Mediterranean Spontaneously Fermented Sausages: Spotlight on Microbiological and Quality Features to Exploit Their Bacterial Biodiversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. pH and Composition Analysis
2.3. Microbial Counts
2.4. DNA Extraction and Sequencing
2.5. Bioinformatic Analysis
2.6. Biogenic Amine Determination
2.7. Aroma Profile Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Geographical Origin and Manufacturing Processes
3.2. Physico-Chemical Characterization
3.3. Microbiological Analysis
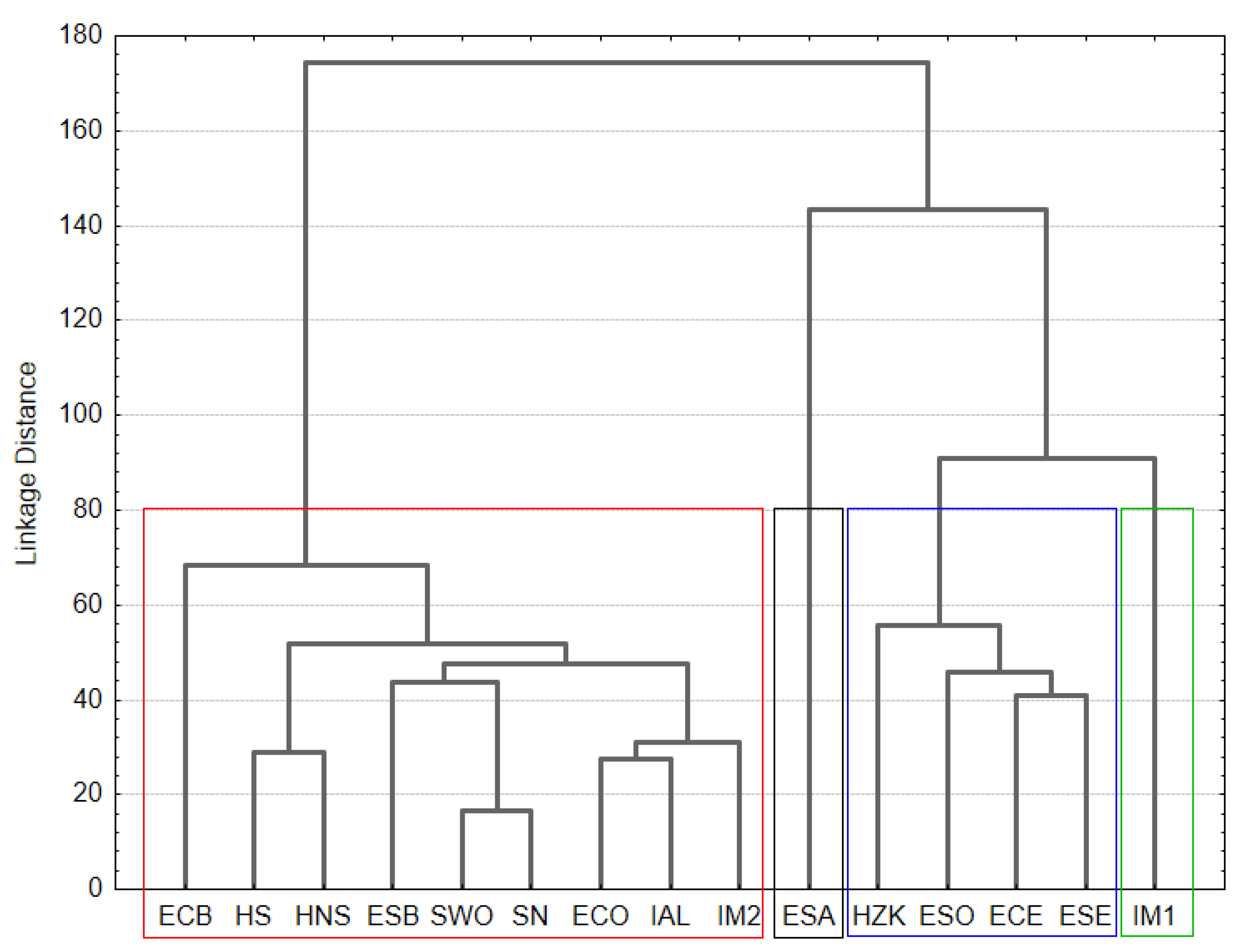
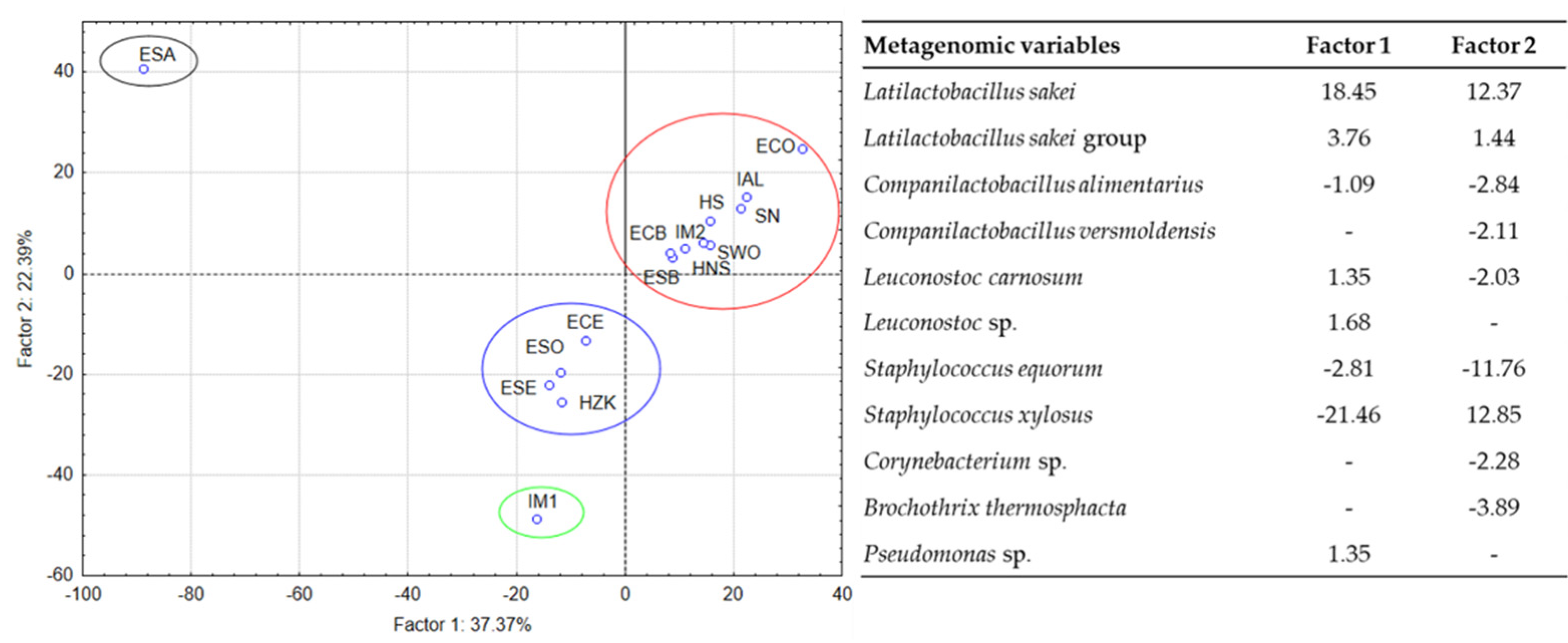
3.4. Metagenomic Analysis
3.5. Biogenic Amine Concentrations
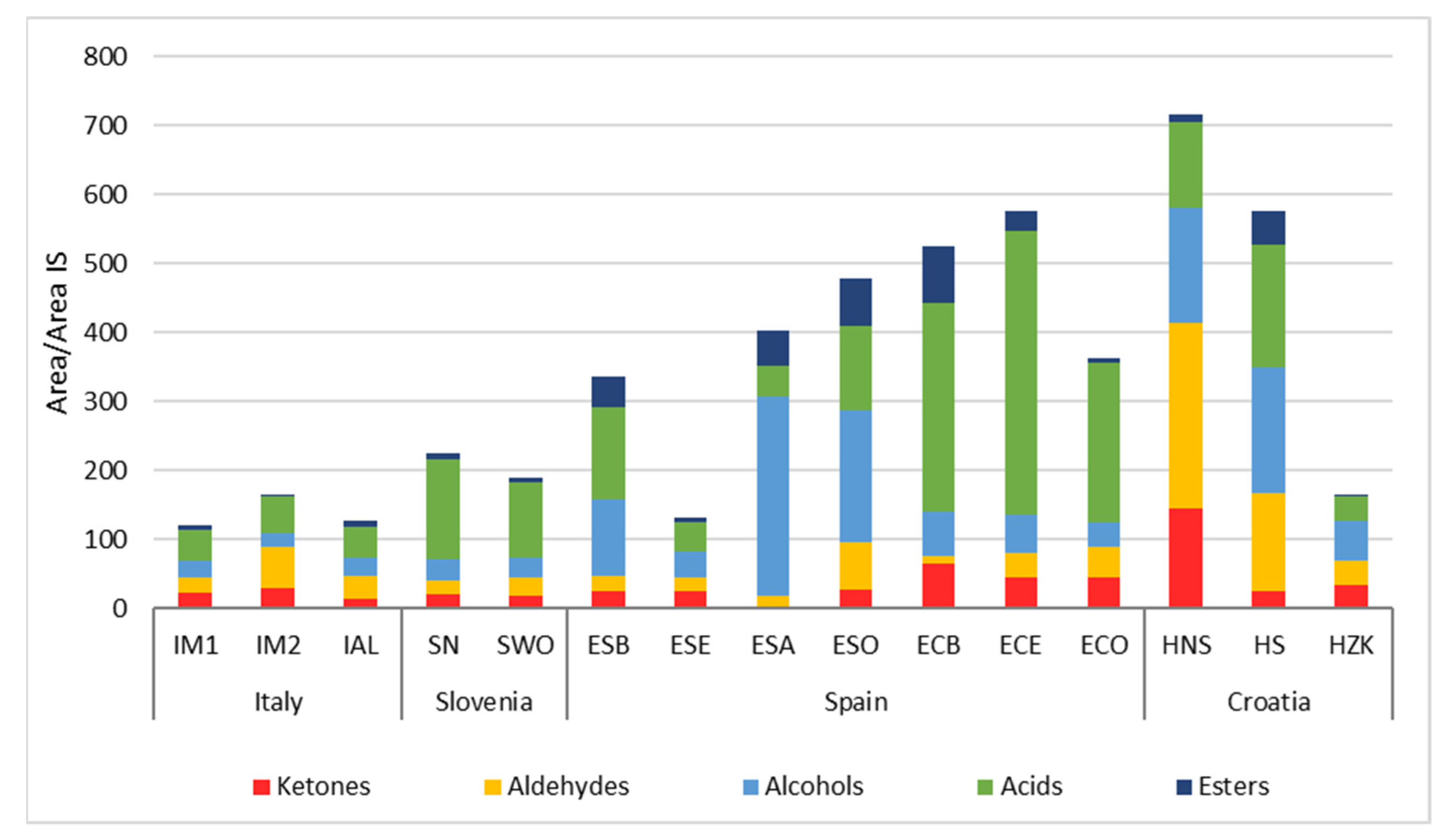
3.6. Sausage Aroma Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leroy, F.; Scholliers, P.; Amilien, V. Elements of innovation and tradition in meat fermentation: Conflicts and synergies. Int. J. Food Sci. 2015, 212, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.S.; Kerry, J.P.; Duffy, G.; Beresford, T.; Tiwari, B.K. Technological advances for enhancing quality and safety of fermented meat products. Trends Food Sci. Technol. 2015, 44, 105–116. [Google Scholar] [CrossRef]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Daniel, H.-M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 2014, 9, e95384. [Google Scholar] [CrossRef]
- Fenger, M.H.; Aschemann-Witzel, J.; Hansen, F.; Grunert, K.G. Delicious word. Assessing the impact of short storytelling messages on consumer preferences for variations of a new processed meat product. Food Qual. Prefer. 2015, 41, 237–244. [Google Scholar] [CrossRef]
- Palla, M.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Identification and characterization of lactic acid bacteria and yeasts of PDO Tuscan bread sourdough by culture dependent and independent methods. Int. J. Food Microbiol. 2017, 250, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Van Reckem, E.; Geeraerts, W.; Charmpi, C.; Van der Veken, D.; De Vuyst, L.; Leroy, F. Exploring the link between the geographical origin of European fermented foods and the diversity of their bacterial communities: The case of fermented meats. Front. Microbiol. 2019, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Vignolo, G.; Fontana, C.; Fadda, S. Semidry and dry fermented sausages. In Handbook of Meat Processing; Toldrá, F., Ed.; Blackwell Publishing: Oxford, UK, 2010; Chapter 22. [Google Scholar]
- Cocconcelli, P.S.; Fontana, C. Starter cultures for meat fermentation. In Handbook of Meat Processing; Toldrá, F., Ed.; Blackwell Publishing: Oxford, UK, 2010; Chapter 10. [Google Scholar]
- Franciosa, L.; Alessandria, V.; Dolci, P.; Rantsiou, K.; Cocolin, L. Sausage fermentation and starter cultures in the era of molecular biology methods. Int. J. Food Microbiol. 2018, 279, 26–32. [Google Scholar] [CrossRef]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Palavecino Prpich, N.Z.; Camprubí, G.E.; Cayré, M.E.; Castro, M.P. Indigenous microbiota to leverage traditional dry sausage production. Int. J. Food Microbiol. 2021, 2021, 15. [Google Scholar]
- ISO. ISO 11290–1:2017. Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.; Part 1: Detection Method; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- ISO. ISO 6579-1: 2017. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. Part 1: Detection of Salmonella spp. Method; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasini, F.; Soglia, F.; Petracci, M.; Caboni, M.F.; Marziali, S.; Montanari, C.; Gardini, F.; Grazia, L.; Tabanelli, G. Effect of fermentation with different lactic acid bacteria starter cultures on biogenic amine content and ripening patterns in dry fermented sausages. Nutrients 2018, 10, 1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martuscelli, M.; Crudele, M.A.; Gardini, F.; Suzzi, G. Biogenic amine formation and oxidation by Staphylococcus xylosus strains from artisanal fermented sausages. Lett. Appl. Microbiol. 2000, 31, 228–232. [Google Scholar] [CrossRef]
- Montanari, C.; Bargossi, E.; Gardini, A.; Lanciotti, R.; Magnani, R.; Gardini, F.; Tabanelli, G. Correlation between volatile profiles of Italian fermented sausages and their size and starter culture. Food Chem. 2016, 192, 736–744. [Google Scholar] [CrossRef]
