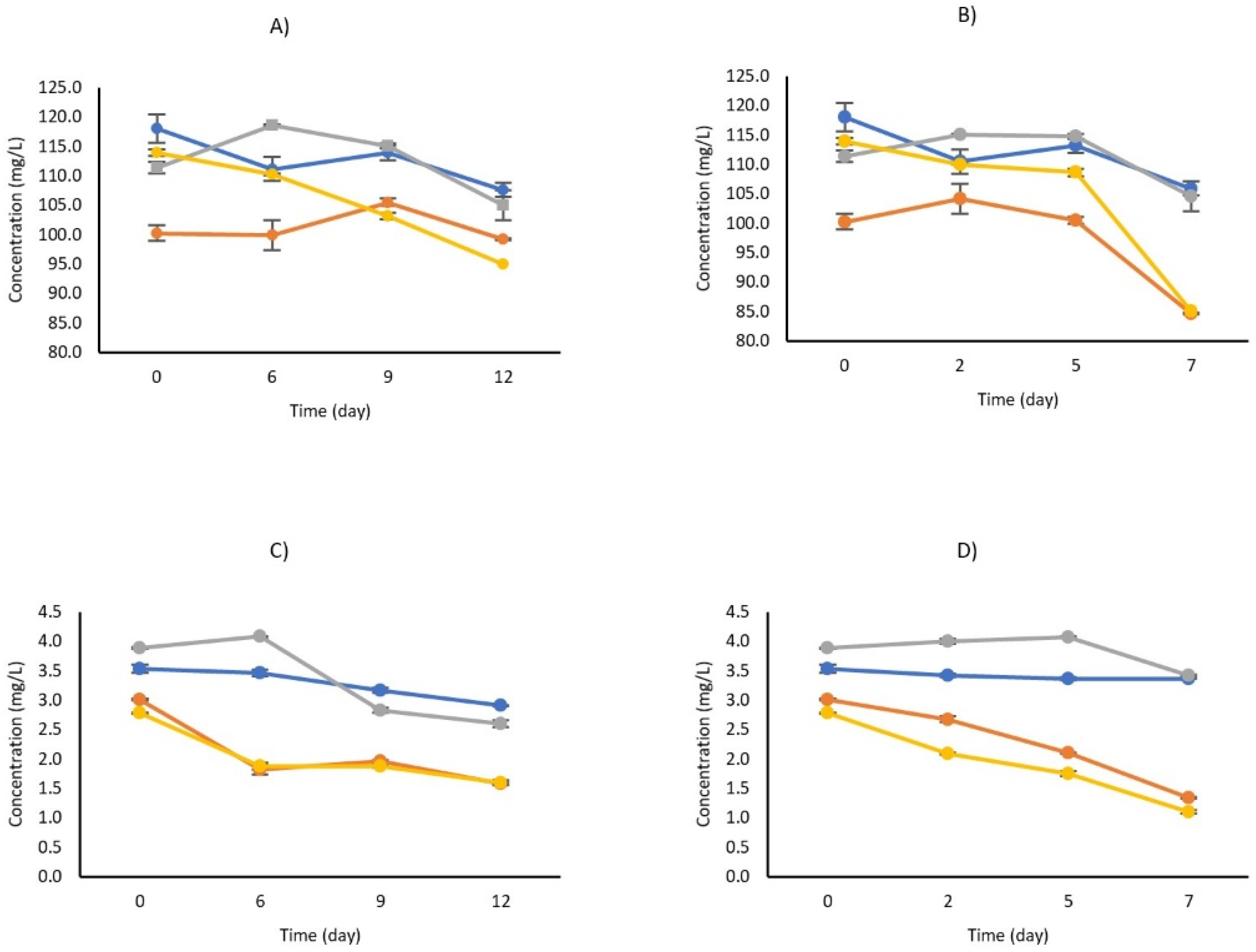
3.2.4. Influence of HPH and HPH Combined with LBG2 on the Stability of Carotenoids and Profile Changes
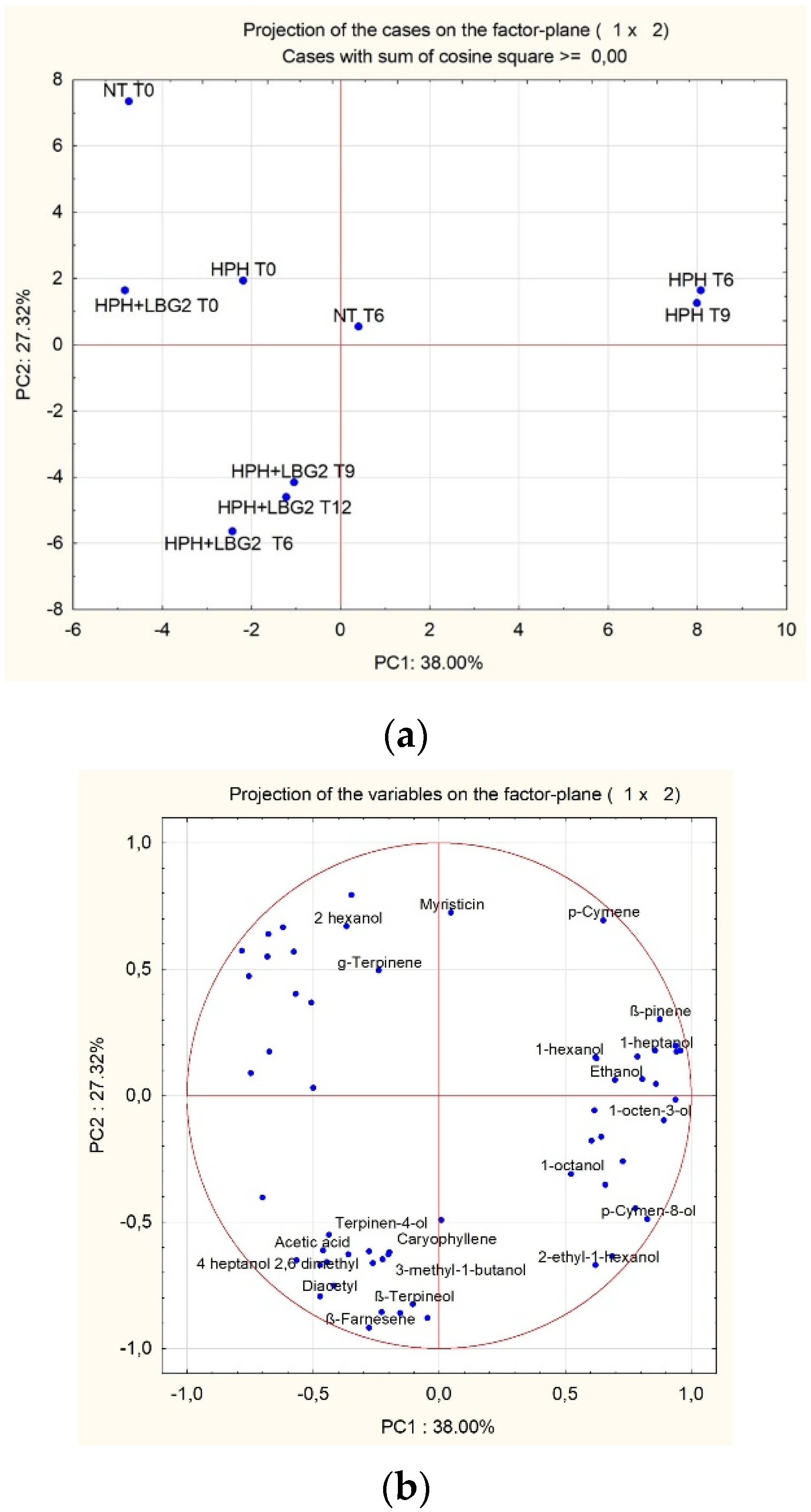
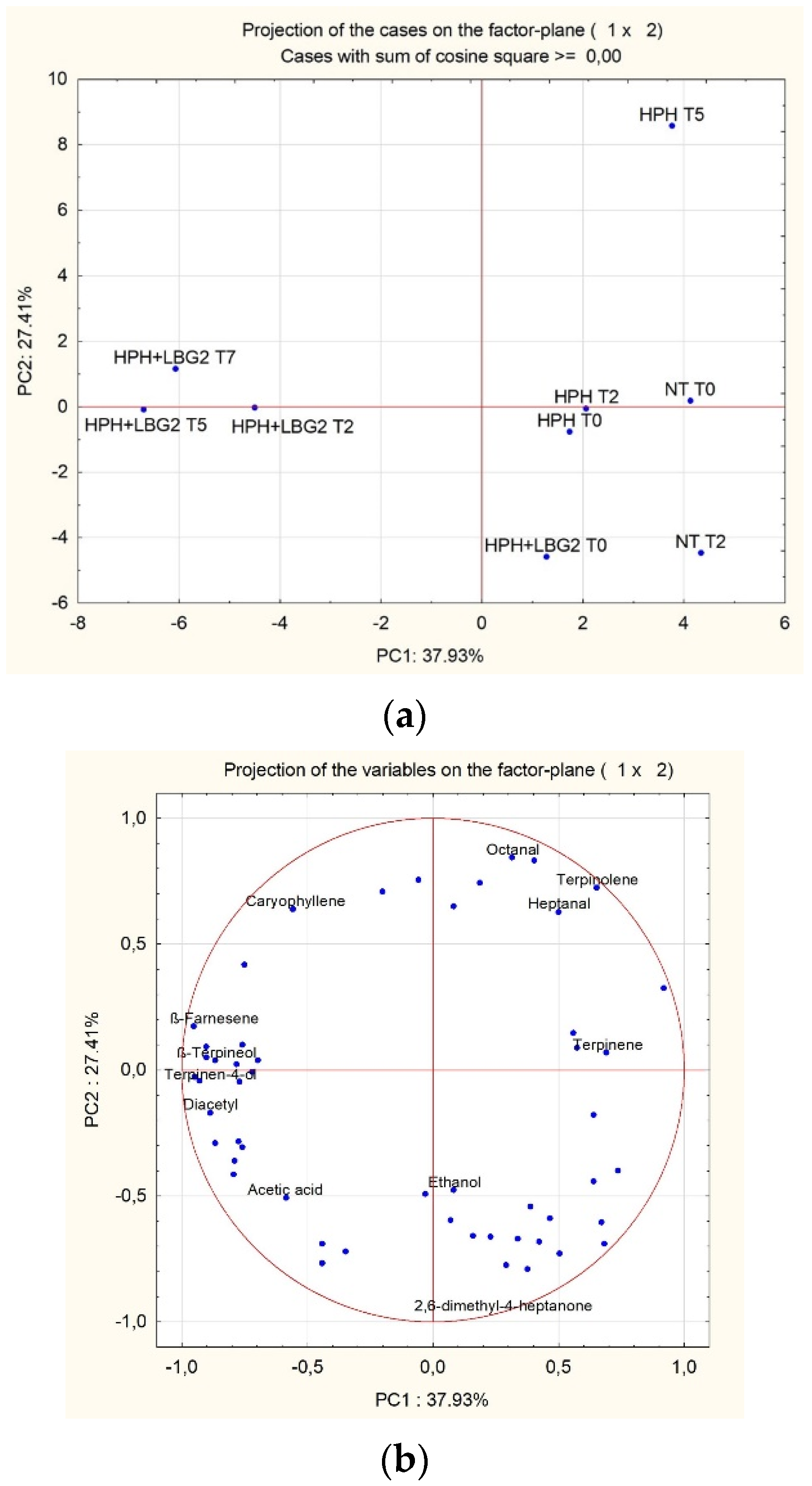
3.2.5. Volatile Molecule Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shahbaz, H.M.; Kim, J.U.; Kim, S.-H.; Park, J. Advances in nonthermal processing technologies for enhanced microbiological safety and quality of fresh fruit and juice products. In Food Processing for Increased Quality and Consumption; Elsevier: Amsterdam, The Netherlands, 2018; pp. 179–217. [Google Scholar]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Wootton-Beard, P.C.; Ryan, L. Improving public health?: The role of antioxidant-rich fruit and vegetable beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pferschy-Wenzig, E.-M.; Getzinger, V.; Kunert, O.; Woelkart, K.; Zahrl, J.; Bauer, R. Determination of falcarinol in carrot (Daucus carota L.) genotypes using liquid chromatography/mass spectrometry. Food Chem. 2009, 114, 1083–1090. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Zhao, F.; Sun, Z.; Liao, X. Quality comparison of carrot juices processed by high-pressure processing and high-temperature short-time processing. Innov. Food Sci. Emerg. Technol. 2016, 33, 135–144. [Google Scholar] [CrossRef]
- Nadeem, M.; Ubaid, N.; Qureshi, T.M.; Munir, M.; Mehmood, A. Effect of ultrasound and chemical treatment on total phenol, flavonoids and antioxidant properties on carrot-grape juice blend during storage. Ultrason. Sonochemistry 2018, 45, 1–6. [Google Scholar] [CrossRef]
- Patrignani, F.; Vannini, L.; Kamdem, S.L.S.; Lanciotti, R.; Guerzoni, M.E. Effect of high pressure homogenization on Saccharomyces cerevisiae inactivation and physico-chemical features in apricot and carrot juices. Int. J. Food Microbiol. 2009, 136, 26–31. [Google Scholar] [CrossRef]
- Siroli, L.; Camprini, L.; Pisano, M.B.; Patrignani, F.; Lanciotti, R. Volatile molecule profiles and anti-Listeria monocytogenes activity of nisin producers Lactococcus lactis strains in vegetable drinks. Front. Microbiol. 2019, 10, 563. [Google Scholar] [CrossRef]
