Impact of Chilling Rate on the Evolution of Volatile and Non-Volatile Compounds in Raw Lamb Meat during Refrigeration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electronic Nose Analysis
2.3. Electronic Tongue Analysis
2.4. Volatile Compounds Analysis
2.4.1. Headspace Solid-Phase Microextraction/Gas Chromatography-Mass Spectrometry (HS-SPME/GC–MS) Analysis
2.4.2. Calculations of Odor Activity Values (OAVs)
2.5. Free Amino Acids Analysis
2.6. Nucleotide Analysis
2.7. Calculations of Equivalent Umami Concentrations (EUCs) and Taste Active Values (TAVs)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Volatile and Non-Volatile Compounds Identification of Raw Meat with Different Chilling Rates and Different Refrigeration Times
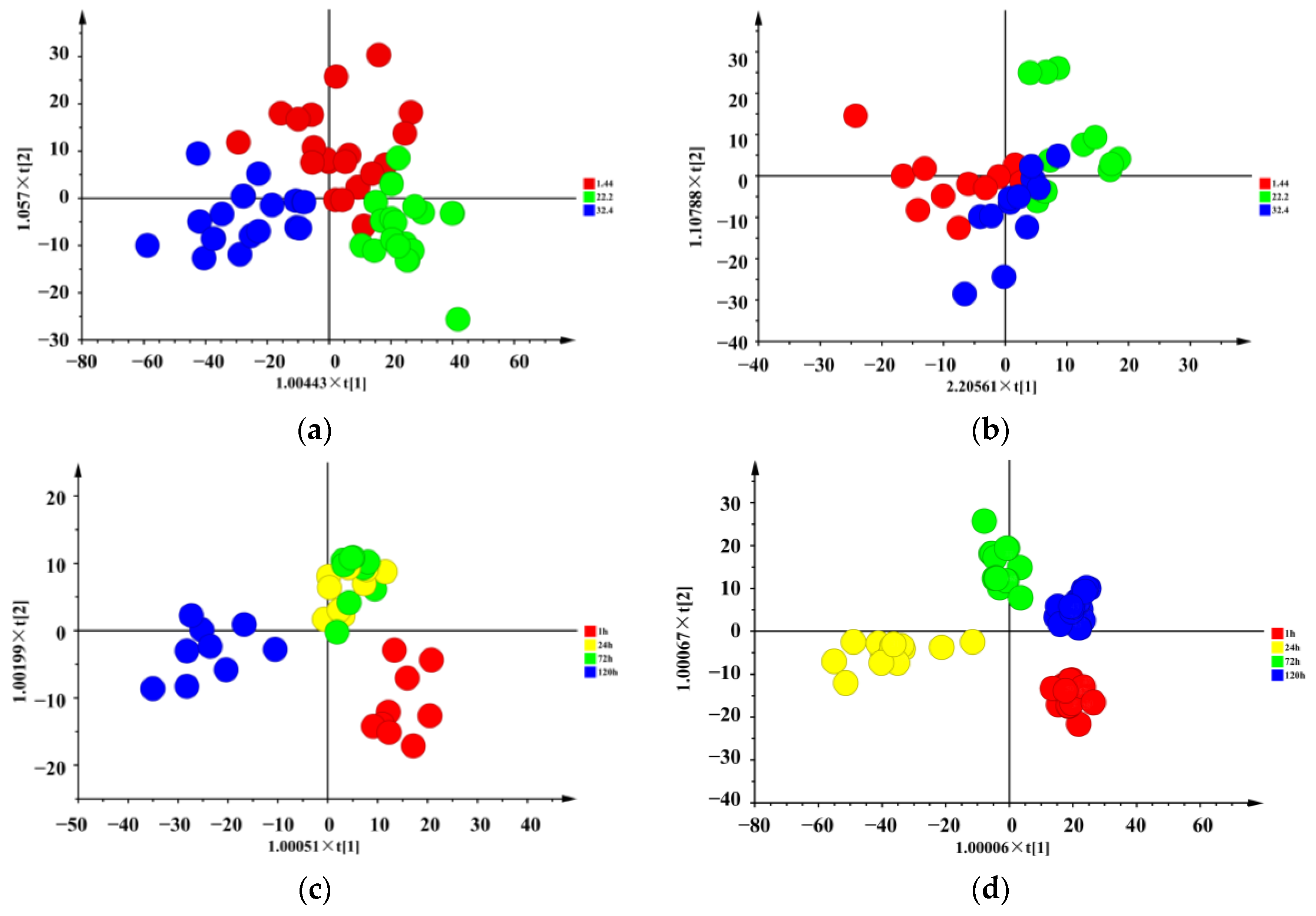
3.1.1. Effect of Chilaling Rates
3.1.2. Effect of Refrigeration Times
3.2. Identification of Key Volatile and Non-Volatile Compounds in Raw Meat during Refrigeration
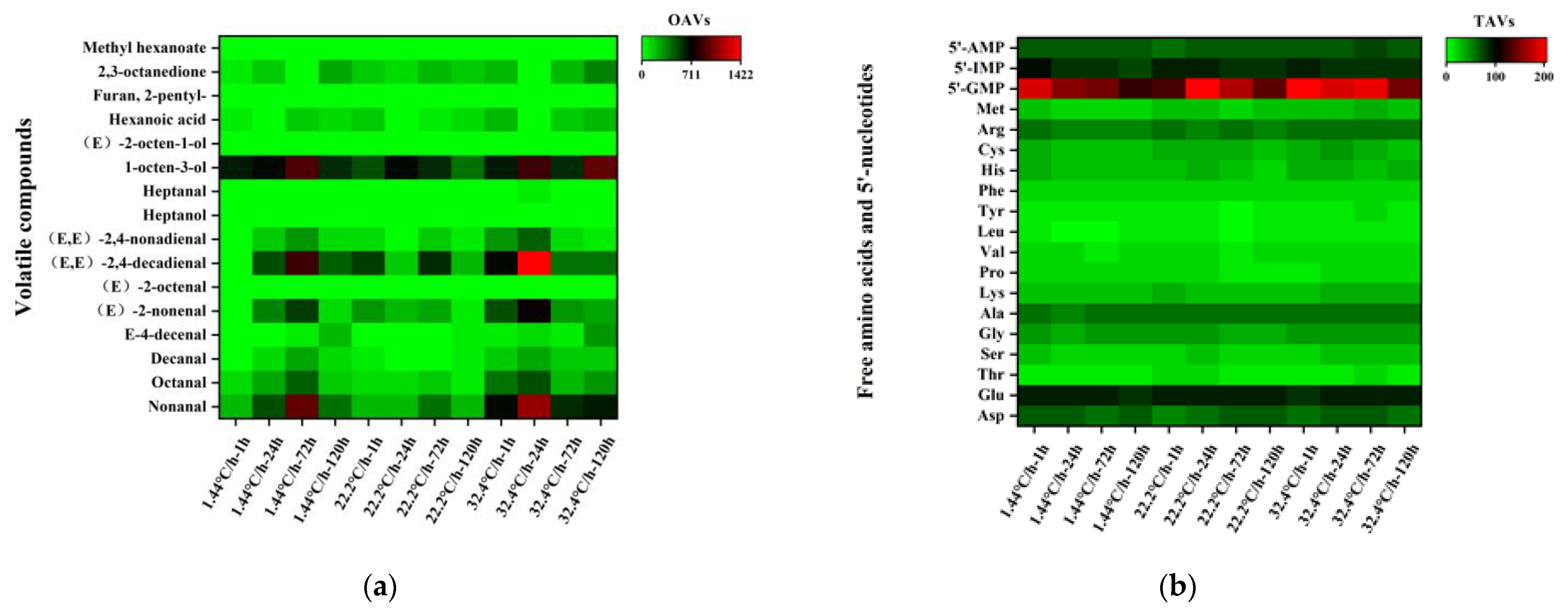
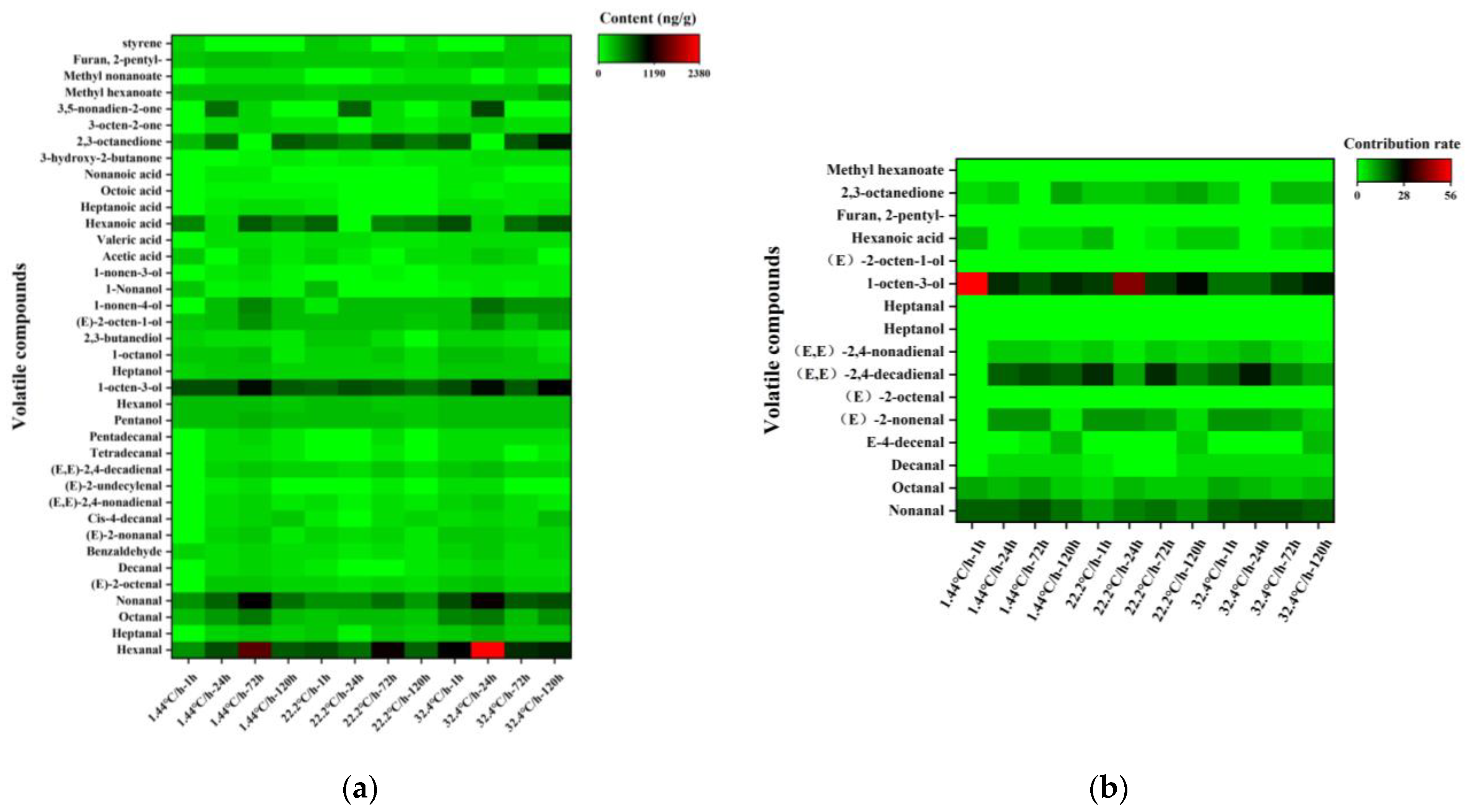
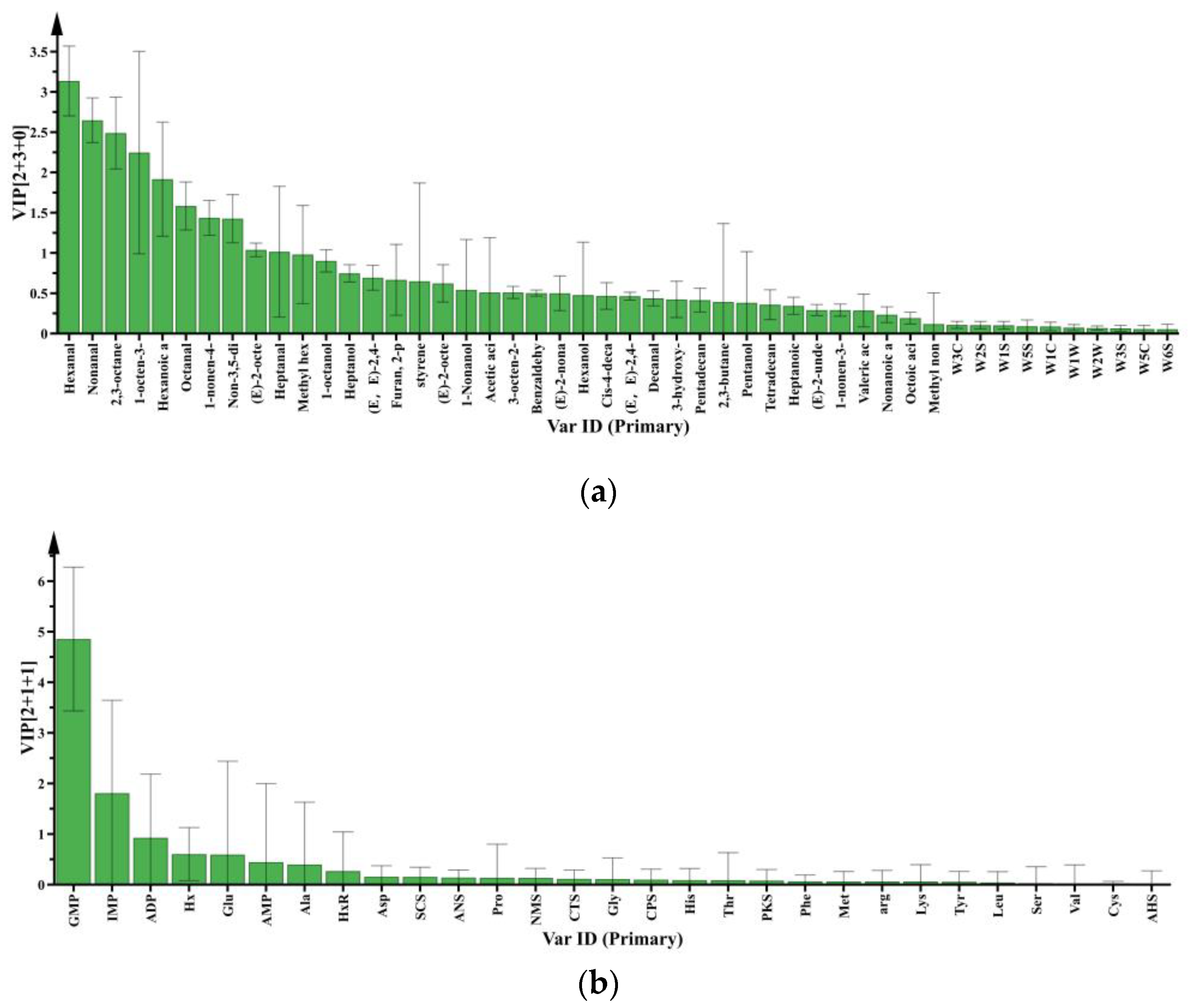
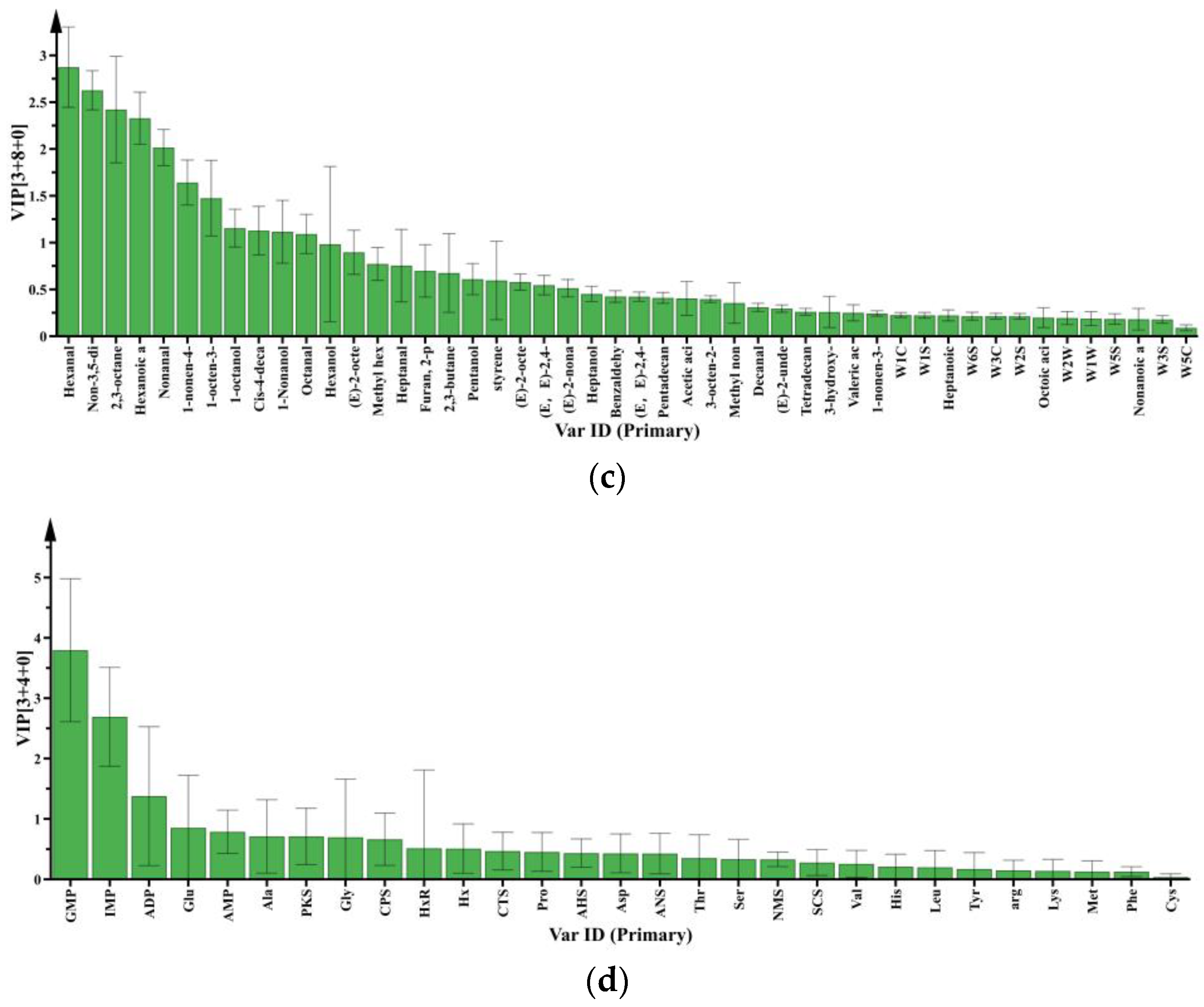
3.2.1. Key Volatile and Non-Volatile Compounds
3.2.2. Differential Metabolites in Raw Meat: Effect of Chilling Rates
3.2.3. Differential Metabolites in Raw Meat: Effect of Refrigeration Time
3.3. Changes in Key Volatile and Non-Volatile Compounds in Raw Meat at Different Chilling Rates and Different Refrigeration Times
3.3.1. Changes in Key Volatile Compounds
3.3.2. Changes in Key Non-Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Ferreira, V.; Escudero, A. Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples. Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbatov, V.M.; Lyaskovskaya, Y.N. Review of the flavour-contributing volatiles and water-soluble non-volatiles in pork meat and derived products. Meat Sci. 1980, 4, 209–225. [Google Scholar] [CrossRef]
- Neethling, J.; Hoffman, L.C.; Muller, M. Factors influencing the flavour of game meat: A review. Meat Sci. 2016, 113, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Li, C.B.; Chen, Y.J.; Xu, X.L.; Huang, M.; Hu, T.J.; Zhou, G.H. Effects of low-voltage electrical stimulation and rapid chilling on meat quality characteristics of Chinese Yellow crossbred bulls. Meat Sci. 2010, 72, 9–17. [Google Scholar] [CrossRef]
- Tomovic, V.M.; Petrovic, L.S.; Dzinic, N.R. Effects of rapid chilling of carcasses and time of deboning on weight loss and technological quality of pork semimembranosus muscle. Meat Sci. 2008, 80, 1188–1193. [Google Scholar] [CrossRef]
- Bertram, H.C.; Donstrup, S.; Karlsson, A.H.; Andersen, H.J.; Stodkilde-Jorgensen, H. Post mortem energy metabolism and pH development in porcine M-longissimus dorsi as affected by two different cooling regimes. A P-31-NMR spectroscopic study. Magn. Reson. Imaging 2001, 19, 993–1000. [Google Scholar] [CrossRef]
- Holmer, S.F.; McKeith, F.K.; Killefer, J. The influence of early post-mortem enhancement and accelerated chilling on pork quality. Meat Sci. 2008, 79, 211–216. [Google Scholar] [CrossRef]