- NIST. NIST/NIH/EPA Mass Spectral Library, Standard Reference Database 1, NIST 11. Standard Reference Data Program; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Lešić, T.; Vahčić, N.; Kos, I.; Zadravec, M.; Sinčić Pulić, B.; Bogdanović, T.; Petričević, S.; Listeš, E.; Škrivanko, M.; Pleadin, J. Characterization of traditional Croatian household-produced dry-fermented sausages. Foods 2020, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, F.; Milanović, V.; Osimani, A.; Aquilanti, L.; Taccari, M.; Garofalo, C.; Polverigiani, S.; Clementi, F.; Franciosi, E.; Tuohy, K.; et al. Microbial dynamics of model Fabriano-like fermented sausages as affected by starter cultures, nitrates and nitrites. Int. J. Food Microbiol. 2018, 278, 61–72. [Google Scholar] [CrossRef]
- Holck, A.; Heir, E.; Johannessen, T.C.; Axelsson, L. Northern European products. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrà, F., Hui, Y.H., Astiasarán, I., Sebranek, J.G., Talon, R., Eds.; John Wiley and Sons: Chichester, UK, 2015; pp. 313–320. [Google Scholar]
- Leroy, F.; Geyzen, A.; Janssens, M.; De Vuyst, L.; Scholliers, P. Meat fermentation at the crossroads of innovation and tradition: A historical outlook. Trends Food Sci. Technol. 2013, 31, 130–137. [Google Scholar] [CrossRef]
- Rodríguez-González, M.; Fonseca, S.; Centeno, J.A.; Carballo, J. Biochemical changes during the manufacture of Galician Chorizo sausage as affected by the addition of autochthonous starter cultures. Foods 2020, 9, 1813. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kozačinski, L.; Zdolec, N.; Hadžiosmanović, M.; Cvrtila, Ž.; Filipović, I.; Majić, T. Microbial flora of the Croatian traditionally fermented sausage. Archiv. Für. Lebensm. 2006, 5710, 141–147. [Google Scholar]
- Aquilanti, L.; Santarelli, S.; Silvestri, G.; Osimani, A.; Petruzzelli, A.; Clementi, F. The microbial ecology of a typical Italian salami during its natural fermentation. Int. J. Food Microbiol. 2007, 120, 136–145. [Google Scholar] [CrossRef]
- Nikodinoska, I.; Tabanelli, G.; Baffoni, L.; Gardini, F.; Di Gioia, D. Characterization of Lactobacillus Strains Isolated form Italian Artisanal Salami: Safety and Techno-Functional Aspects. In Proceedings of the Food Micro 2018 (FM 2018), Berlin, Germany, 3–6 September 2018. [Google Scholar]
- Flores, M.; Corral, S.; Cano-García, L.; Salvador, A.; Belloch, C. Yeast strains as potential aroma enhancers in dry fermented sausages. Int. J. Food Microbiol. 2015, 212, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, J.M.; Córdoba, J.J.; Casado, E.M.; Córdoba, M.G.; Rodríguez, M. Effect of selected strains of Debaryomyces hansenii on the volatile compound production of dry fermented sausage “salchichón”. Meat Sci. 2010, 85, 256–264. [Google Scholar] [CrossRef]
- Roccato, A.; Uyttendaele, M.; Barrucci, F.; Cibin, V.; Favretti, M.; Cereser, A.; Cin, M.D.; Pezzuto, A.; Piovesana, A.; Longo, A.; et al. Artisanal Italian salami and soppresse: Identification of control strategies to manage microbiological hazards. Food Microbiol. 2017, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Gounadaki, A.S.; Skandamis, P.N.; Drosinos, E.H.; Nychas, G.-J.E. Microbial ecology of food contact surfaces and products of small-scale facilities producing traditional sausages. Food Microbiol. 2008, 25, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Talon, R.; Lebert, I.; Lebert, A.; Leroy, S.; Garriga, M.; Aymerich, T.; Drosinos, E.H.; Zanardi, E.; Ianieri, A.; Fraqueza, M.J.; et al. Traditional dry fermented sausages produced in small-scale processing units in Mediterranean countries and Slovakia. 1: Microbial ecosystems of processing environments. Meat Sci. 2007, 77, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Dolci, P.; Rantsiou, K. Biodiversity and dynamics of meat fermentations: The contribution of molecular methods for a better comprehension of a complex ecosystem. Meat Sci. 2011, 89, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Talon, R.; Leroy, S. Diversity and safety hazards of bacteria involved in meat fermentations. Meat Sci. 2011, 89, 303–309. [Google Scholar] [CrossRef]
