Immunomodulatory Potential of the Industrialized Houttuynia cordata Fermentation Product In Vitro and in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HCFP
2.3. In Vitro Phagocytic Activity of Neutrophils
2.4. Animals
2.5. In Vitro Splenocyte Viability and Mitogen-Driven Proliferation
2.5.1. Splenocyte Isolation
2.5.2. MTT Assay
2.6. Dose Selection and Repeated Dose 14-Day Oral Toxicity in Rats
2.7. Immunomodulatory Activity Assays
2.7.1. Antigen Preparation
2.7.2. Treatment
2.7.3. Complete Blood Count (CBC)
2.7.4. Determination of Mitogen-Driven Lymphocyte Proliferation (Ex-Vivo)
2.7.5. Determination of Hemagglutination Assay
2.8. Statistical Analysis
3. Results
3.1. Effect of Soluble Fraction (Lyophilized Powder) of HCFP on In Vitro Phagocytic Activity of Human Neutrophils
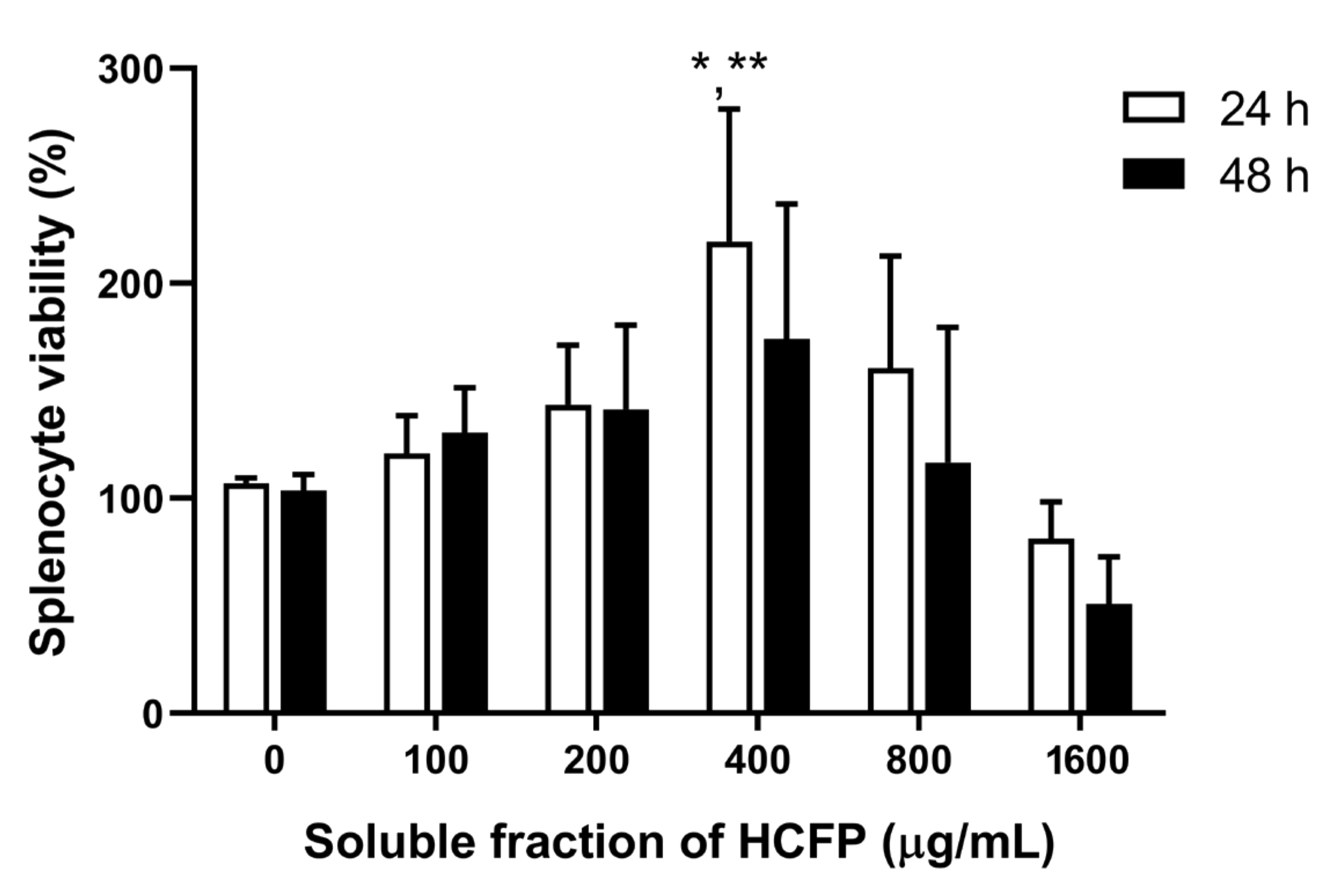
3.2. Effect of Soluble Fraction (Lyophilized Powder) of HCFP on In Vitro Splenocyte Viability and Proliferation
3.3. Oral Toxicity of HCFP in Wistar Rats
3.4. Immunomodulatory Activities of HCFP
3.4.1. Effect of HCFP on Body Weight and Immune Organ of Immunosuppressed Rats
3.4.2. Effect of HCFP on Hematological Parameters
3.4.3. Effect of HCFP on Mitogen-Driven Lymphocyte Proliferation (Ex-Vivo)
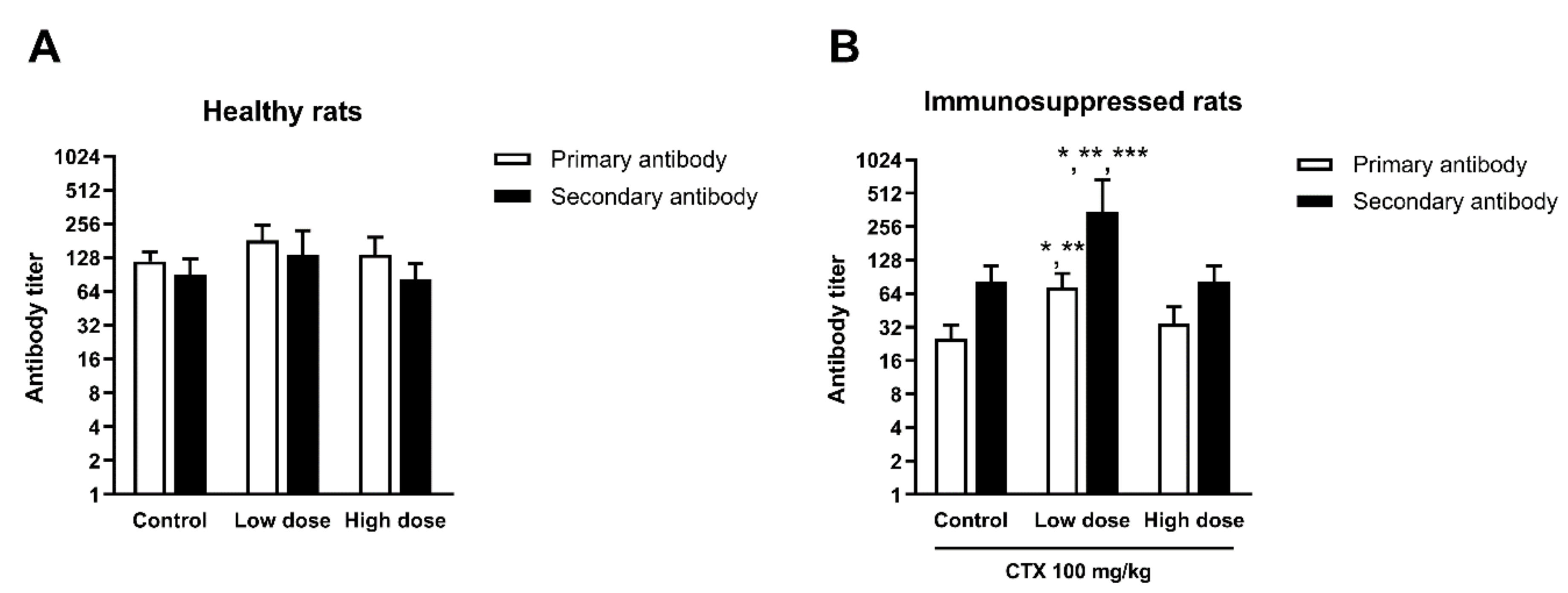
3.4.4. Effect of HCFP on Antibody Production of SRBC-Immunized Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abid, S.; Khajuria, A.; Parvaiz, Q.; Sidiq, T.; Bhatia, A.; Singh, S.; Ahmad, S.; Randhawa, M.K.; Satti, N.K.; Dutt, P. Immunomodulatory studies of a bioactive fraction from the fruit of Prunus cerasus in BALB/c mice. Int. Immunopharmacol. 2012, 12, 626–634. [Google Scholar] [CrossRef]