- Sikes, A.L.; Jacob, R.; D’Arcy, B.; Warner, R. Very fast chilling modifies the structure of muscle fibres in hot-boned beef loin. Food Res. Int. 2017, 93, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.B.; Ma, D.; Setyabrata, D.; Farouk, M.M.; Lonergan, S.M.; Huff-Lonergan, E.; Hunt, M.C. Understanding postmortem biochemical processes and post-harvest aging factors to develop novel smart-aging strategies. Meat Sci. 2018, 144, 74–90. [Google Scholar] [CrossRef]
- Long, V.P.; Tarrant, P.V. The effect of pre-slaughter showering and post-slaughter rapid chilling on meat quality in intact pork sides. Meat Sci. 1990, 27, 181–195. [Google Scholar] [CrossRef]
- Kondjoyan, A.; Daudin, J.D. Optimisation of air-flow conditions during the chilling and storage of carcasses and meat products. J. Food Eng. 1997, 34, 243–258. [Google Scholar] [CrossRef]
- AUS-MEAT. International Red Meat Manual; AUS-MEAT Limited: Queensland, Australia, 2005; p. 110. [Google Scholar]
- Wu, H.; Yue, T.; Xu, Z.; Zhang, C. Sensor array optimization and discrimination of apple juices according to variety by an electronic nose. Anal. Methods 2017, 9, 921–928. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Pan, T.; Suleman, R.; Wang, Z.; Zhang, D. Effects of roasting by charcoal, electric, microwave and superheated steam methods on volatile and non-volatile compounds in oyster cuts of roasted lamb. Meat Sci. 2021, 172, 108324. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of Key Aroma Compounds in Beijing Roasted Duck by Gas Chromatography-Olfactometry-Mass Spectrometry, Odor-Activity Values, and Aroma-Recombination Experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, Q.; Xie, J.; Cheng, J.; Sun, B.; Du, W.; Wang, Y.; Wang, T. Aroma Compounds in Chicken Broths of Beijing Youji and Commercial Broilers. J. Agric. Food Chem. 2018, 66, 10242–10251. [Google Scholar] [CrossRef]
- Zhang, Z.; Elfalleh, W.; He, S.; Tang, M.; Zhao, J.; Wu, Z.; Wang, J.; Sun, H. Heating and cysteine effect on physicochemical and flavor properties of soybean peptide Maillard reaction products. Int. J. Biol. Macromol. 2018, 120, 2137–2146. [Google Scholar] [CrossRef]
- Zou, Y.; Kang, D.; Liu, R.; Qi, J.; Zhou, G.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochemistry 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Offer, G. Modelling of the formation of pale, soft and exudative meat: Effects of chilling regime and rate and extent of glycolysis. Meat Sci. 1991, 30, 157–184. [Google Scholar] [CrossRef]
- Rathgeber, B.M.; Pato, M.D.; Boles, J.A.; Shand, P.J. Rapid post-mortem glycolysis and delay chilling of turkey carcasses cause alterations to protein extractability and degradation of breast muscle proteins. J. Agric. Food Chem. 1999, 47, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Kyla-Puhju, M.; Ruusunen, M.; Puolanne, E. Activity of porcine muscle glycogen debranching enzyme in relation to pH and temperature. Meat Sci. 2005, 69, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Matarneh, S.K.; Yen, C.N.; Bodmer, J.; El-Kadi, S.W.; Gerrard, D.E. Mitochondria influence glycolytic and tricarboxylic acid cycle metabolism under postmortem simulating conditions. Meat Sci. 2021, 172, 108316. [Google Scholar] [CrossRef] [PubMed]