- Chaillou, S.; Champomier-Vergès, M.C.; Cornet, M.; Crutz-Le Coq, A.M.; Dudez, A.M.; Martin, V.; Beaufils, S.; Darbon-Rongère, E.; Bossy, R.; Loux, V.; et al. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 2005, 23, 1527–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, M.; Myter, N.; De Vuyst, L.; Leroy, F. Community dynamics of coagulase-negative staphylococci during spontaneous artisan-type meat fermentations differ between smoking and moulding treatments. Int. J. Food Microbiol. 2013, 166, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, L.; Garofalo, C.; Osimani, A.; Clementi, F. Ecology of lactic acid bacteria and coagulase negative cocci in fermented dry sausages manufactured in Italy and other Mediterranean countries: An overview. Int. Food Res. J. 2016, 23, 429–445. [Google Scholar]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanellia, G.; Gardini, F. Effects of the diameter on physico-chemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- Stavropoulou, D.A.; Filippou, P.; De Smet, S.; De Vuyst, L.; Leroy, F. Effect of temperature and pH on the community dynamics of coagulase-negative staphylococci during spontaneous meat fermentation in a model system. Food Microbiol. 2018, 76, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Janßen, D.; Eisenbach, L.; Ehrmann, M.A.; Vogel, R.F. Assertiveness of Lactobacillus sakei and Lactobacillus curvatus in a fermented sausage model. Int. J. Food Microbiol. 2018, 285, 188–197. [Google Scholar] [CrossRef]
- Rimaux, T.; Vrancken, G.; Pothakos, V.; Maes, D.; De Vuyst, L.; Leroy, F. The kinetics of the arginine deiminase pathway in the meat starter culture Lactobacillus sakei CTC 494 are pH-dependent. Food Microbiol. 2011, 28, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Rimaux, T.; Vrancken, G.; Vuylsteke, B.; De Vuyst, L.; Leroy, F. The pentose moiety of adenosine and inosine is an important energy source for the fermented-meat starter culture Lactobacillus sakei CTC 494. Appl. Environ. Microbiol. 2011, 77, 6539–6550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravyts, F.; Vuyst, L.D.; Leroy, F. Bacterial diversity and functionalities in food fermentations. Eng. Life Sci. 2012, 12, 356–367. [Google Scholar] [CrossRef]
- Montanari, C.; Barbieri, F.; Magnani, M.; Grazia, L.; Gardini, F.; Tabanelli, G. Phenotypic diversity of Lactobacillus sakei strains. Front. Microbiol. 2018, 9, 2003. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Bassi, D.; López, C.; Pisacane, V.; Otero, M.C.; Puglisi, E.; Rebecchi, A.; Cocconcelli, P.S.; Vignolo, G. Microbial ecology involved in the ripening of naturally fermented llama meat sausages. A focus on lactobacilli diversity. Int. J. Food Microbiol. 2016, 236, 17–25. [Google Scholar] [CrossRef]
- Fontán, M.C.G.; Lorenzo, J.M.; Parada, A.; Franco, I.; Carballo, J. Microbiological characteristics of “androlla”, a Spanish traditional pork sausage. Food Microbiol. 2007, 24, 52–58. [Google Scholar]
- Fontán, M.C.G.; Lorenzo, J.M.; Martínez, S.; Franco, I.; Carballo, J. Microbiological characteristics of Botillo, a Spanish traditional pork sausage. LWT 2007, 40, 1610–1622. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Mauriello, G.; Aponte, M.; Moschetti, G.; Villani, F. Microbial succession during ripening of Naples-type salami, a southern Italian fermented sausage. Meat Sci. 2000, 56, 321–329. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Zhang, W.; Domínguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Autochthonous probiotics in meat products: Selection, identification, and their use as starter culture. Microorganisms 2020, 8, 1833. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, R.; Lorenzo, J.M.; Fonseca, S.; Franco, I.; Carballo, J. Strains of Staphylococcus and Bacillus isolated from traditional sausages as producers of biogenic amines. Front. Microbiol. 2012, 3, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greppi, A.; Ferrocino, I.; La Storia, A.; Rantsiou, K.; Ercolini, D.; Cocolin, L. Monitoring of the microbiota of fermented sausages by culture independent rRNA based approaches. Int. J. Food Microbiol. 2015, 212, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Mainar, M.; Stavropoulou, D.; Leroy, F. Exploring the metabolic heterogeneity of coagulase-negative staphylococci to improve the quality and safety of fermented meats: A review. Int. J. Food Microbiol. 2017, 247, 24–37. [Google Scholar] [CrossRef]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef]
- Drosinos, E.H.; Mataragas, M.; Xiraphi, N.; Moschonas, G.; Gaitis, F.; Metaxopoulos, J. Characterization of the microbial flora from a traditional Greek fermented sausage. Meat Sci. 2005, 69, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ravyts, F.; Steen, L.; Goemaere, O.; Paelinck, H.; De Vuyst, L.; Leroy, F. The application of staphylococci with flavour-generating potential is affected by acidification in fermented dry sausages. Food Microbiol. 2010, 27, 945–954. [Google Scholar] [CrossRef]
- Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Mauriello, G.; Villani, F.; Ercolini, D. Spoilage-related activity of Carnobacterium maltaromaticum strains in air-stored and vacuum-packed meat. Appl. Environ. Microbiol. 2011, 77, 7382–7393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercolini, D.; Federica, R.; Torrieri, E.; Masi, P.; Villani, F. Changes in the spoilagerelated microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 2006, 72, 4663–4671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondi, S.; Nappi, M.R.; Sirangelo, T.M.; Leonardi, A.; Amaretti, A.; Ulrici, A.; Magnani, R.; Montanari, C.; Tabanelli, G.; Gardini, F. Bacterial community of industrial raw sausage packaged in modified atmosphere throughout the shelf life. Int. J. Food Microbiol. 2018, 280, 78–86. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393–2486. [Google Scholar] [CrossRef] [Green Version]
- Landete, J.M.; Pardo, I.; Ferrer, S. Regulation of hdc expression and HDC activity by enological factors in lactic acid bacteria. J. Appl. Microbiol. 2008, 105, 1544–1551. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardini, F.; Özogul, Y.; Suzzi, G.; Tabanelli, G.; Özogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalçınkaya, S.; Kılıç, G.B. Isolation, identification and determination of technological properties of the halophilic lactic acid bacteria isolated from table olives. J. Food Sci. Technol. 2019, 56, 2027–2037. [Google Scholar] [CrossRef]
- Straub, B.W.; Kicherer, M.; Schilcher, S.M.; Hammes, W.P. The formation of biogenic amines by fermentation organisms. Z. Lebensm. Unters. Forch. 1995, 201, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.; Berger, R.G. Aroma compounds in fermented sausages of different origins. Lebensm. Wiss. Technol. 1998, 31, 559–567. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P. Studies on essential oil composition of cultivars of black pepper (Piper nigrum L.)-V. J. Essent. Oil Res. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Marongiu, B.; Atzeri, A.; Dessì, M.A.; Falconieri, D.; Porcedda, S. Extraction and separation of volatile and fixed oils from seeds of Myristica fragrans by supercritical CO2: Chemical composition and cytotoxic activity on Caco-2 cancer cells. J. Food Sci. 2012, 77, C448–C453. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Sikorski, Z.E.; Sinkiewicz, I. Principles of smoking. In Handbook of Fermented Meat and Poultry, 2nd ed.; Toldrà, F., Ed.; John Wiley and Sons: Chichester, UK, 2015; Chapter 6. [Google Scholar]
- Pereira, C.; de Guía Córdoba, M.; Aranda, E.; Hernández, A.; Velázquez, R.; Bartolomé, T.; Martín, A. Type of paprika as a critical quality factor in Iberian chorizo sausage manufacture. CyTA-J. Food 2019, 17, 907–916. [Google Scholar] [CrossRef]
- Tabanelli, G.; Montanari, C.; Grazia, L.; Lanciotti, R.; Gardini, F. Effects of aw at packaging time and atmosphere composition on aroma profile, biogenic amine content and microbiological features of dry fermented sausages. Meat Sci. 2013, 94, 177–186. [Google Scholar] [CrossRef]
- Liu, S.Q. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int. J. Food Microbiol. 2003, 83, 115–131. [Google Scholar] [CrossRef]
- Carballo, J. The role of fermentation reactions in the generation of flavor and aroma of foods. In Fermentation, Effects on Food Properties; Mehta, B.M., Kamal-Eldin, A., Iwanski, R.Z., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 51–83. [Google Scholar]
- Smid, E.J.; Kleerebezem, M. Production of aroma compounds in lactic fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- von Wright, A.; Axelsson, L. Lactic acid bacteria: An introduction. In Lactic Acid Bacteria, Microbial and Functional Aspects, 4th ed.; Lahtinen, S., Ouwehand, A.C., Salminen, S., von Wright, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–16. [Google Scholar]
- Olivares, A.; Navarro, J.L.; Flores, M. Establishment of the contribution of volatile compounds to the aroma of fermented sausages at different stages of processing and storage. Food Chem. 2009, 115, 1464–1472. [Google Scholar] [CrossRef]
- Gianelli, M.P.; Olivares, A.; Flores, M. Key aroma components of a dry-cured sausage with high fat content (Sobrassada). Food Sci. Technol. Int. 2011, 17, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics of the Tested Sausages | Italy | Slovenia | Spain | Croatia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IM1 | IM2 | IAL | SN | SWO | ESA | ESB | ESE | ESO | ECB | ECE | ECO | HNS | HS | HZK | |
| Section |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Diameter (cm) | 5 | 3.5 | 6 | 5 | 5 | 4 | 5.5 | 4.5 | 5.5 | 4.5 | 4 | 5 | 3.5 | 3.5 | 2.5 |
