A Multiplex PCR Assay Combined with Capillary Electrophoresis for the Simultaneous Identification of Atlantic Cod, Pacific Cod, Blue Whiting, Haddock, and Alaska Pollock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction
2.3. Primer Design
2.4. Specificity and Sensitivity of Simplex PCR
2.5. Specificity and Sensitivity of Multiplex PCR
3. Results and Discussion
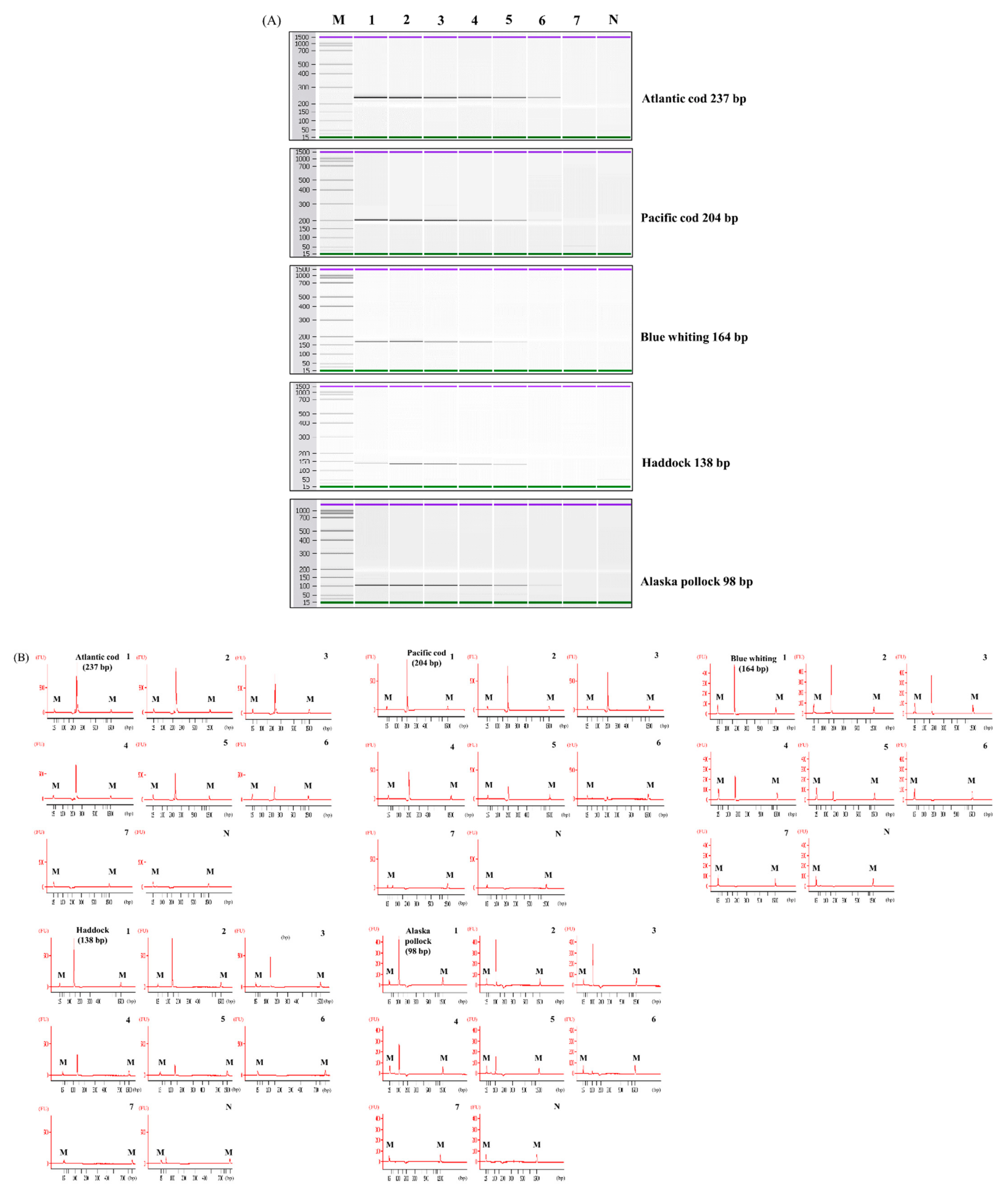
3.1. Specificity and Sensitivity of Simplex PCR
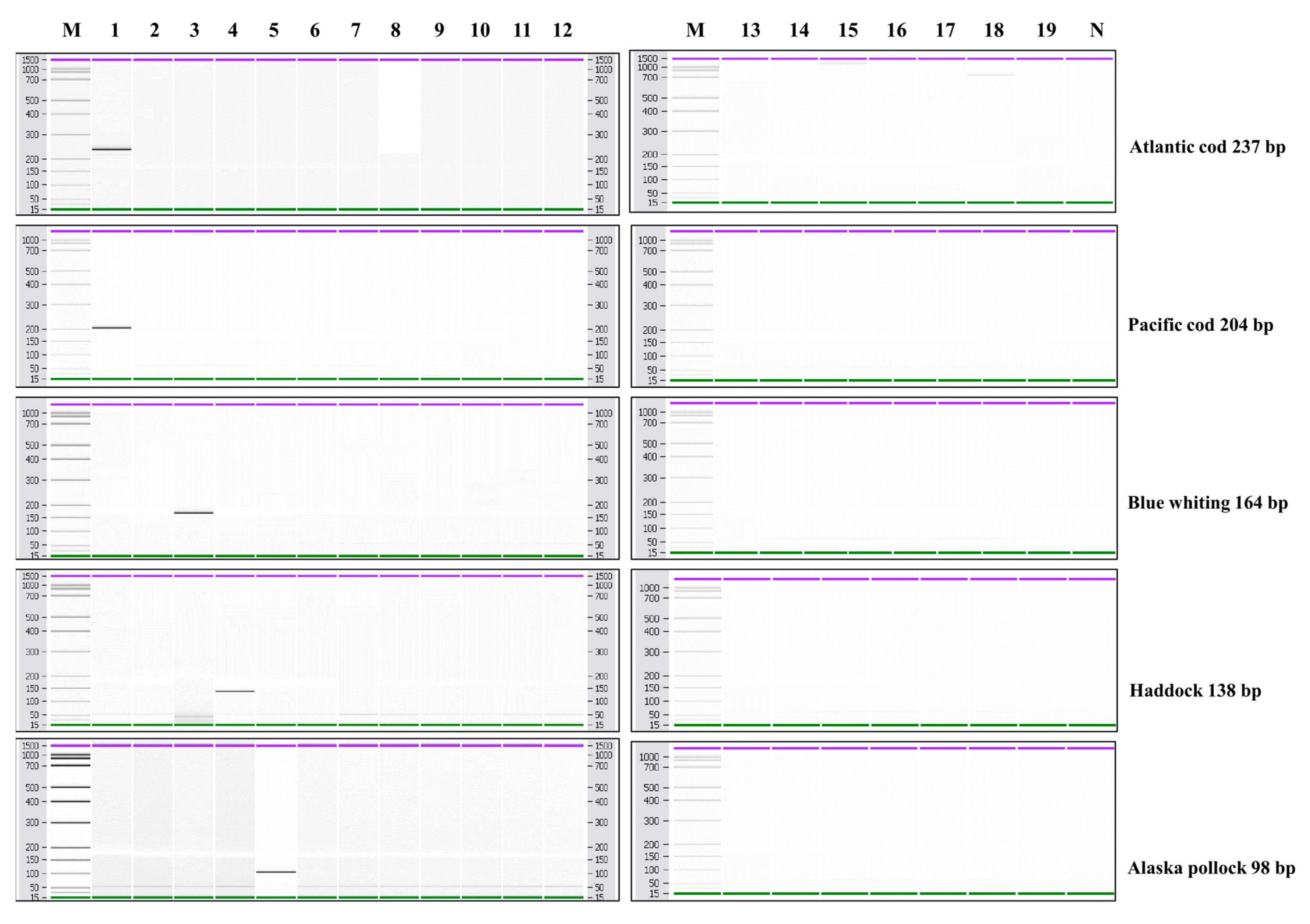
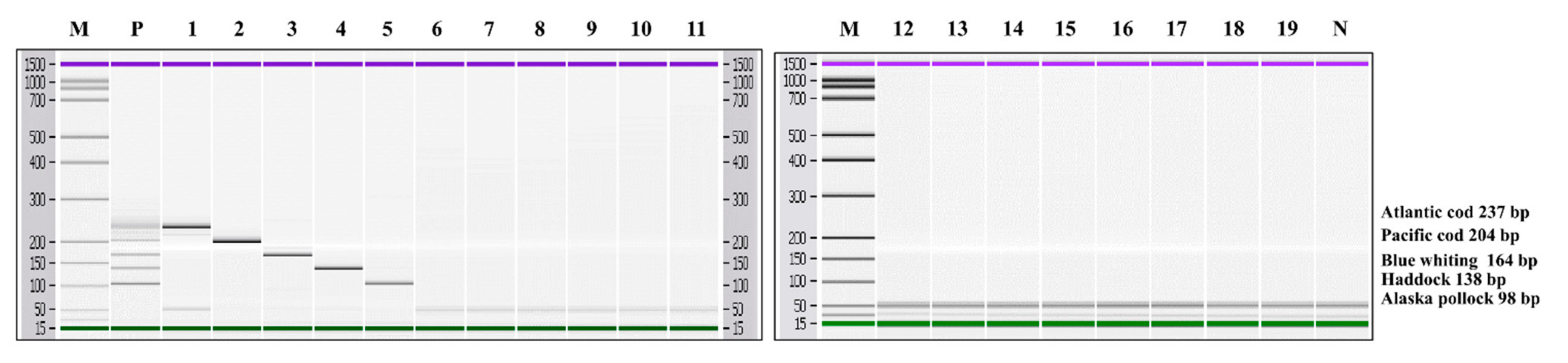
3.2. Specificity and Sensitivity of Multiplex PCR
3.3. Monitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noh, E.S.; Park, Y.J.; Kim, E.M.; Park, J.Y.; Shim, K.B.; Choi, T.J.; Kim, K.H.; Kang, J.H. Quantitative analysis of Alaska pollock in seafood products by droplet digital PCR. Food Chem. 2019, 275, 638–643. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.; Tian, X. Application of loop-mediated isothermal amplification (LAMP) for rapid detection of Atlantic cod (Gadus morhua), Pacific cod (Gadus macrocephalus) and haddock (Melanogrammus aeglefinus). Mol. Cell. Probes 2019, 47, 101420. [Google Scholar] [CrossRef] [PubMed]
- Herreo, B.; Madrinan, M.; Vieites, J.M.; Espineira, M. Authentication of Atlantic cod (Gadus morhua) using Real-Time PCR. Agric. Food Chem. 2010, 58, 4794–4799. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.J.R.; Costa, J.; Oliveria, B.P.P.; Marfra, I. DNA barcoding coupled to HRM analysis as a new and simple tool for the authentication of Gadidae fish species. Food Chem. 2017, 230, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. European Price Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Paracchini, V.; Petrillo, M.; Lievens, A.; Kangkli, D.M.; Loustau, A.A. Nuclear DNA barcodes for cod identification in mildly-treated and processed food products. Food Addit. Contam. 2019, 36, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Helgoe, J.; Oswald, K.J.; Quattro, J.M. A comprehensive analysis of the mislabeling of Atlantic cod (Gadus morhua) products in Spain. Fish. Res. 2020, 222, 105400. [Google Scholar] [CrossRef]
