Active and Robust Composite Films Based on Gelatin and Gallic Acid Integrated with Microfibrillated Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gelatin-Based Films
2.3. Structure Characterizations
2.4. Optical Properties Analyses
2.5. Determination of Physical Properties
2.6. Antioxidant Activity Evaluation
2.7. Statistical Analysis
3. Results and Discussion
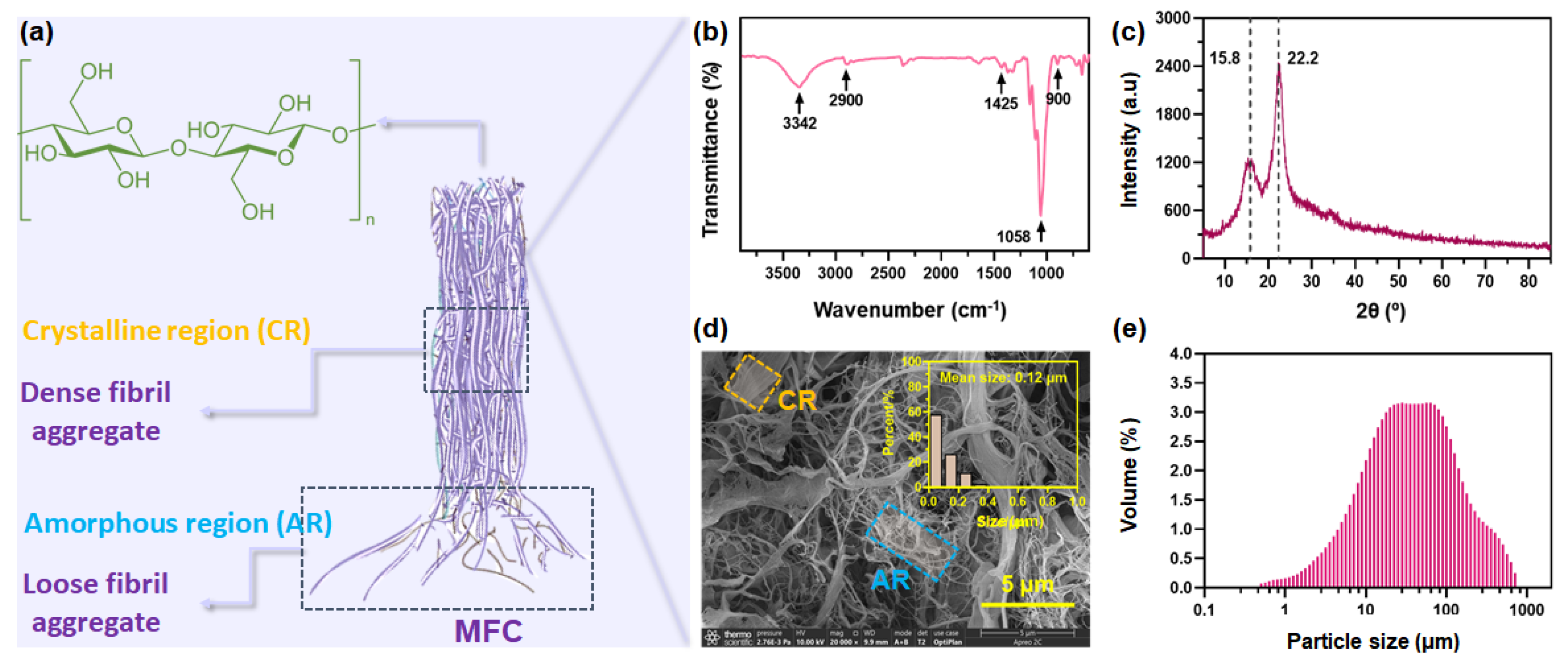
3.1. Structure Features of MFC
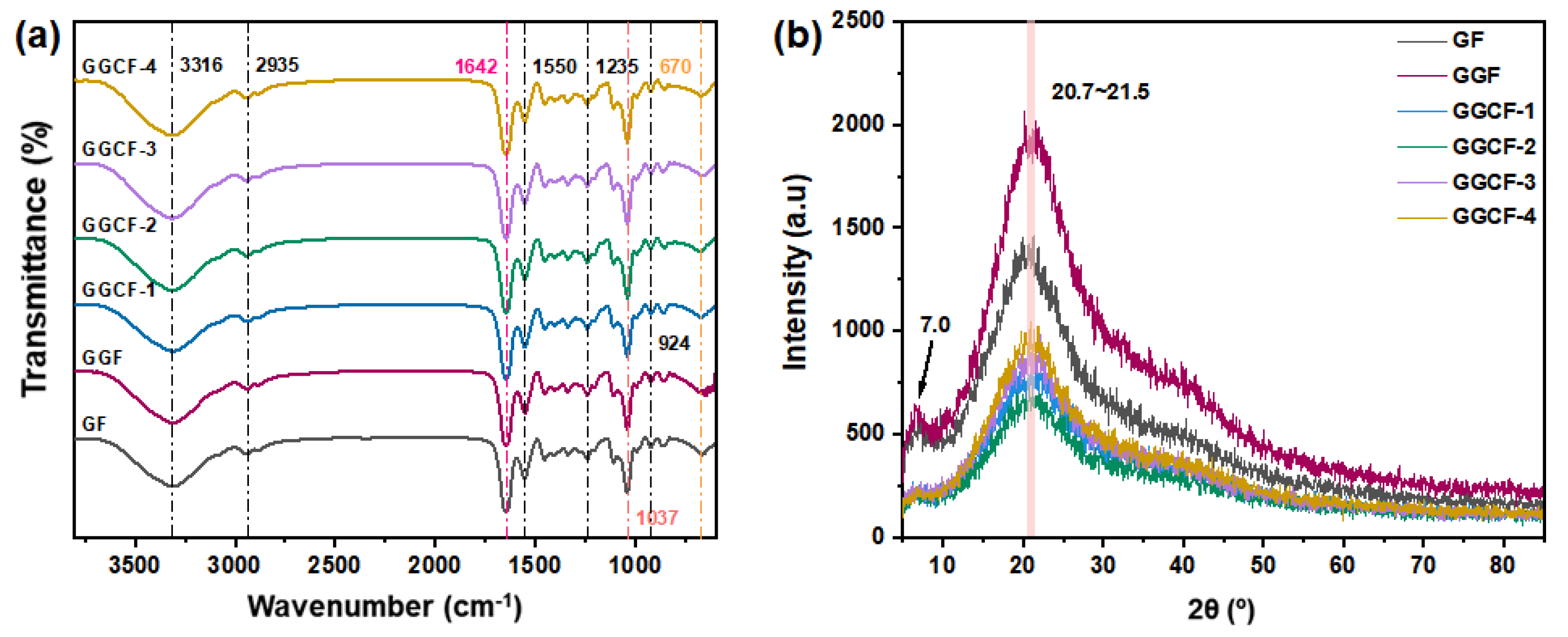
3.2. FTIR and XRD Analyses of GBFs
3.3. Microstructure of GBFs
3.4. Surface Color and Optical Properties
3.5. WVP and WCA of GBFs
3.6. Mechanical Strength
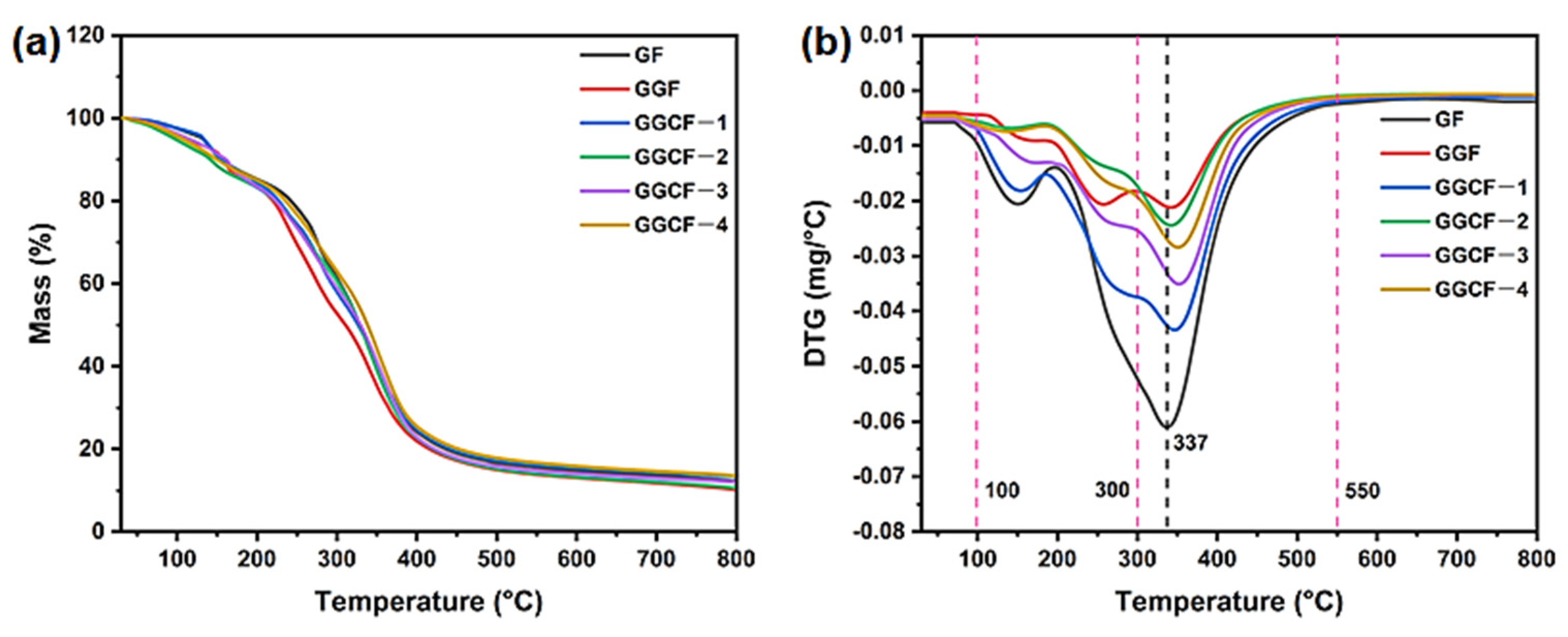
3.7. Thermal Stability
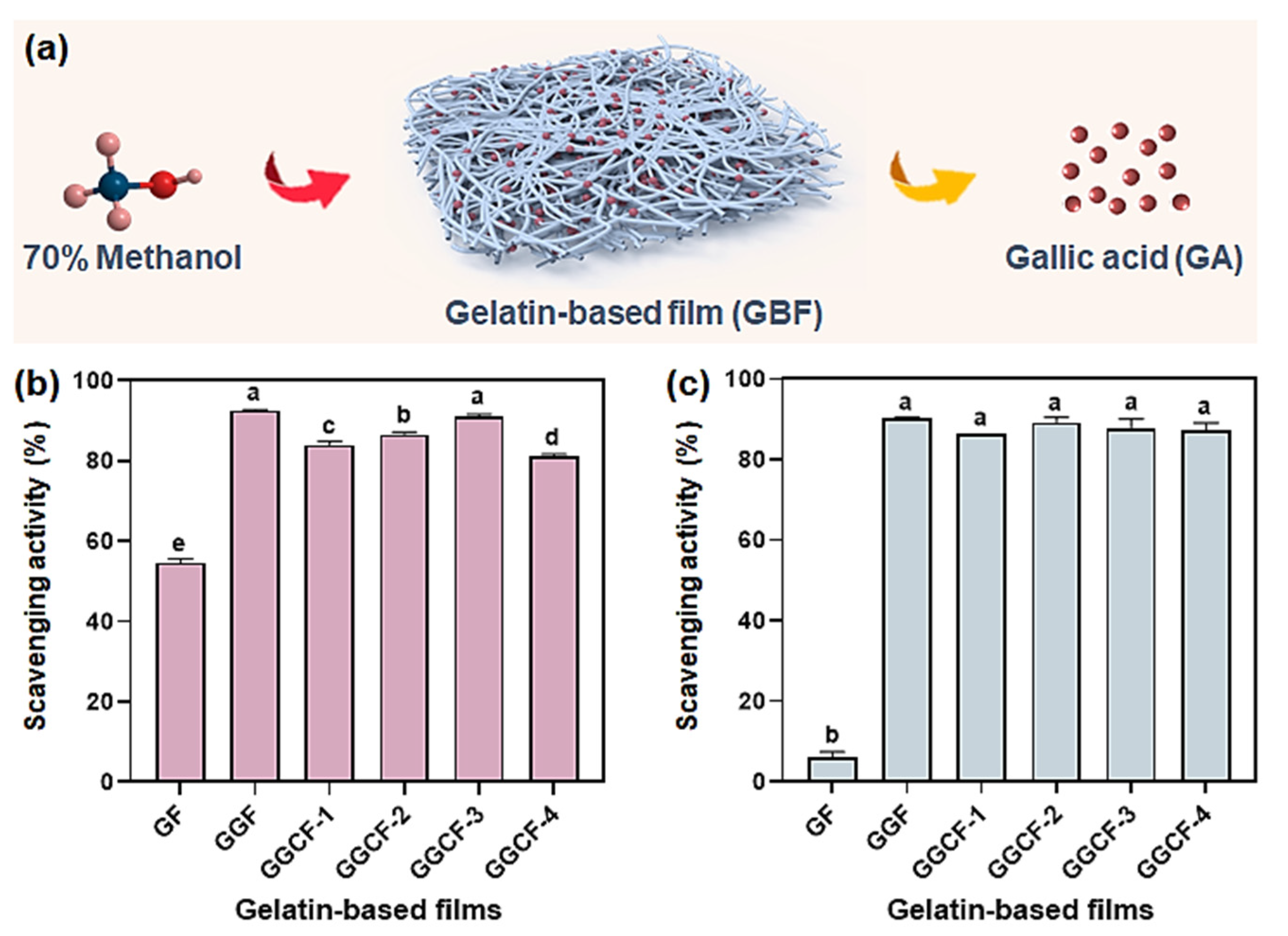
3.8. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Rhim, J. Preparation of antimicrobial and antioxidant gelatin/curcumin composite films for active food packaging application. Colloids Surf. B Biointerfaces 2020, 188, 110761. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Ghorbani, K.; Kouchaki, S.; Sadeghi, N.; Eslamifarsani, E.; Rabbani, F.; Beyramysoltan, S. Distinguishing tissue origin of bovine gelatin in processed products using LC/MS technique in combination with chemometrics tools. Food Chem. 2020, 319, 126302. [Google Scholar]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Shankar, S.; Rhim, J.-W. In situ synthesis of multi-functional gelatin/resorcinol/silver nanoparticles composite films. Food Packag. Shelf Life 2019, 22, 100399. [Google Scholar] [CrossRef]