| Type of lean meat | pork | pork | pork | pork/bovine (3:1) | pork/bovine (3:1) | pork | pork | pork | pork | pork | pork | pork | pork | pork | pork |
| Fat in the meat batter (%) | 8–12 | 8–12 | 20–30 | 19 | 19 | 20–25 | 20–30 | 20–30 | 20–25 | 25–30 | 25–30 | 15–20 | 30–35 | 30–35 | 20 |
| Fat characteristics | cubes | cubes | minced | cubes | cubes | minced | minced | minced | minced | minced | minced | minced | cubes | cubes | cubes |
| Spices | pepper, white wine | pepper, white wine | pepper, cinnamon, nutmeg, cloves | pepper, garlic | pepper, garlic | pepper, nutmeg | pepper, nutmeg | pepper, nutmeg | pepper, nutmeg | paprika, oregano, nutmeg, coriander | paprika, oregano, nutmeg, coriander | paprika, oregano, nutmeg, coriander | pepper, garlic, mild paprika, hot paprika | pepper, garlic, mild paprika, hot paprika | pepper, garlic, wine |
| Nitrate/Nitrite | no | no | yes | yes | no | yes | yes | yes | yes | no | no | no | no | no | no |
| Type of casing | natural | natural | natural (pork or bovine) | collagen | collagen | collagen | collagen | collagen | collagen | collagen | collagen | collagen | natural (pork intestine) | natural (pork intestine) | natural (pork intestine) |
| Smoking | not smoked | not smoked | not smoked | smoked | smoked | not smoked | not smoked | not smoked | not smoked | not smoked | not smoked | not smoked | not smoked | smoked | not smoked |
| Italy | Slovenia | Spain | Croatia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IM1 | IM2 | IAL | SN | SWO | ESA | ESB | ESE | ESO | ECB | ECE | ECO | HNS | HS | HZK | |
| pH | 6.42 ± 0.02 | 6.09 ± 0.01 | 5.88 ± 0.03 | 5.20 ± 0.02 | 5.39 ± 0.01 | 5.83 ± 0.03 | 5.63 ± 0.04 | 5.80 ± 0.02 | 5.13 ± 0.03 | 4.77 ± 0.02 | 5.04 ± 0.01 | 4.52 ± 0.03 | 5.81 ± 0.05 | 5.72 ± 0.04 | 6.05 ± 0.03 |
| aw | 0.824 ± 0.003 | 0.760 ± 0.002 | 0.879 ± 0.002 | 0.823 ± 0.002 | 0.832 ± 0.003 | 0.917 ± 0.002 | 0.811 ± 0.002 | 0.848 ± 0.003 | 0.911 ± 0.004 | 0.895 ± 0.001 | 0.870 ± 0.003 | 0.908 ± 0.001 | 0.928 ± 0.001 | 0.903 ± 0.003 | 0.890 ± 0.001 |
| Humidity (%) | 26.15 ± 0.35 | 20.42 ± 0.38 | 30.11 ± 0.40 | 25.05 ± 0.27 | 26.07 ± 0.38 | 38.54 ± 0.19 | 25.45 ± 0.22 | 27.60 ± 0.18 | 39.04 ± 0.44 | 31.12 ± 0.30 | 30.25 ± 0.41 | 38.54 ± 0.29 | 28.75 ± 0.33 | 25.11 ± 0.38 | 32.69 ± 0.23 |
| Fat (%) | 34.13 ± 0.31 | 35.79 ± 0.33 | 34.18 ± 0.26 | 40.65 ± 0.17 | 41.33 ± 0.41 | 29.75 ± 0.28 | 42.15 ± 0.30 | 36.05 ± 0.35 | 29.42 ± 0.21 | 43.75 ± 0.25 | 40.38 ± 0.29 | 29.71 ± 0.37 | 50.37 ± 0.40 | 47.21 ± 0.46 | 30.21 ± 0.26 |
| Proteins (%) | 34.32 ± 0.21 | 37.73 ± 0.35 | 29.27 ± 0.17 | 27.61 ± 0.60 | 26.15 ± 0.44 | 26.47 ± 0.29 | 26.83 ± 0.33 | 28.48 ± 0.24 | 25.20 ± 0.48 | 18.44 ± 0.41 | 22.53 ± 0.26 | 23.71 ± 0.35 | 15.14 ± 0.40 | 20.08 ± 0.29 | 30.29 ± 0.34 |
| Collagen (%) | 1.12 ± 0.05 | 1.62 ± 0.06 | 2.61 ± 0.07 | 3.25 ± 0.03 | 3.23 ± 0.09 | 1.02 ± 0.10 | 1.28 ± 0.04 | 3.72 ± 0.06 | 2.10 ± 0.09 | 3.34 ± 0.08 | 2.69 ± 0.05 | 3.61 ± 0.04 | 2.81 ± 0.11 | 3.84 ± 0.07 | 2.08 ± 0.10 |
| Salt (%) | 4.31 ± 0.10 | 4.48 ± 0.05 | 3.84 ± 0.09 | 3.44 ± 0.07 | 3.24 ± 0.11 | 4.26 ± 0.02 | 4.24 ± 0.08 | 4.10 ± 0.06 | 4.28 ± 0.12 | 3.31 ± 0.05 | 4.12 ± 0.04 | 4.42 ± 0.08 | 2.94 ± 0.09 | 3.78 ± 0.05 | 4.69 ± 0.07 |
| Italy | Slovenia | Spain | Croatia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IM1 | IM2 | IAL | SN | SWO | ESA | ESB | ESE | ESO | ECB | ECE | ECO | HNS | HS | HZK | |
| LAB | 7.07 ± 0.48 | 8.52 ± 0.11 | 8.26 ± 0.16 | 6.57 ± 0.02 | 7.28 ± 0.28 | 7.85 ± 0.75 | 6.96 ± 0.21 | 6.32 ± 0.30 | 7.78 ± 0.14 | 4.41 ± 0.72 | 5.88 ± 1.02 | 7.73 ± 0.27 | 8.67 ± 0.11 | 8.43 ± 0.09 | 8.54 ± 0.03 |
| CNS | 7.12 ± 0.09 | 7.13 ± 0.12 | 5.22 ± 1.05 | <1 | 1.44 ± 2.03 | 3.65 ± 0.22 | 3.05 ± 4.31 | 4.54 ± 0.16 | 5.40 ± 0.12 | <1 | <1 | <1 | 5.34 ± 0.82 | 5.09 ± 0.01 | 7.24 ± 0.32 |
| Enterococci | <1 | 0.60 ± 0.75 | 0.89 ± 0.71 | <1 | <1 | <1 | 2.37 ± 0.10 | 2.19 ± 0.53 | 2.05 ± 0.38 | <1 | 1.19 ± 0.35 | 0.89 ± 0.46 | 3.74 ± 0.12 | 3.18 ± 0.74 | 4.80 ± 0.16 |
| Yeasts and molds | 5.44 ± 0.05 | 5.01 ± 0.26 | 3.46 ± 0.49 | 0.95 ± 1.35 | 3.26 ± 0.37 | 5.34 ± 0.25 | 3.41 ± 0.80 | 4.10 ± 0.58 | 4.59 ± 0.59 | 3.10 ± 0.27 | 2.60 ± 0.25 | 3.70 ± 0.08 | 2.27 ± 0.31 | 3.85 ± 0.31 | 4.15 ± 0.10 |
| Enterobacteriaceae | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | 3.55 ± 0.50 | 2.71 ± 0.82 | 5.12 ± 0.49 |
| Italy | Slovenia | Spain | Croatia | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IM1 | IM2 | IAL | SN | SWO | ESA | ESB | ESE | ESO | ECB | ECE | ECO | HNS | HS | HZK | ||