- Frylinck, L.; Strydom, P.E.; Webb, E.C.; du Toit, E. Effect of South African beef production systems on post-mortem muscle energy status and meat quality. Meat Sci. 2013, 93, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Straadt, I.K.; Aaslyng, M.D.; Bertram, H.C. An NMR-based metabolomics study of pork from different crossbreeds and relation to sensory perception. Meat Sci. 2014, 96, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Richardson, R.I.; Sheard, P.R. Manipulating meat quality and composition. Proc Nutr. Soc. 1999, 58, 363–370. [Google Scholar] [CrossRef]
- Baron, C.P.; Kjaersgard, I.V.; Jessen, F.; Jacobsen, C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J. Agric. Food Chem. 2007, 55, 8118–8125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tu, J.; Zhou, H.; Lu, A.; Xu, B. A comprehensive insight into the effects of microbial spoilage, myoglobin autoxidation, lipid oxidation, and protein oxidation on the discoloration of rabbit meat during retail display. Meat Sci. 2021, 172, 108359. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Qi, J.; Wang, P.; Xu, X.; Zhou, G. Superchilled storage (−2.5 ± 1 °C) extends the retention of taste-active and volatile compounds of yellow-feather chicken soup. Anim. Sci. J. 2018, 89, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Mohrhauser, D.A.; Lonergan, S.M.; Huff-Lonergan, E.; Underwood, K.R.; Weaver, A.D. Calpain-1 activity in bovine muscle is primarily influenced by temperature, not pH decline. J. Anim. Sci. 2014, 92, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Imafidon, G.I.; Spanier, A.M. Unraveling the secret of meat flavor. Trends Food Sci. Technol. 1994, 5, 315–321. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Wang, H.; Xi, B.; He, X.; Yang, X.; Li, W. Analysis of volatile compounds and flavor fingerprint in Jingyuan lamb of different ages using gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2021, 175, 108449. [Google Scholar] [CrossRef]
- Insausti, K.; Murillo-Arbizu, M.T.; Urrutia, O.; Mendizabal, J.A.; Beriain, M.J.; Colle, M.J.; Bass, P.D.; Arana, A. Volatile Compounds, Odour and Flavour Attributes of Lamb Meat from the Navarra Breed as Affected by Ageing. Foods 2021, 10, 493. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; D’Souza, L.A. Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Crit. Rev. Food Sci. Nutr. 1986, 24, 141–243. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I. Volatile Profile of Raw Lamb Meat Stored at 4 ± 1 °C: The Potential of Specific Aldehyde Ratios as Indicators of Lamb Meat Quality. Foods 2018, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Girard, B.; Durance, T. Headspace volatiles of sockeye and pink salmon as affected by retort process. J. Food Sci. 2000, 65, 34–39. [Google Scholar] [CrossRef]
