Nutritional and Chemical-Physical Characterization of Fresh Pasta Gnocchi Prepared with Sea Water as New Active Ingredient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gnocchi Cooking Process and Water Absorption
2.2. Determination of the Sodium Chloride Content (the Mohr Method)
2.3. Mineralization of Samples and Determination of Micro-Macro Elements: Inductively Coupled Plasma-Optical Emission Spectrometers Analysis ICP-OES
2.4. Determination of Volatile Compounds by Solid Phase Microextraction Gas Chromatography/Mass Spectrometer Analysis (SPME-GC/MS)
2.5. Thermogravimentric Analysis (TGA)
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
3. Results
3.1. Water Absorption
3.2. Evaluation of Sodium Chloride Content in Raw/Cooked GTW and GSW Samples
3.3. Evaluation of Macro and Micro Elements of GTW and GSW Samples by ICP-OES Method
3.4. Evaluation of Volatile Compounds by HS-SPME-GC/MS Analysis
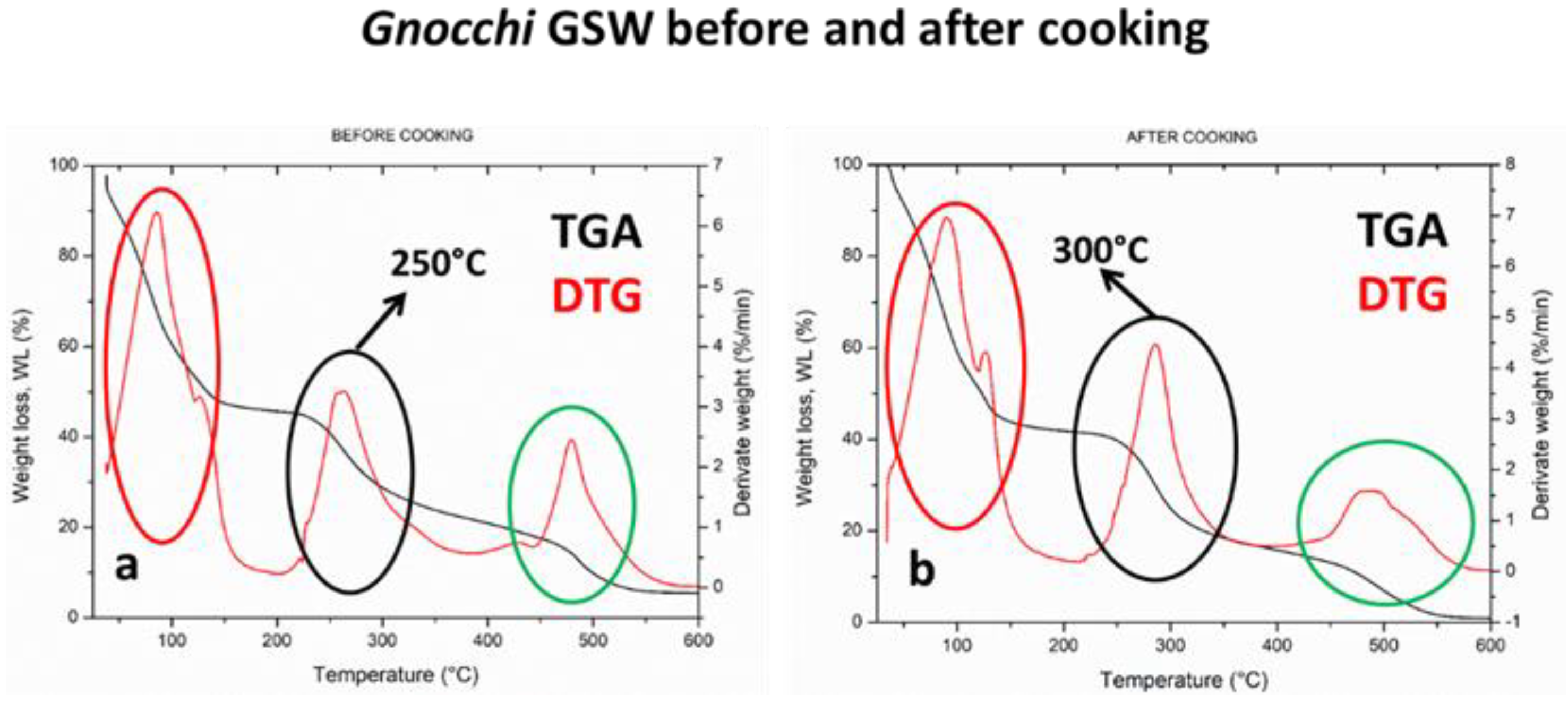
3.5. Thermogravimetric Analysis (TGA, DTG)
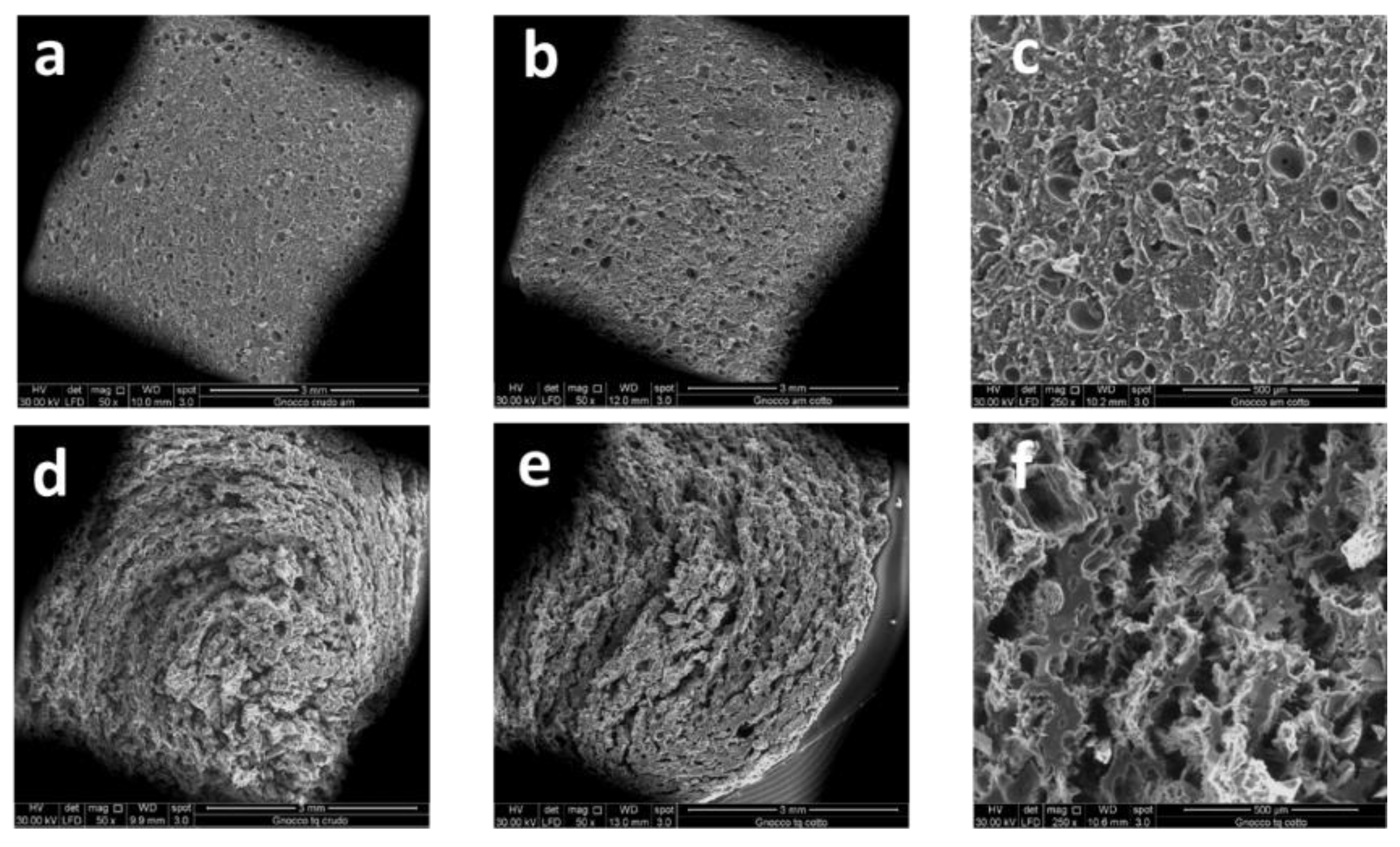
3.6. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ríos, D.; Ghislain, M.; Rodríguez, F.; Spooner, D.M. What Is the Origin of the European Potato? Evidence from Canary Island Landraces. Crop. Sci. 2007, 47, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, L.; Balestra, F.; Romani, S.; Rocculi, P.; Rosa, M.D. Physicochemical and Sensory Properties of Fresh Potato-Based Pasta (Gnocchi). J. Food Sci. 2010, 75, S542–S547. [Google Scholar] [CrossRef] [PubMed]
- Ozkayar, N.; Dede, F.; Ates, I.; Akyel, F.; Yildirim, T.; Altun, B. The Relationship between Dietary Salt Intake and Ambulatory Blood Pressure Variability in Non-Diabetic Hypertensive Patients. Nefrología 2016, 36, 694–700. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. 2—Dietary salt, high blood pressure and other harmful effects on health. In Reducing Salt in Foods; Kilcast, D., Angus, F., Eds.; Woodhead Publishing Series in Food Science; Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2007; pp. 18–54. ISBN 978-1-84569-018-2. [Google Scholar]