- Kaddumukasa, P.P.; Imathiu, S.M.; Mathara, J.M.; Nakavuma, J.L. Influence of physicochemical parameters on storage stability: Microbiological quality of fresh unpasteurized fruit juices. Food Sci. Nutr. 2017, 5, 1098–1105. [Google Scholar] [CrossRef] [Green Version]
- Barzee, T.J.; El-Mashad, H.M.; Zhang, R.; Pan, Z. Carrots. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 297–330. [Google Scholar]
- Bearth, A.; Cousin, M.-E.; Siegrist, M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Prefer. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.D. The impact of nonthermal technologies on the microbiological quality of juices: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef]
- Bello, E.F.T.; Martínez, G.G.; Ceberio, B.F.K.; Rodrigo, D.; López, A.M. High pressure treatment in foods. Foods 2014, 3, 476–490. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, F.; Mannozzi, C.; Tappi, S.; Tylewicz, U.; Pasini, F.; Castellone, V.; Riciputi, Y.; Rocculi, P.; Romani, S.; Caboni, M.F. (Ultra) High pressure homogenization potential on the shelf-life and functionality of kiwifruit juice. Front. Microbiol. 2019, 10, 246. [Google Scholar] [CrossRef]
- Patrignani, F.; Lanciotti, R. Applications of High and Ultra High Pressure Homogenization for Food Safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, F. Physico-chemical and rheological properties of fruit and vegetable juices as affected by high pressure homogenization: A review. Int. J. Food Prop. 2020, 23, 1136–1149. [Google Scholar] [CrossRef]
- Zhou, L.; Guan, Y.; Bi, J.; Liu, X.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Change of the rheological properties of mango juice by high pressure homogenization. LWT-Food Sci. Technol. 2017, 82, 121–130. [Google Scholar] [CrossRef]