- Chen, W.; Li, W.; Yang, Y.; Yu, H.; Zhou, S.; Feng, J.; Li, X.; Liu, Y. Analysis and evaluation of tasty components in the pileus and stipe of Lentinula edodes at different growth stages. J. Agric. Food Chem. 2015, 63, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Fundamental properties of umami taste. J. Agric. Chem. Soc. Jpn. 1991, 65, 903–906. [Google Scholar]
- Sabikun, N.; Bakhsh, A.; Rahman, M.S.; Hwang, Y.H.; Joo, S.T. Volatile and nonvolatile taste compounds and their correlation with umami and flavor characteristics of chicken nuggets added with milkfat and potato mash. Food Chem. 2020, 343, 128499. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the relative taste intensity of some L-α-amino acids and 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Zheng, Y.; Xu, C.H.; Tao, N.P.; Wu, X.; Wang, X. Brackish water improves the taste quality in meat of adult male Eriocheir sinensis during the postharvest temporary rearing. Food Chem. 2021, 343, 128409. [Google Scholar] [CrossRef]
- Liu, C.; Ji, W.; Jiang, H.; Shi, Y.; He, L.; Gu, Z.; Zhu, S. Comparison of biochemical composition and non-volatile taste active compounds in raw, high hydrostatic pressure-treated and steamed oysters Crassostrea hongkongensis. Food Chem. 2020, 342, 128632. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, L.; Li, Q.; Wang, Y.; Chen, Q.; Kong, B. Impacts of different altitudes and natural drying times on lipolysis, lipid oxidation and flavour profile of traditional Tibetan yak jerky. Meat Sci. 2020, 162, 108030. [Google Scholar] [CrossRef]
- Minami, S.; Sato, M.; Shiraiwa, Y.; Iwamoto, K. Molecular characterization of adenosine 5′-monophosphate deaminase--the key enzyme responsible for the umami taste of nori (Porphyra yezoensis Ueda, Rhodophyta). Mar. Biotechnol. 2011, 13, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Jolley, P.D.; Honikel, K.O.; Hamm, R. Influence of temperature on the rate of post-mortem metabolism and water-holding capacity of bovine neck muscles. Meat Sci. 1981, 5, 99–107. [Google Scholar] [CrossRef]
- Stetzer, A.J.; Cadwallader, K.; Singh, T.K.; McKeith, F.K.; Brewer, M.S. Effect of enhancement and ageing on flavor and volatile compounds in various beef muscles. Meat Sci. 2008, 79, 13–19. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Torngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhao, Q.; Yang, Y.; Li, F.; Qin, Y.; Liu, X.; Yue, X.; Zhang, J. Integrated lipidomics and targeted metabolomics analyses reveal changes in flavor precursors in psoas major muscle of castrated lambs. Food Chem. 2020, 333, 127451. [Google Scholar] [CrossRef]
- Honikel, K.O.; Hamm, R. Influence of cooling and freezing of minced pre-rigor muscle on the breakdown of ATP and glycogen. Meat Sci. 1978, 2, 181–188. [Google Scholar] [CrossRef]