| Latilactobacillus sakei | 9.51 | 52.71 | 54.54 | 55.24 | 44.75 | 0.71 | 36.32 | 2.89 | 3.88 | 41.24 | 15.12 | 72.43 | 35.68 | 45.36 | 9.72 | |
| Latilactobacillus sakei group | - * | 9.55 | 17.99 | 4.94 | 2.34 | - | 4.85 | 10.87 | 23.16 | 4.95 | 6.67 | 22.25 | 24.63 | 16.44 | 2.57 | |
| Lactiplantibacillus plantarum group | - | - | - | - | - | - | - | - | 2.64 | - | - | - | - | - | - | |
| Lacticaseibacillus casei group | - | - | - | - | - | - | 1.16 | - | - | - | - | - | - | - | - | |
| Companilactobacillus alimentarius | - | - | - | - | - | - | - | 21.75 | 5.81 | - | 32.32 | 5.32 | - | - | - | |
| Companilactobacillus heilongjiangensis | - | - | - | - | - | - | - | - | 1.32 | - | - | - | - | - | - | |
| Companilactobacillus versmoldensis | - | - | - | - | - | - | - | 33.58 | 3.96 | 44.98 | 2.20 | - | - | - | - | |
| Ligilactobacillus sp. | - | - | - | - | - | - | - | - | - | - | - | - | - | 4.80 | - | |
| Loigolactobacillus rennini | - | - | - | - | - | - | - | - | - | - | - | - | - | 7.94 | - | |
| Dellaglioa algida | - | - | - | - | 3.66 | - | - | - | - | 3.32 | - | - | - | - | - | |
| Levilactobacillus yonginensis group | - | - | - | - | - | - | - | - | 3.36 | - | - | - | - | - | - | |
| Limosilactobacillus mucosae | - | - | - | - | - | - | - | - | - | - | 4.44 | - | - | - | - | |
| Lactobacillus helveticus | - | 3.07 | - | - | - | - | 25.54 | - | 19.45 | 0.94 | - | - | - | - | - | |
| Lactococcus sp. | - | - | 1.12 | - | - | - | - | - | - | - | 2.72 | - | - | - | - | |
| Streptococcus sp. | - | - | - | - | - | - | 3.68 | - | - | 2.09 | 4.61 | - | - | - | - | |
| Leuconostoc carnosum | 15.84 | - | 8.75 | 19.06 | 23.26 | - | 6.70 | - | 1.70 | 0.09 | - | - | - | - | 14.80 | |
| Leuconostoc sp. | 0.71 | - | 17.60 | 2.74 | 10.20 | - | 15.63 | - | - | - | - | - | - | - | 3.65 | |
| Weissella sp. | - | - | - | - | - | - | - | - | 11.40 | - | - | - | - | - | - | |
| Carnobacterium sp. | - | - | - | - | - | - | - | - | - | - | - | - | 9.00 | 19.11 | - | |
| Staphylococcus epidermidis | - | - | - | - | - | - | - | - | - | - | 4.58 | - | - | - | - | |
| Staphylococcus equorum | 67.74 | 11.65 | - | - | - | - | - | - | 4.47 | - | - | - | - | - | 14.04 | |
| Staphylococcus saprophyticus | - | 4.20 | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Staphylococcus succinus | 1.47 | 2.09 | - | - | - | - | - | - | 4.35 | - | - | - | - | - | 5.27 | |
| Staphylococcus xylosus | - | 1.81 | - | - | - | 98.13 | - | - | - | 2.38 | - | - | - | - | - | |
| Kocuria sp. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.25 | |
| Corynebacterium sp. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 27.91 | |
| Corynebacterium variabile | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.21 | |
| Brevibacterium casei | - | - | - | - | - | - | - | - | - | - | 1.59 | - | - | - | - | |
| Rothia sp. | - | - | - | - | - | - | - | - | - | - | 4.10 | - | - | - | - | |
| Brochothrix thermosphacta | 4.73 | 14.91 | - | 1.73 | 5.81 | - | - | 14.23 | 12.77 | - | 7.90 | - | 3.91 | 1.65 | 19.58 | |
| Escherichia/Shigella sp. | - | - | - | - | - | - | 1.45 | - | - | - | 0.68 | - | - | - | - | |
| Klebsiella sp. | - | - | - | - | - | - | 1.21 | - | 1.20 | - | 4.56 | - | - | - | - | |
| Pseudomonas sp. | - | - | - | 14.64 | 7.57 | - | - | 16.68 | - | - | 1.42 | - | 26.79 | 4.70 | - | |
| Photobacterium piscicola | - | - | - | - | - | - | 1.67 | - | - | - | - | - | - | - | - | |
| Acinetobacter sp. | - | - | - | 1.64 | 2.42 | 1.16 | - | - | - | - | 2.26 | - | - | - | - | |
| Erysipelothrix sp. | - | - | - | - | - | - | 1.80 | - | 0.52 | - | 4.84 | - | - | - | - | |
| Italy | Slovenia | Spain | Croatia | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IM1 | IM2 | IAL | SN | SWO | ESA | ESB | ESE | ESO | ECB | ECE | ECO | HNS | HS | HZK | |
| Histamine | - * | - | - | - | - | - | 195.79 ± 27.29 | - | - | - | 170.74 ± 28.54 | - | - | - | - |
| Tyramine | 73.87 ± 21.83 | 47.65 ± 12.93 | 78.66 ± 1.31 | 209.38 ± 0.71 | 180.52 ± 10.34 | 199.24 ± 30.75 | 171.35 ± 37.52 | 149.92 ± 31.19 | 67.96 ± 14.34 | 146.06 ± 48.04 | 173.72 ± 39.46 | 202.50 ± 8.04 | 366.78 ± 38.31 | 312.93 ± 26.46 | 105.31 ± 29.18 |
| Putrescine | - | - | 115.67 ± 3.78 | 59.03 ± 2.70 | 67.58 ± 3.64 | - | 108.07 ± 15.41 | 42.79 ± 8.54 | 110.54 ± 4.26 | 99.28 ± 13.57 | 79.30 ± 10.81 | 155.95 ± 15.52 | 256.59 ± 8.92 | 359.59 ± 80.64 | - |
| Cadaverine | - | - | - | 83.38 ± 2.61 | 100.87 ± 0.44 | 67.94 ± 1.91 | 136.90 ± 20.01 | - | - | - | - | - | 436.03 ± 29.87 | 252.52 ± 30.31 | - |