- Rasheed, H.M.F.; Rasheed, F.; Qureshi, A.W.; Jabeen, Q. Immunostimulant activities of the aqueous methanolic extract of Leptadenia pyrotechnica, a plant from Cholistan desert. J. Ethnopharmacol. 2016, 186, 244–250. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.E.; Huitema, A.D.R.; Rodenhuis, S.; Beijnen, J.H. Clinical Pharmacokinetics of Cyclophosphamide. Clin. Pharmacokinet. 2005, 44, 1135–1164. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Huyan, X.-H.; Lin, Y.-P.; Gao, T.; Chen, R.-Y.; Fan, Y.-M. Immunosuppressive effect of cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice. Int. Immunopharmacol. 2011, 11, 1293–1297. [Google Scholar] [CrossRef]
- Kumar, V.P.; Venkatesh, Y.P. Alleviation of cyclophosphamide-induced immunosuppression in Wistar rats by onion lectin (Allium cepa agglutinin). J. Ethnopharmacol. 2016, 186, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Guo, M.; Feng, C.; Wang, R.; Cheng, D.; Wang, C. Water-soluble polysaccharides from: Grifola Frondosa fruiting bodies protect against immunosuppression in cyclophosphamide-induced mice via JAK2/STAT3/SOCS signal transduction pathways. Food Funct. 2019, 10, 4998–5007. [Google Scholar] [CrossRef] [PubMed]
- Ponnuswamy, S.; Jebasingh Devairrakam, W.E. Comparative study of primary metabolites in different plant parts of Clitoria ternatea Linn. J. Chem. Pharm. Res. 2011, 3, 614–617. [Google Scholar]
- Poonthananiwatkul, B.; Lim, R.H.M.; Howard, R.L.; Pibanpaknitee, P.; Williamson, E.M. Traditional medicine use by cancer patients in Thailand. J. Ethnopharmacol. 2015, 168, 100–107. [Google Scholar] [CrossRef]
- Sharma, U.; Bala, M.; Kumar, N.; Singh, B.; Munshi, R.K.; Bhalerao, S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012, 141, 918–926. [Google Scholar] [CrossRef]
- Fu, J.; Dai, L.; Lin, Z.; Lu, H. Houttuynia cordata Thunb: A Review of Phytochemistry and Pharmacology and Quality Control. Chin. Med. 2013, 4, 101–123. [Google Scholar] [CrossRef]
- Chaliewchalad, P.; Chansakaow, S.; Tragoolpua, Y. Efficacy of Houttuynia cordata Thunb. Extracts Against Herpes Simplex Virus Infection. Chiang Mai J. Sci. 2015, 42, 317–330. [Google Scholar]