- Benet, I.; Guardia, M.D.; Ibanez, C.; Sola, J.; Arnau, J.; Roura, E. Low intramuscular fat (but high in PUFA) content in cooked cured pork ham decreased Maillard reaction volatiles and pleasing aroma attributes. Food Chem. 2016, 196, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.; Ryland, D.; Suh, M.; Aliani, M. The effect of chilled conditioning at 4 °C on selected water and lipid-soluble flavor precursors in Bison bison longissimus dorsi muscle and their impact on sensory characteristics. Meat Sci. 2014, 96, 136–146. [Google Scholar] [CrossRef]
- Resconi, V.C.; Bueno, M.; Escudero, A.; Magalhaes, D.; Ferreira, V.; Campo, M.M. Ageing and retail display time in raw beef odour according to the degree of lipid oxidation. Food Chem. 2018, 242, 288–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ba, H.; Amna, T.; Hwang, I. Significant influence of particular unsaturated fatty acids and pH on the volatile compounds in meat-like model systems. Meat Sci. 2013, 94, 480–488. [Google Scholar] [CrossRef]
- Kosowska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Li, X.; Li, Z.; Shen, Q.; Wang, Y.; Li, G.; Zhang, D. Effects of phosphorylation on mu-calpain activity at different incubation temperature. Food Res. Int. 2017, 100, 318–324. [Google Scholar] [CrossRef]
- Ren, C.; Hou, C.; Li, Z.; Li, X.; Bai, Y.; Zhang, D. Effects of temperature on protein phosphorylation in postmortem muscle. J. Sci. Food Agric. 2020, 100, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Koohmaraie, M.; Whipple, G.; Kretchmar, D.H.; Crouse, J.D.; Mersmann, H.J. Postmortem proteolysis in longissimus muscle from beef, lamb and pork carcasses. J. Anim. Sci. 1991, 69, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Sinz, Q.; Schwab, W. Metabolism of amino acids, dipeptides and tetrapeptides by Lactobacillus sakei. Food Microbiol. 2012, 29, 215–223. [Google Scholar] [CrossRef]
- Chen, L.; Bai, Y.; Everaert, N.; Li, X.; Tian, G.; Hou, C.; Zhang, D. Effects of protein phosphorylation on glycolysis through the regulation of enzyme activity in ovine muscle. Food Chem. 2019, 293, 537–544. [Google Scholar] [CrossRef] [PubMed]
| Compound a | LRI b | Identification e | Compound a | LRI b | Identification e | ||
|---|---|---|---|---|---|---|---|
| Calculated c | Literature d | Calculated c | Literature d | ||||
| Hexanal | 1076 | 1080 | MS, LRI, S | 2,3-Butanediol | 1571 | 1570 | MS, LRI |
| Heptanal | 1168 | 1170 | MS, LRI | 2-Octen-1-ol, (E)- | 1606 | 1603 | MS, LRI, S |
| Octanal | 1269 | 1275 | MS, LRI, S | 1-Nonen-4-ol | 1635 | - f | MS |
| Nonanal | 1376 | 1380 | MS, LRI | 1-Nonanol | 1657 | 1656 | MS, LRI, S |
| (E)-2-Octenal | 1412 | 1416 | MS, LRI, S | 1-Nonen-3-ol | 1747 | 1555 | MS, LRI |
| Decanal | 1481 | 1483 | MS, LRI, S | acetic acid | 1442 | 1441 | MS, LRI |
| Benzaldehyde | 1502 | 1495 | MS, LRI, S | Pentanoic acid | 1729 | 1734 | MS, LRI |
| (E)-2-Nonenal | 1518 | 1514 | MS, LRI, S | Hexanoic acid | 1835 | 1834 | MS, LRI, S |
