Identification of Mammalian and Poultry Species in Food and Pet Food Samples Using 16S rDNA Metabarcoding
Abstract
1. Introduction
- The study included 25 reference samples with known composition, 56 commercial food and 23 pet food products.
- All samples were analyzed by the DNA metabarcoding method published previously [28] as well as by a commercial DNA array and/or by real-time PCR.
- Qualitative and quantitative results obtained by DNA metabarcoding were compared to those obtained by the two PCR methodologies currently playing the most important role in meat species authentication in official food laboratories.
- A subset of seven reference samples was analyzed by using the DNA metabarcoding method in two independent laboratories, yielding information on the robustness and reproducibility of the method.
- We evaluated whether the results obtained by DNA metabarcoding were in line with sample composition (reference samples) or declaration (commercial food and pet food products).
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction and Quantification
2.3. DNA-Library Preparation and NGS
2.4. NGS Data Analysis Using Galaxy
2.5. DNA Array and Real-Time PCR Assays
3. Results and Discussion
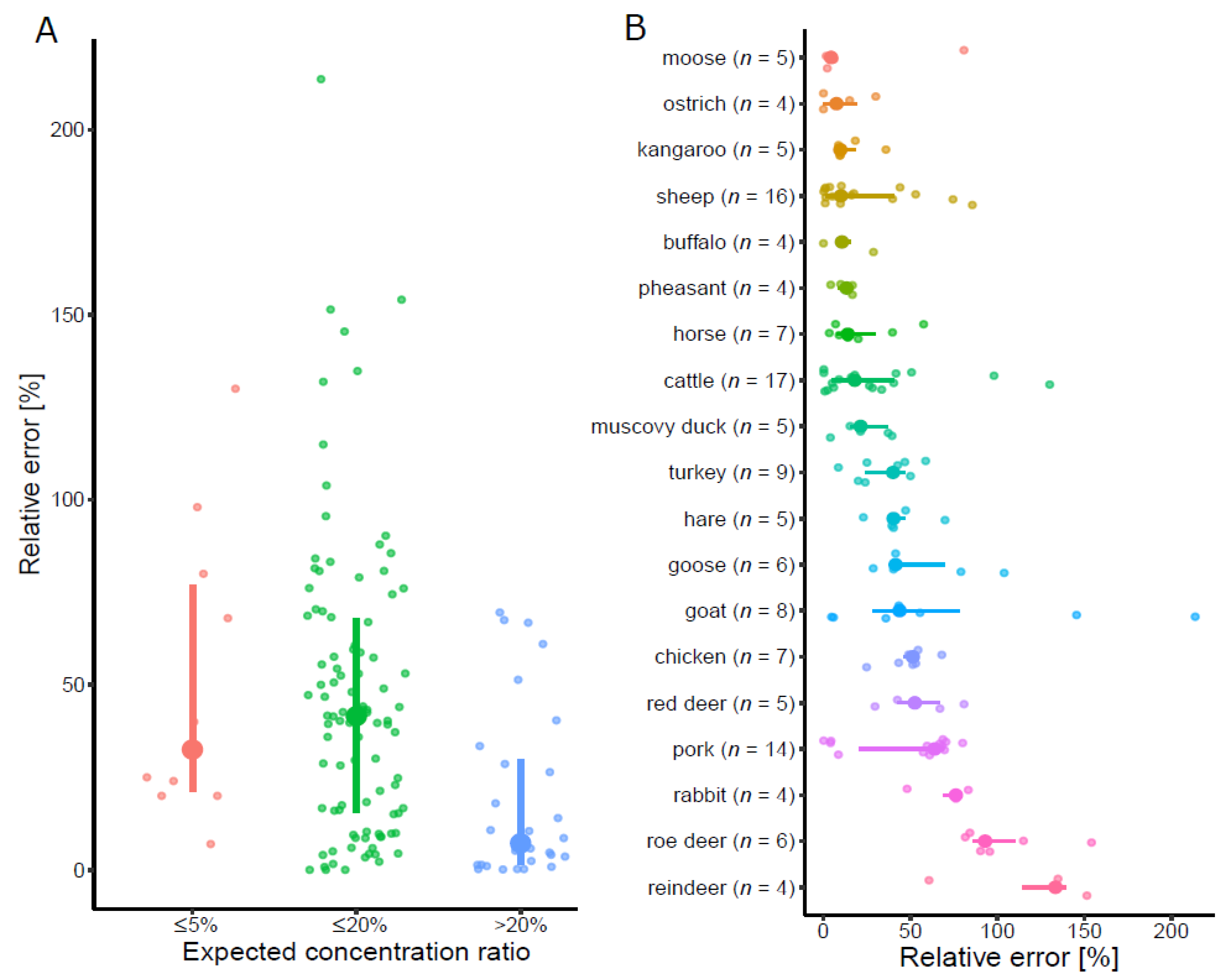
3.1. Reference Samples
3.1.1. Qualitative Results
3.1.2. Quantitative Results
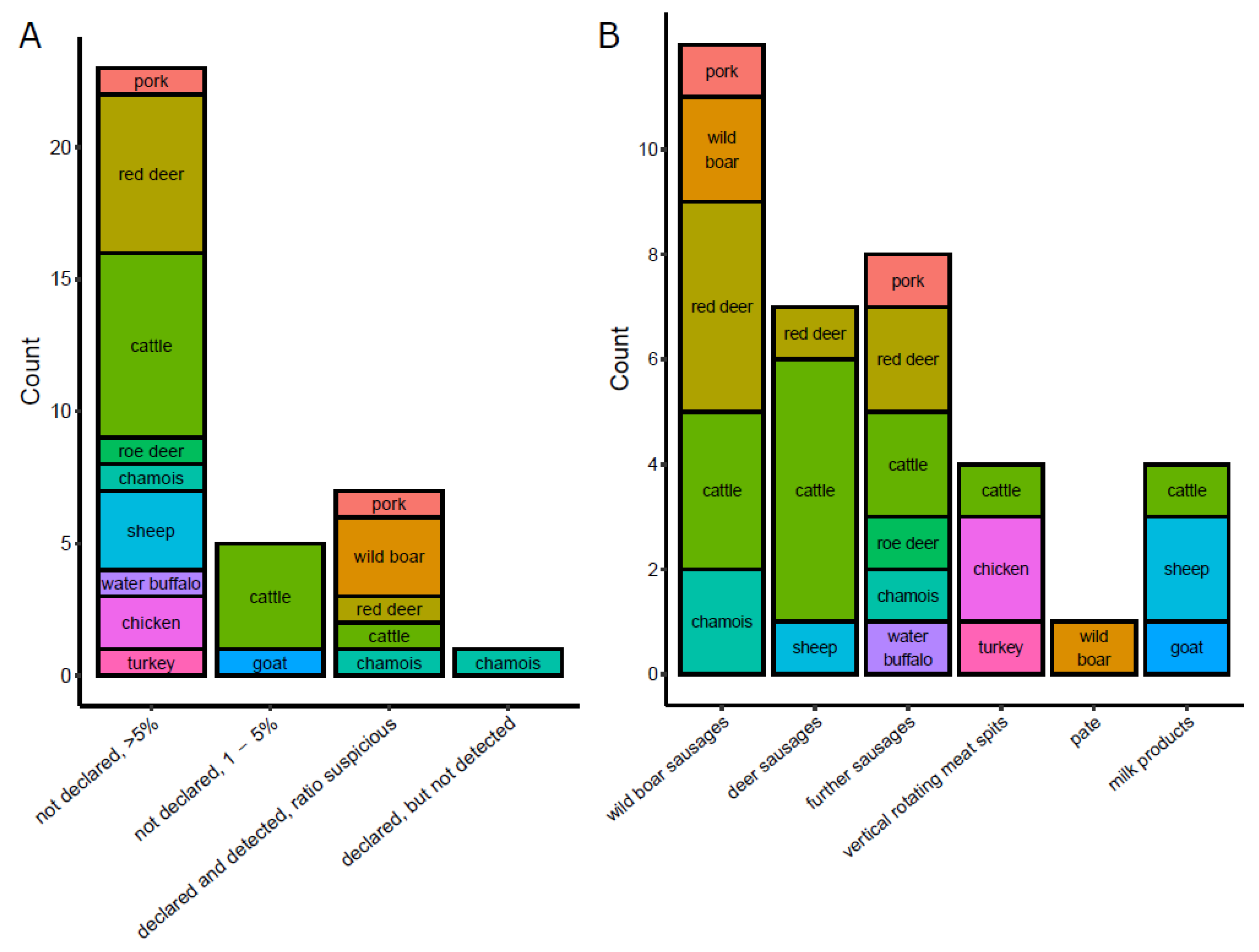
3.2. Commercial Food Products
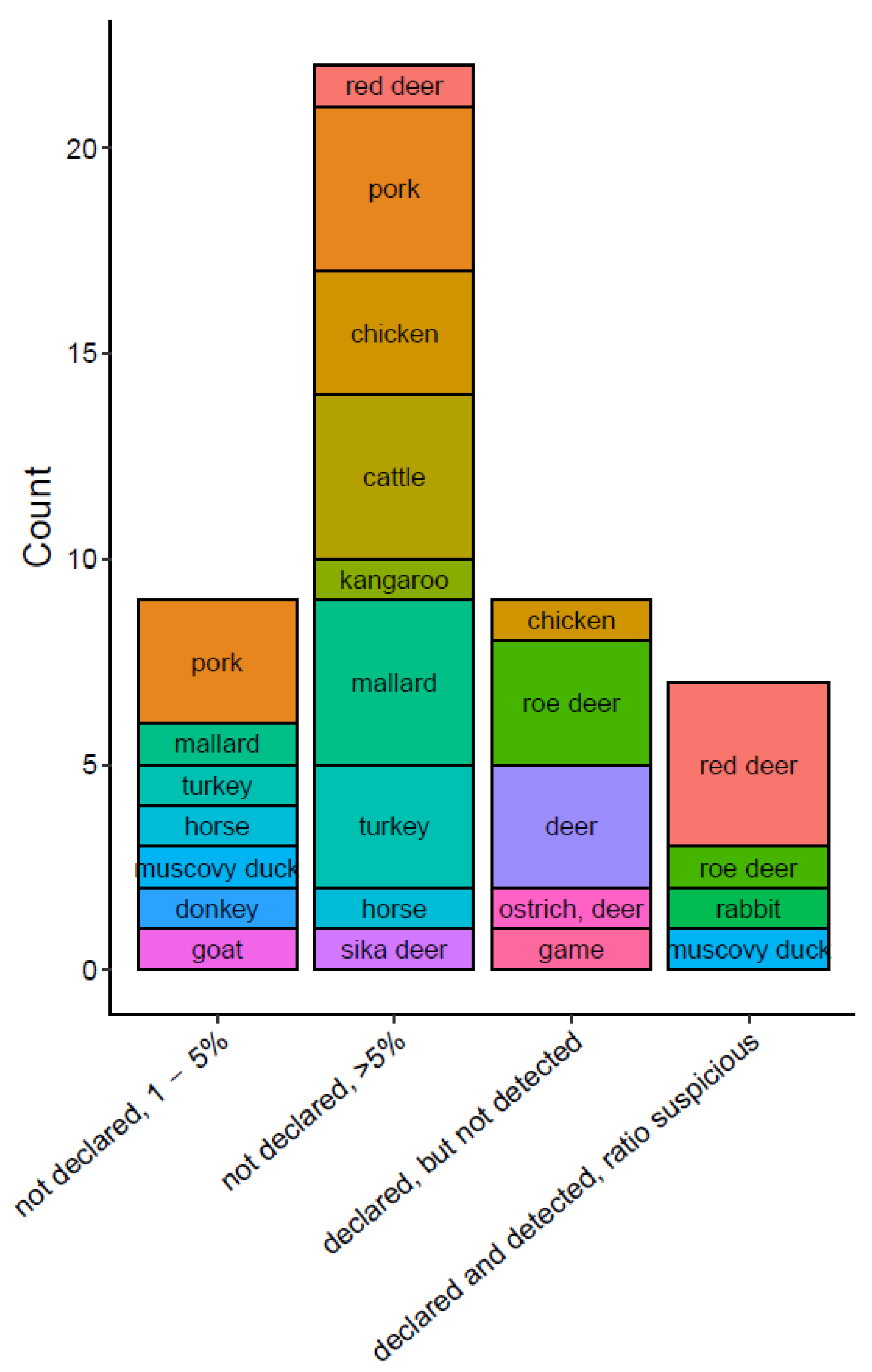
3.3. Commercial Pet Food Products
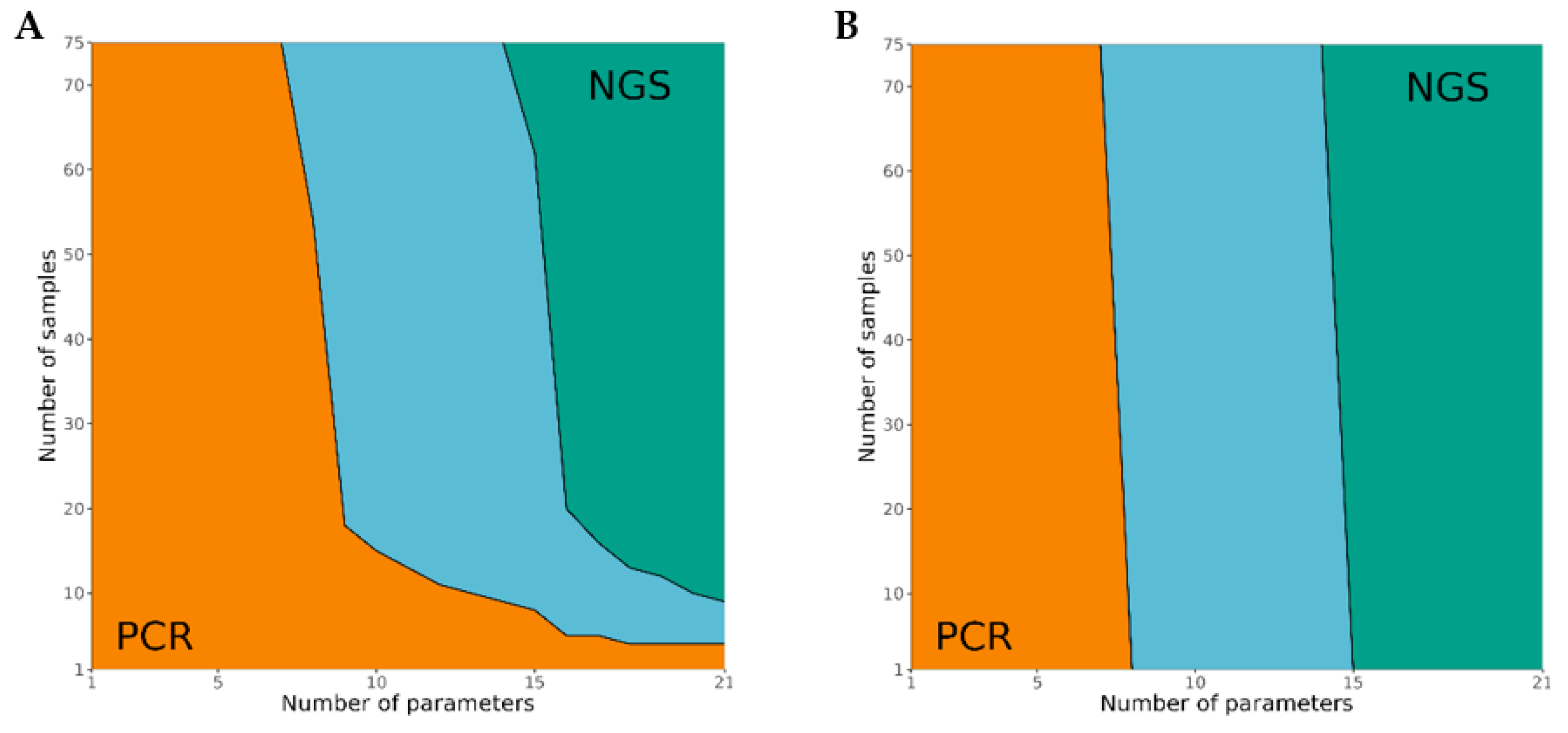
3.4. Cost Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballin, N. Authentication of meat and meat products. Meat Sci. 2010, 86, 577–587. [Google Scholar] [CrossRef]
- Montowska, M.; Pospiech, E. Authenticity Determination of Meat and Meat Products on the Protein and DNA Basis. Food Rev. Int. 2010, 27, 84–100. [Google Scholar] [CrossRef]
- Ballin, N.Z.; Vogensen, F.; Karlsson, A.H. Species determination—Can we detect and quantify meat adulteration? Meat Sci. 2009, 83, 165–174. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.R.; Sharma, B.D.; Gokulakrishnan, P.; Mendiratta, S.K.; Sharma, D. Identification of Species Origin of Meat and Meat Products on the DNA Basis: A Review. Crit. Rev. Food Sci. Nutr. 2013, 55, 1340–1351. [Google Scholar] [CrossRef]
- Amaral, J.; Meira, L.; Oliveira, B.; Mafra, I. Advances in Authenticity Testing for Meat Speciation. In Advances in Food Authenticity Testing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 369–414. [Google Scholar]
- Lo, Y.-T.; Shaw, P.-C. DNA-based techniques for authentication of processed food and food supplements. Food Chem. 2018, 240, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Iwobi, A.N.; Huber, I.; Hauner, G.; Miller, A.; Busch, U. Biochip Technology for the Detection of Animal Species in Meat Products. Food Anal. Methods 2010, 4, 389–398. [Google Scholar] [CrossRef]
- ISO 20813: 2019—Molecular Biomarker Analysis—Methods of Analysis for the Detection and Identification of Animal Species in Foods and Food Products (Nucleic Acid-Based Methods)—General Requirements and Definitions; International Organization for Standardization: Geneva, Switzerland, 2019.
- Eugster, A.; Ruf, J.; Rentsch, J.; Hübner, P.; Köppel, R. Quantification of beef and pork fraction in sausages by real-time PCR analysis: Results of an interlaboratory trial. Eur. Food Res. Technol. 2007, 227, 17–20. [Google Scholar] [CrossRef]
- Eugster, A.; Ruf, J.; Rentsch, J.; Köppel, R. Quantification of beef, pork, chicken and turkey proportions in sausages: Use of matrix-adapted standards and comparison of single versus multiplex PCR in an interlaboratory trial. Eur. Food Res. Technol. 2009, 230, 55–61. [Google Scholar] [CrossRef]
- Köppel, R.; Ruf, J.; Rentsch, J. Multiplex real-time PCR for the detection and quantification of DNA from beef, pork, horse and sheep. Eur. Food Res. Technol. 2011, 232, 151–155. [Google Scholar] [CrossRef]
- Köppel, R.; Eugster, A.; Ruf, J.; Rentsch, J. Quantification of Meat Proportions by Measuring DNA Contents in Raw and Boiled Sausages Using Matrix-Adapted Calibrators and Multiplex Real-Time PCR. J. AOAC Int. 2012, 95, 494–499. [Google Scholar] [CrossRef][Green Version]
