High–Pressure Processing vs. Thermal Treatment: Effect on the Stability of Polyphenols in Strawberry and Apple Products
Abstract
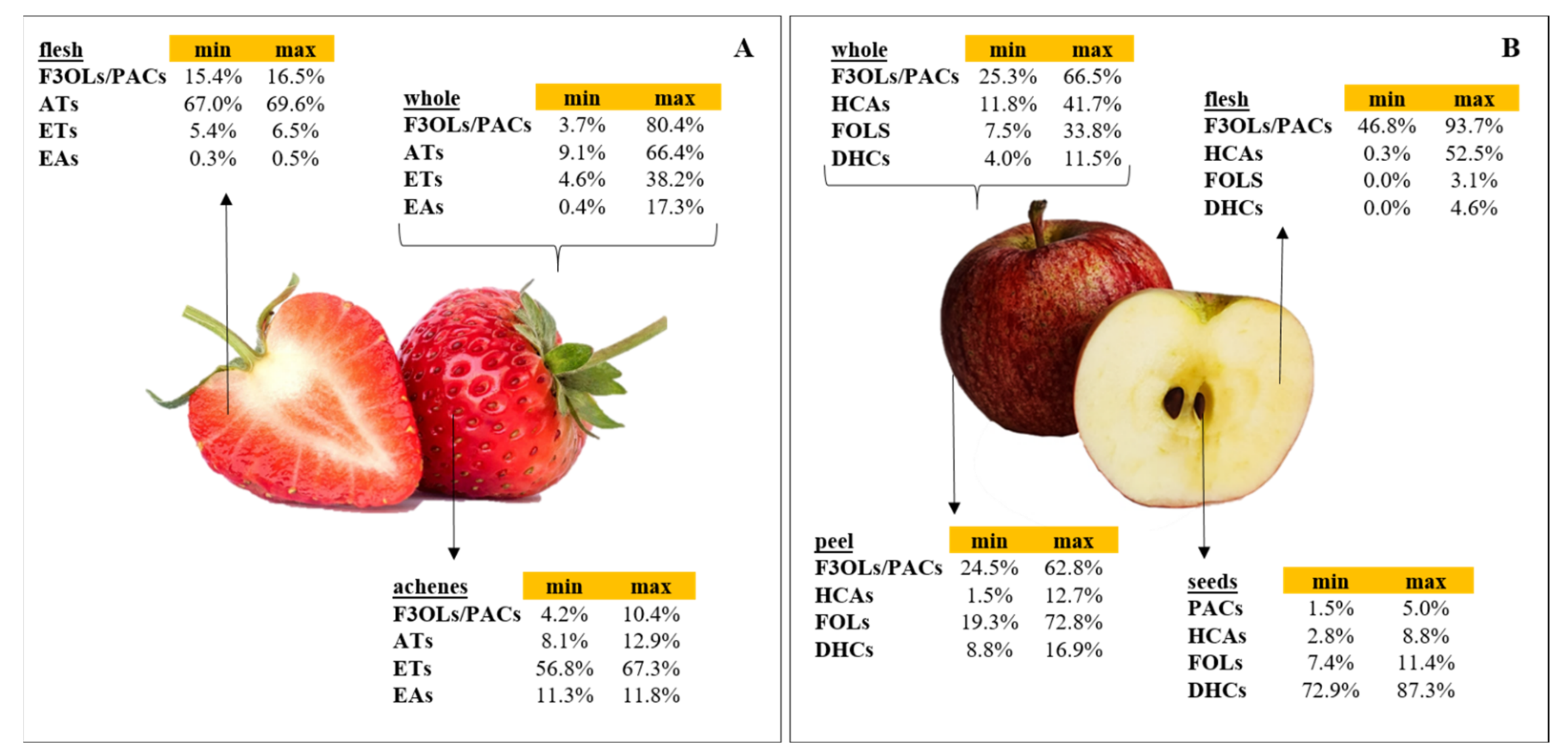
:1. Introduction
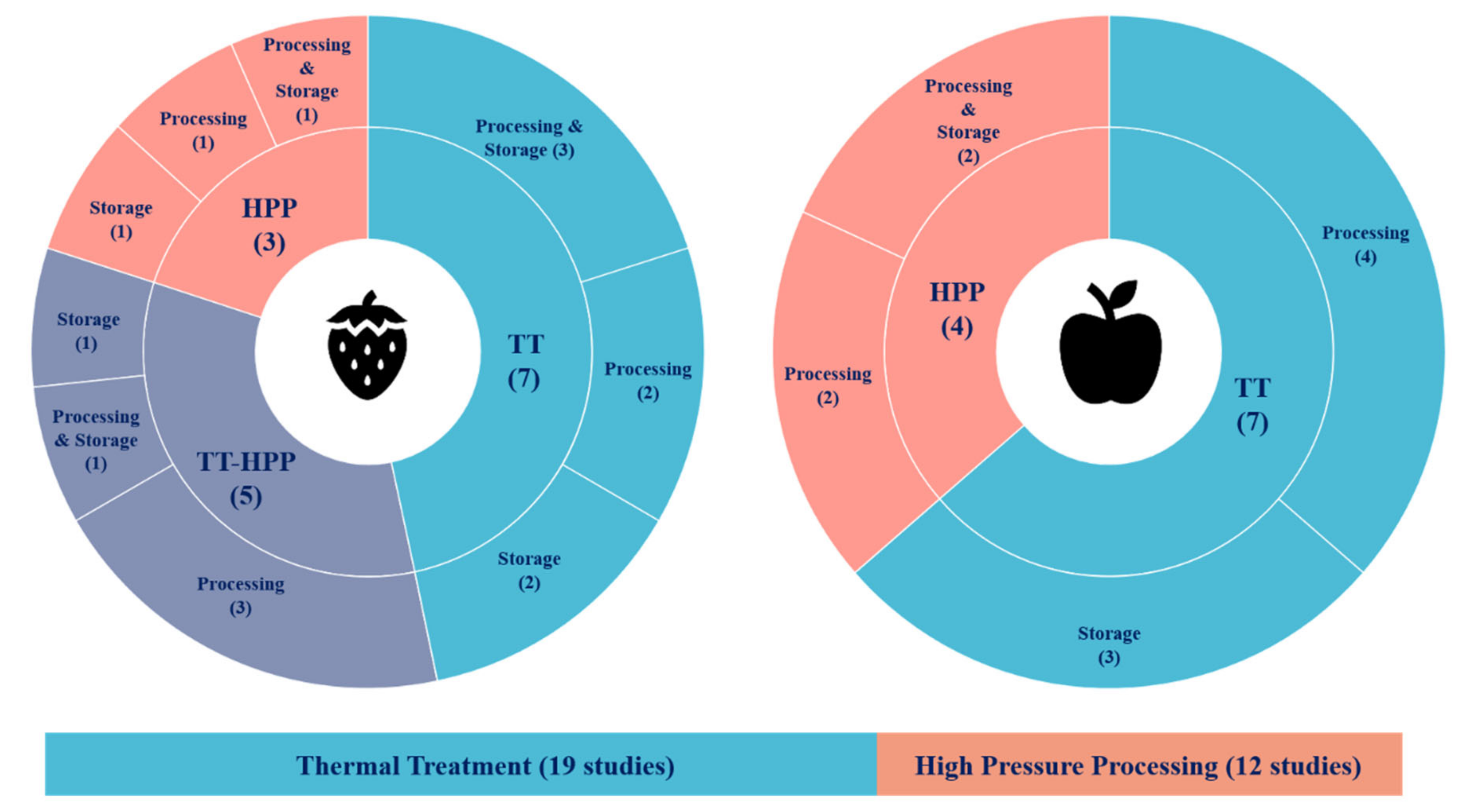
2. Overview of the Studies Included in the Review
- Strawberry was the most studied fruit with 15 articles, whereas 11 were examined for apple products;
- A total of 19 articles reported the effects of thermal treatment and 12 for high-pressure processing. The reviewed studies examined 199 different trials at different processing and storage conditions (Figure 2).