- Guo, L.; Qiang, T.; Ma, Y.; Ren, L.; Zhu, C. Biodegradable Anti-Ultraviolet Film from Modified Gallic Acid Cross-linked Gelatin. ACS Sustain. Chem. Eng. 2021, 9, 8393–8401. [Google Scholar] [CrossRef]
- Shankar, S.; Jaiswal, L.; Selvakannan, P.R.; Ham, K.S.; Rhim, J.W. Gelatin-based dissolvable antibacterial films reinforced with metallic nanoparticles. RSC Adv. 2016, 6, 67340–67352. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.E.; Jin, H.J. Chemical and physical reinforcement of hydrophilic gelatin film with di-aldehyde nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nam, J.; Yun, H.; Jin, H.; Won, H. Aquatic polymer-based edible films of fish gelatin crosslinked with alginate dialdehyde having enhanced physicochemical properties. Carbohydr. Polym. 2021, 254, 117317. [Google Scholar] [CrossRef]
- Scopel, B.S.; Pretto, G.L.; Corrêa, J.I.P.; Baldasso, C.; Dettmer, A.; Santana, R.M.C. Starch-leather waste gelatin films cross-linked with glutaraldehyde. J. Polym. Environ. 2020, 28, 1974–1984. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Effect of date fruit waste extract as an antioxidant additive on the properties of active gelatin films. Food Chem. 2021, 355, 129631. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Loo, C.P.Y.; Sarbon, N.M. Chicken skin gelatin films with tapioca starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- Syahida, N.; Fitry, I.; Zuriyati, A.; Hanani, N. Effects of palm wax on the physical, mechanical