- Laube, I.; Zagon, J.; Spiegelberg, A.; Butschke, A.; Kroh, L.W.; Broll, H. Development and design of a ’ready-to-use’ reaction plate for a PCR-based simultaneous detection of animal species used in foods. Int. J. Food Sci. Technol. 2007, 42, 9–17. [Google Scholar] [CrossRef]
- Druml, B.; Kaltenbrunner, M.; Hochegger, R.; Cichna-Markl, M. A novel reference real-time PCR assay for the relative quantification of (game) meat species in raw and heat-processed food. Food Control 2016, 70, 392–400. [Google Scholar] [CrossRef]
- Iwobi, A.; Sebah, D.; Spielmann, G.; Maggipinto, M.; Schrempp, M.; Kraemer, I.; Gerdes, L.; Busch, U.; Huber, I. A multiplex real-time PCR method for the quantitative determination of equine (horse) fractions in meat products. Food Control 2017, 74, 89–97. [Google Scholar] [CrossRef]
- Köppel, R.; Ruf, J.; Zimmerli, F.; Breitenmoser, A. Multiplex real-time PCR for the detection and quantification of DNA from beef, pork, chicken and turkey. Eur. Food Res. Technol. 2008, 227, 1199–1203. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Hochegger, R.; Cichna-Markl, M. Tetraplex real-time PCR assay for the simultaneous identification and quantification of roe deer, red deer, fallow deer and sika deer for deer meat authentication. Food Chem. 2018, 269, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Dolch, K.; Andrée, S.; Schwägele, F. Comparison of Real-Time PCR Quantification Methods in the Identification of Poultry Species in Meat Products. Foods 2020, 9, 1049. [Google Scholar] [CrossRef]
- Staats, M.; Arulandhu, A.J.; Gravendeel, B.; Holst-Jensen, A.; Scholtens, I.; Peelen, T.; Prins, T.W.; Kok, E. Advances in DNA metabarcoding for food and wildlife forensic species identification. Anal. Bioanal. Chem. 2016, 408, 4615–4630. [Google Scholar] [CrossRef]
- Fernandes, T.J.R.; Amaral, J.S.; Mafra, I. DNA barcode markers applied to seafood authentication: An updated review. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Franco, C.M.; Ambrosio, R.L.; Cepeda, A.; Anastasio, A. Fish intended for human consumption: From DNA barcoding to a next-generation sequencing (NGS)-based approach. Curr. Opin. Food Sci. 2021, 42, 86–92. [Google Scholar] [CrossRef]
- Nehal, N.; Choudhary, B.; Nagpure, A.; Gupta, R.K. DNA barcoding: A modern age tool for detection of adulteration in food. Crit. Rev. Biotechnol. 2021, 41, 767–791. [Google Scholar] [CrossRef]
- Cottenet, G.; Blancpain, C.; Chuah, P.F.; Cavin, C. Evaluation and application of a next generation sequencing approach for meat species identification. Food Control 2020, 110, 107003. [Google Scholar] [CrossRef]
- Druml, B.; Cichna-Markl, M. High resolution melting (HRM) analysis of DNA—Its role and potential in food analysis. Food Chem. 2014, 158, 245–254. [Google Scholar] [CrossRef] [PubMed]
- López-Oceja, A.; Nuñez, C.; Baeta, M.; Gamarra, D.; de Pancorbo, M. Species identification in meat products: A new screening method based on high resolution melting analysis of cyt b gene. Food Chem. 2017, 237, 701–706. [Google Scholar] [CrossRef]
- Parvathy, V.A.; Swetha, V.P.; Sheeja, T.E.; Leela, N.K.; Chempakam, B.; Sasikumar, B. DNA Barcoding to Detect Chilli Adulteration in Traded Black Pepper Powder. Food Biotechnol. 2014, 28, 25–40. [Google Scholar] [CrossRef]
- Chin, T.C.; Adibah, A.; Hariz, Z.D.; Azizah, M.S. Detection of mislabelled seafood products in Malaysia by DNA barcoding: Improving transparency in food market. Food Control 2016, 64, 247–256. [Google Scholar] [CrossRef]
- Dobrovolny, S.; Blaschitz, M.; Weinmaier, T.; Pechatschek, J.; Cichna-Markl, M.; Indra, A.; Hufnagl, P.; Hochegger, R. Development of a DNA metabarcoding method for the identification of fifteen mammalian and six poultry species in food. Food Chem. 2019, 272, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Rentsch, J.; Weibel, S.; Ruf, J.; Eugster, A.; Beck, K.; Köppel, R. Interlaboratory validation of two multiplex quantitative real-time PCR methods to determine species DNA of cow, sheep and goat as a measure of milk proportions in cheese. Eur. Food Res. Technol. 2012, 236, 217–227. [Google Scholar] [CrossRef]
