Water Behavior of Aerogels Obtained from Chemically Modified Potato Starches during Hydration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aerogels
2.3. Aerogel Hydration
2.4. 1H NMR Relaxometry
2.5. Water Activity
2.6. Statistical Analysis
3. Results and Discussion
- A—maximum water activity reached by aerogel,
- B—lowest hydration value at maximum water activity (inflection point),
- C—limit hydration level at which only bound water is present in the system,
- h—the hydration value.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Kenar, J.A.; Eller, F.J.; Felker, F.C.; Jackson, M.A.; Fanta, G.F. Starch aerogel beads obtained from inclusion complexes prepared from high amylose starch and sodium palmitate. Green Chem. 2014, 16, 1921–1930. [Google Scholar] [CrossRef]
- García-González, C.A.; Uy, J.J.; Alnaief, M.; Smirnova, I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydr. Polym. 2012, 88, 1378–1386. [Google Scholar] [CrossRef]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöö, M.; Nilsson, L. (Eds.) Starch in Food: Structure, Function and Applications, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2018; ISBN 9780081008683. [Google Scholar]
- Egharevba, H.O. Chemical Properties of Starch and Its Application in the Food Industry. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch—Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Lewandowicz, G. Physical modification of starch—Really physical? In Proceedings of the 13th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 8–10 November 2017; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2017; pp. 9–14. [Google Scholar]
- Le Thanh-Blicharz, J.; Anioła, J.; Kowalczewski, P.; Przygoński, K.; Zaborowska, Z.; Lewandowicz, G. Type IV resistant starch increases cecum short chain fatty acids level in rats. Acta Biochim. Pol. 2014, 61, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Smigielska, H.; Le Thanh-Blicharz, J. Research on zinc fortified potato starch and on its use in dessert production. Acta Sci. Pol. Technol. Aliment. 2010, 9, 217–226. [Google Scholar]