and water barrier properties of fish gelatin films for food packaging application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, morphological and thermal behaviour characterisations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning structure and properties of gelatin edible films through pullulan dialdehyde crosslinking. LWT 2021, 138, 110607. [Google Scholar] [CrossRef]
- Ding, W.; Wu, Y. Sustainable dialdehyde polysaccharides as versatile building blocks for fabricating functional materials: An overview. Carbohydr. Polym. 2020, 248, 116801. [Google Scholar] [CrossRef]
- Ouyang, J.; Pu, S.; Wang, J.; Deng, Y.; Yang, C.; Naseer, S.; Li, D. Enzymatic hydrolysate of geniposide directly acts as cross-linking agent for enzyme immobilization. Process Biochem. 2020, 99, 187–195. [Google Scholar] [CrossRef]
- Guo, L.; Qiang, T.; Ma, Y.; Ren, L.; Dai, T. Purification and characterization of hydrolysable tannins extracted from Coriaria nepalensis bark using macroporous resin and their application in gallic acid production. Ind. Crops Prod. 2021, 162, 113302. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, L.; Gu, Z.; Yu, X. Feasibility study of the naturally occurring dialdehyde carboxymethyl cellulose for biological tissue fixation. Carbohydr. Polym. 2015, 115, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crop. Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose–Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Zheng, X.; Chen, M.; Tang, K. Soluble soybean polysaccharide/nano zinc oxide antimicrobial nanocomposite films reinforced with microfibrillated cellulose. Int. J. Biol. Macromol. 2020, 159, 793–803. [Google Scholar] [CrossRef]