- Balasubramanian, A. Properties of Seawater. 2011. Available online: https://www.researchgate.net/publication/309785723_Properties_of_Seawater-Documentary (accessed on 20 October 2021). [CrossRef]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining Valuable Minerals from Seawater: A Critical Review. Environ. Sci. Water Res. Technol. 2017, 3, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.G.; Nazzaro, M.; Coppola, R.; Rapuano, F.; Aquino, R.P. Determination and Assessments of Selected Heavy Metals in Eye Shadow Cosmetics from China, Italy, and USA. Microchem. J. 2012, 101, 65–69. [Google Scholar] [CrossRef]
- di Renzo, T.; Reale, A.; Boscaino, F.; Messia, M.C. Flavoring Production in Kamut®, Quinoa and Wheat Doughs Fermented by Lactobacillus Paracasei, Lactobacillus Plantarum, and Lactobacillus Brevis: A SPME-GC/MS Study. Front. Microbiol. 2018, 9, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Suñol, J.J. ICTAC Kinetics Committee Recommendations for Collecting Experimental Thermal Analysis Data for Kinetic Computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of Pectin-Sodium Alginate Based Films for Potential Healthcare Application: Study of Chemico-Physical Interactions between the Components of Films and Assessment of Their Antimicrobial Activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Moeini, A.; Cimmino, A.; Poggetto, G.D.; Biase, M.D.; Evidente, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Leone, A.; Santagata, G.; et al. Effect of PH and TPP Concentration on Chemico-Physical Properties, Release Kinetics and Antifungal Activity of Chitosan-TPP-Ungeremine Microbeads. Carbohydr. Polym. 2018, 195, 631–641. [Google Scholar] [CrossRef]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Chiralt Boix, A.; Malinconico, M.; Rippa, M.; Serio, M.D.; Santagata, G. Poly (Lactic Acid)/Thermoplastic Starch Films: Effect of Cardoon Seed Epoxidized Oil on Their Chemicophysical, Mechanical, and Barrier Properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of Starch–Water Interactions and Their Effects on Two Key Functional Properties: Starch Gelatinization and Retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, H.; Ai, L.; Xiong, W. Insight into Protein-Starch Ratio on the Gelatinization and Retrogradation Characteristics of Reconstituted Rice Flour. Int. J. Biol. Macromol. 2020, 146, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, H.A.A.; Gomez d’Ayala, G.; Santagata, G.; Bosco, F.; Mollea, C.; Larsen, N.; Duraccio, D. Bioactive Films Based on Barley β-Glucans and ZnO for Wound Healing Applications. Carbohydr. Polym. 2021, 272, 118442. [Google Scholar] [CrossRef]