- Górecki, A.; Błaszczak, W.; Lewandowicz, J.; Thanh-Blicharz, J.; Penkacik, K. Influence of High Pressure or Autoclaving-Cooling Cycles and Pullulanase Treatment on Buckwheat Starch Properties and Resistant Starch Formation. Pol. J. Food Nutr. Sci. 2018, 68, 235–242. [Google Scholar] [CrossRef]
- Bemiller, J.N. Starch Modification: Challenges and Prospects. Starch—Stärke 1997, 49, 127–131. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in chemical modifications of starches and their applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh-Blicharz, J.; Lewandowicz, J. Functionality of Native Starches in Food Systems: Cluster Analysis Grouping of Rheological Properties in Different Product Matrices. Foods 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of oxidised starch (E 1404), monostarch phosphate (E 1410), distarch phosphate (E 1412), phosphated distarch phosphate (E 1413), acetylated distarch phosphate (E 1414), acetylated starch (E 1420), acetylated distarch adipate (E 1422), hydrox. EFSA J. 2017, 15, e04911. [Google Scholar] [CrossRef]
- Ačkar, Đ.; Babić, J.; Jozinović, A.; Miličević, B.; Jokić, S.; Miličević, R.; Rajič, M.; Šubarić, D. Starch Modification by Organic Acids and Their Derivatives: A Review. Molecules 2015, 20, 19554–19570. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Acevedo-Fani, A.; McDowell, A.; Barnett, A.; Loveday, S.M.; Singh, H. Nanoemulsion structure and food matrix determine the gastrointestinal fate and in vivo bioavailability of coenzyme Q10. J. Control. Release 2020, 327, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, K.; Kędziora, P.; Le Thanh, J.; Lewandowicz, G. Surface properties of enzymatic hydrolysis products of octenylsuccinate starch derivatives. Food Hydrocoll. 2007, 21, 654–659. [Google Scholar] [CrossRef]