- Sentandreu, E.; Stinco, C.M.; Vicario, I.M.; Mapelli-Brahm, P.; Navarro, J.L.; Meléndez-Martínez, A.J. High-pressure homogenization as compared to pasteurization as a sustainable approach to obtain mandarin juices with improved bioaccessibility of carotenoids and flavonoids. J. Clean. Prod. 2020, 262, 121325. [Google Scholar] [CrossRef]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Zhou, M.; Marszałek, K.; Trych, U. Effect of high pressure homogenization combined with juice ratio on water-soluble pectin characteristics, functional properties and bioactive compounds in mixed juices. Innov. Food Sci. Emerg. Technol. 2020, 60, 102279. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Braschi, G.; Lanciotti, R. Combined use of natural antimicrobial based nanoemulsions and ultra high pressure homogenization to increase safety and shelf-life of apple juice. Food Control 2020, 111, 107051. [Google Scholar] [CrossRef]
- Patrignani, F.; Tabanelli, G.; Siroli, L.; Gardini, F.; Lanciotti, R. Combined effects of high pressure homogenization treatment and citral on microbiological quality of apricot juice. Int. J. Food Microbiol. 2013, 160, 273–281. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal technologies for fruit and vegetable juices and beverages: Overview and advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Dimitrellou, D.; Kandylis, P.; Kokkinomagoulos, E.; Hatzikamari, M.; Bekatorou, A. Emmer-Based Beverage Fortified with Fruit Juices. Appl. Sci. 2021, 11, 3116. [Google Scholar] [CrossRef]
- Serrazanetti, D.I.; Ndagijimana, M.; Miserocchi, C.; Perillo, L.; Guerzoni, M.E. Fermented tofu: Enhancement of keeping quality and sensorial properties. Food Control 2013, 34, 336–346. [Google Scholar] [CrossRef]
- Mauro, C.S.I.; Guergoletto, K.B.; Garcia, S. Development of blueberry and carrot juice blend fermented by Lactobacillus reuteri LR92. Beverages 2016, 2, 37. [Google Scholar] [CrossRef] [Green Version]
- Riciputi, Y.; Serrazanetti, D.I.; Verardo, V.; Vannini, L.; Caboni, M.F.; Lanciotti, R. Effect of fermentation on the content of bioactive compounds in tofu-type products. J. Funct. Foods 2016, 27, 131–139. [Google Scholar] [CrossRef]