- Köppel, R.; Daniels, M.; Felderer, N.; Brünen-Nieweler, C. Multiplex real-time PCR for the detection and quantification of DNA from duck, goose, chicken, turkey and pork. Eur. Food Res. Technol. 2013, 236, 1093–1098. [Google Scholar] [CrossRef]
- Druml, B.; Mayer, W.; Cichna-Markl, M.; Hochegger, R. Development and validation of a TaqMan real-time PCR assay for the identification and quantification of roe deer (Capreolus capreolus) in food to detect food adulteration. Food Chem. 2015, 178, 319–326. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Hochegger, R.; Cichna-Markl, M. Red deer (Cervus elaphus)-specific real-time PCR assay for the detection of food adulteration. Food Control 2018, 89, 157–166. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Hochegger, R.; Cichna-Markl, M. Development and validation of a fallow deer (Dama dama)-specific TaqMan real-time PCR assay for the detection of food adulteration. Food Chem. 2018, 243, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Evaluation Report Proficiency TestDLA 45/2019 Animal Species-Screening III: Buffalo Milk, Cow’s Milk, Sheep’s Milk and Goat’s Milk in Dairy Product (Herder Cheese) (2020) DLA—Proficiency Tests GmbH. Available online: http://www.dla-lvu.de/Auswerteberichte%202019/PT%20-%20DLA%2045-2019%20Final%20Report%20Animal%20Species-Screening%20III.pdf (accessed on 30 January 2020).
- Pirondini, A.; Bonas, U.; Maestri, E.; Visioli, G.; Marmiroli, M.; Marmiroli, N. Yield and amplificability of different DNA extraction procedures for traceability in the dairy food chain. Food Control 2010, 21, 663–668. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; Mayer, W.; Kerkhoff, K.; Epp, R.; Rüggeberg, H.; Hochegger, R.; Cichna-Markl, M. Applicability of a duplex and four singleplex real-time PCR assays for the qualitative and quantitative determination of wild boar and domestic pig meat in processed food products. Sci. Rep. 2020, 10, 117243. [Google Scholar] [CrossRef] [PubMed]
- Goedbloed, D.J.; Megens, H.; Van Hooft, P.; Herrero-Medrano, J.M.; Lutz, W.; Alexandri, P.; Crooijmans, R.P.M.A.; Groenen, M.; Van Wieren, S.E.; Ydenberg, R.C.; et al. Genome-wide single nucleotide polymorphism analysis reveals recent genetic introgression from domestic pigs into Northwest European wild boar populations. Mol. Ecol. 2013, 22, 856–866. [Google Scholar] [CrossRef]
- Dzialuk, A.; Zastempowska, E.; Skórzewski, R.; Twarużek, M.; Grajewski, J. High domestic pig contribution to the local gene pool of free-living European wild boar: A case study in Poland. Mammal Res. 2017, 63, 65–71. [Google Scholar] [CrossRef]
- Böhme, K.; Calo-Mata, P.; Barros-Velázquez, J.; Ortea, I. Review of Recent DNA-Based Methods for Main Food-Authentication Topics. J. Agric. Food Chem. 2019, 67, 3854–3864. [Google Scholar] [CrossRef]

| Reference Sample | Composition | Results | ||
|---|---|---|---|---|
| Species | Ratio (%, w/w) | DNA Metabarcoding Ratio of Reads (%) 4 | Real-Time PCR (Ratio of DNA (%)) or DNA Array (Positive/Negative) | |
| LGC7242 | cattle | 99.0 | 98.2 | 98.9 1 |
| pork | 1.0 | 1.8 | 1.1 1 | |
| LGC7240 | cattle | 99.0 | 98.8 | 95.9 1 |
| horse | 1.0 | 1.2 | 1.3 (Equidae) 1 | |
| LGC7249 | sheep | 95.0 | 90.1 | 90.3 1 |
| cattle | 5.0 | 9.9 | 9.7 1 | |
| LGC7248 | sheep | 99.0 | 97.7 | 97.0 1 |
| cattle | 1.0 | 2.3 | 3.0 1 | |
| LGC7245 | sheep | 95.0 | 98.4 | 93.0 1 |
| chicken | 5.0 | 1.6 | 7.0 1 | |
| LGC7244 | sheep | 99.0 | 100.0 | 99.9 1 |
| chicken | 1.0 | <0.1 | 0.1 1 | |
| LGC7247 | sheep | 95.0 | 96.3 | 94.9 1 |
| turkey | 5.0 | 3.8 | 5.1 1 | |
| LGC7246 | sheep | 99.0 | 98.8 | 98.8 1 |
| turkey | 1.0 | 1.2 | 1.2 1 | |
| DLA44-1, 2019 | pork | 93.4 | 89.6 | 88.5 1 |
| horse | 6.6 | 10.4 | 11.5 (Equidae) 1 | |
| DLA44-3, 2019 | pork | 87.3 | 87.4 | 85.1 1 |
| turkey | 7.0 | 7.6 | 11.3 1 | |
| cattle | 5.6 | 5.1 | 3.6 1 | |
| DLA45-1, 2019 | cattle | 92.0 | 91.8/94.2 5 | 90.7 1 |