3. Effects of Processing and Storage Conditions on the Stability of Polyphenols
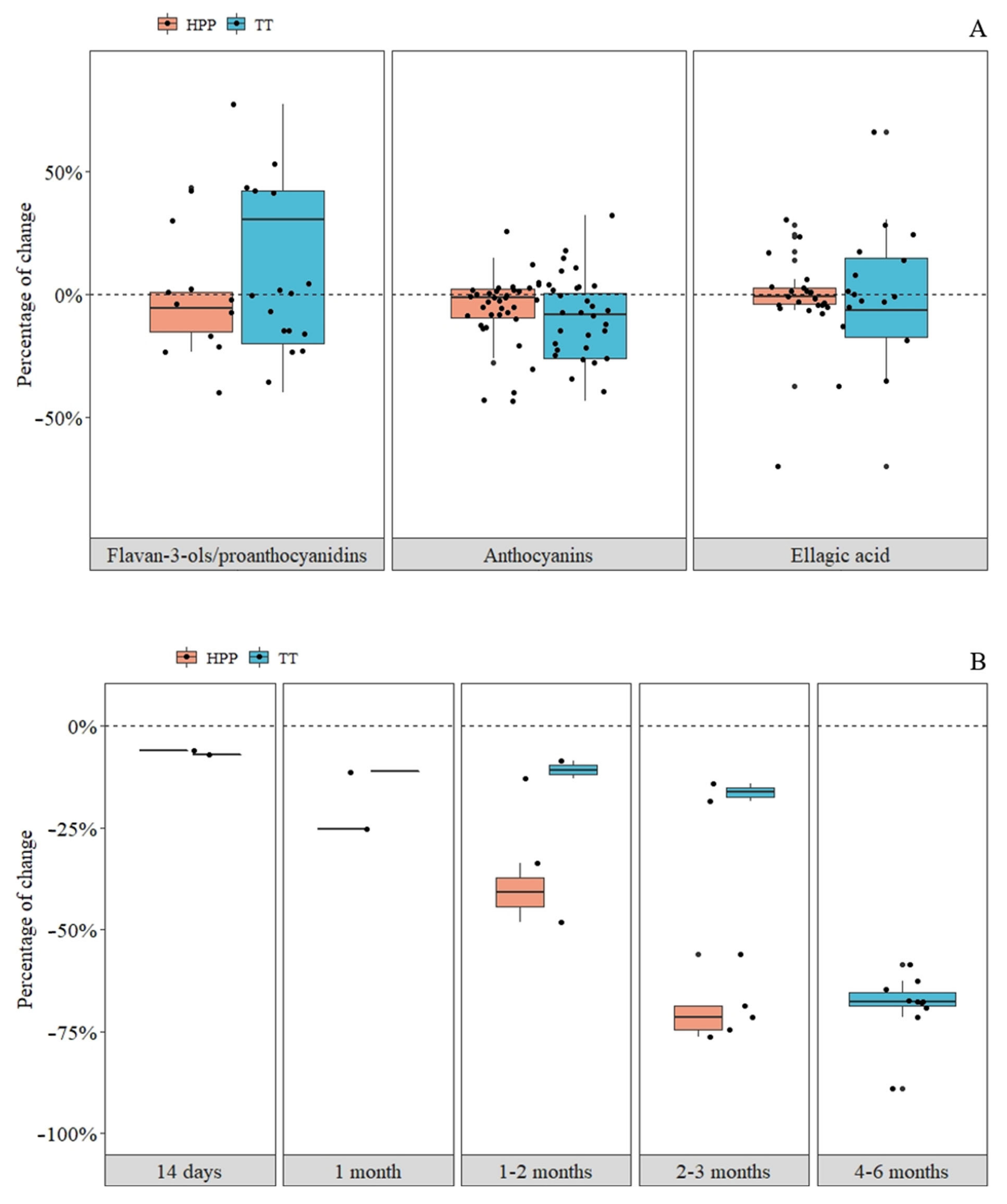
3.1. Effects on the Stability of Proanthocyanidins in Strawberry and Apple Products
3.2. Effects on the Stability of Anthocyanins in Strawberry Products
3.3. Effects on the Stability of Ellagic Acid in Strawberry Products
3.4. Effects on the Stability of Flavonols in Apple Products
3.5. Effects on the Stability of Dihydrochalcones in Apple Products
3.6. Effects on the Stability of Hydroxycinnamic Acids in Apple Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomás-Barberán, F.A.; Espín, J.C. Effect of Food Structure and Processing on (Poly) phenol—Gut Microbiota Interactions and the Effects on Human Health. Annu. Rev. Food Sci. Technol. 2019, 10, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guan, L.; Cao, Y.; Li, C.; Chen, J.; Li, J.; Liu, G.; Li, S.; Wu, B. Diversity of polyphenols in the peel of apple (Malus sp.) germplasm from different countries of origin. Int. J. Food Sci. Technol. 2016, 51, 222–230. [Google Scholar] [CrossRef]
- Aaby, K.; Skrede, G.; Wrolstad, R.E. Phenolic Composition and Antioxidant Activities in Flesh and Achenes of Strawberries (Fragaria ananassa). J. Agric. Food Chem. 2005, 53, 4032–4040. [Google Scholar] [CrossRef]
- Buendía, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F.A. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Bouzerzour, K.; Ginies, C.; Renard, C.M.G.C.; Regis, S.; Ple, Y. Phenolic and polysaccharidic composition of applesauce is close to that of apple flesh. J. Food Compos. Anal. 2011, 24, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Fan, M.; Ran, J.; Zhang, T.; Sun, H.; Dong, M.; Zhang, Z.; Zheng, H. Variation in phenolic compounds and antioxidant activity in apple seeds of seven cultivars. Saudi J. Biol. Sci. 2016, 23, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L.; García-Villalba, R.; Tomás-Barberán, F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Compos. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- Akagić, A.; Vranac, A.; Gaši, F.; Drkenda, P.; Spaho, N.; Žuljević, O.; Kurtović, M.; Musić, O.; Murtić, S.; Hudina, M. Sugars, acids and polyphenols profile of commercial and traditional apple cultivars for processing. Acta Agric. Slov. 2019, 113, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. (Amsterdam) 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; De Ancos, B. Effect of high-pressure processing on flavonoids, hydroxycinnamic acids, dihydrochalcones and antioxidant activity of apple ‘Golden Delicious’ from different geographical origin. Innov. Food Sci. Emerg. Technol. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Masi, E.; Taiti, C.; Vignolini, P.; William, A.; Giordani, E.; Heimler, D.; Romani, A.; Mancuso, S. Polyphenols and aromatic volatile compounds in biodynamic and conventional ‘Golden Delicious’ apples (Malus domestica Bork). Eur. Food Res. Technol. 2017, 243, 1519–1531. [Google Scholar] [CrossRef]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Sanchez-Siles, L.M.; Michel, F.; Román, S.; Bernal, M.J.; Philipsen, B.; Haro, J.F.; Bodenstab, S.; Siegrist, M. The Food Naturalness Index (FNI): An integrative tool to measure the degree of food naturalness. Trends Food Sci. Technol. 2019, 91, 681–690. [Google Scholar] [CrossRef]
- Debelo, H.; Li, M.; Ferruzzi, M.G. Processing influences on food polyphenol profiles and biological activity. Curr. Opin. Food Sci. 2020, 32, 90–102. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; Charoux, C.M.G.; Cullen, P.J.; Tiwari, B.K. Chemical Modifications of Lipids and Proteins by Nonthermal Food Processing Technologies. J. Agric. Food Chem. 2018, 66, 5041–5054. [Google Scholar] [CrossRef]
- Misra, N.N.; Koubaa, M.; Roohinejad, S.; Juliano, P.; Alpas, H.; Inácio, R.S.; Saraiva, J.A.; Barba, F.J. Landmarks in the historical development of twenty fi rst century food processing technologies. Food Res. Int. 2017, 97, 318–339. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A. Thermal processing food-related toxicants: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3579–3596. [Google Scholar] [CrossRef]
- Van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef]
- Fam, S.N. High-Pressure Processing in Food. Biointerface Res. Apllied Chem. 2020, 11, 11553–11561. [Google Scholar]
- Wael, E.; Johari, E.; Yus, Y.; Rosnah, S.; Anvarjon, A. High Pressure Processing Technology and Equipment Evolution: A Review. J. Eng. Sci. Technol. Rev. 2015, 8, 75–83. [Google Scholar]
- Martinez-Monteagudo, S.I.; Saldan, M.D.A. Chemical Reactions in Food Systems at High Hydrostatic Pressure. Food Eng. Rev. 2014, 6, 105–127. [Google Scholar] [CrossRef]