- Lo, R.; Bansal, N.; Turner, M.S. Characterisation of Lactococcus lactis isolates from herbs, fruits and vegetables for use as biopreservatives against Listeria monocytogenes in cheese. Food Control 2018, 85, 472–483. [Google Scholar]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Vannini, L.; Salvetti, E.; Torriani, S.; Gardini, F.; Lanciotti, R. Use of a nisin-producing Lactococcus lactis strain, combined with natural antimicrobials, to improve the safety and shelf-life of minimally processed sliced apples. Food Microbiol. 2016, 54, 11–19. [Google Scholar] [CrossRef]
- Goodburn, C.; Wallace, C.A. The microbiological efficacy of decontamination methodologies for fresh produce: A review. Food Control 2013, 32, 418–427. [Google Scholar] [CrossRef]
- Purkiewicz, A.; Ciborska, J.; Tańska, M.; Narwojsz, A.; Starowicz, M.; Przybyłowicz, K.E.; Sawicki, T. The impact of the method extraction and different carrot variety on the carotenoid profile, total phenolic content and antioxidant properties of juices. Plants 2020, 9, 1759. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, J.; Skąpska, S.; Marszałek, K. Continuous High-pressure Cooling-Assisted Homogenization Process for Stabilization of Apple Juice. Food Bioprocess Technol. 2021, 14, 1101–1117. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Microbial, nutritional, and organoleptic quality of pomegranate juice following high-pressure homogenization and low-temperature pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef]
- Szczepańska, J.; Skąpska, S.; Połaska, M.; Marszałek, K. High pressure homogenization with a cooling circulating system: The effect on physiochemical and rheological properties, enzymes, and carotenoid profile of carrot juice. Food Chem. 2021, 370, 131023. [Google Scholar] [CrossRef]
- Diels, A.M.; Michiels, C.W. High-pressure homogenization as a non-thermal technique for the inactivation of microorganisms. Crit. Rev. Microbiol. 2006, 32, 201–216. [Google Scholar] [CrossRef]