| buffalo | 8.0 | 8.0/5.7 5 | 9.3 1 | |
| DLA45-2, 2019 | buffalo | 81.0 | 72.5/72.3 5 | 71.5 1 |
| cattle | 10.0 | 10.5/11.6 5 | 7.6 1 | |
| sheep | 9.0 | 16.7/15.7 5 | 20.9 1 | |
| goat | not added 6 | 0.3/0.3 5 | negative 3 | |
| DLA45-3, 2019 | cattle | 89.0 | 65.5 / 73.0 5 | 56.2 1 |
| goat | 11.0 | 34.5/27.0 5 | 43.8 1 | |
| DLA45-4, 2019 | goat | 90.0 | 95.2/94.2 5 | 96.9 1 |
| sheep | 10.0 | 4.7/5.6 5 | 3.4 1 | |
| DLAptAUS2-3.1, 2020 | pork | 90.9 | 98.7 | 99.7 1 |
| donkey | 9.1 | 1.1 | positive 3 | |
| horse | not added 6 | 0.2 | 0.3 (Equidae) 1 | |
| Lippold-A, 2013 | cattle | 27.8 | 18.5 | 14.7 2 |
| sheep | 16.7 | 14.0 | 6.6 2 | |
| chicken | 22.2 | 10.8 | 15.3 2 | |
| goose | 11.1 | 15.7 | positive 3 | |
| Muscovy duck | 11.1 | 12.8 | positive 3 | |
| roe deer | 11.1 | 28.2 | 18.1 2 | |
| Lippold-A, 2019 | red deer | 16.0 | 22.8/24.4 5 | 13.2 2 |
| cattle | 15.6 | 9.1/11.2 5 | 22.2 2 | |
| ostrich | 15.3 | 17.6/19.9 5 | positive 3 | |
| hare | 14.4 | 8.6/7.6 5 | positive 3 | |
| kangaroo | 14.2 | 16.8/9.1 5 | positive 3 | |
| sheep | 12.6 | 12.5/13.9 5 | 10.3 2 | |
| pheasant | 12.0 | 12.5/14.0 5 | 10.5 2 | |
| Lippold-B, 2019 | goose | 16.4 | 23.2/23.0 5 | positive 3 |
| rabbit | 15.5 | 3.7/2.6 5 | positive 3 | |
| chicken | 14.9 | 7.6/6.8 5 | 16.6 2 | |
| pork | 13.6 | 21.4/21.7 5 | 2.9 2 | |
| moose | 13.6 | 13.0/13.3 5 | positive 3 | |
| roe deer | 13.5 | 24.5/26.4 5 | 23.8 2 | |
| turkey | 12.4 | 6.6/6.2 5 | 8.7 2 | |
| Lippold-C, 2019 | pork | 28.9 | 9.6/8.8 5 | 8.2 2 |
| horse | 17.8 | 19.4/17.2 5 | 10.6 (Equidae) 2 | |
| Muscovy duck | 16.4 | 19.9/22.5 5 | positive 3 | |
| reindeer | 13.8 | 32.0/32.4 5 | positive 3 | |
| goat | 12.0 | 6.7/6.8 5 | 2.8 2 | |
| fallow deer | 11.1 | - | 12.6 2 | |
| cattle | traces 7 | 1.1/1.2 5 | 1.8 2 | |
| Lippold-A, 2020 | goose | 38.8 | 49.9 | positive 3 |
| horse | 25.0 | 28.5 | 12.9 (Equidae) 2 | |
| pork | 12.5 | 3.7 | 9.1 2 | |
| hare | 11.2 | 6.8 | positive 3 | |
| Muscovy duck | 10.0 | 9.6 | positive 3 | |
| turkey | 2.5 | 1.5 | 2.3 2 | |
| Lippold-B, 2020 | pork | 31.3 | 12.2 | 10.2 2 |
| fallow deer | 24.1 | - | 12.9 2 | |
| reindeer | 17.9 | 45.0 | positive 3 | |
| chicken | 12.5 | 9.4 | 15.9 2 | |
| goat | 11.7 | 7.5 | 3.7 2 | |
| turkey | 2.4 | 1.8 | 1.6 2 | |
| Lippold-C, 2020 | goose | 8.1 | 14.5 | positive 3 |
| red deer | 8.1 | 10.5 | 10.8 2 | |
| cattle | 7.9 | 3.9 | 21.2 2 | |
| rabbit | 7.7 | 4.0 | positive 3 | |
| chicken | 7.4 | 4.2 | 13.0 2 | |
| hare | 7.3 | 2.2 | positive 3 | |
| kangaroo | 7.2 | 6.5 | positive 3 | |
| pork | 6.7 | 11.3 | 2.5 2 | |
| moose | 6.7 | 7.1 | positive 3 | |
| roe deer | 6.7 | 14.4 | 22.4 2 | |
| sheep | 6.3 | 5.2 | 2.8 2 | |
| turkey | 6.1 | 3.5 | 5.4 2 | |
| pheasant | 6.0 | 5.0 | positive 3 | |
| ostrich | 7.7 | 7.7 | positive 3 | |
| Lippold-A, 2021 | cattle | 8.5 | 8.0 | 4.1 2 |
| pork | 6.3 | 10.6 | 3.1 2 | |
| sheep | 7.8 | 4.7 | 6.2 2 | |
| horse | 6.3 | 3.8 | 3.5 (Equidae) 2 | |
| red deer | 7.8 | 14.1 | 7.4 2 | |
| fallow deer | 6.3 | - | 3.8 2 | |
| roe deer | 6.3 | 11.6 | 11.3 2 | |
| moose | 6.3 | 6.4 | positive 3 | |
| kangaroo | 7.4 | 8.1 | positive 3 | |
| rabbit | 7.1 | 1.7 | positive 3 | |
| reindeer | 6.1 | 9.8 | positive 3 | |
| chicken | 9.8 | 4.6 | 12.2 2 | |
| turkey | 6.3 | 2.6 | 5.7 2 | |
| ostrich | 7.8 | 7.8 | positive 3 | |
| Lippold-B, 2021 | cattle | traces 7 | 2.8 | 1.8 2 |
| pork | 32.6 | 10.6 | 14.2 2 | |
| horse | 4.3 | 4.0 | 2.0 (Equidae) 2 | |
| roe deer | 14.4 | 27.4 | 27.4 2 | |
| moose | 10.9 | 19.7 | positive 3 | |
| kangaroo | 13.9 | 12.7 | positive 3 | |
| hare | 10.9 | 8.4 | positive 3 | |
| pheasant | 13.1 | 14.4 | positive 3 | |
| Lippold-C, 2021 | cattle | 25.0 | 14.9 | 6.2 2 |
| pork | 13.9 | 14.5 | 2.3 2 | |
| sheep | 14.3 | 12.9 | 3.9 2 | |
| goat | 16.4 | 7.3 | 2.2 2 | |
| red deer | 12.1 | 20.2 | 6.6 2 | |
| goose | 7.8 | 15.9 | positive 3 | |
| Muscovy duck | 10.4 | 14.5 | positive 3 | |
| Result | |||||
|---|---|---|---|---|---|
| Sample | Animal Species Declared | Animal Species Detected | DNA Metabarcoding Ratio of Reads (%) | Real-Time PCR (Ratio of DNA (%)) or DNA Array (Positive/Negative) | Comment |