- Prochaska, K.; Kędziora, P.; Le Thanh, J.; Lewandowicz, G. Surface activity of commercial food grade modified starches. Colloids Surf. B Biointerfaces 2007, 60, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Agama-Acevedo, E.; Bello-Perez, L.A. Starch as an emulsions stability: The case of octenyl succinic anhydride (OSA) starch. Curr. Opin. Food Sci. 2017, 13, 78–83. [Google Scholar] [CrossRef]
- Fonseca, L.M.; da Silva, F.T.; Bruni, G.P.; Borges, C.D.; da Rosa Zavareze, E.; Dias, A.R.G. Aerogels based on corn starch as carriers for pinhão coat extract (Araucaria angustifolia) rich in phenolic compounds for active packaging. Int. J. Biol. Macromol. 2021, 169, 362–370. [Google Scholar] [CrossRef]
- Zheng, Q.; Tian, Y.; Ye, F.; Zhou, Y.; Zhao, G. Fabrication and application of starch-based aerogel: Technical strategies. Trends Food Sci. Technol. 2020, 99, 608–620. [Google Scholar] [CrossRef]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Sikora, M.; Krystyjan, M.; Dobosz, A.; Tomasik, P.; Walkowiak, K.; Masewicz, Ł.; Kowalczewski, P.Ł.; Baranowska, H.M. Molecular Analysis of Retrogradation of Corn Starches. Polymers 2019, 11, 1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranowska, H.M.; Sikora, M.; Krystyjan, M.; Dobosz, A.; Tomasik, P.; Walkowiak, K.; Masewicz, Ł.; Borczak, B. Analysis of the Retrogradation Processes in Potato Starches Blended with Non-Starchy Polysaccharide Hydrocolloids by LF NMR. Food Biophys. 2020, 15, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Małyszek, Z.; Lewandowicz, J.; Le Thanh-Blicharz, J.; Walkowiak, K.; Kowalczewski, P.Ł.; Baranowska, H.M. Water Behavior of Emulsions Stabilized by Modified Potato Starch. Polymers 2021, 13, 2200. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Baranowska, H.M.; Le Thanh-Blicharz, J.; Makowska, A. Water binding capacity in waxy and normal rice starch pastes. In Proceedings of the 11th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 7–9 October 2015; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2015; pp. 69–72. [Google Scholar]
- Lewandowicz, J. Physicochemical Characteristics and Evaluation of Applicability of Waxy Starches. Ph.D. Thesis, Poznań University of Economics and Business, Poznań, Poland, 2017. [Google Scholar]
- Luo, F.; Huang, Q.; Fu, X.; Zhang, L.; Yu, S. Preparation and characterisation of crosslinked waxy potato starch. Food Chem. 2009, 115, 563–568. [Google Scholar] [CrossRef]
- Le Thanh-Blicharz, J.; Małyszek, Z.; Walkowski, A.; Drożdzyńska, A.; Lewandowicz, G. Rheological properties and texture of new RS4 type starch pastes. Postępy Nauk. Technol. Przem. Rolno-Spożywczego 2011, 66, 53–65. [Google Scholar]
- Jeon, Y.-S.; Lowell, A.V.; Gross, R.A. Studies of Starch Esterification: Reactions with Alkenylsuccinates in Aqueous Slurry Systems. Starch—Stärke 1999, 51, 90–93. [Google Scholar] [CrossRef]
- Chang, P.R.; Yu, J.; Ma, X. Preparation of porous starch and its use as a structure-directing agent for production of porous zinc oxide. Carbohydr. Polym. 2011, 83, 1016–1019. [Google Scholar] [CrossRef]
- Ruegg, M. Calculation of the activity of water in sulfuric acid solutions at various temperatures. Leb. Technol. 1980, 13, 22–24. [Google Scholar]
- Makowska, A.; Baranowska, H.M.; Michniewicz, J.; Chudy, S.; Kowalczewski, P.Ł. Triticale extrudates—Changes of macrostructure, mechanical properties and molecular water dynamics during hydration. J. Cereal Sci. 2017, 74, 250–255. [Google Scholar] [CrossRef]
- Brosio, E.; Gianferri, R.R. An analytical tool in foods characterization and traceability. In Basic NMR in Foods Characterization; Research Signpost: Kerala, India, 2009; pp. 9–37. [Google Scholar]