- Zamora, A.; Guamis, B. Opportunities for ultra-high-pressure homogenisation (UHPH) for the food industry. Food Eng. Rev. 2015, 7, 130–142. [Google Scholar] [CrossRef]
- Pinho, C.R.; Franchi, M.A.; Tribst, A.A.; Cristianini, M. Effect of ultra high pressure homogenization on alkaline phosphatase and lactoperoxidase activity in raw skim milk. Procedia Food Sci. 2011, 1, 874–878. [Google Scholar] [CrossRef] [Green Version]
- Floury, J.; Bellettre, J.; Legrand, J.; Desrumaux, A. Analysis of a new type of high pressure homogeniser. A study of the flow pattern. Chem. Eng. Sci. 2004, 59, 843–853. [Google Scholar] [CrossRef]
- Donsì, F.; Esposito, L.; Lenza, E.; Senatore, B.; Ferrari, G. Production of shelf-stable annurca apple juice with pulp by high pressure homogenization. Int. J. Food Eng. 2009, 5, 12. [Google Scholar] [CrossRef]
- Stannard, C. Development and use of microbiological criteria for foods. Food Sci. Technol. Today 1997, 11, 137–177. [Google Scholar]
- Food Safety Authority of Ireland. Report on a Total Diet Study Carried out by the Food Safety Authority of Ireland in the Period 2012–2014; Monitoring & Surveillance Series, Chemical; Food Safety Authority of Ireland: Dublin, Ireland, 2016. [Google Scholar]
- Calligaris, S.; Foschia, M.; Bartolomeoli, I.; Maifreni, M.; Manzocco, L. Study on the applicability of high-pressure homogenization for the production of banana juices. LWT-Food Sci. Technol. 2012, 45, 117–121. [Google Scholar] [CrossRef]
- Tribst, A.A.L.; Franchi, M.A.; de Massaguer, P.R.; Cristianini, M. Quality of mango nectar processed by high-pressure homogenization with optimized heat treatment. J. Food Sci. 2011, 76, M106–M110. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Bi, J.; Cao, F.; Ding, Y.; Peng, J. Effects of high pressure homogenization on physical stability and carotenoid degradation kinetics of carrot beverage during storage. J. Food Eng. 2019, 263, 63–69. [Google Scholar] [CrossRef]
- AIJN. Code of Practice for Evaluation of Fruit and Vegetable Juices. 2001. Available online: https://aijn.eu/en/the-aijn-code-of-practice (accessed on 3 December 2021).