- Feldmann, F.; Ardura, A.; Fernandez, C.B.; Vazquez, E.G. DNA Analysis detects different mislabeling trend by country in European cod fillets. Foods 2021, 10, 1515. [Google Scholar] [CrossRef]
- Pardo, M.A.; Jimenez, E.; Vioarsson, J.R.; Olafsson, K.; Olafsdottir, G.; Danielsdottir, A.K.; Villareal, B.P. DNA barcoding revealing mislabeling of seafood in European mass caterings. Food Control 2018, 92, 7–16. [Google Scholar] [CrossRef]
- Carvalho, D.C.; Palhares, R.M.; Drummond, M.G.; Gadanho, M. Food metagenomics: Next generation sequencing identifies species mixtures and mislabeling within highly processed cod products. Food Control 2017, 80, 183–186. [Google Scholar] [CrossRef]
- Taboada, L.; Sanchez, A.; Velasco, A.; Santaclara, F.J.; Perez-Martin, R.I.; Sotelo, C.G. Identification of Atlantic Cod (Gadus morhua), Ling (Molva molva), and Alaska Pollock (Gadus chalcogrammus) by PCR-ELISA using duplex PCR. Agric. Food Chem. 2014, 62, 5699–5706. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Yuan, F.; Huang, M.; Cao, M.; Xiong, X. Development of a rapid method for codfish identification in processed fish products based on SYBR Green real-time PCR. Food Sci. Technol. 2020, 55, 1843–1850. [Google Scholar] [CrossRef]
- Herrero, B.; Vieites, J.M.; Espineira, M. Authentication of Atlantic salmon (Salmo salar) using real-time PCR. Food Chem. 2011, 127, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Catanese, G.; Manchado, M.; Trujillo, A.F.; Infante, C. A multiplex-PCR assay for the authentication of mackerels of the genus Scomber in processed fish products. Food Chem. 2010, 122, 319–326. [Google Scholar] [CrossRef]