- Cozzolino, C.A.; Cerri, G.; Brundu, A.; Farris, S. Microfibrillated cellulose (MFC): Pullulan bionanocomposite films. Cellulose 2014, 21, 4323–4335. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.; Yin, S.; Yang, X.; Wang, Q.; Liu, F.; Wei, Z. Characterization of gelatin-based edible fi lms incorporated with olive oil. Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Quero, F.; Padilla, C.; Campos, V.; Luengo, J.; Caballero, L.; Melo, F.; Li, Q.; Eichhorn, S.J.; Enrione, J. Stress transfer and matrix-cohesive fracture mechanism in microfibrillated cellulose-gelatin nanocomposite films. Carbohydr. Polym. 2018, 195, 89–98. [Google Scholar] [CrossRef]
- Ding, W.; Guo, S.; Liu, H.; Pang, X.; Ding, Z. Synthesis of an amino-terminated waterborne polyurethane-based polymeric dye for high-performance dyeing of biomass-derived aldehyde-tanned chrome-free leather. Mater. Today Chem. 2021, 21, 100508. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, O.Y.; Hwang, S. The effect of chemical surface treatment on the fracture toughness of micro fi brillated cellulose reinforced epoxy composites. J. Ind. Eng. Chem. 2017, 45, 301–306. [Google Scholar] [CrossRef]
- Khazraji, A.C.; Robert, S. Self-Assembly and Intermolecular Forces When Cellulose and Water Interact Using Molecular Modeling. J. Nanomater. 2013, 2013, 745979. [Google Scholar] [CrossRef]