| TOTAL | 73.87 | 47.65 | 194.33 | 268.41 | 348.98 | 267.18 | 612.10 | 192.72 | 178.50 | 245.34 | 423.76 | 358.46 | 1059.40 | 925.04 | 105.31 |
| VOCs | Italy | Slovenia | Spain | Croatia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IM1 | IM2 | IAL | SN | SWO | ESB | ESE | ESA | ESO | ECB | ECE | ECO | HNS | HS | HZK | |
| Acetone | 5.85 | 3.00 | 2.61 | 0.87 | 1.31 | 3.70 | 3.20 | - * | 1.79 | 2.26 | 13.48 | 4.85 | 1.86 | 0.66 | 1.38 |
| 2-butanone | 0.55 | 1.08 | 1.13 | - | 0.44 | 1.42 | 0.86 | - | 0.97 | 47.36 | 2.70 | 2.51 | 94.05 | 3.50 | 1.30 |
| 2,3-butanedione | 1.87 | 1.54 | 0.23 | 0.41 | 0.48 | 0.50 | 2.44 | 0.18 | 0.52 | 5.93 | 3.68 | 1.32 | 2.14 | 3.42 | 2.03 |
| 2-pentanone | 0.73 | 0.52 | 0.51 | - | - | - | 1.45 | - | - | - | 1.53 | - | - | - | - |
| Methyl Isobutyl Ketone | 0.89 | 0.81 | 0.70 | 0.62 | 0.50 | 0.64 | 0.52 | 0.61 | 2.87 | 0.79 | 2.46 | 3.00 | 2.97 | 3.94 | 3.90 |
| 4-methyl-3-penten-2-one | 3.61 | 3.42 | 3.51 | 2.87 | 2.87 | 2.98 | 2.96 | 2.03 | 7.43 | 1.84 | 6.52 | 7.72 | 7.48 | 5.96 | 10.08 |
| 2,6-dimethyl-4-heptanone | - | - | - | - | - | - | - | - | - | 0.65 | 5.39 | 6.87 | 3.20 | 3.42 | - |
| 2-heptanone | 1.71 | 4.15 | 1.99 | 1.39 | 1.02 | 4.94 | 2.44 | - | 3.14 | - | 4.32 | 1.70 | 2.76 | - | - |
| 3-octanone | 1.10 | 0.87 | 0.72 | 0.41 | 0.28 | 0.48 | 0.34 | - | 2.88 | - | 2.65 | 3.23 | 1.55 | - | 1.76 |
| 2-octanone | 1.13 | 1.44 | 0.72 | 0.86 | 1.64 | 0.97 | 1.08 | 1.01 | 1.60 | 1.32 | 2.37 | 2.57 | 2.03 | 1.06 | 0.96 |
| 3-hydroxy-2-butanone | 3.72 | 2.85 | 0.71 | 10.72 | 7.98 | 1.27 | 9.37 | - | 1.57 | 4.19 | - | 10.74 | 9.03 | - | 11.78 |
| 2,5-octanedione | 0.39 | 4.64 | 0.72 | 1.34 | 0.78 | - | - | - | 2.84 | - | - | - | 14.68 | - | - |
| 2-nonanone | 1.06 | 4.40 | 0.81 | - | - | 5.74 | 0.30 | - | 1.58 | - | - | 0.70 | 1.49 | - | - |
| 2-undecanone | 0.84 | 0.65 | - | 0.78 | 0.71 | 3.06 | 0.40 | - | - | - | - | - | 1.47 | 2.88 | - |
| Ketones | 23.46 | 29.36 | 14.35 | 20.26 | 18.02 | 25.70 | 25.37 | 3.83 | 27.18 | 64.33 | 45.09 | 45.21 | 144.71 | 24.84 | 33.19 |
| 3-methyl-butanal | 1.03 | 0.38 | 0.73 | - | 0.29 | 1.65 | 1.74 | - | - | - | - | - | 1.20 | - | - |
| Pentanal | 0.44 | 2.01 | 0.69 | 0.70 | 0.90 | 0.95 | 0.74 | 0.21 | 4.22 | - | - | 0.65 | 6.56 | - | - |
| Hexanal | 4.90 | 38.66 | 6.59 | 3.38 | 3.51 | 1.56 | 5.77 | 1.09 | 48.94 | 0.81 | 3.29 | 3.44 | 110.21 | - | 2.92 |
| Heptanal | - | - | - | - | - | - | - | - | - | - | - | - | 6.53 | - | - |
| Octanal | - | - | - | - | - | - | - | - | - | - | 2.46 | - | 5.10 | 1.47 | - |
| 2-heptenal | - | - | - | - | - | - | - | - | - | - | - | - | 51.97 | - | - |
| Nonanal | 4.01 | 6.33 | 4.60 | 6.75 | 3.97 | 4.37 | 3.45 | 3.92 | 6.11 | 1.03 | 11.14 | 8.97 | 14.11 | 7.47 | 7.85 |
| Decanal | 1.72 | 2.59 | 1.57 | - | - | 2.63 | 0.91 | 1.27 | 2.01 | 0.78 | - | - | 5.16 | - | - |
| Benzaldehyde | 3.64 | 3.99 | 1.58 | 2.06 | 3.18 | 3.70 | 1.37 | 0.56 | 1.14 | 3.80 | 8.98 | 25.37 | 38.39 | 6.26 | 2.24 |
| Benzeneacetaldehyde | 5.47 | 4.03 | 14.61 | 5.30 | 13.70 | 6.28 | 5.66 | 6.27 | 4.85 | 1.30 | 4.82 | 2.49 | 30.31 | 127.99 | 23.42 |
| Hexadecanal | 1.31 | 2.93 | 2.63 | 1.40 | 1.61 | 1.39 | 1.03 | 1.10 | 1.30 | 3.49 | 5.58 | 3.26 | - | - | - |
| Aldehydes | 22.52 | 60.92 | 33.00 | 19.59 | 27.17 | 22.54 | 20.67 | 14.42 | 68.57 | 11.21 | 36.27 | 44.18 | 269.54 | 143.19 | 36.43 |
| Ethyl alcohol | 15.46 | 6.17 | 20.31 | 24.81 | 18.94 | 101.21 | 20.17 | 270.32 | 173.69 | 41.81 | 29.99 | 18.04 | 71.23 | 118.85 | 52.12 |
| 2-butanol | - | - | - | - | - | - | - | - | - | 0.49 | - | - | 4.72 | 3.87 | 0.83 |
| 1-propanol | - | - | - | - | - | - | - | - | - | 9.77 | 4.51 | - | 6.49 | 14.93 | - |
| 2-propen-1-ol | - | - | - | 0.67 | 0.46 | - | - | - | - | 0.33 | 1.42 | 1.29 | 3.22 | - | 0.41 |
| Isoamyl alcohol | 0.66 | 0.44 | 0.35 | 0.49 | 0.40 | 2.10 | 3.45 | 6.19 | 2.66 | 0.51 | 2.13 | 1.03 | 3.72 | - | 1.73 |
| 1-pentanol | 0.43 | 1.04 | 0.87 | 0.79 | 0.41 | 2.07 | 0.91 | - | 1.21 | - | - | 0.67 | 5.46 | - | - |
| 1-hexanol | 2.08 | 3.88 | 2.00 | 1.69 | 2.26 | 1.89 | 5.01 | 2.68 | 8.71 | 6.98 | 5.48 | 3.96 | - | 3.00 | - |
| 1-octen-3-ol | 2.85 | 5.01 | 1.30 | 0.62 | 1.08 | 0.49 | 1.41 | - | 2.81 | 0.44 | 2.70 | 1.19 | 19.92 | 1.52 | 0.84 |
| 1-octanol | 0.69 | 0.88 | 0.67 | 0.81 | 0.62 | 0.55 | 0.94 | 0.52 | 0.61 | 0.41 | 1.36 | 0.93 | 3.09 | 1.26 | 1.03 |
| Benzyl alcohol | 0.94 | - | 0.37 | 0.85 | 0.93 | 1.06 | 0.91 | 0.82 | 1.08 | 3.43 | 2.12 | 4.85 | 43.88 | 11.04 | - |