| wild boar sausage 1 | wild boar, pork, pork bacon | pork | 83.0 4 | 52.5 1 | |
| wild boar | 35.2 1 | ||||
| red deer | 15.1 | 3.9 1 | not declared, >5% | ||
| cattle | 1.7 | 8.4 1 | not declared, 1%–5% | ||
| wild boar sausage 2 | wild boar, pork, pork bacon | wild boar | 86.9 4 | 23.7 1 | |
| pork | 64.1 1 | ||||
| red deer | 13.1 | 12.3 1 | not declared, >5% | ||
| wild boar sausage 3 | 55% wild boar, 36% roe deer | roe deer | 60.7 | 40.8 1 | |
| pork | 25.1 4 | 50.5 1 | |||
| wild boar | <1.0 1 | declared and detected 3 | |||
| cattle | 14.0 | 8.7 1 | not declared, >5% | ||
| wild boar sausage 4 | 74% red deer, 22% wild boar bacon | cattle | 30.2 | 46.4 1 | not declared, >5% |
| pork | 28.8 4 | 43.5 1 | not declared, >5% | ||
| wild boar | <1.0 1 | declared and detected, r.s. | |||
| red deer | 22.8 | 10.1 1 | declared and detected, r.s. | ||
| chamois | 18.2 | - | not declared, >5% | ||
| wild boar sausage 5 | chamois, wild boar, roe deer, pork bacon | pork | 48.8 4 | 8.9 1 | |
| wild boar | 38.2 1 | ||||
| red deer | 36.8 | 42.0 1 | not declared, >5% | ||
| roe deer | 14.0 | 10.9 1 | |||
| chamois | 0.0 | - | declared, but not detected | ||
| wild boar sausage 6 | no declaration | pork | 62.2 4 | 28.5 1 | |
| wild boar | 26.1 1 | ||||
| roe deer | 24.4 | 16.0 1 | |||
| cattle | 13.4 | 29.4 1 | |||
| wild boar sausage 7 | game, cattle, pork bacon | pork | 70.2 4 | 60.3 1 | |
| wild boar | 16.0 1 | ||||
| cattle | 28.7 | 23.7 1 | |||
| roe deer | <1.0 | <1.0 1 | |||
| sheep | <1.0 | <1.0 1 | |||
| deer sausage 1 | deer, pork, pork bacon | red deer | 72.0 | 52.6 1 | |
| pork | 19.5 | 41.6 1 | |||
| cattle | 8.5 | 5.8 1 | not declared, >5% | ||
| deer sausage 2 | roe deer, pork, pork bacon | roe deer | 52.0 | 28.3 1 | |
| pork | 22.8 4 | 54.3 1 | |||
| wild boar | <1.0 | ||||
| cattle | 10.8 | 7.9 1 | not declared, >5% | ||
| red deer | 14.3 | 9.5 1 | not declared, >5% | ||
| deer sausage 3 | roe deer, pork | roe deer | 89.9 | 75.1 1 | |
| pork | 5.9 | 23.0 1 | |||
| cattle | 4.2 | 1.9 1 | not declared, 1%–5% | ||
| deer sausage 4 | deer, pork | red deer | 67.0 | 52.3 1 | |
| pork | 33.0 4 | 47.7 1 | |||
| wild boar | <1.0 1 | ||||
| deer sausage 5 | roe deer, pork, pork bacon | roe deer | 81.5 | 78.5 1 | |
| pork | 9.3 | 15.8 1 | |||
| cattle | 9.0 | 5.7 1 | not declared, >5% | ||
| red deer | < 1.0 | <1.0 1 | |||
| deer sausage 6 | game, pork | red deer | 83.8 | 66.9 1 | |
| cattle | 9.3 | 14.9 1 | not declared, >5% | ||
| pork | 5.2 4 | 15.3 1 | |||
| wild boar | <1.0 | ||||
| roe deer | 1.7 | 3.0 1 | |||
| deer sausage 7 | 70% red deer, 30% pork | red deer | 70.4 | 72.2 1 | |
| pork | 29.6 4 | <1.0 1 | |||
| wild boar | 27.8 1 | ||||
| deer sausage 8 | deer, pork, pork bacon | red deer | 68.0 | 46.7 1 | |
| pork | 31.7 | 53.3 1 | |||
| sika deer | <1.0 | - | |||
| deer sausage 9 | deer, pork, pork bacon | roe deer | 79.0 | 58.7 1 | |
| pork | 20.9 | 41.3 1 | |||
| deer sausage 10 | deer, pork, pork bacon | red deer | 74.1 | 38.5 1 | |
| pork | 25.3 | 61.5 1 | |||
| deer sausage 11 | deer, pork, pork bacon | red deer | 72.0 | 36.4 1 | |
| pork | 27.8 | 63.6 1 | |||
| deer sausage 12 | pork, red deer | pork | 66.6 | 51.9 2 | |
| red deer | 33.4 | 48.1 2 | |||
| deer sausage 13 | roe deer, pork, pork bacon | roe deer | 81.6 | 67.4 1 | |
| pork | 18.3 4 | 32.6 1 | |||
| wild boar | <1.0 1 | ||||
| deer sausage 14 | deer, pork, pork bacon, cattle casing 5 | red deer | 70.6 | 48.7 1 | |
| pork | 25.7 | 50.0 1 | |||
| sika deer | 2.6 | 1.3 1 | |||
| roe deer | <1.0 | <1.0 1 | |||
| deer sausage 15 | pork, deer, cattle | red deer | 51.4 | 39.9 1 | |
| pork | 31.1 | 45.3 1 | |||
| cattle | 16.9 | 14.8 1 | |||
| roe deer | < 1.0 | <1.0 1 | |||
| deer sausage 16 | deer, pork, pork bacon, cattle casing 5 | red deer | 53.6 | 27.2 1 | |
| pork | 24.2 | 61.3 1 | |||
| sheep | 21.6 | 11.4 1 | not declared, >5% | ||
| roe deer | <1.0 | <1.0 1 | |||
| fallow deer | - | <1.0 1 | |||
| deer sausage 17 | deer, pork, cattle | roe deer | 55.8 | 59.0 1 | |