- Sozer, N.; Kaya, A. The Effect of Cooking Water Composition on Textural and Cooking Properties of Spaghetti. Int. J. Food Prop. 2008, 11, 351–362. [Google Scholar] [CrossRef]
- Kloss, L.; Meyer, J.D.; Graeve, L.; Vetter, W. Sodium Intake and Its Reduction by Food Reformulation in the European Union—A Review. NFS J. 2015, 1, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Gie Liem, D.; Miremadi, F.; J Keast, R.S. Reducing Sodium in Foods: The Effect on Flavor. Nutrients 2011, 3, 694–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, M.K.; Butt, M.S.; Sharif, H.R.; Nasir, M. Sensory Evaluation and Consumer Acceptability. Handb. Food Sci. Technol. 2017, 3, 362–386. [Google Scholar]
- Moeini, A.; Mallardo, S.; Cimmino, A.; Poggetto, G.D.; Masi, M.; Biase, M.D.; van Reenen, A.; Lavermicocca, P.; Valerio, F.; Evidente, A.; et al. Thermoplastic Starch and Bioactive Chitosan Sub-Microparticle Biocomposites: Antifungal and Chemico-Physical Properties of the Films. Carbohydr. Polym. 2020, 230, 115627. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of Secondary Compounds as Additives of Biopolymer-Based Food Packaging: A Review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Delcour, J.A.; Vansteelandt, J.; Hythier, M.-C.; Abécassis, J. Fractionation and Reconstitution Experiments Provide Insight into the Role of Starch Gelatinization and Pasting Properties in Pasta Quality. J. Agric. Food Chem. 2000, 48, 3774–3778. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C.; Harper, K.J. Potassium, Magnesium, and Calcium: Their Role in Both the Cause and Treatment of Hypertension. J. Clin. Hypertens. 2008, 10, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Crook, M. Handbook of Toxicologic Pathology, 2nd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2001; ISBN 0 12 330215 3. [Google Scholar]
- Nalepa, B.; Siemianowska, E.; Skibniewska, K.A. Influence of Bifidobacterium Bifidum on Release of Minerals from Bread with Differing Bran Content. J. Toxicol. Environ. Heal. Part A 2012, 75, 1–5. [Google Scholar] [CrossRef]
