Development and Characterization of Semi-Refined Iota Carrageenan/SiO2-ZnO Bionanocomposite Film with the Addition of Cassava Starch for Application on Minced Chicken Meat Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticle Suspension
2.3. Preparation of Bionanocomposite Films
2.4. Characterization of Bionanocomposite Films
2.4.1. Particle Size Distribution and Zeta Potential
2.4.2. FTIR Analysis
2.4.3. Thickness
2.4.4. Optical Properties
2.4.5. Film Morphology Analysis
2.4.6. Contact Angle and Critical Surface Tension
2.4.7. Water Vapor Permeability (WVP)
2.4.8. Water Solubility
2.4.9. Mechanical Properties
2.4.10. Thermal Stability
2.4.11. Antimicrobial Activity
2.4.12. Biodegradability
2.5. Application of Bionanocomposite Film for Minced Chicken Meat Packaging
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bionanocomposite Films
3.1.1. Particle Size Distribution
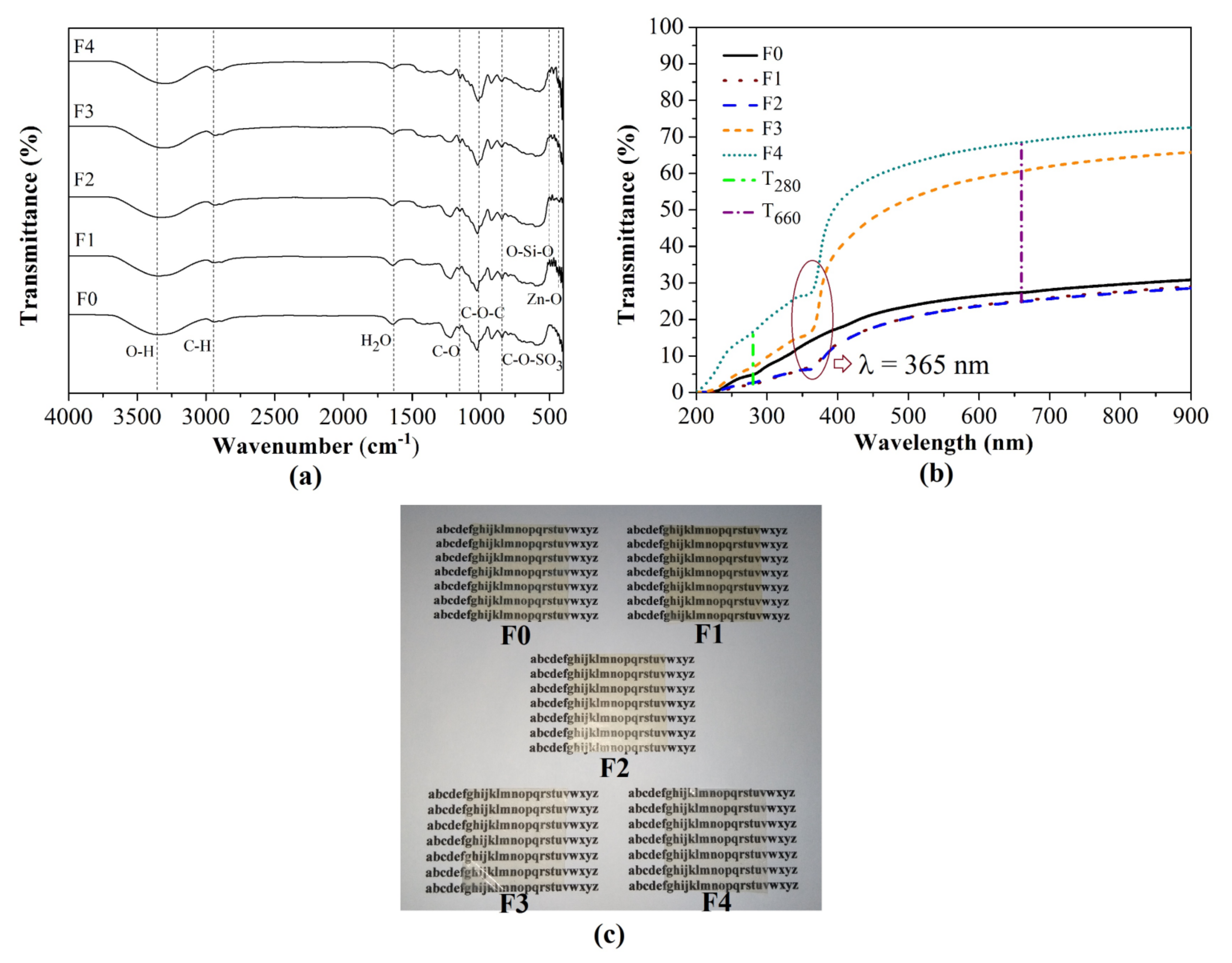
3.1.2. FTIR Analysis
3.1.3. Appearance, Thickness, and Optical Properties of Bionanocomposite Films
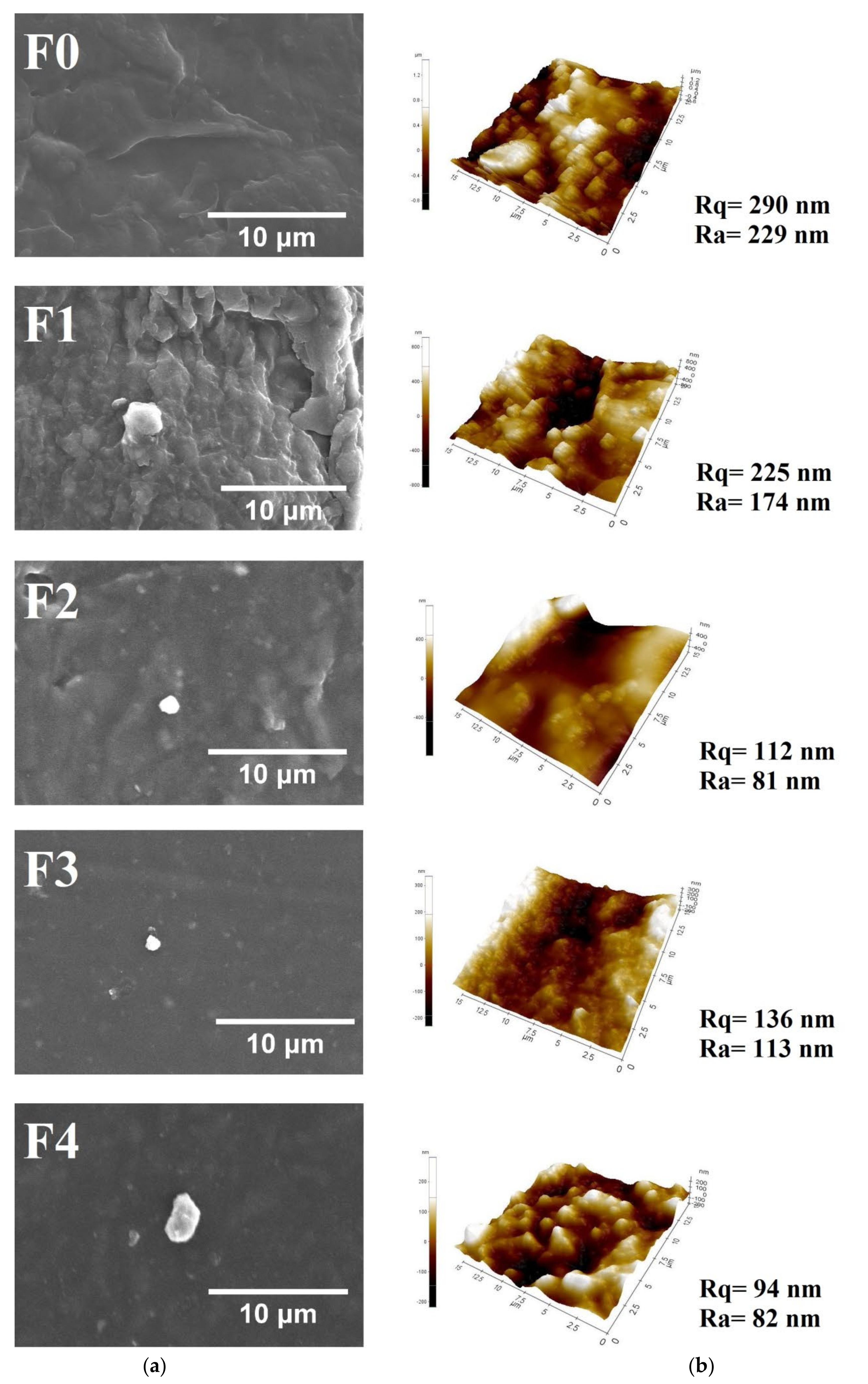
3.1.4. Film Morphology Analysis
3.1.5. Contact Angle and Critical Surface Tension
3.1.6. Water Vapor Permeability
3.1.7. Water Solubility
3.1.8. Mechanical Properties
3.1.9. Thermal Stability
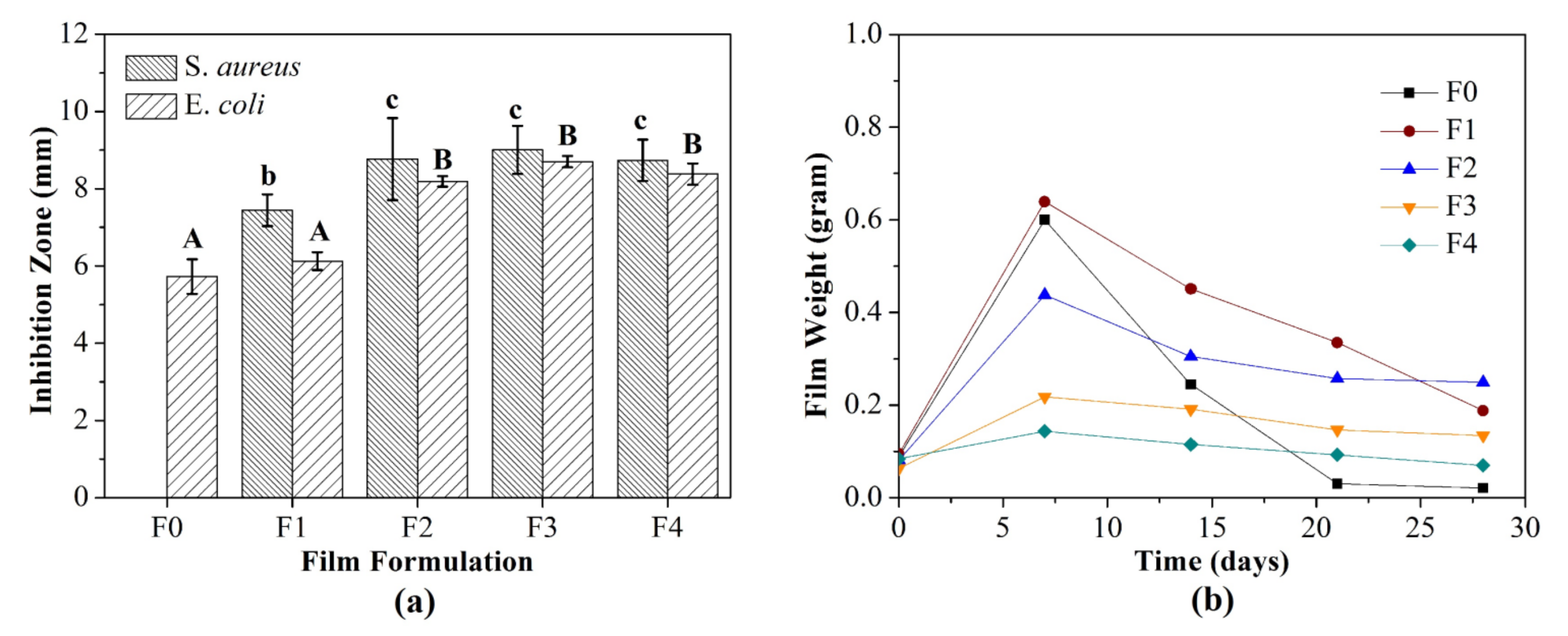
3.1.10. Antimicrobial Activity
3.1.11. Biodegradability
3.2. Application of Bionanocomposite Films for Minced Chicken Meat Packaging
3.2.1. Total Plate Count
3.2.2. pH
3.2.3. Weight Loss
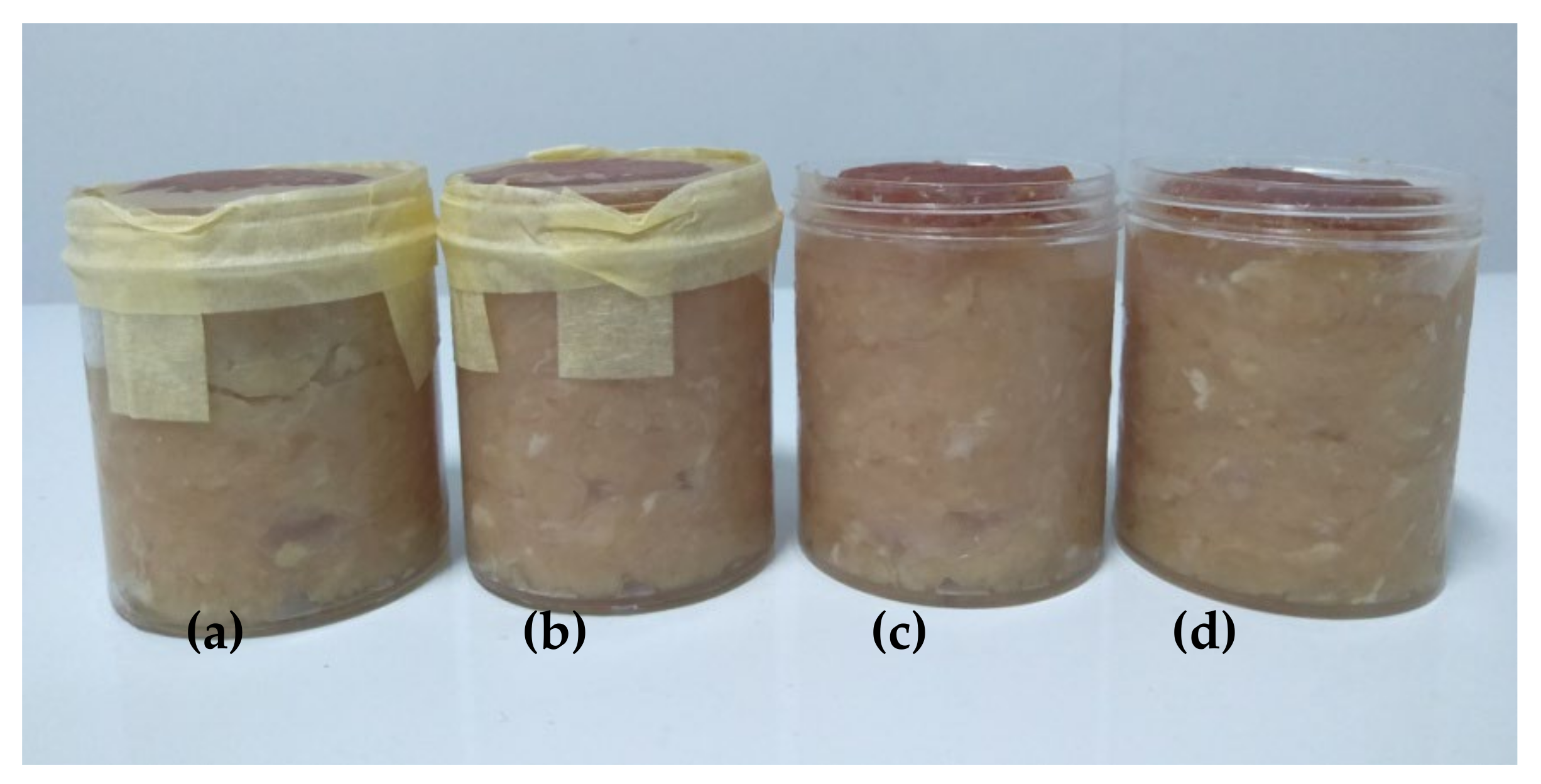
3.2.4. Meat Color
3.2.5. Thiobarbituric Acid Analysis
3.2.6. Total Volatile Base Nitrogen Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope. Plastics—The Facts 2019; PlasticsEurope: Brussels, Belgium, 2019. [Google Scholar]
- Wang, L.; Wu, W.-M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A Review of Property Enhancement Techniques for Carrageenan-based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef]