- Weglarz, W.P.; Haranczyk, H. Two-dimensional analysis of the nuclear relaxation function in the time domain: The program CracSpin. J. Phys. D Appl. Phys. 2000, 33, 1909–1920. [Google Scholar] [CrossRef]
- Stangierski, J.; Rezler, R.; Baranowska, H.M.; Poliszko, S. Effect of enzymatic modification on chicken surimi. Czech J. Food Sci. 2012, 30, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Masewicz, L.; Lewandowicz, J.; Le Thanh-Blicharz, J.; Kempka, M.; Baranowska, H.M. Diffusion of water in potato starch pastes. In Proceedings of the 12th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 19–21 October 2016; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2016; pp. 193–195. [Google Scholar]
- Przybył, K.; Samborska, K.; Koszela, K.; Masewicz, L.; Pawlak, T. Artificial neural networks in the evaluation of the influence of the type and content of carrier on selected quality parameters of spray dried raspberry powders. Measurement 2021, 186, 110014. [Google Scholar] [CrossRef]
- Ocieczek, A.; Ruszkowska, M. Selected physico-mechanical properties of instant puddings. Milchwissenschaft 2012, 67, 185–188. [Google Scholar]
- Ocieczek, A.; Zieba, M. Comparison of the Sorption Properties of Fruit Powder Shampoos Using the BET, GAB, and Peleg Models. ACS Omega 2020, 5, 14354–14359. [Google Scholar] [CrossRef]
- Šárka, E.; Dvořáček, V. New processing and applications of waxy starch (a review). J. Food Eng. 2017, 206, 77–87. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Production, structure, physicochemical and functional properties of maize, cassava, wheat, potato and rice starches. Starch—Stärke 2015, 67, 14–29. [Google Scholar] [CrossRef]