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- O’sullivan, L.; Ross, R.; Hill, C. Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 2002, 84, 593–604. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhunia, A.K.; Yao, Y. Impact of starch-based emulsions on the antibacterial efficacies of nisin and thymol in cantaloupe juice. Food Chem. 2017, 217, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Demir, N.; Acar, J.; Bahçeci, K.S. Effects of storage on quality of carrot juices produced with lactofermentation and acidification. Eur. Food Res. Technol. 2004, 218, 465–468. [Google Scholar] [CrossRef]
- Yu, L.J.; Rupasinghe, H.V. Effect of acidification on quality and shelf-life of carrot juice. Can. J. Plant Sci. 2012, 92, 1113–1120. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef] [Green Version]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Fukuda, T.; Tanaka, H.; Ihori, H.; Okazaki, K.; Shinano, T.; Fukumori, Y. Identification of important volatiles in fresh and processing carrot varieties: Using kuroda and flakee types. Food Sci. Technol. Res. 2013, 19, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Dolan, L.C.; Matulka, R.A.; Burdock, G.A. Naturally occurring food toxins. Toxins 2010, 2, 2289–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
| TMC Post Treat. | |||
|---|---|---|---|
| Pressure (MPa) | N° Passes | Inlet T° (°C) | Δ log CFU/mL |
| 0.1 | 1 | 25 | 0.03 ± 0.29 a |
| 150 | 1 | 25 | 1.04 ± 0.29 b |
| 150 | 3 | 25 | 1.95 ± 0.07 c |
| 150 | 5 | 25 | 2.21 ± 0.77 c |
| 0.1 | 1 | 50 | 0.50 ± 0.15 a |
| 150 | 1 | 50 | 1.67 ± 0.16 c |
| 150 | 3 | 50 | 2.08 ± 0.64 c |
| 150 | 5 | 50 | 2.38 ± 0.85 c |
| Storage at 4 °C | Storage at 10 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 9 | 12 | 0 | 1 | 2 | 5 | 7 | |
| Control | 5.1 ± 0.2 a | 4.6 ± 0.2 a | 6.7 ± 0.4 a | 7.7 ± 0.2 a | - | 5.1 ± 0.2 a | 5.7 ± 0.4 a | 6.3 ± 0.2 a | 9.0 ± 0.2 a | - |
| HPH | 3.6 ± 0.2 b | 3.9 ± 0.1 b | 3.5 ± 0.1 b | 5.6 ± 0.3 b | 7.2 ± 0.2 a | 3.6 ± 0.2 b | 3.9 ± 0.2 b | 4.7 ± 0.2 b | 6.4 ± 0.8 b | 9.1 ± 0.5 a |
| HPH + LBG2 | 3.5 ± 0.1 b | 3.7 ± 0.1 b | 3.8 ± 0.0 b | 3.7 ± 0.1 c | 4.4 ± 0.2 b | 3.5 ± 0.1 b | 3.9 ± 0.2 b | 3.7 ± 0.2 c | 3.8 ± 0.4 c | 3.9 ± 0.1 b |
| Storage at 10 °C | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 5 | 7 | |
| Control | 3.4 ± 0.1 a | 4.1 ± 0.1 a | 3.4 ± 0.1 a | 3.7 ± 0.1 a | - |