- Maciel, D.J.; de Mello Ferreira, I.L.; da Costa, G.M.; da Silva, M.R. Nanocomposite hydrogels based on iota-carrageenan and maghemite: Morphological, thermal and magnetic properties. Eur. Polym. J. 2016, 76, 147–155. [Google Scholar] [CrossRef]
- Aji, A.I.; Praseptiangga, D.; Rochima, E.; Joni, I.M.; Panatarani, C. Optical transparency and mechanical properties of semi-refined iota carrageenan film reinforced with SiO2 as food packaging material. AIP Conf. Proc. 2018, 1927, 030039. [Google Scholar]
- Khoirunnisa, A.R.; Joni, I.M.; Panatarani, C.; Rochima, E.; Praseptiangga, D. UV-screening, transparency and water barrier properties of semi refined iota carrageenan packaging film incorporated with ZnO nanoparticles. AIP Conf. Proc. 2018, 1927, 030041. [Google Scholar]
- Saputri, A.E.; Praseptiangga, D.; Rochima, E.; Panatarani, C.; Joni, I.M. Mechanical and solubility properties of bio-nanocomposite film of semi refined kappa carrageenan/ZnO nanoparticles. AIP Conf. Proc. 2018, 1927, 030040. [Google Scholar]
- Praseptiangga, D.; Mufida, N.; Panatarani, C.; Joni, I.M. Enhanced multi functionality of semi-refined iota carrageenan as food packaging material by incorporating SiO2 and ZnO nanoparticles. Heliyon 2021, 7, e06963. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.; Mohammadzadeh Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Friedrich, D. How regulatory measures towards biobased packaging influence the strategic behaviour of the retail industry: A microempirical study. J. Clean. Prod. 2020, 260, 121128. [Google Scholar] [CrossRef]
- Sapper, M.; Talens, P.; Chiralt, A. Improving Functional Properties of Cassava Starch-Based Films by Incorporating Xanthan, Gellan, or Pullulan Gums. Int. J. Polym. Sci. 2019, 2019, 5367164. [Google Scholar] [CrossRef] [Green Version]
- Loo, C.P.Y.; Sarbon, N.M. Chicken skin gelatin films with tapioca starch. Food Biosci. 2020, 35, 100589. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2017–2026; OECD-FAO Agricultural Outlook; OECD: Paris, France, 2017; ISBN 9789264275478. [Google Scholar]