| cis-4-Decenal | 1525 | 1542 | MS, LRI | Heptanoic acid | 1966 | 1967 | MS, LRI |
| (E, E)-2,4-Nonadienal | 1682 | 1687 | MS, LRI, S | Octanoic acid | 2068 | 2072 | MS, LRI |
| 2-Undecenal | 1739 | 1755 | MS, LRI | Nonanoic acid | 2171 | 2174 | MS, LRI |
| (E, E)-2,4-Decadienal | 1752 | 1763 | MS, LRI, S | 3-hydroxy-2-butanone | 1265 | 1259 | MS, LRI |
| Tetradecanal | 1900 | 1908 | MS, LRI | 2,3-Octanedione | 1311 | 1325 | MS, LRI |
| Pentadecanal | 2037 | 2042 | MS, LRI | 3-Octen-2-one | 1393 | 1414 | MS, LRI |
| 1-Pentanol | 1262 | 1261 | MS, LRI | 3,5-Nonadien-2-one | 1801 | - f | MS |
| 1-Hexanol | 1354 | 1356 | MS, LRI, S | Hexanoic acid, methyl ester | 1170 | 1177 | MS, LRI |
| 1-Octen-3-ol | 1447 | 1447 | MS, LRI, S | Nonanoic acid, methyl ester | 1477 | 1481 | MS, LRI |
| 1-Heptanol | 1452 | 1458 | MS, LRI, S | Furan, 2-pentyl- | 1202 | 1213 | MS, LRI, S |
| 1-Octanol | 1553 | 1554 | MS, LRI, S | styrene | 1234 | 1242 | MS, LRI |
| Content (mg/100 g) | Flavor Trait | Chilling Rate | Storage Time | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 72 h | 120 h | Storage Time | Chilling Rate | Storage Time × Chilling Rate | |||
| Asp | Umami | 1.44 °C/h | 4.6 ± 0.74 Aa | 3.52 ± 1.23 Aa | 3.36 ± 0.35 ABa | 2.77 ± 0.09 Ba | 0.004 | 0.004 | 0.000 |
| 22.2 °C/h | 3.21 ± 0.17 Ab | 1.1 ± 0.17 Cc | 3.46 ± 0.28 Aa | 2.85 ± 1.00 Ba | |||||
| 32.4 °C/h | 1.63 ± 0.14 Ac | 2.2 ± 0.54 Ab | 1.37 ± 0.10 Bb | 1.78 ± 0.17 Ab | |||||
| Glu | Umami | 1.44 °C/h | 40.67 ± 4.75 Bb | 33.81 ± 1.55 Cc | 57.08 ± 6.69 Aa | 62.76 ± 10.62 Aa | 0.000 | 0.000 | 0.000 |
| 22.2 °C/h | 66.2 ± 4.44 Aa | 67.72 ± 4.05 Aa | 41.78 ± 3.23 Bb | 67.17 ± 3.48 Aa | |||||
| 32.4 °C/h | 48.66 ± 0.22 Ab | 55.38 ± 8.30 Ab | 24.85 ± 6.05 Bc | 44.21 ± 7.59 Ab | |||||
| Thr | Sweet | 1.44 °C/h | 2.83 ± 0.36 ABa | 2.27 ± 0.37 Bb | 1.85 ± 0.02 Cb | 3.29 ± 0.64 Ab | 0.000 | 0.001 | 0.017 |
| 22.2 °C/h | 2.69 ± 0.24 Ca | 3.55 ± 0.32 Ba | 2.94 ± 0.12 Ca | 4.19 ± 0.16 Aa | |||||
| 32.4 °C/h | 2.47 ± 0.07 Ba | 3.49 ± 0.72 Aa | 1.81 ± 0.35 Cb | 3.03 ± 0.48 Ab | |||||
| Gly | Sweet | 1.44 °C/h | 14.68 ± 0.97 Aa | 11.8 ± 1.48 Bc | 9.38 ± 0.12 Bb | 12.83 ± 2.09 ABb | 0.010 | 0.000 | 0.002 |
| 22.2 °C/h | 10.47 ± 0.66 Bb | 14.68 ± 0.86 Ab | 8.45 ± 0.49 Cb | 14.63 ± 0.54 Aa | |||||
| 32.4 °C/h | 14.33 ± 0.23 Ba | 18.35 ± 2.66 Aa | 17.38 ± 2.95 Aa | 14.94 ± 2.19 ABa | |||||
| Ala | Sweet | 1.44 °C/h | 41.55 ± 3.66 Aa | 34.14 ± 3.52 Bb | 30.81 ± 0.13 Ca | 34.43 ± 5.69 Ba | 0.086 | 0.001 | 0.014 |
| 22.2 °C/h | 22.67 ± 1.23 Bc | 30.07 ± 2.12 Ac | 27.61 ± 1.46 Ab | 30.65 ± 1.93 Ab | |||||
| 32.4 °C/h | 27.84 ± 0.42 Bb | 38.54 ± 6.05 Aa | 29.14 ± 4.28 ABab | 31.12 ± 5.13 ABab | |||||
| Lys | Sweet/Bitter | 1.44 °C/h | 2.29 ± 0.29 Aa | 1.72± 0.19 Bb | 1.91 ± 0.02 Bb | 2.98 ± 0.53 Ab | 0.000 | 0.004 | 0.012 |
| 22.2 °C/h | 2.11 ± 0.15 Ca | 2.82 ± 0.11 Ba | 2.45 ± 0.41 Ba | 3.54 ± 0.04 Aa | |||||
| 32.4 °C/h | 1.84 ± 0.19 Bb | 2.53 ± 0.42 Aa | 2.48 ± 0.23 Aa | 2.73 ± 0.45 Ab | |||||