| HPH | 0.9 ± 0.1 c | 1.8 ± 0.2 b | 1.9 ± 0.1 b | 2.0 ± 0.1 b | 1.9 ± 0.2 a |
| HPH + LBG2 | 1.6 ± 0.1 b | 0.9 ± 0.2 c | 0.8 ± 0.1 c | 0.9 ± 0.1 c | 1.1 ± 0.1 b |
| Storage at 4 °C | Storage at 10 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 9 | 12 | 0 | 1 | 2 | 5 | 7 | |
| Control | 3.8 ± 0.3 a | 4.4 ± 0.2 a | 4.3 ± 0.5 a | 4.5 ± 0.1 a | - | 3.8 ± 0.3 a | 4.0 ± 0.1 a | 4.4 ± 0.2 a | 6.1 ± 0.1 a | - |
| HPH | 2.5 ± 0.2 b | 3.8 ± 0.2 b | 2.9 ± 0.2 b | 4.1 ± 0.2 ab | 4.5 ± 0.2 a | 2.5 ± 0.2 b | 3.0 ± 0.2 b | 3.7 ± 0.2 b | 3.9 ± 0.3 b | 3.5 ± 0.2 a |
| HPH + LBG2 | 2.5 ± 0.2 b | 3.1 ± 0.1 c | 3.3 ± 0.1 b | 3.8 ± 0.1 b | 3.9 ± 0.1 b | 2.5 ± 0.2 b | 2.9 ± 0.2 b | 3.5 ± 0.1 b | 3.7 ± 0.2 b | 3.6 ± 0.2 a |
| Days of Storage at 4 °C | Days of Storage at 10 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 9 | 12 | 0 | 1 | 2 | 5 | 7 | ||
| L* | Control | 50.6 ± 2.5 a | 48.1 ± 2.3 ab | 49.8 ± 0.5 a | 49.0 ± 1.6 a | 45.5 ± 0.2 a | 50.5 ± 2.5 a | 50.3 ± 1.3 | 49.6 ± 3.0 ab | 49.5 ± 0.1 a | - |
| HPH | 46.5 ± 0.8 b | 46.7 ± 0.6 a | 46.6 ± 2.2 b | 45.5 ± 0.3 b | 45.1 ± 0.3 a | 46.5 ± 0.8 b | 48.7 ± 0.6 | 47.9 ± 2.1 a | 44.8 ± 0.1 b | 47.7 ± 2.4 | |
| HPH + LBG2 | 47.9 ± 0.6 ab | 49.6 ± 0.5 b | 48.7 ± 0.5 ab | 47.8 ± 0.2 a | 49.4 ± 0.7 b | 47.9 ± 0.6 ab | 49.3 ± 1.2 | 51.5 ± 0.1 b | 44.7 ± 0.1 b | 45.9 ± 1.0 | |
| a* | Control | 14.8 ± 3.2 a | 16.7 ± 2.3 | 16.8 ± 0.4 a | 17.3 ± 1.2 a | 17.1 ± 0.5 a | 14.8 ± 3.2 a | 15.6 ± 1.4 a | 15.6 ± 2.4 | 16.7 ± 2.1 a | - |
| HPH | 17.2 ± 0.7 b | 16.7 ± 0.2 | 15.3 ± 1.9 a | 16.8 ± 0.1 a | 16.1 ± 0.4 b | 17.2 ± 0.7 b | 13.9 ± 1.0 a | 14.7 ± 2.6 | 17.9 ± 0.1 a | 18.4 ± 0.2 a | |
| HPH + LBG2 | 20.1 ± 0.4 c | 17.7 ± 0.8 | 21.3 ± 0.4 b | 21.9 ± 0.1 b | 21.2 ± 1.0 c | 20.1 ± 0.4 c | 18.6 ± 2.6 b | 13.5 ± 0.1 | 22.7 ± 0.1b | 21.6 ± 0.5 b | |
| b* | Control | 38.8 ± 3.0 a | 37.3 ± 1.1 a | 39.2 ± 0.1 a | 37.2 ± 1.0 a | 33.7 ± 1.1 a | 38.8 ± 3.0 a | 38.9 ± 1.3 | 38.9 ± 1.2 a | 39.2 ± 2.1 a | - |
| HPH | 43.7 ± 2.4 b | 42.5 ± 0.9 b | 40.7 ± 0.6 b | 40.0 ± 0.6 b | 39.2 ± 0.5 b | 43.7 ± 2.4 b | 41.8 ± 1.4 | 41.6 ± 2.7 a | 41.9 ± 0.1 a | 38.8 ± 3.4 | |
| HPH + LBG2 | 47.9 ± 0.9 c | 43.2 ± 1.4 b | 43.4 ± 0.7 c | 43.1 ± 0.5 c | 43.5 ± 0.6 c | 47.9 ± 0.9 c | 43.1 ± 3.4 | 34.9 ± 0.0 b | 43.1 ± 0.1 b | 40.9 ± 0.5 | |
| Storage at 4 °C | Storage at 10 °C | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (Days) | 0 | 6 | 9 | 12 | 2 | 5 | 7 | ||||||||