- Dobosz, A.; Sikora, M.; Krystyjan, M.; Tomasik, P.; Lach, R.; Borczak, B.; Berski, W.; Lukasiewicz, M. Short- and long-term retrogradation of potato starches with varying amylose content. J. Sci. Food Agric. 2019, 99, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Tortoe, C.; Akonor, P.T.; Koch, K.; Menzel, C.; Adofo, K. Amylose and amylopectin molecular fractions and chain length distribution of amylopectin in 12 varieties of Ghanaian sweet potato (Ipomoea batatas) flours. Int. J. Food Prop. 2017, 20, 3225–3233. [Google Scholar] [CrossRef] [Green Version]
- Fredriksson, H.; Björck, I.; Andersson, R.; Liljeberg, H.; Silverio, J.; Eliasson, A.-C.; Åman, P. Studies on α-amylase degradation of retrograded starch gels from waxy maize and high-amylopectin potato. Carbohydr. Polym. 2000, 43, 81–87. [Google Scholar] [CrossRef]
- Lewandowicz, G.; Soral-Śmietana, M. Starch modification by iterated syneresis. Carbohydr. Polym. 2004, 56, 403–413. [Google Scholar] [CrossRef]
- Tao, H.; Yan, J.; Zhao, J.; Tian, Y.; Jin, Z.; Xu, X. Effect of multiple freezing/thawing cycles on the structural and functional properties of waxy rice starch. PLoS ONE 2015, 10, e0127138. [Google Scholar] [CrossRef]
- Karim, A.A.; Toon, L.C.; Lee, V.P.L.; Ong, W.Y.; Fazilah, A.; Noda, T. Effects of Phosphorus Contents on the Gelatinization and Retrogradation of Potato Starch. J. Food Sci. 2007, 72, C132–C138. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Compendium of Food Additive Specifications; Modified Starches; FAO JECFA Monographs 22: Geneva, Switzerland, 2018; pp. 53–86. ISBN 978-92-5-131100-4. [Google Scholar]
- Harańczyk, H.; Leja, A.; Nowak, P.; Baran, E.; Strzałka, K. The Effect of Mild Rehydration on Freeze-Dried Dipalmitoylphosphatidylcholine (DPPC) Multilamellar Membranes as Observed by Proton NMR and Sorption Isotherm. Acta Phys. Pol. A 2016, 129, 179–184. [Google Scholar] [CrossRef]