- Qin, P.; Hong, Y.; Kim, H.Y. Multiplex-PCR assay for simultaneous identification of lamb, beef and duck in raw and heat-treated meat mixtures. J. Food Saf. 2016, 36, 367–374. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, Y.M.; Suh, S.M.; Kim, H.Y. Species identification of red deer (Cervus elaphus), roe deer (Capreolus capreolus), and water deer (Hydropotes inermis) using capillary electrophoresis-based multiplex PCR. Foods 2020, 9, 982. [Google Scholar] [CrossRef]
- Suh, S.M.; Kim, M.J.; Kim, H.I.; Kim, H.J.; Kim, H.Y. A multiplex PCR assay combined with capillary electrophoresis for the simultaneous detection of tropomyosin allergens from oyster, mussel, abalone, and clam mollusk species. Food Chem. 2020, 317, 126451. [Google Scholar] [CrossRef] [PubMed]
- Sul, S.Y.; Kim, M.J.; Kim, H.Y. Development of a direct loop-mediated isothermal amplification (LAMP) assay for rapid and simple on-site detection of chicken in processed meat products. Food Control 2019, 98, 194–199. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, M.J.; Hong, Y.; Kim, H.Y. Development of a rapid on-site detection method for pork in processed meat products using real-time loop-mediated isothermal amplification. Food Control 2016, 66, 53–61. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.J.; Jung, S.K.; Kim, H.Y. Development of a fast real-time PCR assay based on TaqMan probe for identification of edible rice grasshopper (Oxya chinensis) in processed food products. Food Res. Int. 2019, 116, 441–446. [Google Scholar] [CrossRef]
- Kim, N.Y.S.; Park, E.J.; Lee, S.H.; Mun, K.H.; Yang, J.Y.; Kim, J.B. Development and validation of multiplex PCR assay for differentiating tunas and billfishes. Food Sci. Technol. 2021, 30, 497–503. [Google Scholar]
- Hulley, E.N.; Tharmalingam, S.; Zarnke, A.; Boreham, D.R. Development and validation of probe-based multiplex real-time PCR assays for the rapid and accurate detection of freshwater fish species. PLoS ONE 2019, 14, e0210165. [Google Scholar] [CrossRef]
- Sangthong, P.; Ngernsiri, L.; Sangthong, D. Identification of Puffer fish of the genus Lagocephalus: L. lunaris, L. spadiceus and L. inermis, using multiplex PCR. Food Biotechnol. 2014, 28, 216–231. [Google Scholar] [CrossRef]