| Pro | Sweet/Bitter | 1.44 °C/h | 4.4 ± 0.06 Aa | 4.33 ± 0.22 Ab | 3.93 ± 0.15 Ba | 4.94 ± 0.86 Ab | 0.000 | 0.000 | 0.002 |
| 22.2 °C/h | 4.51 ± 0.14 Ba | 6.04 ± 0.54 Aa | 3.61 ± 0.49 Bab | 6.7 ± 0.55 Aa | |||||
| 32.4 °C/h | 4.17 ± 0.23 Bb | 5.4 ± 0.81 Aab | 3.22 ± 0.1 Cb | 4.33 ± 0.27 Bb | |||||
| Val | Sweet/Bitter | 1.44 °C/h | 3.03 ± 0.42 Aa | 2.53 ± 0.32 Bb | 1.33 ± 0.21 Cb | 2.81 ± 0.47 Bb | 0.000 | 0.000 | 0.003 |
| 22.2 °C/h | 2.44 ± 0.13 Cb | 3.08 ± 0.2 Ba | 2.35 ± 0.06 Ca | 3.43 ± 0.2 Aa | |||||
| 32.4 °C/h | 1.86 ± 0.14 Bc | 2.29 ± 0.41 Ab | 2.12 ± 0.31 Aa | 2.38 ± 0.4 Ab | |||||
| Leu | Bitter | 1.44 °C/h | 3.82 ± 0.4 Aa | 3.15 ± 0.37 Aa | 1.82 ± 0.15 Bc | 3.73 ± 0.64 Ab | 0.000 | 0.231 | 0.000 |
| 22.2 °C/h | 2.51 ± 0.22 Bb | 3.35 ± 0.25 Aa | 2.91 ± 0.12 Bb | 4.95 ± 0.34 Aa | |||||
| 32.4 °C/h | 2.28 ± 0.15 Bb | 3.18 ± 0.48 Aa | 3.6 ± 0.48 Aa | 3.6 ± 0.66 Ab | |||||
| Tyr | Bitter | 1.44 °C/h | 2.62 ± 0.24 Aa | 2.22 ± 0.1 Bb | 1.66 ± 0.04 Cb | 2.85 ± 0.45 Ab | 0.000 | 0.004 | 0.002 |
| 22.2 °C/h | 2.09 ± 0.14 Cb | 2.91 ± 0.19 Ba | 2.7 ± 0.16 Ba | 3.82 ± 0.19 Aa | |||||
| 32.4 °C/h | 2.05 ± 0.3 Bb | 2.97 ± 0.36 Aa | 3.08 ± 0.58 Aa | 3.06 ± 0.52 Ab | |||||
| Phe | Bitter | 1.44 °C/h | 2.48 ± 0.33 Ba | 2.68 ± 0.06 Ba | 2.57 ± 0.34 Bb | 3.27 ± 0.34 Aab | 0.000 | 0.183 | 0.034 |
| 22.2 °C/h | 2.25 ± 0.17 Ca | 2.88 ± 0.16 Ba | 2.87 ± 0.25 Bab | 3.72 ± 0.29 Aa | |||||
| 32.4 °C/h | 2.39 ± 0.12 Ba | 2.96 ± 0.13 Aa | 3.18 ± 0.12 Aa | 3.14 ± 0.34 Ab | |||||
| His | Bitter | 1.44 °C/h | 1.74 ± 0.25 Aa | 0.88 ± 0.32 Bb | 0.53 ± 0.21 Bb | 1.41 ± 0.51 ABa | 0.015 | 0.005 | 0.006 |
| 22.2 °C/h | 0.63 ± 0.39 Bb | 1.02 ± 0.22 Aab | 0.33 ± 0.03 Cb | 0.93 ± 0.05 Ab | |||||
| 32.4 °C/h | 0.74 ± 0.49 Bb | 1.37 ± 0.14 Aa | 1.17 ± 0.17 Aa | 1.24 ± 0.23 Aa | |||||
| Arg | Bitter/Sweet | 1.44 °C/h | 5.02 ± 0.56 Aa | 3.97 ± 0.47 Cb | 4.51 ± 0.13 Ba | 5.29 ± 0.91 Aab | 0.009 | 0.162 | 0.008 |
| 22.2 °C/h | 3.53 ± 0.24 Bb | 4.91 ± 0.36 Aa | 3.38 ± 0.25 Bb | 5.45 ± 0.32 Aa | |||||
| 32.4 °C/h | 3.86 ± 0.86 Ab | 4.22 ± 0.71 Aab | 4.52 ± 0.49 Aa | 4.37 ± 0.58 Ab | |||||
| Cys | Bitter/Sweet/Sulfurous | 1.44 °C/h | 0.13 ± 0.04 Aa | 0.08 ± 0.03 Ac | 0.19 ± 0 Aa | 0.23 ± 0.07 Aa | 0.043 | 0.061 | 0.001 |
| 22.2 °C/h | 0.06 ± 0.05 Ac | 0.13 ± 0.03 Ab | 0.07 ± 0.08 Ab | 0.18 ± 0.07 Ab | |||||
| 32.4 °C/h | 0.09 ± 0 Db | 0.19 ± 0.03 Aa | 0.12 ± 0 Bb | 0.07 ± 0 Cc | |||||
| Met | Bitter/Sweet/Sulfurous | 1.44 °C/h | 0.95 ± 0.06 Aa | 0.79 ± 0.16 Ab | 0.45 ± 0.05 Bc | 1.12 ± 0.18 Ab | 0.000 | 0.000 | 0.000 |
| 22.2 °C/h | 0.65 ± 0.21 Cb | 1.11 ± 0.11 Ba | 0.9 ± 0.04 Db | 2.29 ± 0.18 Aa | |||||
| 32.4 °C/h | 0.79 ± 0.09 Bb | 1.2 ± 0.17 Aa | 1.28 ± 0.12 Aa | 1.46 ± 0.29 Ab | |||||
| FAA | UN | 1.44 °C/h | 135.18 ± 13.53 Aa | 111.48 ± 6.93 Bb | 123.98 ± 5.93 Aa | 148.89 ± 24.62 Ab | 0.001 | 0.445 | 0.016 |
| 22.2 °C/h | 128.77 ± 7.69 Bb | 149.35 ± 9.17 Aa | 108.93 ± 6.03 Cb | 159.9 ± 7.23 Aa | |||||
| 32.4 °C/h | 118.59 ± 0.75 Bc | 148.82 ± 22.3 Aa | 102.98 ± 15.4 Cb | 125.65 ± 19.67 Ac | |||||