- Witkowski, M.; Hubert, J.; Mazur, A. Methods of Assessment of Magnesium Status in Humans: A Systematic Review. Magnes. Res. 2011, 24, 163–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The Metal of Life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B.; Beck, F.W.J.; Kucuk, O.; Sarkar, F.H. Antioxidant Effect of Zinc in Humans. Free. Radic. Biol. Med. 2004, 37, 1182–1190. [Google Scholar] [CrossRef]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, s3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Al-MussaliI, M.S.; Al-Gahri, M.A. Nutritive Value of Commonly Consumed Bread in Yemen. E-J. Chem. 1900, 6, 975960. [Google Scholar] [CrossRef] [Green Version]
- Głąbska, D.; Malowaniec, E.; Guzek, D. Validity and Reproducibility of the Iodine Dietary Intake Questionnaire Assessment Conducted for Young Polish Women. Int. J. Environ. Res. Public Health 2017, 14, 700. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; di Domenico, A.; Férnandez-Cruz, M.L.; Fürst, P.; Fink-Gremmels, J.; Galli, C.L.; et al. Cadmium in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2009, 980, 1–139. [Google Scholar] [CrossRef]
- Panel, E.; Chain, F. Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1–151. [Google Scholar] [CrossRef]
- Giannetti, V.; Boccacci Mariani, M.; Mannino, P.; Testani, E. Furosine and Flavour Compounds in Durum Wheat Pasta Produced under Different Manufacturing Conditions: Multivariate Chemometric Characterization. LWT—Food Sci. Technol. 2014, 56, 15–20. [Google Scholar] [CrossRef]
- Dresow, J.F.; Böhm, H. The Influence of Volatile Compounds of the Flavour of Raw, Boiled and Baked Potatoes: Impact of Agricultural Measures on the Volatile Components. Landbauforsch. Volkenrode 2009, 59, 309–338. [Google Scholar]
- Beleggia, R.; Platani, C.; Spano, G.; Monteleone, M.; Cattivelli, L. Metabolic Profiling and Analysis of Volatile Composition of Durum Wheat Semolina and Pasta. J. Cereal Sci. 2009, 49, 301–309. [Google Scholar] [CrossRef]
- Kermasha, S.; van de Voort, F.R.; Metche, M. Changes in Carbonyl Compounds in the French Bean as a Function of Cooking and Enzyme Treatment. J. Agric. Food Chem. 1988, 36, 133–137. [Google Scholar] [CrossRef]
- Yang, N.; Luan, J.; Ashton, J.; Gorczyca, E.; Kasapis, S. Effect of Calcium Chloride on the Structure and in Vitro Hydrolysis of Heat Induced Whey Protein and Wheat Starch Composite Gels. Food Hydrocoll. 2014, 42, 260–268. [Google Scholar] [CrossRef]