- Cheng, D.; Sun, L.; Zou, S.; Chen, J.; Mao, H.; Zhang, Y.; Liao, N.; Zhang, R.; Cheng, D.; Sun, L.; et al. Antiviral effects of Houttuynia cordata polysaccharide extract on Murine Norovirus-1 (MNV-1)—A human norovirus surrogate. Molecules 2019, 24, 1835. [Google Scholar] [CrossRef] [PubMed]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.H.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac. J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Hung, P.Y.; Ho, B.C.; Lee, S.Y.; Chang, S.Y.; Kao, C.L.; Lee, S.S.; Lee, C.N. Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS ONE 2015, 10, e0115475. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Park, J.H.; Kim, J.Y.; Jeong, H.G. Houttuynia cordata water extract suppresses anaphylactic reaction and IgE-mediated allergic response by inhibiting multiple steps of FcεRI signaling in mast cells. Food Chem. Toxicol. 2009, 47, 1659–1666. [Google Scholar] [CrossRef]
- Li, G.Z.; Chai, O.H.; Lee, M.S.; Han, E.H.; Kim, H.T.; Song, C.H. Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biol. Pharm. Bull. 2005, 28, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Chiu, Y.J.; Tang, Y.J.; Lin, K.L.; Chiang, J.H.; Jiang, Y.L.; Jen, H.F.; Kuo, Y.H.; Agamaya, S.; Chung, J.G.; et al. Houttuynia cordata Thunb. extract inhibits cell growth and induces apoptosis in human primary colorectal cancer cells. Anticancer Res. 2010, 30, 3549–3556. [Google Scholar] [PubMed]
- Yanarojana, M.; Nararatwanchai, T.; Thairat, S.; Tancharoen, S. Antiproliferative activity and induction of apoptosis in human melanoma cells by Houttuynia cordata Thunb. extract. Anticancer Res. 2017, 37, 6619–6628. [Google Scholar] [CrossRef]
- Shim, S.Y.; Seo, Y.K.; Park, J.R. Down-regulation of FcepsilonRI expression by Houttuynia cordata Thunb. extract in human basophilic KU812F cells. J. Med. Food 2009, 12, 383–388. [Google Scholar] [CrossRef]
- Lu, H.M.; Liang, Y.Z.; Yi, L.Z.; Wu, X.J. Anti-inflammatory effect of Houttuynia cordata injection. J. Ethnopharmacol. 2006, 104, 245–249. [Google Scholar] [CrossRef]