- Makita, T. Application of high pressure and thermophysical properties of water to biotechnology. Fluid Phase Equilib. 1992, 76, 87–95. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of high pressure and thermal processing on shelf life and quality of strawberry purée and juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- Benito-Román, O.; Sanz, M.T.; Illera, A.E.; Melgosa, R.; Beltrán, S. Polyphenol oxidase (PPO) and pectin methylesterase (PME) inactivation by high pressure carbon dioxide (HPCD) and its applicability to liquid and solid natural products. Catal. Today. 2019, 346, 112–120. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [Green Version]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116. [Google Scholar] [CrossRef]
- Oliveira, A.; Coelho, M.; Alexandre, E.M.C.; Almeida, D.P.F.; Pintado, M. Long-Term Frozen Storage and Pasteurization Effects on Strawberry Polyphenols Content. Food Bioprocess Technol. 2015, 8, 1838–1844. [Google Scholar] [CrossRef]
- Oliveira, A.; Almeida, D.P.F.; Pintado, M. Changes in Phenolic Compounds During Storage of Pasteurized Strawberry. Food Bioprocess Technol. 2014, 7, 1840–1846. [Google Scholar] [CrossRef]
- Garzoli, S.; Cairone, F.; Carradori, S.; Mocan, A.; Menghini, L.; Paolicelli, P.; Ak, G.; Zengin, G.; Cesa, S. Effects of Processing on Polyphenolic and Volatile Composition and Fruit Quality of Clery Strawberries. Antioxidants 2020, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, M.A.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; García-Parrilla, M.C. Effects of the strawberry (fragaria ananassa) purée elaboration process on non-anthocyanin phenolic composition and antioxidant activity. Food Chem. 2014, 164, 104–112. [Google Scholar] [CrossRef]
- Howard, L.R.; Brownmiller, C.; Prior, R.L. Improved color and anthocyanin retention in strawberry puree by oxygen exclusion. J. Berry Res. 2014, 4, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, M.; Korhummel, S.; Kammerer, D.R.; Carle, R. Thermal inactivation of strawberry polyphenoloxidase and its impact on anthocyanin and color retention in strawberry (Fragaria x ananassa Duch) pure. Eur. Food Res. Technol. 2012, 235, 1171–1180. [Google Scholar] [CrossRef]
- Teleszko, M.; Nowicka, P.; Wojdyło, A. Effect of cultivar and storage temperature on identification and stability of polyphenols in strawberry cloudy juices. J. Food Compos. Anal. 2016, 54, 10–19. [Google Scholar] [CrossRef]
- Bodelón, O.G.; Avizcuri, J.; Dizy, M. Pressurization and cold storage of strawberry purée: Colour, anthocyanins, ascorbic acid and pectin methylesterase. LWT Food Sci. Technol. 2013, 52, 123–130. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, L.; Skąpska, S. The application of high pressure–mild temperature processing for prolonging the shelf-life of strawberry purée. High Press. Res. 2016, 36, 220–234. [Google Scholar] [CrossRef]
- Stübler, A.S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of pilot-scale processing (thermal, PEF, HPP) on the stability and bioaccessibility of polyphenols and proteins in mixed protein- and polyphenol-rich juice systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innov. Food Sci. Emerg. Technol. 2015, 27, 48–56. [Google Scholar] [CrossRef]
- Terefe, N.S.; Kleintschek, T.; Gamage, T.; Fanning, K.J.; Netzel, G.; Versteeg, C.; Netzel, M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov. Food Sci. Emerg. Technol. 2013, 19, 57–65. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. High pressure processing and thermal pasteurization of strawberry purée: Quality parameters and shelf life evaluation during cold storage. J. Food Sci. Technol. 2017, 54, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef]
- Cao, X.; Bi, X.; Huang, W.; Wu, J.; Hu, X.; Liao, X. Changes of quality of high hydrostatic pressure processed cloudy and clear strawberry juices during storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 181–190. [Google Scholar] [CrossRef]
- Kim, A.N.; Lee, K.Y.; Rahman, M.S.; Kim, H.J.; Kerr, W.L.; Choi, S.G. Thermal treatment of apple puree under oxygen-free condition: Effect on phenolic compounds, ascorbic acid, antioxidant activities, color, and enzyme activities. Food Biosci. 2021, 39, 100802. [Google Scholar] [CrossRef]
- Murtaza, A.; Iqbal, A.; Marszałek, K.; Iqbal, M.A.; Ali, S.W.; Xu, X.; Pan, S.; Hu, W. Enzymatic, phyto-, and physicochemical evaluation of apple juice under high-pressure carbon dioxide and thermal processing. Foods 2020, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Alongi, M.; Verardo, G.; Gorassini, A.; Anese, M. Effect of pasteurization on in vitro α -glucosidase inhibitory activity of apple juice. LWT Food Sci. Technol. 2018, 98, 366–371. [Google Scholar] [CrossRef]