- Ali, M.E.; Razzak, M.A.; Hamid, S.B.; Rahman, M.M.; Amin, M.A.; Rashid, N.R. Asing.; Multiplex PCR assay for the detection of five meat species forbidden in Islamic foods. Food Chem. 2015, 177, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, J.; Zhang, J.; Pan, A.; Quan, S.; Zhan, D.; Kim, H.Y.; Li, X.; Zhou, S.; Yang, L. Development and inter-laboratory transfer of a decaplex polymerase chain reaction assay combined with capillary electrophoresis for the simultaneous detection of ten food allergens. Food Chem. 2016, 199, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Taboada, L.; Sanchez, A.; Perez-Martin, R.I.; Sotelo, C.G. A new method for the rapid detection of Atlantic cod (Gadus morhua), Pacific cod (Gadus macrocephalus), Alaska pollock (Gadus chalogrammus) and ling (Molva molva) using a lateral flow dipstick assay. Food Chem. 2017, 233, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.J.R.; Amaral, J.S.; Mafra, I. DNA barcode markers applied to seafood authentication: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 25, 1–32. [Google Scholar] [CrossRef]
- Filonizi, L.; Chiesa, S.; Vaghi, M.; Marzano, F.N. Molecular barcoding reveals mislabeling of commercial fish products in Italy. Food Res. Int. 2010, 43, 1383–1388. [Google Scholar] [CrossRef]
- Helyar, S.J.; Lloyd, H.D.; Bruyn, M.; Leake, J.; Bennett, N.; Carvalho, G.R. Fish product mislabelling: Failings of traceability in the production chain and implications for Illegal, Unreported and Unregulated (IUU) fishing. PLoS ONE 2014, 9, e98691. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xie, R.; Yu, W.; Wang, N.; Chen, A. Rapid identification of cod and oil fish components based on loop-mediated isothermal amplification. Aquaculture 2021, 545, 737209. [Google Scholar] [CrossRef]