| Content (mg/100 g) | Flavor Trait | Chilling Rate | Storage Time | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 72 h | 120 h | Storage Time | Chilling Rate | Storage Time × Chilling Rate | |||
| ADP | UN | 1.44 °C/h | 64.72 ± 6.13 Cb | 103.76 ± 7.11 Ba | 113.22 ± 6.96 Ba | 140.56 ± 0.89 Aa | 0.000 | 0.048 | 0.002 |
| 22.2 °C/h | 106.13 ± 2.11 Ba | 71.98 ± 0.83 Cb | 85.12 ± 0.98 Cb | 146.69 ± 14.59 Aa | |||||
| 32.4 °C/h | 106.76 ± 12.69 Ba | 76.95 ± 8.73 Cb | 102.29 ± 11.1 Ba | 140.09 ± 11.13 Aa | |||||
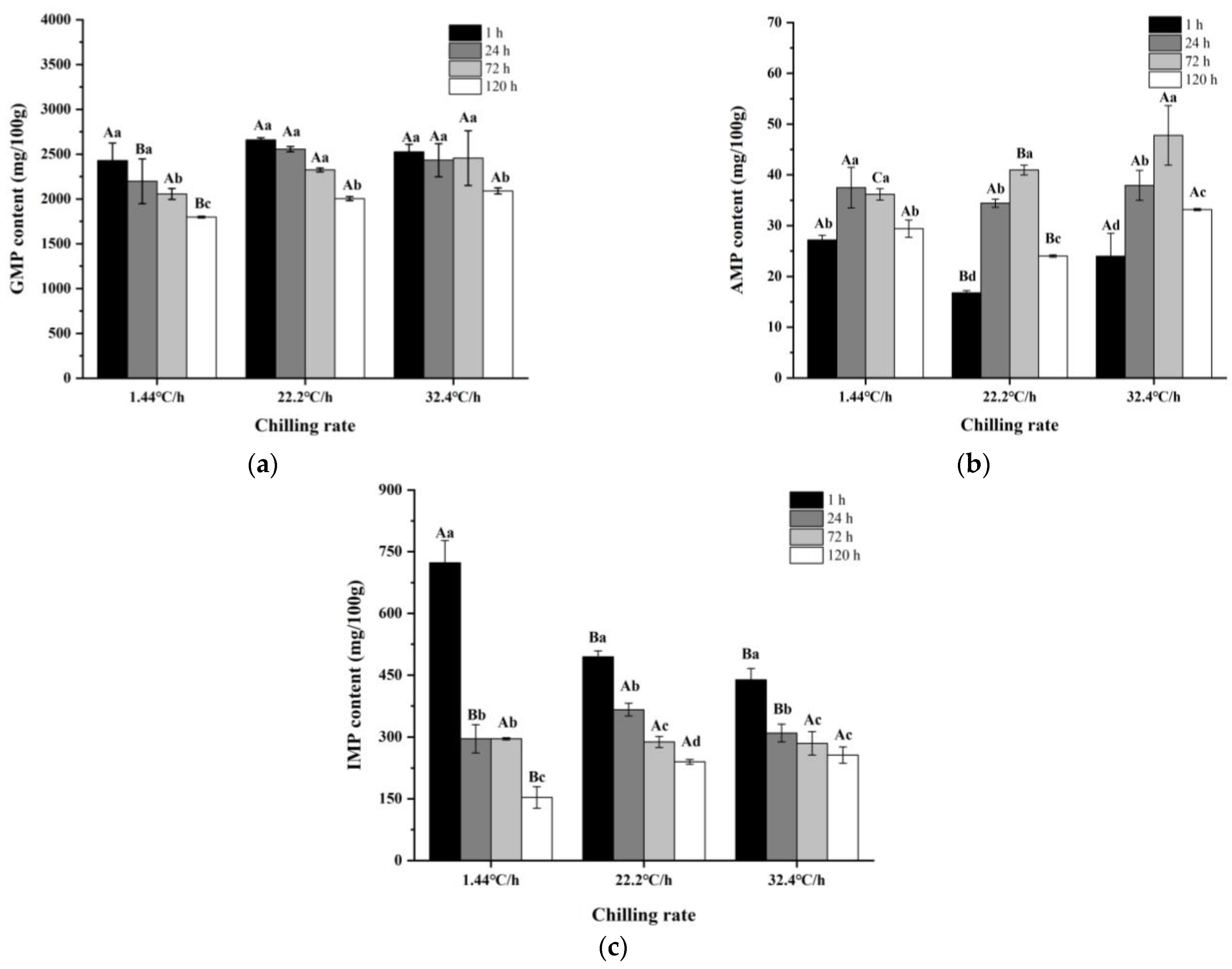
| AMP | Umami | 1.44 °C/h | 27.19 ± 0.9 Ca | 37.48 ± 3.98 Aa | 36.17 ± 1.12 Ac | 29.41 ± 1.69 Bb | 0.000 | 0.000 | 0.000 |
| 22.2 °C/h | 16.78 ± 0.41 Dc | 34.42 ± 0.82 Ba | 40.97 ± 0.97 Ab | 24 ± 0.24 Cc | |||||
| 32.4 °C/h | 23.99 ± 4.49 Db | 37.94 ± 2.95 Ba | 47.79 ± 5.87 Aa | 33.16 ± 0.21 Ca | |||||
| GMP | Umami/Sweet | 1.44 °C/h | 2430.04 ± 194.1 Ac | 2197.66 ± 249.65 Ac | 2056.68 ± 61.18 Bb | 1797.17 ± 8.92 Cb | 0.000 | 0.000 | 0.000 |
| 22.2 °C/h | 2660.13 ± 22.5 Aa | 2555.08 ± 29.53 Ba | 2324.4 ± 22.27 Ca | 2003.88 ± 22.97 Da | |||||
| 32.4 °C/h | 2526.15 ± 83.82 Ab | 2432.85 ± 185.17 Ab | 2457.26 ± 305.49 Aa | 2090.01 ± 33.49 Ba | |||||
| IMP | Umami | 1.44 °C/h | 722.99 ± 54.07 Aa | 296 ± 34.4 Bb | 295.93 ± 2.99 Ba | 153.41 ± 26.25 Cb | 0.000 | 0.000 | 0.000 |
| 22.2 °C/h | 494.55 ± 14.17 Ab | 366.11 ± 15.58 Ba | 288.36 ± 13.61 Ca | 239.94 ± 5.81 Da | |||||
| 32.4 °C/h | 438.62 ± 27.43 Ab | 309.9 ± 21.54 Ba | 284.74 ± 28.52 Ca | 256.37± 19.75 Da | |||||
| Hx | Bitter | 1.44 °C/h | 23.61 ± 1.05 Aa | 25.87 ± 5.42 Aa | 23.98 ± 2.34 Ac | 24.94 ± 0.21 Ab | 0.000 | 0.000 | 0.054 |
| 22.2 °C/h | 22.21 ± 6.07 Ba | 31.95 ± 1.3 Aa | 33.35 ± 0.75 Aa | 23.39 ± 0.22 Bb | |||||
| 32.4 °C/h | 25.97 ± 1.34 Aa | 28.26 ± 2.06 Aa | 27.2 ± 2.87 Ab | 28.65 ± 7.99 Aa | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, C.; Li, S.; Liu, H.; Liang, C.; Fang, F.; Zhang, D.; Wang, Z. Impact of Chilling Rate on the Evolution of Volatile and Non-Volatile Compounds in Raw Lamb Meat during Refrigeration. Foods 2021, 10, 2792. https://doi.org/10.3390/foods10112792
Xiang C, Li S, Liu H, Liang C, Fang F, Zhang D, Wang Z. Impact of Chilling Rate on the Evolution of Volatile and Non-Volatile Compounds in Raw Lamb Meat during Refrigeration. Foods. 2021; 10(11):2792. https://doi.org/10.3390/foods10112792
Chicago/Turabian StyleXiang, Can, Shaobo Li, Huan Liu, Ce Liang, Fei Fang, Dequan Zhang, and Zhenyu Wang. 2021. "Impact of Chilling Rate on the Evolution of Volatile and Non-Volatile Compounds in Raw Lamb Meat during Refrigeration" Foods 10, no. 11: 2792. https://doi.org/10.3390/foods10112792
APA StyleXiang, C., Li, S., Liu, H., Liang, C., Fang, F., Zhang, D., & Wang, Z. (2021). Impact of Chilling Rate on the Evolution of Volatile and Non-Volatile Compounds in Raw Lamb Meat during Refrigeration. Foods, 10(11), 2792. https://doi.org/10.3390/foods10112792