- Knebel, T.; Braun, P.; Dietrich, H. Degradation kinetics of anthocyanins and polyphenols during storage of red apple juice produced from red-fleshed apples. Eur. Food Res. Technol. 2018, 244, 1741–1750. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, L.; Yang, Y.; Gou, X.; Niu, P.; Guo, Y. Changes in the physicochemical properties, aromas and polyphenols of not from concentrate (NFC) apple juice during production. CyTA J. Food 2018, 16, 755–764. [Google Scholar] [CrossRef] [Green Version]
- De Paepe, D.; Valkenborg, D.; Coudijzer, K.; Noten, B.; Servaes, K.; De Loose, M.; Voorspoels, S.; Diels, L.; Van Droogenbroeck, B. Thermal degradation of cloudy apple juice phenolic constituents. Food Chem. 2014, 162, 176–185. [Google Scholar] [CrossRef]
- Szczepańska, J.; Pinto, C.A.; Skąpska, S.; Saraiva, J.A.; Marszałek, K. Effect of static and multi-pulsed high pressure processing on the rheological properties, microbial and physicochemical quality, and antioxidant potential of apple juice during refrigerated storage. LWT Food Sci. Technol. 2021, 150, 112038. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Balderas, C.; Sánchez-Moreno, C.; De Ancos, B. Impact of an in vitro dynamic gastrointestinal digestion on phenolic compounds and antioxidant capacity of apple treated by high-pressure processing. Innov. Food Sci. Emerg. Technol. 2020, 66, 102486. [Google Scholar] [CrossRef]
- Marszalek, K.; Szczepańska, J.; Starzonek, S.; Wozniak, L.; Trych, U.; Skapska, S.; Rzoska, S.; Saraiva, J.A.; Lorenzo, J.M.; Barba, F.J. Enzyme inactivation and evaluation of physicochemical properties, sugar and phenolic profile changes in cloudy apple juices after high pressure processing, and subsequent refrigerated storage. Food Process. Eng. 2019, 42, e13034. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Bueno, J.M.; Sáez-Plaza, P.; Ramos-Escudero, F.; Jiménez, A.M.; Fett, R.; Asuero, A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Motilva, M.; Bars-cortina, D.; Sakhawat, A.; Pi, C. Chemopreventive effects of anthocyanins on colorectal and breast cancer: A review. Semin. Cancer Biol. 2021, 6, 1203. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Skrede, G.; Wrolstad, R.E.; Lea, P.; Enersen, G. Color Stability of Strawberry and Blackcurrant Syrups. J. Food Sci. 1992, 57. [Google Scholar] [CrossRef]
- Nikkhah, E.; Khaiamy, M.; Heidary, R.; Azar, A.S. The effect of ascorbic acid and H2O2 treatment on the stability of anthocyanin pigments in berries. Turk J. Biol. 2010, 34, 47–53. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Hawken, P.; Talcott, S.T. Phytochemical, antioxidant and pigment stability of ac (Euterpe oleracea Mart) as affected by clarification, ascorbic acid fortification and storage. Food Res. Int. 2007, 40, 620–628. [Google Scholar] [CrossRef]
- Bakkalbasi, E.; Mentes, O.; Artik, N. Food ellagitannins-occurrence, effects of processing and storage. Crit. Rev. Food Sci. Nutr. 2009, 49, 283–298. [Google Scholar] [CrossRef]
- Williner, M.R.; Pirovani, M.E.; Güemes, D.R. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003, 83, 842–845. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Al-Harbi, S.A.; Abdulrahman, A.O.; Zamzami, M.A.; Khan, M.I. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front. Nutr. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Ellagitannins as active constituents of medicinal plants. Planta Med. 1989, 55, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Truchado, P.; Larrosa, M.; García-Conesa, M.T.; Cerdá, B.; Vidal-Guevara, M.L.; Tomás-Barberán, F.A.; Espín, J.C. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J. Agric. Food Chem. 2012, 60, 5749–5754. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Polish J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Kamiloglu, S. Effect of different freezing methods on the bioaccessibility of strawberry polyphenols. Int. J. Food Sci. Technol. 2019, 54, 2652–2660. [Google Scholar] [CrossRef]
- Herrmann, K. Flavonols and flavones in food plants: A review. Int. J. Food Sci. Technol. 1976, 11, 433–448. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Pinta, D.; Majki, T.; Bekvalac, K.; Or, D.; Mimica-duki, N. Antioxidant and anti-in fl ammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Panthi, V.K.; Kaushal, S.; Adhikari, B.; Basnet, N.; Chaudary, D.; Parajuli, R.; Pokhrel, P. A Review of Quercetin: Anticancer Activity. Int. J. Innov. Res. Rev. 2020, 4, 1–7. [Google Scholar]