- Park, E.; Kum, S.; Wang, C.; Park, S.Y.; Kim, B.S.; Schuller-Levis, G. Anti-inflammatory activity of herbal medicines: Inhibition of nitric oxide production and tumor necrosis factor-α secretion in an activated macrophage-like cell line. Am. J. Chin. Med. 2005, 33, 415–424. [Google Scholar] [CrossRef]
- Shin, S.; Soo Joo, S.; Jeon, J.H.; Park, D.; Jang, M.-J.; Kim, T.-O.; Kim, H.-K.; Hwang, Y.; Kim, K.-Y.; Kim, Y.-B. Anti-inflammatory effects of a Houttuynia cordata supercritical extract. J. Vet. Sci. 2010, 11, 273–275. [Google Scholar] [CrossRef]
- Lau, K.M.; Lee, K.M.; Koon, C.M.; Cheung, C.S.F.; Lau, C.P.; Ho, H.M.; Lee, M.Y.H.; Au, S.W.N.; Cheng, C.H.K.; Lau, C.B.S.; et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008, 118, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kim, I.S.; Kim, J.-H.; Kim, J.S.; Kim, D.-H.; Yun, C.-Y. Suppressive effects of Houttuynia cordata Thunb. (Saururaceae) extract on Th2 immune response. J. Ethnopharmacol. 2008, 117, 34–40. [Google Scholar] [CrossRef]
- Satthakarn, S.; Chung, W.; Promsong, A.; Nittayananta, W. Houttuynia cordata modulates oral innate immune mediators: Potential role of herbal plant on oral health. Oral Dis. 2015, 21, 512–518. [Google Scholar] [CrossRef]
- Wigraiboon, S.; Nomura, N.P.; Whangchai, N. Effect of essential oils from Houttuynia cordata Thunb. supplemented diets on growth performance and immune response of Hybrid red tilapia. Int. J. Fish. Aquat. Stud. 2016, 4, 677–684. [Google Scholar]
- Chaiyasut, C.; Sivamaruthi, B.S.; Makhamrueang, N.; Peerajan, S.; Kesika, P. A survey of consumer’ opinion about consumption and health benefits of fermented plant beverages in Thailand. Food Sci. Technol. 2018, 38, 299–309. [Google Scholar] [CrossRef]