- Cardona, M.; Gorriz, A.; Barat, J.M.; Fernández-Segovia, I. Perception of fat and other quality parameters in minced and burger meat from Spanish consumer studies. Meat Sci. 2020, 166, 108138. [Google Scholar] [CrossRef]
- Williams, H.; Lindström, A.; Trischler, J.; Wikström, F.; Rowe, Z. Avoiding food becoming waste in households—The role of packaging in consumers’ practices across different food categories. J. Clean. Prod. 2020, 265, 121775. [Google Scholar] [CrossRef]
- Panatarani, C.; Mufida, N.; Widyaastuti, D.; Praseptiangga, D.; Joni, I.M. Dispersion of SiO2 and ZnO nanoparticles by bead milling in the preparation of carrageenan bio-nanocomposite film. AIP Conf. Proc. 2020, 2219, 100002. [Google Scholar]
- Lamour, G.; Hamraoui, A.; Buvailo, A.; Xing, Y.; Keuleyan, S.; Prakash, V.; Eftekhari-Bafrooei, A.; Borguet, E. Contact Angle Measurements Using a Simplified Experimental Setup. J. Chem. Educ. 2010, 87, 1403–1407. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Water Vapor Transmission of Materials, E 96/E 96M-05; ASTM Annual Book of American Standard Testing Methods: Philadelphia, PA, USA, 2005; pp. 1–8. [Google Scholar]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. Int. J. Biol. Macromol. 2017, 98, 348–356. [Google Scholar] [CrossRef]
- Rhim, J. Effect of PLA lamination on performance characteristics of agar/κ-carrageenan/clay bio-nanocomposite film. Food Res. Int. 2013, 51, 714–722. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Degradation behavior of biocomposites based on cassava starch buried under indoor soil conditions. Carbohydr. Polym. 2014, 101, 20–28. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Agricultural Chemists 16th Edition; AOAC International: Arlington, Virginia, USA, 1995. [Google Scholar]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the Physicochemical and Structural Properties and the Sensory Characteristics of Meat Analogues Prepared with Various Non-Animal Based Liquid Additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanone, L.A.; Roche, L.A.; Salvadori, V.O.; Mascheroni, R.H. Monitoring of Weight Losses in Meat Products during Freezing and Frozen Storage. Food Sci. Technol. Int. 2002, 8, 229–238. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kamkar, A.; Misaghi, A. Nanocomposite films based on CMC, okra mucilage and ZnO nanoparticles: Physico mechanical and antibacterial properties. Carbohydr. Polym. 2018, 181, 351–357. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Senapati, M.; Sahu, P.P. Meat quality assessment using Au patch electrode Ag-SnO2/SiO2/Si MIS capacitive gas sensor at room temperature. Food Chem. 2020, 324, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Praseptiangga, D.; Zahara, H.L.; Widjanarko, P.I.; Joni, I.M.; Panatarani, C. Preparation and FTIR spectroscopic studies of SiO2-ZnO nanoparticles suspension for the development of carrageenan-based bio-nanocomposite film. AIP Conf. Proc. 2020, 2219, 100005. [Google Scholar]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Joni, I.M.; Nulhakim, L.; Vanitha, M.; Panatarani, C. Characteristics of crystalline silica (SiO2) particles prepared by simple solution method using sodium silicate (Na2SiO3) precursor. J. Phys. Conf. Ser. 2018, 1080, 012006. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Tensile Properties of Thin Plastic Sheeting 1 D 882-02; ASTM International: West Conshohocken, PA, USA, 1989; Volume 14. [Google Scholar]
- Roy, S.; Shankar, S.; Rhim, J.-W. Melanin-mediated synthesis of silver nanoparticle and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Silva, O.A.; Pellá, M.G.; Pellá, M.G.; Caetano, J.; Simões, M.R.; Bittencourt, P.R.S.; Dragunski, D.C. Synthesis and characterization of a low solubility edible film based on native cassava starch. Int. J. Biol. Macromol. 2019, 128, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J. Carrageenan-based antimicrobial bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Rhim, J.W. Preparation of carrageenan-based nanocomposite films incorporated with functionalized halloysite using AgNP and sodium dodecyl sulfate. Food Hydrocoll. 2020, 106, 105934. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; de Oliveira, V.R.L.; dos Santos, F.K.G.; de Barros, N.E.L.; de Lima, L.R.H.; Aroucha, E.M.M.; de Oliveira, S.K.N. Synergistic effect of the sequential intercalation of three types of surfactants in the exfoliation degree of bentonite clay in films of cassava. J. Mol. Liq. 2018, 266, 770–780. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kim, S.S.; Lee, J.; Lee, J. Effect of TiO2 on highly elastic, stretchable UV protective nanocomposite films formed by using a combination of k-Carrageenan, xanthan gum and gellan gum. Int. J. Biol. Macromol. 2019, 123, 1020–1027. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Jamroz, E.; Behalova, H.; Tremlova, B. Chemical and physical characteristics of edible films, based on κ- and ι-carrageenans with the addition of lapacho tea extract. Foods 2020, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- JIS. Japanese Industrial Standard Z 1707; Japanese Standard Association: Tokyo, Japan, 1975. [Google Scholar]
- Padhi, J.R.; Nayak, D.; Nanda, A.; Rauta, P.R.; Ashe, S.; Nayak, B. Development of highly biocompatible Gelatin & i-Carrageenan based composite hydrogels: In depth physiochemical analysis for biomedical applications. Carbohydr. Polym. 2016, 153, 292–301. [Google Scholar] [CrossRef]
- Xiong, H.; Tang, S.; Tang, H.; Zou, P. The structure and properties of a starch-based biodegradable film. Carbohydr. Polym. 2008, 71, 263–268. [Google Scholar] [CrossRef]
- Sullivan, W.G.; Wicks, E.M.; Koelling, C.P. Engineering Economy, 16th ed.; Pearson Education USA: Upper Saddle River, NJ, USA, 2015. [Google Scholar]
- BSN. SNI 3924:2009 Standard Quality of Carcass and Chicken Meat; Badan Standarisasi Nasional: Jakarta, Indonesia, 2009.