- Islam, S.; Quispe, C.; Hossain, R.; Islam, M.T. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Alongi, M.; Verardo, G.; Gorassini, A.; Lemos, M.A.; Hungerford, G.; Cortella, G.; Anese, M. Phenolic content and potential bioactivity of apple juice as affected by thermal and ultrasound pasteurization. Food Funct. 2019, 10, 7366–7377. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.; Garcia-viguera, C.; Nieto, J.L.; Ferreres, F. Dihydrochalcones from apple juices and jams. Food Chem. 1993, 46, 33–36. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The bioavailability, extraction, biosynthesis and distribution of natural dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Viet, M.H.; Chen, C.Y.; Hu, C.K.; Chen, Y.R.; Li, M.S. Discovery of dihydrochalcone as potential lead for Alzheimer’s disease: In Silico and in Vitro study. PLoS ONE 2013, 8, e79151. [Google Scholar] [CrossRef] [Green Version]
- Raphaelli, C.d.O.; Azevedo, J.G.; Pereira, E.d.S.; Vinholes, J.R.; Camargo, T.M.; Hoffmann, J.F.; Ribeiro, J.A.; Vizzotto, M.; Rombaldi, C.V.; Wink, M.R.; et al. Phenolic-rich apple extracts have photoprotective and anti-cancer effect in dermal cells. Phytomedicine Plus 2021, 1, 100112. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.; Clifford, M. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wolniak, M.; Wojdylo, A.; Wawer, I. Influence of apple puree preparation and storage on polyphenol contents and antioxidant activity. Food Chem. 2008, 107, 1473–1484. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic Acid Antioxidants: An Electrochemical Overview. Biomed. Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Sova, M. Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Vinholes, J.; Silva, B.M.; Silva, L.R. Hydroxycinnamic acids (HCAS): Structure, Biological properties and health effects. Adv. Med. Biol. 2019, 88, 1–33. [Google Scholar]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. Soc. Chem. Ind. 2019. [Google Scholar] [CrossRef]
- Rocha, L.D.; Monteiro, M.C.; Teodoro, A.J. Anticancer Properties of Hydroxycinnamic Acids -A Review. Cancer Clin. Oncol. 2012, 1, 109–121. [Google Scholar] [CrossRef]

| Material | Treatment: Conditions | Storage | Impact of Processing Conditions on Polyphenols a | Impact of Storage Conditions on Polyphenols b | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Strawberry fresh | TT: 90 °C/5 min | 360 days −20 °C | F3OLs/PACs: ↑30% CA, ↑73% EGC; ↑45% EC ATs: ↓16% pel-3-glu; ↓5% pel-3-rut; ≈cya-3-glu EA: ↑143% | F3OLs/PACs: ↓19% CA, ↓39% EGC ATs: ↓pel-3-glu; ↓pel-3-rut; ↑cya-3-glu EA: ↓65% | ↑F3OLs/PACs: cleavage → release of dimers and monomers; release from cellular tissue ↓ATs: cleavage of covalent bonds, polymerization, and derivatization; ↑pH in the food matrix; enzymatic oxidation after pasteurization and during storage ↑EA: ETs hydrolysis | [31] d |
| Strawberry fresh | TT: 90 °C/5 min | 90 days 23 °C | F3OLs/PACs: ↑34% CA; ↑134% EC; ↑119% EGCG; ↑30% T.F3OLs/PACs ATs: ↓30% cya-3-glu; ↓35% pel-3-glu; ≈ pel-3-rut; ↓30% T.ATs EA: ↑66% | F3OLs/PACs: ↓42% CA; ↓62% EGCG; ↓67% EC ATs: ↓87% cya-3-glu, ↓97% pel-3-glu; ↓92%pel-3-rut EA: ↓32% | TT ↑F3OLs/PACs: PACs cleavage → release of dimers and monomers; release from cellular tissue ↓ATs: cleavage of covalent bonds, polymerization, and derivatization ↑EA: release from cell walls; hydrolysis from ETs to EA Storage ↓F3OLs/PACs, ↓ATs and ↓EA: oxidation; non-ezymatic and enzymatic oxidation | [32] |
| Strawberry puree | TT1 (SB): 85 °C/3 min TT2 (P): 85 °C/3 min | No | SB: F3OLs/PACs: from ↑42% to↓16% ATs: ↓34–43% P: F3OLs/PACs: from ↑53% to ↓35% ATs: ↑2–18% | - | ↓F3OLs/PACs: TT conditions degraded heat-labile flavan-3-ols ↓ATs: SB may influence the ATs stability | [33] |
| Strawberry puree (2 yearsharvest ) | TT: 90 °C/2 min | No | Puree with seeds EA: ↓8% (2011 harvest), ↓13% (2012 harvest) Puree without seeds EA: ↓35% (2011 harvest) ↓19% EA (2012 harvest) | - | ↓EA: oxidation by membrane breakage | [34] |
| Strawberry puree | TT: 100 °C/10 min | 8 weeks 25 °C | - | ATs: ↓80–88% pel-3-glu (8 weeks); ↓53–74% pel-3-rut (2 weeks); ↓63–78% pel-3-mal-glu (2 weeks); ↓70–86% pel-3-ace-glu (2 weeks); ↓90–93% T.ATs (8 weeks) | ↓ATs: oxidation by PPO; formation of dark condensation products | [35] |
| Strawberry puree (2 varieties) | TT1 (SB): 100 °C/3 min TT2 (P): 60, 75, 90 °C/3 min | 28 days 20 °C | Ats—Elsanta var.: from ≈ to ↓5% (P60; P75); ↓10–12% (SB; P90) ATs—Everest var.: ≈ (P90); ↓8% (SB, P60, P75) | Elsanta var.: ↓62–65% T.ATs (all treatments) Everest var.: ↓64–73% T.ATs (all treatments) | SB and P ↓ATs: cleavage of covalent bonds, polymerization, and derivatization Storage ↓ATs: PPO partial reactivation during storage → oxidation | [36] |
| Strawberry juice (7 varieties) | TT: 90 °C/2 min | 6 months at 4 and 20 °C | - | 4 °C: F3OLs/PACs: from↓0.3% to ↑27% polymeric PACs ATs: ↓69% pel-3-glu; ↓73% pel-3-mal-lglu; ↓68% cya-3-glu; ↓56% cya-3-mal-glu; ↓59–89% T.ATs 20 °C: F3OLs/PACs: from ↓10% to ↑11% polymeric PACs ATs: ↓97% pel-3-glu; ↓99% pel-3-malonylglu; ↓98% cya-3-glu; ↓85% cya-3-mal-glu; ↓94–99% T.ATs | ↑F3OLs/PACs: protective effect of colloidal suspensions ↓F3OLs/PACs and ↓ATs: cleavage of covalent bonds, polymerization, and derivatization; enzymatic oxidation after pasteurization and during storage | [37] |