- Larsson, P.A.; Riazanova, A.V.; Cinar Ciftci, G.; Rojas, R.; Øvrebø, H.H.; Wågberg, L.; Berglund, L.A. Towards optimised size distribution in commercial microfibrillated cellulose: A fractionation approach. Cellulose 2019, 26, 1565–1575. [Google Scholar] [CrossRef] [Green Version]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Pereda, M.; Ponce, A.G.; Marcovich, N.E.; Ruseckaite, R.A.; Martucci, J.F. Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll. 2011, 25, 1372–1381. [Google Scholar] [CrossRef]
- Shankar, S.; Teng, X.; Li, G.; Rhim, J.-W. Preparation, characterization, and antimicrobial activity of gelatin/ZnO nanocomposite films. Food Hydrocoll. 2015, 45, 264–271. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Xiao, J.; Liu, Y.; Zhao, Y.; Liu, A. Performance of high amylose starch-composited gelatin films influenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Hilal, N.; Lim, Y.P.; Amin, I.N.H.M.; Raslan, R. Atomic force microscopy as a tool for asymmetric polymeric membrane characterization. Sains Malaysiana 2011, 40, 237–244. [Google Scholar]
- Wang, W.; Liu, Y.; Jia, H.; Liu, Y.; Zhang, H.; He, Z.; Ni, Y.H. Effects of Cellulose Nanofibers Filling and Palmitic Acid Emulsions Coating on the Physical Properties of Fish Gelatin Films. Food Biophys. 2017, 12, 23–32. [Google Scholar] [CrossRef]