- Pathak, V.M.; Navneet. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 1–31. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Active edible films based on semi-refined κ-carrageenan: Antioxidant and color properties and application in chicken breast packaging. Food Packag. Shelf Life 2020, 24, 100476. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Maimuni, B.H.; Manuhara, G.J.; Muhammad, D.R.A. Mechanical and Barrier Properties of Semi Refined Kappa Carrageenan-based Composite Edible Film and Its Application on Minimally Processed Chicken Breast Fillet. IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 012086. [Google Scholar] [CrossRef] [Green Version]
- Instruments, P. Colour Meter PCE-TCD 100 Instruction Manual; PCE Instruments: Jupiter, FL, USA, 2010. [Google Scholar]
- O’Sullivan, M.G.; Kerry, J.P. Sensory and quality properties of packaged fresh and processed meats. In Advances in Meat, Poultry and Seafood Packaging; Elsevier: Amsterdam, The Netherlands, 2012; pp. 86–111. ISBN 9781845697518. [Google Scholar]

| Film Type | Thickness (μm) | T280 (%) | T660 (%) | TS (MPa) | EAB (%) |
|---|---|---|---|---|---|
| F0 | 73.375 ± 3.365 b | 4.890 | 24.834 | 9.131 ± 2.676 d | 24.129 ± 9.210 a |
| F1 | 83.675 ± 3.430 c | 2.238 | 25.078 | 7.323 ± 0.595 cd | 25.529 ± 2.180 a |
| F2 | 69.150 ± 0.777 ab | 2.679 | 27.362 | 5.194 ± 1.604 bc | 27.962 ± 5.768 a |
| F3 | 73.425 ± 3.860 b | 6.908 | 60.603 | 4.192 ± 0.905 ab | 19.616 ± 7.494 a |
| F4 | 65.200 ± 4.090 a | 16.579 | 68.456 | 1.907 ± 0.380 a | 16.897 ± 9.388 a |
| Film Type | θwater (°) | γc (m N/m) | WVP (10−10 g/m·Pa·s) | WS (%) |
|---|---|---|---|---|
| F0 | 100.967 ± 10.950 a | 18.064 ± 1.750 ab | 3.072 ± 0.080 a | 65.976 ± 2.092 c |
| F1 | 94.083 ± 8.228 a | 18.635 ± 1.438 ab | 2.951 ± 0.067 a | 76.341 ± 3.563 d |
| F2 | 105.017 ± 5.229 a | 18.427 ± 1.092 ab | 3.877 ± 0.465 b | 81.381 ± 4.340 d |
| F3 | 105.250 ± 7.957 a | 21.693 ± 2.523 b | 4.105 ± 0.378 b | 39.210 ± 7.680 a |
| F4 | 90.817 ± 5.849 a | 16.910 ± 3.680 a | 3.847 ± 0.552 b | 51.895 ± 7.162 b |
| Parameter | Day | A0 | A1 | A2 | A3 |
|---|---|---|---|---|---|
| TPC (log CFU/g) | 0 | 5.559 ± 0.004 aA | 5.559 ± 0.004 aA | 5.559 ± 0.004 aA | 5.559 ± 0.004 aA |
| 3 | 6.145 ± 0.470 aA | 5.903 ± 0.269 aA | 5.678 ± 0.017 aA | 5.698 ± 0.049 aAB | |