- Surowka, K.; Krokosz, D.; Rychlicka-Rybska, J.; Witek, M.; Nieckarz, P.; Maciejaszek, I.; Fiutak, G. Molecular mobility changes during hydration of carrageenan studied by NMR relaxation. In Proceedings of the 14th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 7–9 November 2018; Rapkova, R., Hinkova, A., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2018; pp. 377–379. [Google Scholar]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Smarzyński, K.; Thanh-Blicharz, J.L.; Kačániová, M.; Baranowska, H.M. LF NMR spectroscopy analysis of water dynamics and texture of Gluten-Free bread with cricket powder during storage. Food Sci. Technol. Int. 2021, 27, 776–785. [Google Scholar] [CrossRef]
- Smith, I.C.P. Magnetic resonance spectroscopy in biology and medicine. Clin. Biochem. 1989, 22, 69–76. [Google Scholar] [CrossRef]
- Baranowska, H.M.; Olek, W.; Guzenda, R. Fiber saturation point identification in wood by means of NMR relaxometry experiments. Mol. Phys. Rep. 2000, 29, 150–152. [Google Scholar]
- Masewicz, L.; Pers, K.; Le Thanh-Blicharz, J.; Lewandowicz, J.; Baranowska, H.M. The effect of degree of substitution on dynamics of molecules of hydration water in acetylated distarch adipate powders. In Proceedings of the 13th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 8–10 November 2017; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Prague, Czech Republic, 2017; pp. 29–32. [Google Scholar]

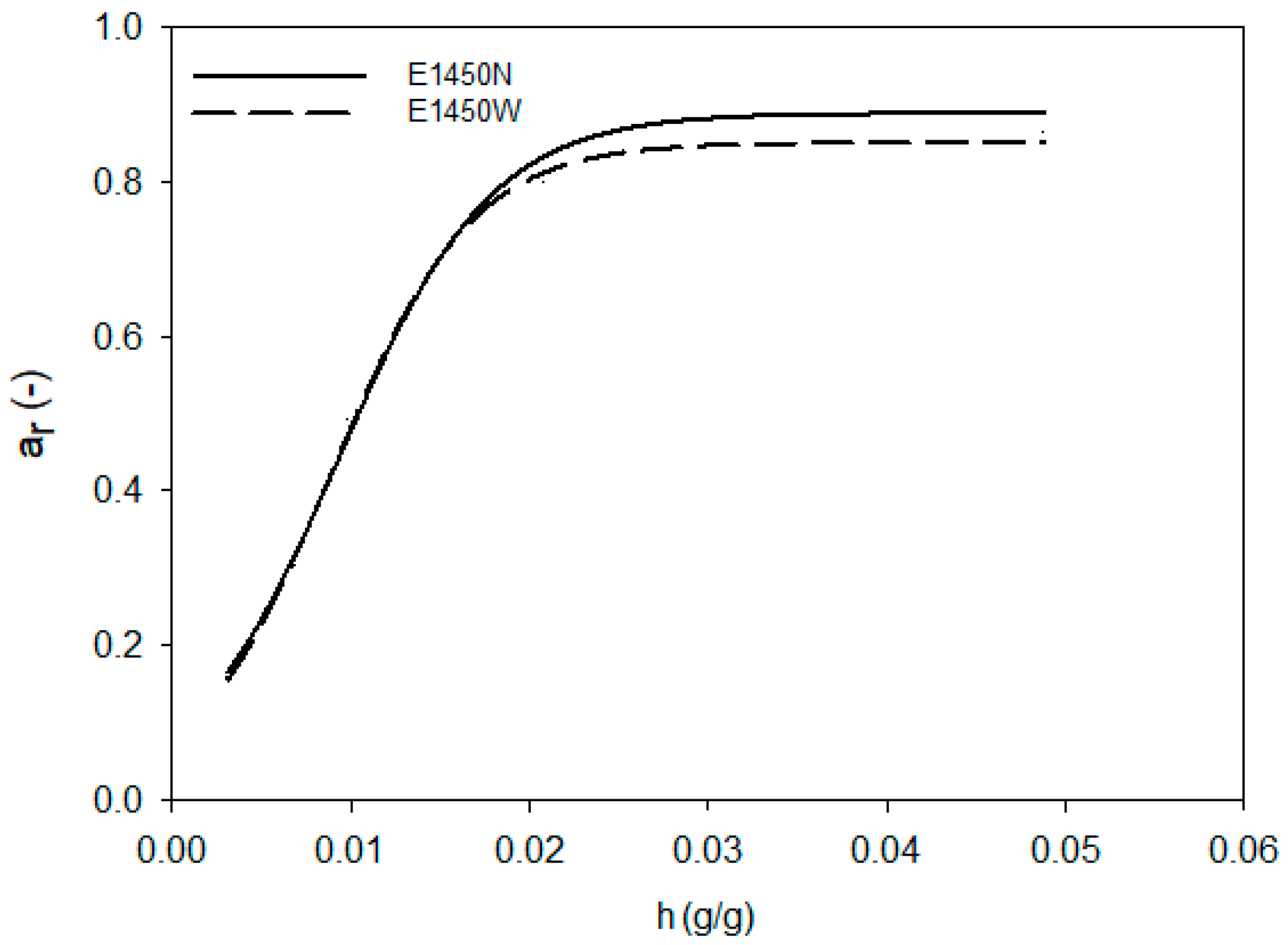

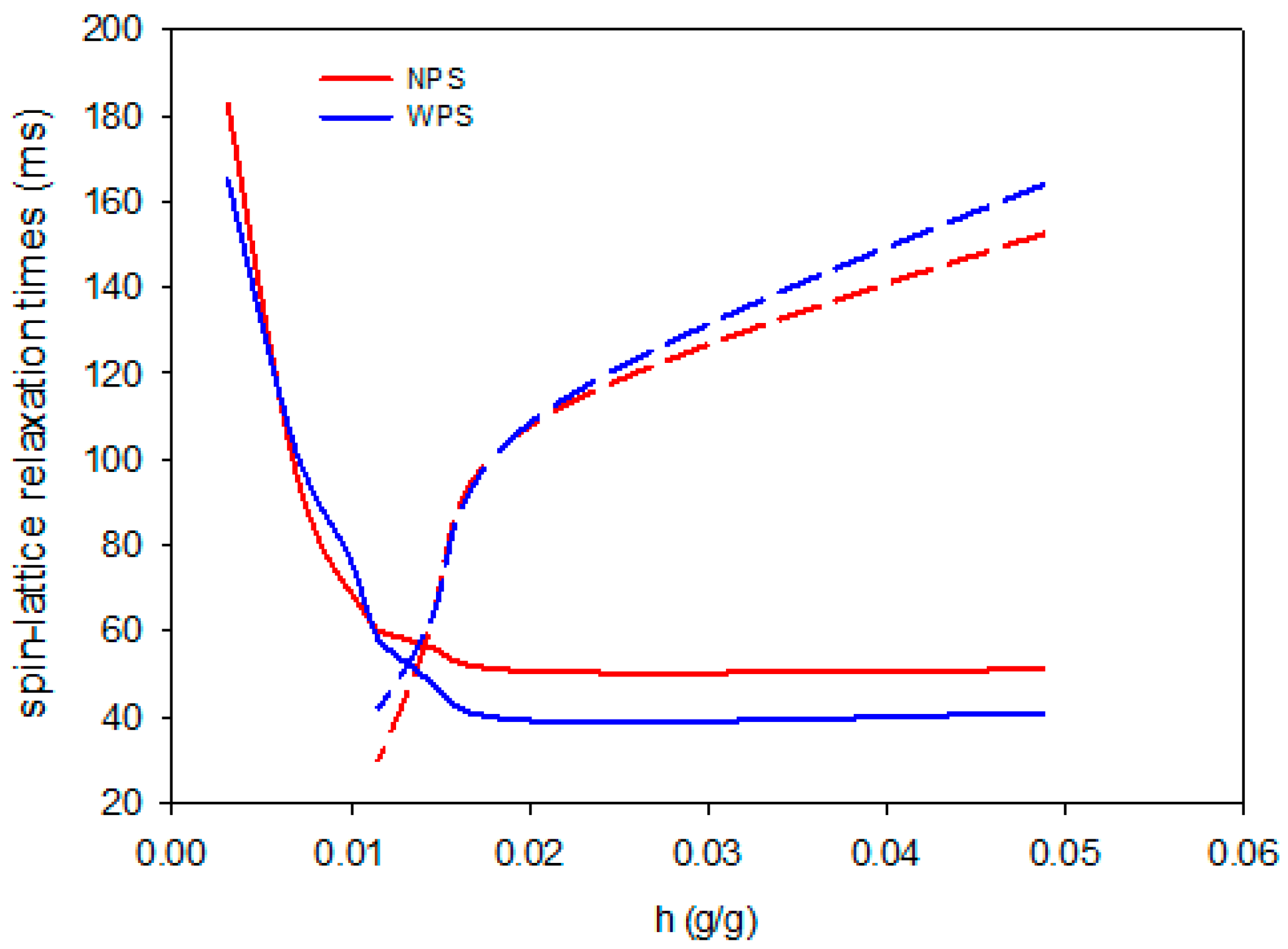

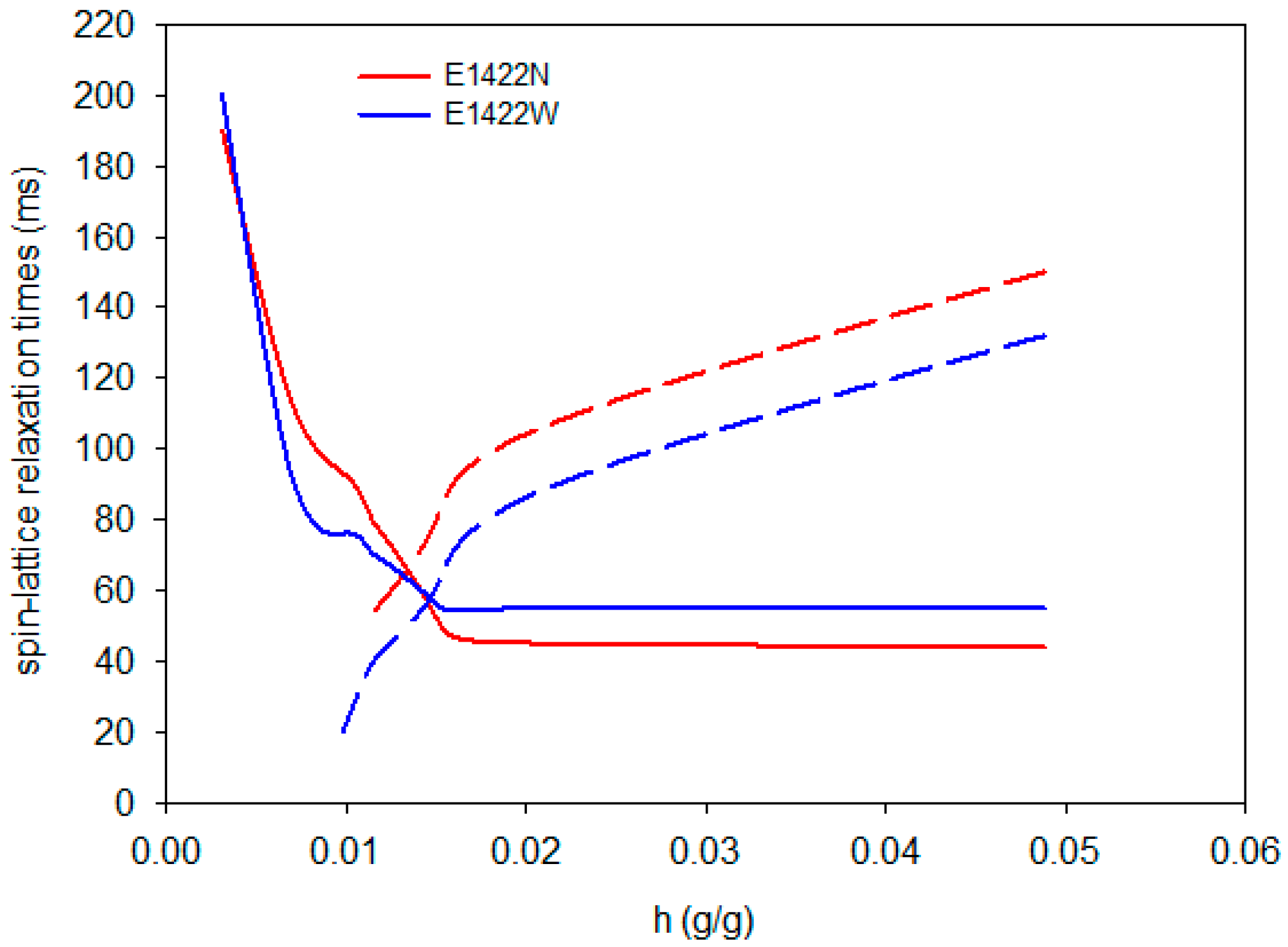
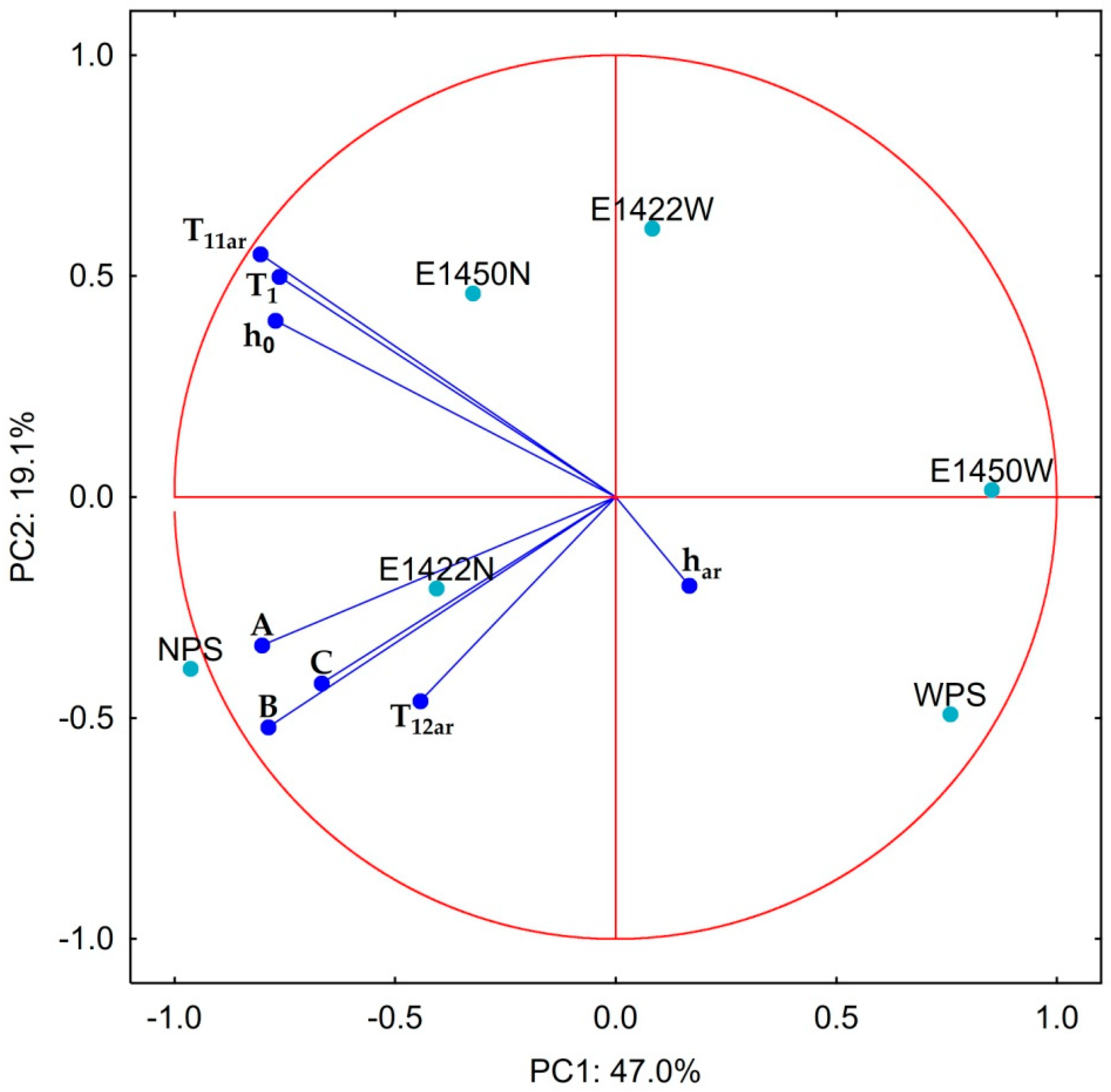
| Scheme | A | B | C |
|---|---|---|---|
| NPS | 0.904 ± 0.002 | 0.006 ± 0.001 a | 0.010 ± 0.001 a |
| WPS | 0.858 ± 0.001 a | 0.004 ± 0.001 a | 0.009 ± 0.001 a |
| E1450N | 0.888 ± 0.003 | 0.004 ± 0.001 a | 0.009 ± 0.001 a |
| E1450W | 0.858 ± 0.002 a | 0.004 ± 0.001 a | 0.009 ± 0.001 a |
| E1422N | 0.916 ± 0.001 | 0.005 ± 0.001 a | 0.009 ± 0.001 a |
| E1422W | 0.850 ± 0.002 | 0.004 ± 0.001 a | 0.009 ± 0.001 a |