- Kwon, R.H.; Ha, B.J. Increased flavonoid compounds from fermented Houttuynia cordata using isolated six of bacillus from traditionally fermented Houttuynia cordata. Toxicol. Res. 2012, 28, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Philip, K.; Sinniah, S.K.; Muniandy, S. Antimicrobial peptides in aqueous and ethanolic extracts from microbial, plant and fermented sources. Biotechnology 2009, 8, 248–253. [Google Scholar] [CrossRef][Green Version]
- Maqbool, M.; Vidyadaran, S.; George, E.; Ramasamy, R. Optimisation of laboratory procedures for isolating human peripheral blood derived neutrophils. Med. J. Malays. 2011, 66, 296–299. [Google Scholar]
- Linden, J.R.; Maccani, M.A.; Laforce-Nesbitt, S.S.; Bliss, J.M. High efficiency opsonin-independent phagocytosis of Candida parapsilosis by human neutrophils. Med. Mycol. 2010, 48, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Mengi, S. Immunostimulant activity of noni (Morinda citrifolia) on T and B lymphocytes. Pharm. Biol. 2010, 48, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, L.; Ravinder, S.S.; Johnson, P.; Padmavathi, R.; Rajagopalan, B.; Kindo, A.J. Assessment of phagocytic activity of neutrophils in chronic obstructive pulmonary disease. Lung India 2015, 32, 437–440. [Google Scholar] [CrossRef]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Hong, H. Do Immunostimulatory effects of polysaccharides isolated from Makgeolli (traditional korean rice wine). Molecules 2014, 19, 5266–5277. [Google Scholar] [CrossRef]
- Nayak, S.; Mengi, S. Immunostimulant activity of the extracts and bioactives of the fruits of Morinda citrifolia. Pharm. Biol. 2009, 47, 248–254. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Senawong, T.; Khaopha, S.; Misuna, S.; Komaikul, J.; Senawong, G.; Wongphakham, P.; Yunchalard, S. Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. Sci. Asia 2015, 40, 420–427. [Google Scholar] [CrossRef]
- Chanda, S.; Juvekar, A.R. In vitro anti-inflammatory activity of syringic acid. Int. J. Pharm. Pharm. Sci. 2018, 11, 71–73. [Google Scholar] [CrossRef]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Watari, A.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on CCl4-Induced Liver Injury. Biol. Pharm. Bull. 2010, 33, 983–987. [Google Scholar] [CrossRef]