| 6 | 5.980 ± 0.079 aA | 6.290 ± 0.059 bA | 6.241 ± 0.071 bA | 6.191 ± 0.053 bAB | |
| 9 | 6.069 ± 0.381 aA | 6.029 ± 0.106 aA | 6.295 ± 0.078 aA | 7.025 ± 0.087 bAB | |
| 12 | 6.444 ± 0.878 aA | 6.307 ± 0.336 aA | 7.734 ± 0.157 aA | 7.513 ± 0.826 aB | |
| pH | 0 | 5.987 ± 0.025 aAB | 5.987 ± 0.025 aA | 5.987 ± 0.025 aA | 5.987 ± 0.025 aA |
| 3 | 5.791 ± 0.460 aA | 6.047 ± 0.011 aB | 6.001 ± 0.021 aA | 6.018 ± 0.008 aB | |
| 6 | 6.062± 0.015 aAB | 6.080 ± 0.008 aBC | 6.078 ± 0.008 aB | 6.071 ± 0.017 aC | |
| 9 | 6.107 ± 0.003 aAB | 6.301 ± 0.054 dD | 6.237 ± 0.018 cC | 6.182 ± 0.003 bE | |
| 12 | 6.163 ± 0.011 abB | 6.127 ± 0.037 aC | 6.210 ± 0.084 bC | 6.123 ± 0.005 aD | |
| WHC (%) | 0 | 108.611 ± 2.678 aD | 108.611 ± 2.678 aC | 108.611 ± 2.678 aD | 108.611 ± 2.678 aC |
| 3 | 87.783 ± 2.163 aB | 96.398 ± 1.282 bB | 98.405 ± 0.806 bC | 86.753 ± 1.941 aAB | |
| 6 | 90.153 ± 1.165 bBC | 89.278 ± 1.772 bB | 83.636 ± 1.616 aB | 88.190 ± 2.804 bAB | |
| 9 | 83.312 ± 3.244 bA | 79.629 ± 2.423 bA | 72.138 ± 1.995 aA | 84.653 ± 4.818 bA | |
| 12 | 92.502 ± 2.622 aC | 90.508 ± 9.359 aB | 86.200 ± 1.004 aB | 91.291 ± 2.395 aB | |
| WL (%) | 0 | 0 aA | 0 aA | 0 aA | 0 aA |
| 3 | 9.148 ± 2.504 bB | 3.545 ± 0.599 aB | 4.269 ± 0.310 aB | 3.880 ± 0.516 aB | |
| 6 | 13.405 ± 0.639 cC | 4.668 ± 0.193 aC | 5.517 ± 0.287 bC | 5.994 ± 0.625 bC | |
| 9 | 19.044 ± 0.669 cD | 5.431 ± 0.227 aD | 6.593 ± 0.423 bD | 7.402 ± 0.903 bD | |
| 12 | 23.498 ± 1.111 dE | 6.810 ± 0.164 aE | 8.228 ± 0.246 bE | 10.172 ± 1.014 cE | |
| ΔE | 0 | - | - | - | - |
| 3 | 3.412 ± 0.137 bA | 2.526 ± 0.276 aB | 3.300 ± 0.292 bB | 2.795 ± 0.389 aA | |
| 6 | 3.005 ± 0.724 aA | 3.886 ± 0.612 aC | 3.620 ± 0.109 aB | 2.976 ± 0.715 aA | |
| 9 | 5.081 ± 0.066 bB | 3.061 ± 0.681 aB | 2.310 ± 0.209 aA | 3.033 ± 1.790 aA | |
| 12 | 8.987 ± 0.011 dC | 1.265 ± 0.277 aA | 2.146 ± 0.978 bA | 3.717 ± 0.336 cA | |
| TBA (mg MDA/kg sample) | 0 | 0.293 ± 0.130 aA | 0.293 ± 0.130 aA | 0.293 ± 0.130 aA | 0.293 ± 0.130 aA |
| 3 | 0.650 ± 0.061 aB | 0.656 ± 0.060 aBC | 0.561 ± 0.115 aB | 0.578 ± 0.104 aB | |