- Hu, Y.; He, C.; Zhang, M.; Zhang, L.; Xiong, H.; Zhao, Q. Inhibition from Whey Protein Hydrolysate on the Retrogradation of Gelatinized Rice Starch. Food Hydrocoll. 2020, 108, 105840. [Google Scholar] [CrossRef]
- Mohamed, I.O. Effects of Processing and Additives on Starch Physicochemical and Digestibility Properties. Carbohydr. Polym. Technol. Appl. 2021, 2, 100039. [Google Scholar] [CrossRef]
- Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B.; Klapiszewski, Ł. Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers 2021, 13, 1280. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.M.; Borges, S.V.; Marconcini, J.M.; Yoshida, M.I.; Neto, A.R.S.; Pereira, T.C.; Pereira, C.F.G. Effect of Replacement of Corn Starch by Whey Protein Isolate in Biodegradable Film Blends Obtained by Extrusion. Carbohydr. Polym. 2017, 157, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turco, R.; Tesser, R.; Cucciolito, M.E.; Fagnano, M.; Ottaiano, L.; Mallardo, S.; Malinconico, M.; Santagata, G.; Di Serio, M. Cynara Cardunculus Biomass Recovery: An Eco-Sustainable, Nonedible Resource of Vegetable Oil for the Production of Poly(Lactic Acid) Bioplasticizers. ACS Sustain. Chem. Eng. 2019, 7, 4069–4077. [Google Scholar] [CrossRef]
- Turco, R.; Zannini, D.; Mallardo, S.; Dal Poggetto, G.; Tesser, R.; Santagata, G.; Malinconico, M.; Di Serio, M. Biocomposites Based on Poly(Lactic Acid), Cynara Cardunculus Seed Oil and Fibrous Presscake: A Novel Eco-Friendly Approach to Hasten PLA Biodegradation in Common Soil. Polym. Degrad. Stab. 2021, 188, 109576. [Google Scholar] [CrossRef]

| Sample | % NaCl Content | % NaCl Gain (+), Losing (−) |
|---|---|---|
| Raw GTW | 1.81 | - |
| GTW cooked in 10% w/v salted water | 3.00 | +65.7 |
| GTW cooked in unsaltedWater | 0.50 | −72.4 |
| Raw GSW | 2.00 | - |
| GSW cooked in unsaltedWater | 1.40 | −30.0 |
| Sample/Elements | Na | Ca | K | Mg | Fe | Cu | Sr | Zn | I |
|---|---|---|---|---|---|---|---|---|---|
| GTW | 80 | 240 | 1870 | 285 | 11.735 | 2.179 | 1.970 | 11.130 | 0.010 |
| GSW | 50 | 340 | 2500 | 700 | 17.690 | 2.905 | 4.607 | 14.095 | 0.041 |
| Sample/Elements | Raw GTW | Raw GSW | GTW Cooked in Unsalted Water | GSW Cooked in Unsalted Water |