| Strawberry puree | HPP: 100–400 MPa /15 min at 20 and 50 °C | No | ATs: ≈ pel-3-glu; ≈cya-3-glu; ≈pel-3-rut (all HPP); from ≈ to ↑15% T.ATs | - | ≈ ATs: Sufficient enzyme inactivation | [38] |
| Strawberry puree (2 years harvest) | HPP: 300 and 600 MPa/ 15 min at 50 °C | 28 weeks 6 °C | ATs - 300 MPa harvest 2011: ↓12% cy-3-glu; ↓13% pel-3-glu; ↓36% pel-3-rut; ↓15% T.ATs ATs - 600 MPa harvest 2012: ↓22% cy-3-glu; ↓21% pel-3-glu; ↓10% pel-3-rut; ↓21% T.ATs | ATs–300 MPa harvest 2011: 86 days half-life ATs–600 MPa harvest 2012: 62 days half-life | ↓ATs: residual PPO activity → oxidation | [39] |
| Strawberry pure | TT: 72 °C/1 min HPP: 600 MPa/1 min | No | TT F3OLs/PACs: ↑122% CA; ↑33% proanthocyanidin B1; ↑78% T.F3OLs/PACs ATs: ↑40% cya-3-O-glu; ↑26% pel-3-O-glu; ↑22% pel-3-O-rut; ↑34% pel-3-O-mal-glu; ↑39% pel-3-O-acetylglu; ↑32% T.ATs EA: ↑8% HPP F3OLs/PACs: ↑68% CA; ↑19% proanthocyanidin B1; ↑43% T.F3OLs/PACs ATs: ↑12% cya-3-O-glu; ↑8% pel-3-O-glu; ↑10% pel-3-O-rut; ↑13% pel-3-O-mal-glu; ↑12% pel-3-O-ace-glu; ↑11% T.ATs EA: ≈ | - | TT ↑F3OLs/PACs: cleavage → release of dimers and monomers ↑ATs: higher extraction from cell matrix favored by TT HPP: ↑F3OLs/PACs: release from the disrupted cell walls | [40] |
| Strawberry puree | TT: 90 °C for 15 min HPP: 300 and 500 MPa/ 1, 5, 15 min at 0 °C | No | TT ATs: ↓44% cya-3-glu; ↓43% pel-3-glu; ↓49% pel-3-rut; ↓44% T.ATs EA: ↑30.5% EA HPP – 0 °C (all HPP conditions) ATs: ↓5% cya-3-glu; ↓7% pel-3-glu; ↓15% pel-3-rut; ↓7% T. ATs EA: ≈ EA (300 and 600 MPa) HPP + 50 °C ATs: ↓14% cya-3-glu; ↓13% pel-3-glu; ↓31% pel-3-rut; ↓14% T.ATs EA: ↑28.4% (300 MPa); ↑15.5% (600 MPa) | - | TT ↓ATs: cleavage of covalent bonds, polymerization, and derivatization ↑EA: release from the achenes favored by TT HPP – 0 °C ↓ATs: insufficient enzyme inactivation (PPO and POD) → oxidation HPP + 50 °C ↓ATs: formation of colorless chalcones ↑EA: release from ETs | [41] |
| Strawberry Puree c (3 varieties) | TT: 88 °C/2 min HPP: 600 MPa/5 min at 20 °C | 3 months 4 °C | TT Camarosa var. ATs: ↓16% cya-3-glu; ↓23% pel-3-glu; ↓26% pel-3-rut; ↓22% T.ATs Rubygem var. ATs: ↓42% cya-3-glu; ↓24% pel-3-glu; ↓29% pel-3-rut; ↓25% T.ATs Festival ATs: ↓27% cya-3-glu; ↓26% pel-3-glu; ↓26% pel-3-rut; ↓26% T.ATs HPP Camarosa var. ATs: ↓22% cya-3-glu; ↓26% pel-3-glu; ↓28% pel-3-rut; ↓26% T.ATs Rubygem var. ATs: ↓42% cya-3-glu; ↓27% pel-3-glu; ↓32% pel-3-rut; ↓28% T.ATs Festival ATs: ↓17% cya-3-glu; ↓20% pel-3-glu; ↓18% pel-3-rut; ↓20% T.ATs | TT Camarosa var. ATs: ↓66% cya-3-glu; ↓69% pel-3-glu; ↓60% pel-3-rut; ↓68% T.ATs Rubygem var. ATs: ↓52% cya-3-glu; ↓69% pel-3-glu; ↓59% pel-3-rut; ↓68% T.ATs Festival ATs: ↓59% cya-3-glu; ↓65% pel-3-glu; ↓59% pel-3-rut; ↓65% T.ATs HPP Camarosa var. ATs: ↓69% cya-3-glu; ↓72% pel-3-glu; ↓69% pel-3-rut; ↓72% T.ATs Rubygem var. ATs: ↓62% cya-3-glu; ↓75% pel-3-glu; ↓71% pel-3-rut; ↓75% T.ATs Festival ATs: ↓73% cya-3-glu; ↓77% pel-3-glu; ↓76% pel-3-rut; ↓76% T.ATs | ↓ATs: partially due to variety effect PPO and POD; oxidation and co-oxidation; non-enzymatic reactions; cleavage of covalent bonds | [42] |
| Strawberry pure | TT: 90 °C for 15 min HPP: 500 MPa/15 min at 50 °C | 12 weeks 6 °C | - | TT ATs: ↓17% cya-3-glu; ↓19% pel-3-glu; ↓19% pel-3-rut; ↓19% T.ATs EA: ↑ 56% EA until week 10 HPP+50 °C ATs: ↓72% cya-3-glu; ↓68% pel-3-glu; ↓72% pel-3-rut; ↓69% T.ATs EA: ↑43% EA until the end of storage | TT-Storage ↑EA: release from the achenes; ETs hydrolysis; low pH increased ETs hydrolysis HPP Storage ↓ATs: not enough enzyme inactivation PPO and POD → oxidation | [43] |
| Strawberry pulp | TT: 70 °C/2 min HPP: 400, 500, 600 MPa/ 5, 10, 15, 20, 25 min at 25 °C | No | TT F3OLs/PACs: ↑42% CA ATs: ↓17% cy-3-glu, ↓23% pel-3-glu, ↓21% pel-3-rut; ↓22% T.ATs EA: ↑17% HPP F3OLs/PACs: ≈CA (500 MPa/20, 25 min; 600 MPa/5–25 min); ↓7–23% CA (400 MPa/5–25 min; 500 MPa/5–15 min); from ≈ to ↓23% T.F3OLs/PACs ATs: ≈cy-3-glu, ≈pel-3-glu, ≈pel-3-rut, ≈T.ATs (all HPP conditions) EA: ↓2–37% (400MPa/20 min; 500 MPa/5, 20 min; 600 MPa/10, 20, 25 min) | - | TT ↑F3OLs/PACs: extraction from the achenes favored by TT ↓ATs: condensation reactions with other phenols → browning; PPO and POD oxidation ↑EA: release from the achenes; ETs hydrolysis HPP ↓F3OLs/PACs: no complete enzyme inactivation (PPO and POD) → oxidation | [44] |
| Clear and cloud strawberry juices | TT (SB): 100 °C/1 min HPP: 600 MPa/4 min at 43 °C | 6 months at 4 and 25 °C | - | Clear juice at 4 °C ATs: ↓10% cy-3-glu, ↓6% pel-3-glu, ↓9% pel-3-rut; ↓7% T.ATs Cloudy juice at 4 °CATs: ↓26% cy-3-glu, ↓33% pel-3-glu, ↓21% pel-3-rut; ↓30% T.ATs Clear and cloudy juice at 25 °C: T.ATs: ↓> 95% | ↓ATs: PPO and POD oxidation; condensation with other phenols → colorless compounds; oxidative degradation of ascorbic acid (especially at higher storage temperature) | [45] |