| red deer | 24.5 | 24.6 1 | |||
| pork | 10.2 4 | 8.9 1 | |||
| wild boar | <1.0 1 | ||||
| cattle | 9.4 | 7.5 1 | |||
| deer sausage 18 | deer, cattle | red deer | 66.1 | 53.8 1 | |
| cattle | 32.2 | 46.2 1 | |||
| sika deer | 1.3 | <1.0 1 | |||
| deer sausage 19 | deer, cattle | red deer | 76.9 | 43.9 1 | |
| cattle | 20.1 | 56.1 1 | |||
| sika deer | 2.9 | <1.0 1 | |||
| deer sausage 20 | game, cattle, pork bacon | red deer | 78.3 | 80.9 1 | |
| roe deer | 14.5 | 7.0 1 | |||
| pork | 5.4 | 10.4 1 | |||
| cattle | 1.8 | 1.7 1 | |||
| sausage 1 | chamois, cattle, pork bacon | red deer | 35.8 | 11.9 1 | not declared, >5% |
| pork | 29.2 | 70.0 1 | declared and detected, r.s. | ||
| cattle | 19.8 | 8.9 1 | |||
| roe deer | 13.4 | 9.2 1 | not declared, >5% | ||
| chamois | 1.6 | - | declared and detected, r.s. | ||
| sausage 2 | 60% sheep, 35% pork, 5% goat | sheep | 44.5 | 35.7 1 | |
| pork | 27.0 | 49.7 1 | |||
| red deer | 12.5 | 3.9 1 | not declared, >5% | ||
| cattle | 8.5 | 9.4 1 | not declared, >5% | ||
| goat | 7.4 | 1.4 1 | |||
| sausage 3 | cattle | water buffalo | 67.0 | - | not declared, >5% |
| cattle | 33.0 | 22.9 2 | |||
| sausage 4 | 42% cattle, 35% chicken | chicken | 86.0 | 96.4 1 | |
| cattle | 13.5 | 3.6 1 | declared and detected, r.s. | ||
| sausage 5 | 40% poultry, 15% cattle, cattle fat | turkey | 44.4 | 36.4 1 | |
| chicken | 30.1 | 32.9 1 | |||
| cattle | 25.0 | 30.7 1 | |||
| sausage 6 | pork, cattle or lamb | cattle | 53.6 | 59.5 1 | |
| pork | 46.1 | 40.5 1 | |||
| sausage 7 | lamb, pork | sheep | 80.1 | 71.7 1 | |
| pork | 19.8 | 28.3 1 | |||
| vertical rotating meat spit 1 | 95% beef | cattle | 64.9 | 85.4 1 | |
| turkey | 35.1 | 14.6 1 | not declared, >5% | ||
| vertical rotating meat spit 2 | 75% veal, 20% turkey | cattle | 57.5 | 76.0 1 | |
| turkey | 35.4 | 21.1 1 | |||
| chicken | 7.1 | 2.9 1 | not declared, >5% | ||
| vertical rotating meat spit 3 | 70% veal, 20% turkey | cattle | 59.2 | 74.1 1 | |
| turkey | 33.7 | 21.5 1 | |||
| chicken | 7.2 | 4.4 1 | not declared, >5% | ||
| vertical rotating meat spit 4 | turkey | turkey | 98.2 | 94.8 1 | |
| cattle | 1.7 | 5.2 1 | not declared, 1%–5% | ||
| vertical rotating meat spit 5 | 55% cattle, 10% turkey, 25% chicken | cattle | 58.8 | 29.1 1 | |
| chicken | 23.0 | 35.0 1 | |||
| turkey | 18.1 | 35.9 1 | |||
| vertical rotating meat spit 6 | 55% cattle, 35% poultry | cattle | 56.0 | 41.9 1 | |
| chicken | 43.5 | 58.1 1 | |||
| turkey | < 1.0 | <1.0 1 | |||
| pâté 1 | wild boar, pork | pork | 99.8 4 | 100.0 1 | |
| wild boar | <1.0 1 | declared and detected, r.s. | |||
| pâté 2 | game, pork | pork | 57.6 | 77.5 1 | |
| red deer | 42.1 | 22.5 1 | |||
| pâté 3 | 49% pork, lamb liver | pork | 66.6 | 71.9 1 | |
| sheep | 33.4 | 28.1 1 | |||
| pâté 4 | pork neck and liver, rabbit meat | pork | 96.2 | 46.5 2 | |
| rabbit | 3.8 | positive 3 | |||
| pâté 5 | duck meat and breast, poultry liver | turkey | 49.1 | 21.3 2 | |
| mallard | 28.1 | positive 3 | |||
| Muscovy duck | 22.8 | positive 3 | |||
| pâté 6 | 50% pork meat, 20% red deer meat | pork | 57.9 | 84.4 2 | |
| red deer | 42.0 | 15.6 2 | |||
| pâté 7 | 33% pork meat, 20% roe deer meat | roe deer | 59.7 | 59.9 2 | |
| pork | 40.3 | 40.1 2 | |||
| minced meat product 1 | chicken, cattle | chicken | 76.2 | 81.9 2 | |
| cattle | 23.0 | 18.1 2 | |||
| buffalo, kangaroo, fish | <1.0 | positive 3 | |||
| minced meat product 2 | lamb, cattle | cattle | 70.3 | 51.6 1 | |
| sheep | 29.4 | 48.4 1 | |||
| steak | reindeer | reindeer | 100.0 | positive 3 | |
| convenience food 1 | 37% pork and cattle, cattle soup | pork | 67.9 | 82.0 1 | |
| cattle | 32.1 | 18.0 1 | |||
| convenience food 2 | 25% pork, cattle soup | pork | 87.2 | 93.9 1 | |
| cattle | 12.5 | 6.1 1 | |||
| milk product 1 | goat | goat | 97.4 | positive 3 | |
| cattle | 2.6 | positive 3 | not declared, 1%–5% | ||
| milk product 2 | goat milk | goat | 62.8 | positive 3 | |
| sheep | 36.2 | positive 3 | not declared, >5% | ||
| ibex | <1.0 | - | |||
| cattle | <1.0 | positive 3 | |||
| milk product 3 | goat milk | goat | 62.9 | positive 3 | |