|---|---|---|---|---|
| Aldehydes | ||||
| 2-methylbutanal | 0.54 ± 0.02 | 1.09 ± 0.02 | 1.17 ± 0.02 | 0.21 ± 0.01 |
| 3-methylbutanal | 1.10 ± 0.07 | 2.11 ± 0.28 | 1.71 ± 0.02 | 1.96 ± 0.08 |
| Pentanal | 2.69 ± 0.14 | Nd | 2.93 ± 0.38 | 1.55 ± 0.06 |
| Hexanal Octanal | 56.31 ± 0.31 | 14.28 ± 0.03 | 66.36 ± 0.50 | 39.88 ± 1.53 |
| 2-heptenal | 0.31 ± 0.01 | 1.12 ± 0.00 | Nd | 3.64 ± 0.16 |
| Nonanal | Nd | Nd | Nd | 3.34 ± 0.08 |
| 2-octenal | 2.33 ± 0.04 | 5.11 ± 0.15 | 3.51 ± 0.09 | 6.51 ± 0.18 |
| decanal | Nd | Nd | Nd | 0.94 ± 0.0 |
| Benzaldehyde | Nd | Nd | Nd | 2.13 ± 0.01 |
| Tot | 1.98 ± 0.07 | 5.07 ± 0.09 | 3.51 ± 0.02 | 5.87 ± 0.28 |
| Ketones | ||||
| 2-Propanone | 3.52 ± 0.07 | 2.63 ± 0.22 | 2.23 ± 0.09 | 5.85 ± 0.42 |
| 2,3-butanedione | Nd | 1.33 ± 0.01 | Nd | Nd |
| 2-heptanone | Nd | 2.45 ± 0.10 | Nd | 3.02 ± 0.03 |
| 1-hepten-3-one | Nd | Nd | 0.47 ± 0.02 | Nd |
| Acetoin | Nd | 1.69 ± 0.00 | 0.84 ± 0.02 | 2.05 ± 0.08 |
| 2,3-octanedione | 0.17 ± 0.00 | Nd | 0.34 ± 0.03 | Nd |
| 6-methyl-5-hepten-2-one | 0.22 ± 0.00 | 0.49 ± 0.02 | 0.27 ± 0.04 | 0.77 ± 0.04 |
| 3,5-octadien-2-one | 0.61 ± 0.01 | 2.04 ± 0.10 | 1.07 ± 0.03 | 2.54 ± 0.08 |
| 3-octen-2-one | Nd | Nd | 0.55 ± 0.02 | Nd |
| Tot | 4.52 ± 0.07 | 10.62 ± 0.23 | 5.77 ± 0.09 | 14.23 ± 0.63 |
| Alcohols | ||||
| ethanol | 0.18 ± 0.01 | 0.17 ± 0.00 | Nd | Nd |
| 1-Pentanol | 3.92 ± 0.13 | 4.68 ± 0.14 | 3.05 ± 0.18 | 4.00 ± 0.15 |
| 1-Hexanol | 3.56 ± 0.00 | 6.42 ± 0.14 | 2.43 ± 0.01 | 5.77 ± 0.15 |
| 3,5-octadien-2-ol | 0.36 ± 0.01 | Nd | 0.23 ± 0.01 | Nd |
| 1-octen-3-ol | 1.20 ± 0.05 | 1.73 ± 0.02 | 1.36 ± 0.10 | 1.49 ± 0.03 |
| Benzenemethanol | Nd | 0.58 ± 0.01 | Nd | Nd |
| Tot | 9.22 ± 0.15 | 13.58 ± 0.26 | 7.07 ± 0.06 | 11.26 ± 0.02 |
| Furans | ||||
| 2-methylfuran | 0.52 ± 0.00 | 4.90 ± 0.08 | Nd | Nd |
| 2-ethylfuran | 1.49 ± 0.02 | 3.93 ± 0.14 | 1.33 ± 0.05 | 2.09 ± 0.02 |
| 2-pentylfuran | 4.70 ± 0.21 | 8.73 ± 0.42 | 4.91 ± 0.12 | 3.34 ± 0.02 |
| Tot | 6.70 ± 0.22 | 17.57 ± 0.20 | 6.24 ± 0.16 | 5.43 ± 0.04 |
| Acids | ||||
| Acetic acid | 3.58 ± 0.11 | 5.61 ± 0.13 | Nd | Nd |
| 2-methylbutanoic acid | 0.24 ± 0.01 | Nd | Nd | Nd |
| pentanoic acid | Nd | 0.48 ± 0.03 | Nd | Nd |
| Hexanoic acid | 1.21 ± 0.07 | 1.88 ± 0.01 | 0.82 ± 0.01 | Nd |