| Material | Treatment: Conditions | Storage | Impact of Processing Conditions on Polyphenols a | Impact of Storage Conditions on Polyphenols b | Mechanisms | Ref. |
|---|---|---|---|---|---|---|
| Apple pureec | TTwith O2: 90 °C/30 min + O2 TT ∅ O2: 90 °C/30 min ∅ O2 | No | TTwith O2 F3OLs/PACs: ≈ EC; ≈procyanidin-dimer 1; ↓45% proanthocyanidin trimer; ↓62% CA; ↓30% T.F3OLs/PACs HCAs: ↓44% chlorogenic acid TT ∅ O2 F3OLs/PACs: ≈EC, ↑35% procyanidin-dimer 1, ≈proanthocyanidin trimer; ≈CA; ↑7% T.F3OLs/PACs HCAs: ≈chlorogenic acid | - | ↓F3OLs/PACs and HCAs: oxidation reactions during heating | [46] |
| Clear apple juice | TT: 25, 35, 45, 55, 65 and 75 °C/20 min | No | F3OLs/PACs: ↓EC and CA HCAs: ↓chlorogenic acid | - | ↓F3OLs/PACs and HCAs: enzymatic oxidation | [47]d |
| Apple juice | TT1: 71.7 °C/0.4 min TT2: 90 °C/14.8 min | No | TT1 F3OLs/PACs: ↑71% T.F3OLs/PACs HCAs: ↑244% chlorogenic acid; ↑156% p-coumaoylquinic acid; ↑205% T.HCAs DHCs: ↑156% phloretin xyloglucoside; ↑192% phloridzin; ↑165% T.DHCs FOLs: ↑39% que-3-O-gal; ↑988% que-3-O-hex; ↑50% que-3-O-xyl; ↑7% que-3-O-rha; ↑33% que-3-O-pen; ↑49% T.FOLs TT2 F3OLs/PACs: ↑1800% T.F3OLs/PACs HCAs: ↑1352% chlorogenic acid; ↑389% p-coumaoylquinic acid; ↑925% T.HCAs DHCs: ↑752% phloretin xyloglucoside; ↑808% phloridzin; ↑767% total DHCs FOLs: ↑67% que-3-O-gal; ↑1113% que-3-O-hex; ↑92% que-3-O-xyl; ↑14% que-3-O-rha; ↑48% que-3-O-pen; ↑69% T.FOLs | - | ↑F3OLs/PACs: cleavage → release of dimers and monomers ↑FOLs: release from cells walls; ↓ PPO activity ↑DHCs: enhanced release from peel and seeds ↑HCAs: release from cells walls favored by TT; enzyme inactivation | [48] |
| Apple juice (2 years of harvest) | TT: 85 °C | 360 days 4, 20, and 37 °C | - | 4 and 20 °C: ≈ ∑ flavanols, DHCs, FOLs, and phenol carboxylic acids 37 °C: ↓ ∑ flavanols, DHCs, FOLs, and phenol carboxylic acids | ↓ ∑ flavanols, DHCs, FOLs, and phenol carboxylic acids: Higly influenced by storage temperature | [49]d |
| Apple juice | TT1: 98 °C/30 sec TT2: 98 °C/30 sec | No | TT1 F3OLs/PACs: ↓32% CA; ↓31% EC; ↓33% EGC; ↓43% ECG; ↓18% procyanidin B2; ↓18% T.F3OLs/PACs FOLs: ↓25% rutin; ↓50% hyperin; ↓27% que; ↓32% T.FOLs DHCs: ↓ 18% phloridzin HCAs: ↓16% chlorogenic acid TT1+TT2 F3OLs/PACs: ↓58% CA; ↓56% EC; ↓59% EGC; ↓70% ECG; ↓37% procyanidin B2; ↓48% T.F3OLs/PACs FOLs: ↓64% rutin; ↓86% hyperin; ↓55% que; ↓63% T.FOLs DHCs: ↓ 48% phloridzin HCAs: ↓30% chlorogenic acid | - | ↓F3OLs/PACs: TT conditions degraded heat-labile flavan-3-ols ↓FOLs: due to the discard of peel solids from the juice; the remaining enzyme activity ↓DHCs: due to thermal degradation | [50] |
| Apple sauce c (12 varieties) | TT1: 95 °C/2 min TT2: 95 °C/5 min | No | F3OLs/PACs: ↓20–85% procyanidins oligomers; ↓22–59% CA; ↓13–74% EC; ↓20–75% T.F3OLs/PACs FOLs: from ↑6–63% to ↓4–57% T.FOLs; DHCs: from ↓50% to ↑54% phloridzin; ↑1–1285% phloretin-2-xyloglucoside; from ↓8–14% to ↑%8–325% T.DHCs HCAs: ↓1–47% 5’-caffeoylquinic acid (9 varieties); ↑4–30% 5’-caffeoylquinic acid (4 varieties); from ↓4–49% to ↑7–27% T. HCAs | ↑ and ↓ of phenols highly related to the apple variety ↓F3OLs/PACs: due to oxidation ↑ FOLs: diffusion of quercetin glycosides from the peel to the applesauce ↑DHCs: diffusion from the seeds to the applesauce ↑HCAs: release from cells walls favored by TT; Enzyme inactivation | [7] | |
| Cloudy apple juicec | TT: 80–145 °C/over 7200 sec | No | F3OLs/PACs: ↓7 procyanidin oligomers (mainly B type); ↑ CA; ↑EC; ↑dimeric compounds | - | ↓F3OLs/PACs oligomers: cleavage → release of dimers and monomers ↓FOLs: glycosidic bond hydrolisis in an acidic matrix | [51]d |
| Apple juice | HPP: 300, 300 (x3), 450, 600 MPa/5 min at 20 °C | 12 weeks 4 °C | F3OLs/PACs: ≈CA (all HPP); ↓EC (all HPP); ↑8% procyanidin B2 (300 and 400 MPa); ↑18% procyanidin B2 (300 x3 and 600 MPa); from ≈ to ↑8% T. F3OLs/PACs DHCs: ≈ phloridzin (300, 300x3, 450 MPa; 600 MPa) FOLs: ≈ que (300, 300x3, 450 MPa); ↑25% que (600 MPa); ↑1–25% T. FOLss HCAs: ≈chlorogenic acid (300 and 300x3 MPa); ↑5% chlorogenic acid (450 and 600 MPa) | F3OLs/PACs: ∅ C and procyanidin B2 after 6 weeks (all HPP); ↓77% EC (except in 300 MPa) FOLs: ∅ quercetin after 2 weeks (all HPP) DHCs: ↓71–84% phloridzin (all HPP) HCAs: ↓66–77% chlorogenic acid (300x3, 450, 600 MPa) | HPP: higher pressurization → higher extraction from apple tissue ↑FOLs: release from the disrupted cell walls Storage ↓F3OLs/PACs and FOLs: oxidation reactions | [52] |