- Hietala, M.; Mathew, A.P.; Oksman, K. Bionanocomposites of thermoplastic starch and cellulose nanofibers manufactured using twin-screw extrusion. Eur. Polym. J. 2013, 49, 950–956. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.; Liu, Y. Physicochemical properties of gelatin films containing tea polyphenol-loaded chitosan nanoparticles generated by electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, C.M.; Salgado, P.R.; Dufresne, A.; Mauri, A.N. Microfibrillated cellulose addition improved the physicochemical and bioactive properties of biodegradable films based on soy protein and clove essential oil. Food Hydrocoll. 2018, 79, 416–427. [Google Scholar] [CrossRef]
- Li, J.; Miao, J.; Wu, J.; Chen, S.; Zhang, Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Bitencourt, C.M.; Fávaro-Trindade, C.S.; Sobral, P.J.A.; Carvalho, R.A. Gelatin-based films additivated with curcuma ethanol extract: Antioxidant activity and physical properties of films. Food Hydrocoll. 2014, 40, 145–152. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Zhou, Y.; Luo, J.; Li, J.; Li, X.; Shi, S.Q.; Li, J. Bioinspired interface design of multifunctional soy protein-based biomaterials with excellent mechanical strength and UV-blocking performance. Compos. Part B 2021, 224, 109187. [Google Scholar] [CrossRef]
- Jin, S.; Li, K.; Xia, C.; Li, J. Sodium alginate-assisted route to antimicrobial biopolymer film combined with aminoclay for enhanced mechanical behaviors. Ind. Crops Prod. 2019, 135, 271–282. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Jiang, S.; Zhou, Y.; Li, J.; Li, K.; Shi, S.Q.; Li, J. Full Bio-Based Soy Protein Isolate Film Enhanced by Chicken Feather Keratin. Macromol. Mater. Eng. 2021, 306, 2100004. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Pang, H.; Li, Z.; Zhang, W.; Zhang, S.; Li, J.; Li, L. Designing Biomimetic Microphase-Separated Motifs to Construct Mechanically Robust Plant Protein Resin with Improved Water-Resistant Performance. Macromol. Mater. Eng. 2020, 305, 1900462. [Google Scholar] [CrossRef]
- You, X.; Wang, X.; Zhang, H.J.; Cui, K.; Zhang, A.; Wang, L.; Yadav, C.; Li, X. Supertough lignin hydrogels with multienergy dissipative structures and ultrahigh antioxidative activities. ACS Appl. Mater. Interfaces 2020, 12, 39892–39901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Yang, D.; Qiu, X. Biomimetic supertough and strong biodegradable polymeric materials with improved thermal properties and excellent UV-blocking performance. Adv. Funct. Mater. 2019, 29, 1806912. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Antioxidative activity and properties of fish skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll. 2008, 22, 449–458. [Google Scholar] [CrossRef]


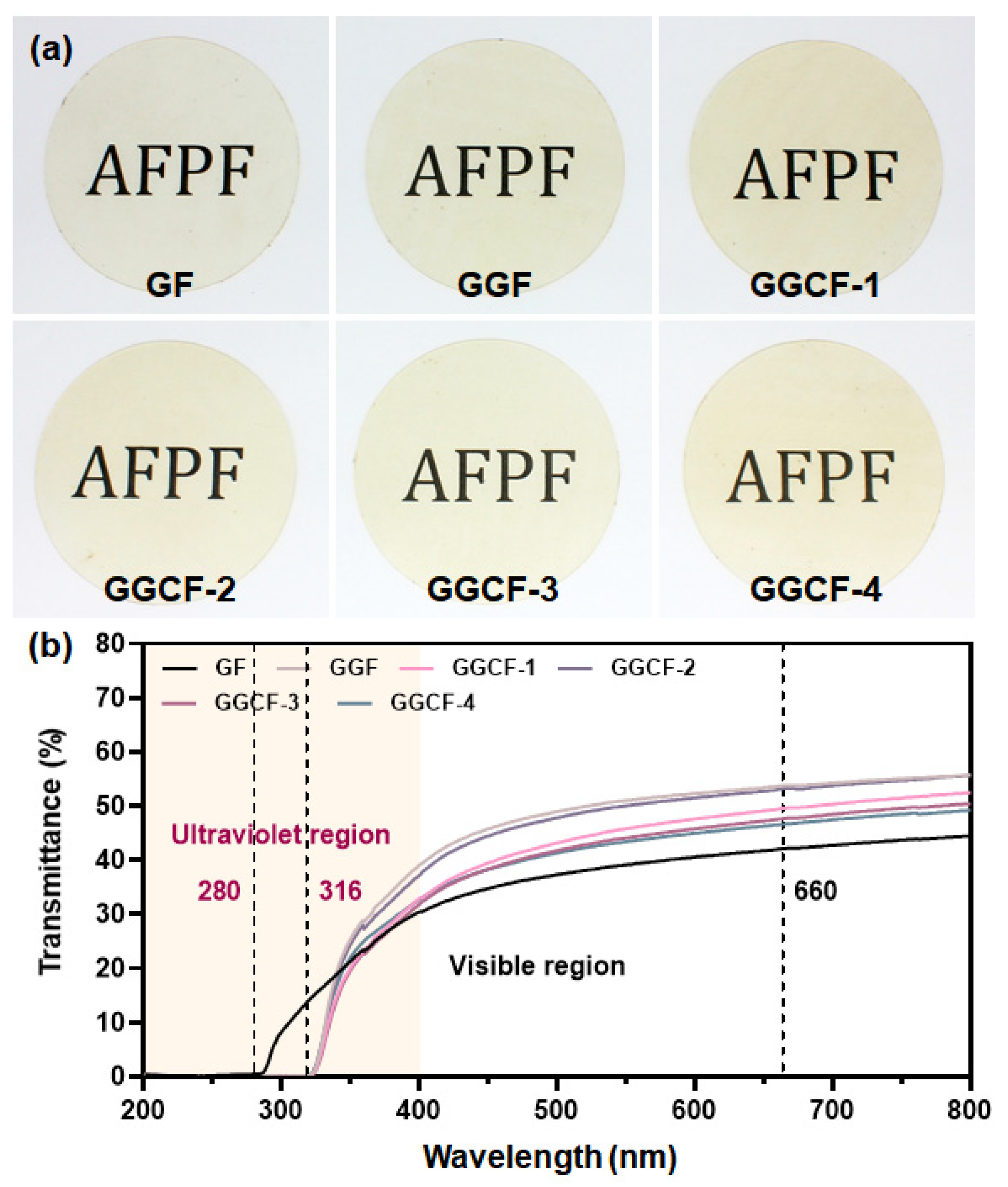

| Films | L * | A * | B * | ∆E | T280/% | T660/% |
|---|---|---|---|---|---|---|
| GF | 89.90 ± 0.44 a | −0.84 ± 0.31 a | 8.29 ± 0.58 c | 8.30 | 0.4 | 42.0 |
| GGF | 88.16 ±0.44 b | −0.69 ± 0.43 a | 10.77 ± 0.20 b | 11.25 | 0.3 | 53.0 |
| GGCF-1 | 87.86 ± 0.16 b | −0.63 ± 0.32 a | 12.08 ± 0.52 ab | 12.47 | 0.3 | 49.4 |