| Target Species | Primer Name | Sequence (5′→3′) | Target Gene | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| Gadus morhua | G.MOR_F | TCA ATG GAT CTG AGG AGG T | cyt b | 237 | This study |
| G.MOR_R | GTT AAG CCC AGA AGC ATC | ||||
| Gadus macrocephalus | G.MAC_F | CAG TAG ATA ATG CCA CCT TG | cyt b | 204 | This study |
| G.MAC_R | GAA GCA TTA CAG CAA AGC CG | ||||
| Micromesistius poutassou | M. POU_F | TCT CCT AGG CCT TTG CTT GG | cyt b | 164 | This study |
| M. POU_R | AGA AAG AGG CAC CGT TAG CG | ||||
| Melanogrammus aeglefinus | M. AEG_F | GCA CTT GTT GAT CTT CCC AC | cyt b | 138 | This study |
| M. AEG_R | GGC TGT TTC AAT GTC TGA AGT A | ||||
| Gadus chalcogrammus | G. CHA_F | CCC TTT CAC CCA TAT TTC ACG | cyt b | 98 | This study |
| G. CHA_R | CCA AGC AAA TTA GGT GCG AAA |
| No | Product Type | Labeled Species | Origin | Multiplex PCR Results | ||||
|---|---|---|---|---|---|---|---|---|
| Atlantic Cod | Pacific Cod | Blue Whiting | Haddock | Alaska Pollock | ||||
| 1 | Dried | Cod | Russia | +++ | ||||
| 2 | Dried | Cod | Russia | +++ | ||||
| 3 | Egg | Cod | USA | +++ | ||||
| 4 | Egg | Cod | USA | +++ | ||||
| 5 | Minced | Cod | Korea | +++ | ||||
| 6 | Pancake | Cod | Russia | +++ | ||||
| 7 | Pancake | Alaska pollock | Russia | +++ | ||||
| 8 | Dried | Alaska pollock | Russia | +++ | ||||
| 9 | Dried | Alaska pollock | Russia | +++ | ||||
| 10 | Roasted | Alaska pollock | Russia | +++ | ||||
| 11 | Dried | Alaska pollock | Russia | +++ | ||||
| 12 | Dried | Alaska pollock | Russia | +++ | ||||
| 13 | Salted | Blue whiting | Vietnam | +++ | ||||
| 14 | Cutlet | Blue whiting | China | +++ | ||||
| 15 | Cutlet | Blue whiting | China | +++ | ||||
| 16 | Dried | Blue whiting | China | +++ | ||||
| 17 | Fried | Alaska pollock | Russia | +++ | ||||
| 18 | Fried | Haddock | USA | +++ | ||||
| 19 | Egg | Alaska pollock | USA | +++ | ||||
| 20 | Fried | Pacific cod | USA | +++ | ||||
| 21 | Salted | Alaska pollock | Russia | +++ | ||||
| 22 | Salted | Alaska pollock | Russia | +++ | ||||
| 23 | Egg | Alaska pollock | Russia | +++ | ||||