| Heptanoic acid | 0.41 ± 0.02 | 0.78 ± 0.02 | 0.24 ± 0.01 | Nd |
| Octanoic acid | 0.53 ± 0.01 | 1.60 ± 0.01 | Nd | Nd |
| Nonanoic acid | 0.53 ± 0.02 | 2.41 ± 0.09 | Nd | Nd |
| Dodecanoic acid | 0.49 ± 0.02 | Nd | Nd | Nd |
| Tetradecanoic acid | 1.32 ± 0.09 | Nd | 0.21 ± 0.00 | Nd |
| Hexadecanoic acid | 3.21 ± 0.13 | 4.55 ± 0.06 | 0.22 ± 0.00 | Nd |
| Tot | 11.51 ± 0.23 | 17.31 ± 0.09 | 1.49 ± 0.00 | Nd |
| Terpenes | ||||
| alpha pinene | 0.86 ± 0.00 | 1.42 ± 0.04 | Nd | 1.04 ± 0.03 |
| delta 3-carene | 1.55 ± 0.08 | 3.35 ± 0.14 | Nd | Nd |
| p-cymene | Nd | 0.68 ± 0.02 | Nd | Nd |
| tot | 2.41 ± 0.08 | 5.45 ± 0.08 | Nd | 1.04 ± 0.02 |
| Esters | ||||
| Ethyl Acetate | Nd | 5.81 ± 0.04 | Nd | Nd |
| Tot | Nd | 5.81 ± 0.04 | Nd | Nd |
| Phenols | ||||
| 3-ethylphenol | 0.31 ± 0.01 | 0.61 ± 0.01 | Nd | Nd |
| Phenol | 0.08 ± 0.00 | 0.28 ± 0.02 | 0.25 ± 0.02 | Nd |
| Tot | 0.38 ± 0.01 | 0.89 ± 0.07 | 0.25 ± 0.02 | Nd |
| Terpenes | ||||
| alpha pinene | 0.86 ± 0.00 | 1.42 ± 0.04 | Nd | 1.04 ± 0.03 |
| delta 3-carene | 1.55 ± 0.08 | 3.35 ± 0.14 | Nd | Nd |
| p-cymene | Nd | 0.68 ± 0.02 | Nd | Nd |
| Tot | 0.38 ± 0.01 | 0.89 ± 0.07 | 0.25 ± 0.02 | Nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santagata, G.; Zannini, D.; Mallardo, S.; Boscaino, F.; Volpe, M.G. Nutritional and Chemical-Physical Characterization of Fresh Pasta Gnocchi Prepared with Sea Water as New Active Ingredient. Foods 2021, 10, 2585. https://doi.org/10.3390/foods10112585
Santagata G, Zannini D, Mallardo S, Boscaino F, Volpe MG. Nutritional and Chemical-Physical Characterization of Fresh Pasta Gnocchi Prepared with Sea Water as New Active Ingredient. Foods. 2021; 10(11):2585. https://doi.org/10.3390/foods10112585
Chicago/Turabian StyleSantagata, Gabriella, Domenico Zannini, Salvatore Mallardo, Floriana Boscaino, and Maria Grazia Volpe. 2021. "Nutritional and Chemical-Physical Characterization of Fresh Pasta Gnocchi Prepared with Sea Water as New Active Ingredient" Foods 10, no. 11: 2585. https://doi.org/10.3390/foods10112585
APA StyleSantagata, G., Zannini, D., Mallardo, S., Boscaino, F., & Volpe, M. G. (2021). Nutritional and Chemical-Physical Characterization of Fresh Pasta Gnocchi Prepared with Sea Water as New Active Ingredient. Foods, 10(11), 2585. https://doi.org/10.3390/foods10112585