| GGCF-2 | 87.30 ± 0.29 bc | −0.30 ± 0.58 a | 12.30 ± 0.88 ab | 12.98 | 0.3 | 53.1 |
| GGCF-3 | 86.78 ± 0.02 c | −0.90 ± 0.34 a | 12.71 ± 0.13 a | 13.62 | 0.3 | 47.5 |
| GGCF-4 | 87.41 ± 0.31 bc | −0.72 ± 0.24 a | 12.78 ± 0.32 a | 13.30 | 0.3 | 46.5 |
| Films | WVP (×10−8 g·m−1·Pa−1·s−1) * | WCA (deg.) * |
|---|---|---|
| GF | 3.985 ± 0.001 abc | 69.30 ± 1.41 b |
| GGF | 4.379 ± 0.225 ab | 75.16 ± 0.49 a |
| GGCF-1 | 3.768 ± 0.194 bc | 76.35 ± 0.81 a |
| GGCF-2 | 3.894 ± 0.082 bc | 79.25 ± 1.61 a |
| GGCF-3 | 4.093 ± 0.079 ab | 79.48 ± 2.21 a |
| GGCF-4 | 3.634 ± 0.189 c | 80.22 ± 2.27 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Wu, Y.; Wang, Y.; Yu, L. Active and Robust Composite Films Based on Gelatin and Gallic Acid Integrated with Microfibrillated Cellulose. Foods 2021, 10, 2831. https://doi.org/10.3390/foods10112831
Luo Y, Wu Y, Wang Y, Yu L. Active and Robust Composite Films Based on Gelatin and Gallic Acid Integrated with Microfibrillated Cellulose. Foods. 2021; 10(11):2831. https://doi.org/10.3390/foods10112831
Chicago/Turabian StyleLuo, Yinghua, Yanbei Wu, Yali Wang, and Liangli (Lucy) Yu. 2021. "Active and Robust Composite Films Based on Gelatin and Gallic Acid Integrated with Microfibrillated Cellulose" Foods 10, no. 11: 2831. https://doi.org/10.3390/foods10112831
APA StyleLuo, Y., Wu, Y., Wang, Y., & Yu, L. (2021). Active and Robust Composite Films Based on Gelatin and Gallic Acid Integrated with Microfibrillated Cellulose. Foods, 10(11), 2831. https://doi.org/10.3390/foods10112831