| 24 | Egg | Atlantic cod | Sweden | +++ | +++ | |||
| 25 | Fried | Alaska pollock | Russia | +++ | ||||
| 26 | Dried | Alaska pollock | Russia | +++ | ||||
| 27 | Dried | Alaska pollock | Russia | +++ | ||||
| 28 | Fried | Cod | Russia | +++ | ||||
| 29 | Fillet | Cod | USA | +++ | ||||
| 30 | Minced | Cod | Korea | +++ | ||||
| 31 | Dried | Atlantic cod | Norway | +++ | ||||
| 32 | Roasted | Atlantic cod | Norway | +++ | ||||
| 33 | Boiled | Atlantic cod | Norway | +++ | ||||
| 34 | Dried | Atlantic cod | Norway | +++ | ||||
| 35 | Roasted | Atlantic cod | Norway | +++ | ||||
| 36 | Boiled | Haddock | USA | +++ | ||||
| 37 | Boiled | Haddock | USA | +++ | ||||
| 38 | Roasted | Haddock | USA | +++ | ||||
| 39 | Roasted | Haddock | USA | +++ | ||||
| 40 | Dried | Haddock | USA | +++ | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-M.; Lee, S.; Kim, H.-Y. A Multiplex PCR Assay Combined with Capillary Electrophoresis for the Simultaneous Identification of Atlantic Cod, Pacific Cod, Blue Whiting, Haddock, and Alaska Pollock. Foods 2021, 10, 2631. https://doi.org/10.3390/foods10112631
Lee Y-M, Lee S, Kim H-Y. A Multiplex PCR Assay Combined with Capillary Electrophoresis for the Simultaneous Identification of Atlantic Cod, Pacific Cod, Blue Whiting, Haddock, and Alaska Pollock. Foods. 2021; 10(11):2631. https://doi.org/10.3390/foods10112631
Chicago/Turabian StyleLee, Yu-Min, Shinyoung Lee, and Hae-Yeong Kim. 2021. "A Multiplex PCR Assay Combined with Capillary Electrophoresis for the Simultaneous Identification of Atlantic Cod, Pacific Cod, Blue Whiting, Haddock, and Alaska Pollock" Foods 10, no. 11: 2631. https://doi.org/10.3390/foods10112631
APA StyleLee, Y.-M., Lee, S., & Kim, H.-Y. (2021). A Multiplex PCR Assay Combined with Capillary Electrophoresis for the Simultaneous Identification of Atlantic Cod, Pacific Cod, Blue Whiting, Haddock, and Alaska Pollock. Foods, 10(11), 2631. https://doi.org/10.3390/foods10112631