| Apples (Spain and Italy) | HPP: 400, 500, 600 MPa/5 min at 35 °C | No | Spanish apples: 400 MPa (best treatment) F3OLs/PACs: ≈CA; ≈EC; ≈dimers; ≈trimers; ↑4% procyanidin B2; ≈ T.F3OLs/PACs FOLs: ≈que-3-rut; ↑35% que-3-gal; ↑22% que-3-glu; ↑30% que-3-ara; ↑32% que-3-xyl; ↑33% que-3-rha; ↑30% T.FOLs DHCs: ≈phloridzin; ↓9% phloretin-2-xyloglucoside; ↓2% T.DHCs HCAs: ↓44% chlorogenic acid; ↓9% neochlorogenic acid; ↓10% cryptochlorogenic acid; ↓17% coumaroyl quinic acid; ↓39% T. HCAs 500–600 MPa F3OLs/PACs: ≈CA; ↓8–13% procyanidin B2; ≈trimers; ≈dimers; ↓11–17% EC; ↓11% T.F3OLs/PACs FOLs:↓40–50% que-3-rut; ↓33–53% que-3-gal; ↓24–46% que-3-glu; ↓3–16% que-3-ara; ↓6–23% que-3-xyl; ↓15% que-3-rha; ↓16–33% T.FOLs DHCs: ↓16–20% phloridzin; ↓14–17% phloretin-2-xyloglucoside; ↓15–19% T.DHCs HCAs: ↓15–24% chlorogenic acid; ↓4% neochlorogenic acid; ↓12–14% cryptochlorogenic acid; ↓12–19% coumaroyl quinic acid; ↓14–22% T. HCAs Italian apples: 600 MPa (best treatment) F3OLs/PACs: ↑30% CA; ↑39% procyanidin B2; ↑70% trimers; ↑242% dimers; ↑45% EC; ↑58% T.F3OLs/PACs FOLs: ↑ 88% que-3-rut; ↑107% que-3-gal; ↑78% que-3-glu; ↑59% que-3-ara; ↑68% que-3-xyl; ↑61% que-3-rha; ↑75% T.FOLs DHCs: ↑ 67% phloridzin; ↑ 51% phloretin-2-xyloglucoside; ↑63% T.DHCs HCAs: ↑31% chlorogenic acid; ↑4% neochlorogenic acid; ↑5% cryptochlorogenic acid; ↑51% coumaroyl quinic acid; ↑29% T. HCAs 400–500 MPa F3OLs/PACs: ↑2–13% CA; ↓10–19% procyanidin B2; ≈trimers; ↑131–161% dimers; ↓4–14% EC; ≈T.F3OLs/PACs FOLs: ↑24% que-3-rut (400 MPa); ↑10–44% que-3-gal; ↓35% que-3-glu (400 MPa); ↓7–21% que-3-ara; ↓7–25% que-3-xyl; ↓7–23% que-3-rha; ↑5–29% T.FOLs DHCs: ↓15% phloridzin; ↓1–14% phloretin-2-xyloglucoside; ↓11–↑8% T.DHCs HCAs: ↓14–17% chlorogenic acid; ↓8–13% neochlorogenic acid; ↓8–16% cryptochlorogenic acid; ↓3–7% coumaroyl quinic acid; ↓13–16% T. HCAs | - | Differences highly influenced by apple origin ↓Oligomers PACs → epimerization changes and depolymerization ↑FOLs: enhanced extraction by higher permeability or disruption of cell walls ↓FOLs: residual enzyme activity (PPO and POD) → oxidation | [12] |
| Apples (Spain) | HPP: 400 MPa-/5 min at 35 °C | No | F3OLs/PACs: ≈procyanidin B1, EC trimers and tetramers; ↓4% EC; ↑10% CA, ↑4% procyanidin B2; ↑65% EC-dimer; ↑3% T.F3OLs/PACs FOLs: ↑9% que-3-rut; ↑35% que-3-gal; ↑22% que-3-glu; ↑30% que-3-ara; ↑32% que-3-xyl; ↑33% que-3-rha; ↑30% T.FOLs DHCs: ≈phloridzin; ↓9% phloretin-2-xyloglucoside; ≈T.DHCs HCAs: ↓13% chlorogenic acid; ↓9% neochlorogenic acid; ↓10% cryptochlorogenic acid; ↓17% coumaroyl quinic acid; ↓12% T. HCAs | ↓Oligomers PACs → epimerization changes and depolymerization | [53] | |
| Cloudy apple juice | HPP: 600 MPa/5 minat 25 °C | 12 weeks 4 ºC | F3OLs/PACs: ≈ CA; ↓13% EC; ↓45% procyanidin B1; ↓15% T. F3OLs/PACs DHCs: ↓18% phloridzin HCAs: ≈ chlorogenic acid | F3OLs/PACs: ↓92% procyanidin B1; ↓61%; ↓15% CA DHCs: ↓71% phloridzin HCAs: ↓53% chlorogenic acid | ↓F3OLs/PACs: residual PPO and POD activity → oxidation ↓DHCs: residual enzyme activity → oxidation | [54] |
| TT | HPP | |||
|---|---|---|---|---|
| F3OLs/ PACs | 8 studies/30 trials | 6 studies/28 trials | ||
| Positive (10) | Negative (20) | Positive (13) | Negative (15) | |
|
|
|
| |
| ATs | 7 studies/23 trials | 6 studies/40 trials | ||
| Positive (5) | Negative (18) | Positive (16) | Negative (24) | |
|
|
|
| |
| EA | 5 studies/10 trials | 3 studies/27 trials | ||
| Positive (6) | Negative (4) | Positive (13) | Negative (14) | |
|
|
|
| |
| FOLs | 3 studies/17 trials | 3 studies/11 trials | ||
| Positive (14) | Negative (3) | Positive (9) | Negative (2) | |
|
|
|
| |
| DHCs | 3 studies/16 trials | 6 studies/12 trials | ||
| Positive (12) | Negative (4) | Positive (6) | Negative (6) | |
|
|
|
| |
| HCAs | 4 studies/19 trials | 4 studies/12 trials | ||
| Positive (5) | Negative (14) | Positive (5) | Negative (7) | |
|
|
|
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Orbea, G.L.; García-Villalba, R.; Tomás-Barberán, F.A.; Sánchez-Siles, L.M. High–Pressure Processing vs. Thermal Treatment: Effect on the Stability of Polyphenols in Strawberry and Apple Products. Foods 2021, 10, 2919. https://doi.org/10.3390/foods10122919
Salazar-Orbea GL, García-Villalba R, Tomás-Barberán FA, Sánchez-Siles LM. High–Pressure Processing vs. Thermal Treatment: Effect on the Stability of Polyphenols in Strawberry and Apple Products. Foods. 2021; 10(12):2919. https://doi.org/10.3390/foods10122919
Chicago/Turabian StyleSalazar-Orbea, Gabriela Lorena, Rocío García-Villalba, Francisco A. Tomás-Barberán, and Luis Manuel Sánchez-Siles. 2021. "High–Pressure Processing vs. Thermal Treatment: Effect on the Stability of Polyphenols in Strawberry and Apple Products" Foods 10, no. 12: 2919. https://doi.org/10.3390/foods10122919
APA StyleSalazar-Orbea, G. L., García-Villalba, R., Tomás-Barberán, F. A., & Sánchez-Siles, L. M. (2021). High–Pressure Processing vs. Thermal Treatment: Effect on the Stability of Polyphenols in Strawberry and Apple Products. Foods, 10(12), 2919. https://doi.org/10.3390/foods10122919