- Zhao, C.; Li, M.; Luo, Y.; Wu, W. Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Aconitum carmichaeli. Carbohydr. Res. 2006, 341, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, W.; Zhou, J.; Dai, J.; Li, Y.; Zhao, Y. Computational analysis of naturally occurring aristolochic acid analogues and their biological sources. Biomolecules 2021, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, F.; Cordeiro-Da-Silva, A.; Gaspar-Marques, C.; Simões, F.; Pinto, M.M.M.; Nascimento, M.S.J. Effect of abietane diterpenes from Plectranthus grandidentatus on T- and B-lymphocyte proliferation. Bioorg. Med. Chem. 2004, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Tunkamnerdthai, O.; Auvichayapat, P.; Chaiwiriyakul, S. Effect of Houttuynia cordata Thunb. extract in high-fat diet-induced obese rats. Srinagarind Med. J. 2019, 34, 461–467. [Google Scholar]
- Aghili, T.; Arshami, J.; Tahmasbi, A.M.; Haghparast, A.R. Effects of Hypericum perforatum extract on IgG titer, leukocytes subset and spleen index in rats. Avicenna J. Phytomed. 2014, 4, 413–420. [Google Scholar]
- Chaiyasut, C.; Sivamaruthi, B.S.; Duangjitcharoen, Y.; Kesika, P.; Sirilun, S.; Chaiyasut, K.; Peerajan, S. Assessment of subchronic toxicity of fermented Houttuynia cordata Thunb. using rodent model system. Asian J. Pharm. Clin. Res. 2018, 11, 307–311. [Google Scholar] [CrossRef]
- Mukinda, J.T.; Syce, J.A. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J. Ethnopharmacol. 2007, 112, 138–144. [Google Scholar] [CrossRef]
- Solanki, Y.B.; Jain, S.M. Immunostimolatory activities of Vigna mungo L. extract in male Sprague-Dawley rats. J. Immunotoxicol. 2010, 7, 213–218. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Huang, L.; Gebreselassie, N.G.; Gagliardo, L.F.; Ruyechan, M.C.; Lee, N.A.; Lee, J.J.; Appleton, J.A. Eosinophil-derived IL-10 supports chronic nematode infection. J. Immunol. 2014, 193, 4178–4187. [Google Scholar] [CrossRef]