| sheep | 36.0 | positive 3 | not declared, >5% | ||
| ibex | <1.0 | - | |||
| cattle | <1.0 | negative 3 | |||
| milk product 4 | sheep milk | sheep | 95.4 | positive 3 | |
| goat | 4.5 | positive 3 | not declared, 1%–5% | ||
| Result | |||||
|---|---|---|---|---|---|
| Sample | Animal Species Declared | Animal Species Detected | DNA Metabarcoding Ratio of Reads (%) | Real-Time PCR (Ratio of DNA (%)) or DNA Array (Positive/Negative) | Comment |
| 1 | 65% deer (heart, liver, lung, rumen) | red deer | 96.3 | 92.9 1 | |
| pork | 1.7 | <1.0 1 | not declared, 1%–5% | ||
| fallow deer | - | 6.0 1 | |||
| sheep | <1.0 | <1.0 1 | |||
| chicken | <1.0 | <1.0 1 | |||
| cattle | <1.0 | <1.0 1 | |||
| kangaroo | <1.0 | positive 2 | |||
| 2 | 60% deer meat | pork | 47.1 | 32.6 1 | not declared, >5% |
| roe deer | 36.0 | 55.0 1 | |||
| red deer | 16.9 | 12.4 1 | |||
| 3 | 51% deer meat, <2.5% chicken liver | red deer | 96.2 | 95.9 1 | |
| roe deer | 2.4 | 3.1 1 | |||
| pork | 1.0 | <1.0 1 | not declared, 1%–5% | ||
| rabbit | <1.0 | positive 2 | |||
| chicken | negative | negative 1 | declared, but not detected | ||
| 4 | 59% fresh meat from deer and roe deer, 1.2% eggshell powder | red deer | 62.4 | 53.0 1 | |
| mallard | 29.8 | positive 2 | not declared, >5% | ||
| chicken | 6.5 | 16.9 1 | |||
| fallow deer | - | 2.3 1 | |||
| roe deer | <1.0 | <1.0 1 | declared and detected, r.s. | ||
| pork, sheep, cattle | <1.0 | <1.0 1 | |||
| 5 | 10% deer meat (dried and ground) | chicken | 38.1 | 25.7 1 | not declared, >5% |
| turkey | 12.3 | 7.3 1 | not declared, >5% | ||
| mallard | 10.7 | positive 2 | not declared, >5% | ||
| horse | 33.0 | 15.8 (Equidae) 1 | not declared, >5% | ||
| Muscovy duck | 4.6 | positive 2 | not declared, 1%–5% | ||
| donkey | 1.1 | positive 2 | not declared, 1%–5% | ||
| cattle | <1.0 | <1.0 1 | |||
| deer | negative | negative 1, 2 | declared, but not detected | ||
| 6 | 28% fresh and 26% dried deer meat, 9% chicken fat, 2% dried eggs, 2% fresh and 2% dried herrings, 1% fish oil | pork | 92.2 | 39.4 1 | not declared, >5% |
| fish | - | positive 2 | |||
| chicken | 3.9 | 36.0 1 | |||
| red deer | 2.7 | 2.3 1 | declared and detected, r.s. | ||
| turkey | <1.0 | 22.0 1 | |||
| sheep | <1.0 | <1.0 1 | |||
| 7 | 18% dried Muscovy duck meat, 9.4% dried and ground deer meat, 6.3% dried whiting, 6.3% ground wild bones, egg yolk powder | cattle | 67.8 | 59.9 1 | not declared, >5% |
| chicken | 9.7 | 13.3 1 | not declared, >5% | ||
| mallard | 7.1 | positive 2 | not declared, >5% | ||
| red deer | 7.5 | 2.8 1 | |||
| turkey | 5.0 | 2.3 1 | not declared, >5% | ||
| Muscovy duck | 1.8 | positive 2 | declared and detected, r.s. | ||
| sheep | <1.0 | <1.0 1 | |||
| sika deer | <1.0 | - | |||
| goat | <1.0 | positive 2 | |||
| fish | - | positive2 | |||
| 8 | 50% meat and animal byproducts, 4% ostrich and deer | pork | 52.4 | 3.8 1 | |
| cattle | 30.5 | 69.6 1 | |||
| chicken | 16.8 | 26.5 1 | |||
| turkey | <1.0 | <1.0 1 | |||
| mallard | <1.0 | negative 2 | |||
| ostrich, deer | negative | negative 2 | declared, but not detected | ||
| 9 | 35% cattle, 31% poultry, 4% deer | cattle | 71.4 | 43.6 1 | |
| turkey | 9.0 | 8.3 1 | |||
| reindeer | 12.3 | positive 2 | |||
| chicken | 6.0 | 6.4 1 | |||
| mallard | <1.0 | positive 2 | |||
| pork, sheep, horse | <1.0 | <1.0 1 | |||
| red deer | negative | negative 1 | |||
| 10 | lung, meat, kidney, liver, udder, 5% deer | pork | 89.3 | 88.8 1 | |
| cattle | 9.7 | 10.5 1 | |||
| red deer | <1.0 | <1.0 1 | declared and detected, r.s. | ||
| chicken | <1.0 | <1.0 1 | |||
| 11 | 48% fresh deer meat, 4% entrails of deer | mallard | 96.3 | 31.0 1 | not declared, >5% |
| goat | 1.7 | <1.0 1 | not declared, 1%–5% | ||
| turkey, chicken | <1.0 | <1.0 1 | |||
| pork, sheep | <1.0 | <1.0 1 | |||
| deer | negative | negative 1 | declared, but not detected | ||
| 12 | 50% roe deer (60% meat, 25% heart, 10% lung, 5% liver) | red deer | 98.1 | 97.9 1 | not declared, >5% |
| horse | 1.6 | <1.0 (Equidae) 1 | not declared, 1%–5% | ||
| cattle | <1.0 | 1.7 1 | |||