| Sample | T1 (ms) | h0 (g/g) | har (g/g) | T11ar (ms) | T12ar (ms) |
|---|---|---|---|---|---|
| NPS | 57.5 ± 0.4 a | 0.014 ± 0.002 a | 0.021 ± 0.001 ab | 56.8 ± 0.5 b | 111.1 ± 0.5 |
| WPS | 30.3 ± 0.1 | 0.012 ± 0.005 a | 0.024 ± 0.002 ab | 38.9 ± 0.7 a | 119.1 ± 0.3 |
| E1450N | 71.4 ± 0.5 | 0.013 ± 0.001 a | 0.020 ± 0.002 a | 59.9 ± 0.7 | 113.2 ± 0.2 |
| E1450W | 43.2 ± 0.5 | 0.011 ± 0.003 a | 0.021 ± 0.001 ab | 38.6 ± 0.5 a | 81.5 ± 0.7 |
| E1422N | 66.4 ± 0.3 | 0.013 ± 0.003 a | 0.025 ± 0.002 b | 49.6 ± 0.7 | 116.7 ± 0.6 |
| E1422W | 56.5 ± 0.2 a | 0.015 ± 0.002 a | 0.024 ± 0.001 ab | 57.4 ± 0.5 b | 92.8 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Thanh-Blicharz, J.; Lewandowicz, J.; Małyszek, Z.; Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Baranowska, H.M. Water Behavior of Aerogels Obtained from Chemically Modified Potato Starches during Hydration. Foods 2021, 10, 2724. https://doi.org/10.3390/foods10112724
Le Thanh-Blicharz J, Lewandowicz J, Małyszek Z, Kowalczewski PŁ, Walkowiak K, Masewicz Ł, Baranowska HM. Water Behavior of Aerogels Obtained from Chemically Modified Potato Starches during Hydration. Foods. 2021; 10(11):2724. https://doi.org/10.3390/foods10112724
Chicago/Turabian StyleLe Thanh-Blicharz, Joanna, Jacek Lewandowicz, Zuzanna Małyszek, Przemysław Łukasz Kowalczewski, Katarzyna Walkowiak, Łukasz Masewicz, and Hanna Maria Baranowska. 2021. "Water Behavior of Aerogels Obtained from Chemically Modified Potato Starches during Hydration" Foods 10, no. 11: 2724. https://doi.org/10.3390/foods10112724
APA StyleLe Thanh-Blicharz, J., Lewandowicz, J., Małyszek, Z., Kowalczewski, P. Ł., Walkowiak, K., Masewicz, Ł., & Baranowska, H. M. (2021). Water Behavior of Aerogels Obtained from Chemically Modified Potato Starches during Hydration. Foods, 10(11), 2724. https://doi.org/10.3390/foods10112724