| 6 | 0.865 ± 0.138 bBC | 1.059 ± 0.029 cD | 1.014 ± 0.142 bcC | 0.703 ± 0.060 aB | |
| 9 | 0.984 ± 0.240 bC | 0.802 ± 0.205 abC | 0.680 ± 0.032 aB | 0.676 ± 0.004 aB | |
| 12 | 0.712 ± 0.213 aB | 0.566 ± 0.014 aB | 0.719 ± 0.064 aB | 1.131 ± 0.236 bC | |
| TVBN (mg N/100 g) | 0 | 5.423 ± 0.063 aA | 5.538 ± 0.109 aA | 5.423 ± 0.063 aA | 5.538 ± 0.109 aA |
| 3 | 25.248 ± 1.300 Cb | 19.570 ± 0.008 aB | 21.445 ± 0.073 bB | 24.478 ± 0.105 cB | |
| 6 | 23.848 ± 1.074 cB | 21.018 ± 1.518 aB | 21.965 ± 0.303 abB | 23.273 ± 0.587 bcC | |
| 9 | 35.878 ± 2.065 cC | 29.663 ± 0.120 bC | 23.478 ± 0.428 aC | 31.035 ± 0.450 bD | |
| 12 | 67.875 ± 1.738 cD | 54.993 ± 1.994 bD | 45.635 ± 1.658 aD | 45.458 ± 0.587 aE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praseptiangga, D.; Widyaastuti, D.; Panatarani, C.; Joni, I.M. Development and Characterization of Semi-Refined Iota Carrageenan/SiO2-ZnO Bionanocomposite Film with the Addition of Cassava Starch for Application on Minced Chicken Meat Packaging. Foods 2021, 10, 2776. https://doi.org/10.3390/foods10112776
Praseptiangga D, Widyaastuti D, Panatarani C, Joni IM. Development and Characterization of Semi-Refined Iota Carrageenan/SiO2-ZnO Bionanocomposite Film with the Addition of Cassava Starch for Application on Minced Chicken Meat Packaging. Foods. 2021; 10(11):2776. https://doi.org/10.3390/foods10112776
Chicago/Turabian StylePraseptiangga, Danar, Dea Widyaastuti, Camellia Panatarani, and I Made Joni. 2021. "Development and Characterization of Semi-Refined Iota Carrageenan/SiO2-ZnO Bionanocomposite Film with the Addition of Cassava Starch for Application on Minced Chicken Meat Packaging" Foods 10, no. 11: 2776. https://doi.org/10.3390/foods10112776
APA StylePraseptiangga, D., Widyaastuti, D., Panatarani, C., & Joni, I. M. (2021). Development and Characterization of Semi-Refined Iota Carrageenan/SiO2-ZnO Bionanocomposite Film with the Addition of Cassava Starch for Application on Minced Chicken Meat Packaging. Foods, 10(11), 2776. https://doi.org/10.3390/foods10112776