| fallow deer | - | <1.0 1 | |||
| roe deer | negative | negative 1 | declared, but not detected | ||
| 13 | 99% deer meat | chicken | 71.4 | 51.8 1 | not declared, >5% |
| kangaroo | 17.6 | positive 2 | not declared, >5% | ||
| red deer | 10.3 | 3.8 1 | declared and detected, r.s. | ||
| rabbit | <1.0 | positive 2 | |||
| pork, cattle | <1.0 | <1.0 1 | |||
| 14 | 75% deer (meat, heart, lung) | pork | 84.3 | 45.1 1 | not declared, >5% |
| cattle | 6.4 | 10.0 1 | not declared, >5% | ||
| roe deer | 4.2 | 38.9 1 | |||
| mallard | 2.2 | 1.5 1 | not declared, 1%–5% | ||
| turkey | 1.4 | <1.0 1 | not declared, 1%–5% | ||
| red deer | 1.5 | 1.7 1 | |||
| 15 | 100% deer meat | roe deer | 65.8 | 12.6 1 | |
| cattle | 31.2 | 87.0 1 | not declared, >5% | ||
| chicken | <1.0 | <1.0 1 | |||
| pork | 1.9 | <1.0 1 | not declared, 1%–5% | ||
| red deer | 1.0 | <1.0 1 | |||
| 16 | 50% roe deer | turkey | 98.2 | 99.6 1 | not declared, >5% |
| red deer, horse | <1.0 | <1.0 1 | |||
| pork | <1.0 | <1.0 1 | |||
| roe deer | negative | negative 1 | declared, but not detected | ||
| 17 | 60% deer | red deer | 40.4 | 25.3 1 | |
| cattle | 36.3 | 73.0 1 | not declared, >5% | ||
| pork | 22.9 | 1.7 1 | not declared, >5% | ||
| chicken | <1.0 | <0.1 1 | |||
| 18 | 46% poultry meat, 8% roe deer | chicken | 55.7 | 83.8 1 | |
| turkey | 26.7 | 13.0 1 | |||
| sika deer | 16.6 | - | not declared, >5% | ||
| cattle | <1.0 | <1.0 1 | |||
| red deer, pork | <1.0 | <1.0 1 | |||
| fallow deer | - | 3.0 1 | |||
| roe deer | negative | negative 1 | declared, but not detected | ||
| 19 | 51% meat and animal byproducts, 12% chicken, turkey, duck | pork | 58.5 | 55.0 1 | |
| chicken | 26.1 | 28.4 1 | |||
| turkey | 9.0 | 10.1 1 | |||
| cattle | 5.3 | 4.6 1 | |||
| mallard | <1.0 | <1.0 1 | |||
| guinea fowl | <1.0 | <1.0 1 | |||
| 20 | 51% meat and animal byproducts, 12% cattle, sheep, chicken | chicken | 45.2 | 53.6 1 | |
| pork | 40.2 | 15.3 1 | |||
| cattle | 10.9 | 26.8 1 | |||
| sheep | 3.5 | 4.3 1 | |||
| turkey | <1.0 | positive 2 | |||
| 21 | 33% meat and animal byproducts, 4% poultry, 4% deer | pork | 94.4 | 83.6 1 | |
| chicken | 3.9 | 15.3 1 | |||
| guinea fowl | <1.0 | <1.0 1 | |||
| turkey | <1.0 | 1.0 1 | |||
| deer | negative | negative 2 | declared, but not detected | ||
| 22 | meat and animal byproducts (4% turkey, 4% duck, 4% game) | chicken | 49.4 | 57.6 1 | |
| pork | 25.1 | 13.4 1 | |||
| cattle | 12.6 | 7.3 1 | |||
| turkey | 6.3 | 19.5 1 | |||
| duck | 4.1 | <1.0 1 | |||
| sheep | 1.9 | <1.0 1 | |||
| horse | <1.0 | <1.0 (Equidae) 1 | |||
| fish | - | positive 2 | |||
| game | negative | negative 2 | declared, but not detected | ||
| 23 | 40% chicken (heart, meat, liver, stomachs, necks), 28.7% broth, 28% rabbit | chicken | 99.1 | positive 2 | |
| cattle | <1.0 | positive 2 | |||
| rabbit | <1.0 | positive 2 | declared and detected, r.s. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preckel, L.; Brünen-Nieweler, C.; Denay, G.; Petersen, H.; Cichna-Markl, M.; Dobrovolny, S.; Hochegger, R. Identification of Mammalian and Poultry Species in Food and Pet Food Samples Using 16S rDNA Metabarcoding. Foods 2021, 10, 2875. https://doi.org/10.3390/foods10112875
Preckel L, Brünen-Nieweler C, Denay G, Petersen H, Cichna-Markl M, Dobrovolny S, Hochegger R. Identification of Mammalian and Poultry Species in Food and Pet Food Samples Using 16S rDNA Metabarcoding. Foods. 2021; 10(11):2875. https://doi.org/10.3390/foods10112875
Chicago/Turabian StylePreckel, Laura, Claudia Brünen-Nieweler, Grégoire Denay, Henning Petersen, Margit Cichna-Markl, Stefanie Dobrovolny, and Rupert Hochegger. 2021. "Identification of Mammalian and Poultry Species in Food and Pet Food Samples Using 16S rDNA Metabarcoding" Foods 10, no. 11: 2875. https://doi.org/10.3390/foods10112875
APA StylePreckel, L., Brünen-Nieweler, C., Denay, G., Petersen, H., Cichna-Markl, M., Dobrovolny, S., & Hochegger, R. (2021). Identification of Mammalian and Poultry Species in Food and Pet Food Samples Using 16S rDNA Metabarcoding. Foods, 10(11), 2875. https://doi.org/10.3390/foods10112875