| Molecules | CTRL 1 | HPH | HPH + LBG2 | CTRL 1 | HPH | HPH + LBG2 | HPH | HPH + LBG2 | HPH + LBG2 | CTRL | HPH | HPH + LBG2 | HPH | HPH + LBG2 | HPH + LBG2 |
| Aldehydes | 0.4 | 1.0 | 0.9 | 0.9 | 0.9 | 1.7 | 1.0 | 1.5 | 1.3 | 0.8 | 0.7 | 1.3 | 0.8 | 1.4 | 1.7 |
| Ketones | 11.0 | 5.5 | 14.8 | 5.4 | 2.0 | 6.1 | 2.4 | 3.8 | 4.8 | 11.0 | 6.8 | 6.6 | 0.8 | 4.9 | 2.9 |
| Alcohols | 0.7 | 0.7 | 2.1 | 2.3 | 2.6 | 4.1 | 1.9 | 4.9 | 5.0 | 1.7 | 0.6 | 4.3 | 0.9 | 4.1 | 5.5 |
| Acids | 0.0 | 0.3 | 0.4 | 0.0 | 0.2 | 0.4 | 0.2 | 0.3 | 0.3 | 0.1 | 0.1 | 0.3 | 0.0 | 0.2 | 0.4 |
| Esters | 9.8 | 5.3 | 5.5 | 7.3 | 4.3 | 4.2 | 4.4 | 4.5 | 4.9 | 7.6 | 4.1 | 5.1 | 5.0 | 4.9 | 4.7 |
| Terpenes and Terpenoids | 73.3 | 84.5 | 71.7 | 79.4 | 84.7 | 80.6 | 85.0 | 81.4 | 79.5 | 75.0 | 84.9 | 74.4 | 89.6 | 80.6 | 80.9 |
| Others | 2.4 | 1.3 | 1.0 | 1.8 | 1.6 | 1.1 | 1.7 | 1.1 | 1.2 | 1.9 | 1.0 | 1.1 | 1.1 | 1.1 | 1.3 |
| Total area 3 | 17,500 | 32,800 | 17,600 | 21,400 | 22,500 | 28,000 | 21,700 | 26,300 | 23,000 | 18,600 | 26,800 | 22,400 | 18,900 | 22,600 | 24,400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottardi, D.; Siroli, L.; Braschi, G.; Rossi, S.; Ferioli, F.; Vannini, L.; Patrignani, F.; Lanciotti, R. High-Pressure Homogenization and Biocontrol Agent as Innovative Approaches Increase Shelf Life and Functionality of Carrot Juice. Foods 2021, 10, 2998. https://doi.org/10.3390/foods10122998
Gottardi D, Siroli L, Braschi G, Rossi S, Ferioli F, Vannini L, Patrignani F, Lanciotti R. High-Pressure Homogenization and Biocontrol Agent as Innovative Approaches Increase Shelf Life and Functionality of Carrot Juice. Foods. 2021; 10(12):2998. https://doi.org/10.3390/foods10122998
Chicago/Turabian StyleGottardi, Davide, Lorenzo Siroli, Giacomo Braschi, Samantha Rossi, Federico Ferioli, Lucia Vannini, Francesca Patrignani, and Rosalba Lanciotti. 2021. "High-Pressure Homogenization and Biocontrol Agent as Innovative Approaches Increase Shelf Life and Functionality of Carrot Juice" Foods 10, no. 12: 2998. https://doi.org/10.3390/foods10122998
APA StyleGottardi, D., Siroli, L., Braschi, G., Rossi, S., Ferioli, F., Vannini, L., Patrignani, F., & Lanciotti, R. (2021). High-Pressure Homogenization and Biocontrol Agent as Innovative Approaches Increase Shelf Life and Functionality of Carrot Juice. Foods, 10(12), 2998. https://doi.org/10.3390/foods10122998