| Phenylethyl alcohol | 1.16 | 1.17 | 0.59 | 2.26 | 3.40 | 1.62 | 3.01 | 7.85 | 1.70 | - | 4.11 | 2.84 | 5.05 | 28.23 | 1.45 |
| Alcohols | 24.27 | 18.59 | 26.46 | 32.99 | 28.50 | 111.00 | 35.81 | 288.37 | 192.46 | 64.17 | 53.81 | 34.81 | 166.79 | 182.70 | 58.40 |
| Acetic acid | 11.28 | 25.95 | 27.28 | 95.75 | 79.25 | 87.10 | 14.38 | 11.72 | 65.64 | 203.95 | 349.10 | 185.04 | 73.10 | 114.92 | 16.40 |
| Propanoic acid | 0.43 | 0.71 | 0.43 | 2.93 | 0.87 | 1.10 | 0.48 | - | - | 7.07 | 7.65 | 0.94 | 14.39 | 22.71 | - |
| Butanoic acid | 10.88 | 5.70 | 4.23 | 10.48 | 8.37 | 15.04 | 9.36 | 19.13 | 20.17 | 4.91 | 14.94 | 20.07 | 4.68 | 8.52 | 3.43 |
| Isovaleric acid | 8.56 | 3.06 | 0.76 | 1.39 | 1.43 | 4.11 | 2.89 | 1.03 | 3.99 | 2.48 | 4.35 | 2.74 | - | - | 3.38 |
| Pentanoic acid | 0.68 | 0.78 | 0.59 | 1.08 | 0.87 | 1.07 | 0.91 | 0.68 | 1.17 | 1.21 | 2.90 | 1.11 | 0.95 | 2.82 | 1.00 |
| Hexanoic acid | 2.98 | 3.25 | 2.62 | 5.04 | 4.12 | 5.30 | 6.03 | 2.74 | 13.22 | 6.21 | 16.35 | 6.68 | 16.21 | 5.94 | 2.60 |
| 4-hexenoic acid | - | - | - | - | - | - | - | - | - | 54.84 | - | - | - | - | - |
| Heptanoic acid | 0.89 | 1.18 | 0.74 | 1.32 | 1.08 | 1.41 | 0.98 | 0.66 | 1.45 | 1.60 | 2.22 | 1.32 | 1.30 | 1.86 | 1.06 |
| Octanoic acid | 2.55 | 3.14 | 2.65 | 6.45 | 4.63 | 3.84 | 3.89 | 2.63 | 6.19 | 7.34 | 5.93 | 4.90 | 4.57 | 6.79 | 2.76 |
| Nonanoic acid | 1.99 | 2.01 | 1.75 | 2.64 | 1.91 | 2.45 | 1.22 | 1.94 | 2.27 | 1.81 | 2.28 | 3.03 | 3.58 | 3.62 | 2.01 |
| n-decanoic acid | 1.82 | 2.98 | 2.56 | 6.68 | 5.18 | 5.58 | 2.52 | 2.35 | 4.38 | 7.37 | 5.86 | 5.09 | 4.84 | 6.10 | 1.79 |
| Dodecanoic acid | 1.70 | 4.24 | 1.99 | 8.87 | 1.99 | 4.81 | 0.89 | 2.23 | 2.36 | 4.10 | 1.66 | 1.87 | 1.50 | 2.67 | 0.84 |
| Acids | 43.75 | 52.98 | 45.61 | 142.64 | 109.69 | 131.80 | 43.55 | 45.12 | 120.82 | 302.91 | 413.24 | 232.80 | 125.11 | 175.96 | 35.27 |
| Acetic acid, methyl ester | - | - | - | - | - | - | - | - | - | 2.74 | 9.55 | 2.73 | - | 1.72 | - |
| Ethyl Acetate | 1.30 | 1.02 | 2.93 | 2.78 | 2.42 | 23.96 | 1.60 | 12.85 | 22.98 | 14.30 | 6.52 | 3.31 | 2.90 | 13.65 | 1.07 |
| Butanoic acid, ethyl ester | - | - | 0.33 | 0.91 | 0.72 | 1.94 | 0.78 | 4.71 | 5.68 | 0.90 | 0.97 | 0.46 | 1.00 | 8.84 | - |
| Hexanoic acid, ethyl ester | 2.58 | 1.80 | 1.73 | 1.79 | 1.76 | 4.17 | 2.53 | 6.97 | 17.18 | 2.72 | 1.71 | - | - | 7.58 | - |
| 4-Hexenoic acid, ethyl ester | - | - | - | - | - | - | - | - | - | 37.68 | - | - | - | - | - |
| Octanoic acid, ethyl ester | - | - | 1.14 | 1.60 | 1.07 | 7.61 | 1.12 | 14.60 | 13.55 | 6.24 | 4.20 | - | 2.89 | 7.18 | 1.00 |
| Dodecanoic acid, methyl ester | - | - | - | - | - | - | - | - | - | 1.45 | 2.28 | 0.61 | - | - | - |
| Dodecanoic acid, ethyl ester | 1.75 | - | 1.35 | 1.84 | 1.03 | 7.95 | 0.62 | 13.02 | 6.98 | 4.65 | 2.56 | - | 2.63 | 9.86 | - |
| Benzoic acid, ethyl ester | - | - | - | - | - | - | - | - | 2.82 | 11.28 | - | - | - | - | - |
| Esters | 5.62 | 2.82 | 7.48 | 8.93 | 7.00 | 45.64 | 6.65 | 52.15 | 69.20 | 81.96 | 27.79 | 7.11 | 9.42 | 48.82 | 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, F.; Tabanelli, G.; Montanari, C.; Dall’Osso, N.; Šimat, V.; Smole Možina, S.; Baños, A.; Özogul, F.; Bassi, D.; Fontana, C.; et al. Mediterranean Spontaneously Fermented Sausages: Spotlight on Microbiological and Quality Features to Exploit Their Bacterial Biodiversity. Foods 2021, 10, 2691. https://doi.org/10.3390/foods10112691
Barbieri F, Tabanelli G, Montanari C, Dall’Osso N, Šimat V, Smole Možina S, Baños A, Özogul F, Bassi D, Fontana C, et al. Mediterranean Spontaneously Fermented Sausages: Spotlight on Microbiological and Quality Features to Exploit Their Bacterial Biodiversity. Foods. 2021; 10(11):2691. https://doi.org/10.3390/foods10112691
Chicago/Turabian StyleBarbieri, Federica, Giulia Tabanelli, Chiara Montanari, Nicolò Dall’Osso, Vida Šimat, Sonja Smole Možina, Alberto Baños, Fatih Özogul, Daniela Bassi, Cecilia Fontana, and et al. 2021. "Mediterranean Spontaneously Fermented Sausages: Spotlight on Microbiological and Quality Features to Exploit Their Bacterial Biodiversity" Foods 10, no. 11: 2691. https://doi.org/10.3390/foods10112691
APA StyleBarbieri, F., Tabanelli, G., Montanari, C., Dall’Osso, N., Šimat, V., Smole Možina, S., Baños, A., Özogul, F., Bassi, D., Fontana, C., & Gardini, F. (2021). Mediterranean Spontaneously Fermented Sausages: Spotlight on Microbiological and Quality Features to Exploit Their Bacterial Biodiversity. Foods, 10(11), 2691. https://doi.org/10.3390/foods10112691