- Wong, T.W.; Doyle, A.D.; Lee, J.J.; Jelinek, D.F. Eosinophils regulate peripheral B cell numbers in both mice and humans. J. Immunol. 2014, 192, 3548–3558. [Google Scholar] [CrossRef] [PubMed]
- Yuseff, M.I.; Pierobon, P.; Reversat, A.; Lennon-Duménil, A.M. How B cells capture, process and present antigens: A crucial role for cell polarity. Nat. Rev. Immunol. 2013, 13, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Nfambi, J.; Sembajwe, L.F. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 603–611. [Google Scholar] [CrossRef] [PubMed]
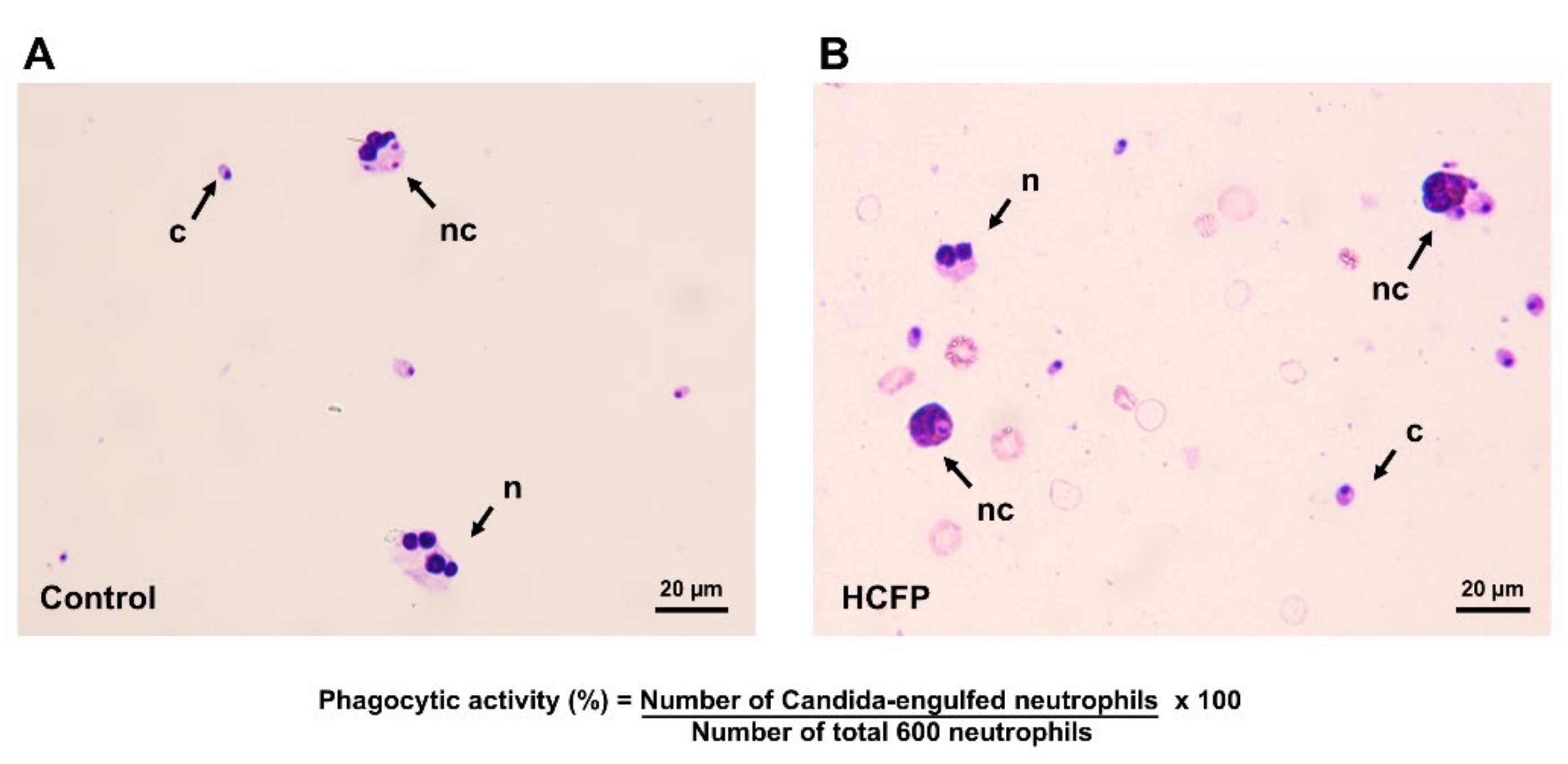


| Treatments | Concentrations (µg/mL) | Phagocytic Activity (%) |
|---|---|---|
| Control (deionized water) | - | 47.74 ± 8.36 |
| Soluble fraction | 100 | 50.47 ± 7.42 |
| 200 | 68.88 ± 5.36 * | |
| 400 | 69.22 ± 5.77 * | |
| 800 | 76.07 ± 6.15 *, ** |
| Gender | Group | Body Weight Gain (g) | Spleen Index |
|---|---|---|---|
| Male | Control | 78.00 ± 17.06 | 2.54 ± 0.32 |
| Low dose | 101.67 ± 7.10 | 2.66 ± 0.20 | |
| High dose | 32.00 ± 12.73 * | 3.37 ± 0.08 * | |
| Female | Control | 38.33 ± 5.86 | 3.38 ± 0.02 |
| Low dose | 26.67 ± 8.51 | 3.15 ± 0.46 | |
| High dose | 27.67 ± 14.01 | 3.26 ± 0.15 |
| Treatment | Body Weight Gain (g) | Spleen Index | |
|---|---|---|---|
| Healthy rats | Control | 86.84 ± 5.89 | 2.51 ± 0.31 |
| Low dose | 68.91 ± 11.43 * | 2.46 ± 0.22 | |
| High dose | 65.40 ± 13.71 ** | 2.82 ± 0.42 | |
| Immunosuppressed rats | Control | 47.71 ± 7.96 ### | 2.79 ± 0.28 |
| Low dose | 51.39 ± 9.32 | 2.44 ± 0.35 | |
| High dose | 39.46 ± 7.81 | 2.79 ± 0.21 |
| Hematological Parameters | Healthy Rats a | Immunosuppressed Rats a | ||||
|---|---|---|---|---|---|---|
| Control | Low Dose | High Dose | Control | Low Dose | High Dose | |
| WBC (103/µL) | 5.30 ± 0.73 | 5.67 ± 0.64 | 5.53 ± 1.54 | 3.50 ± 0.66 ## | 4.21 ± 0.82 | 4.08 ± 0.86 |
| RBC (106/µL) | 8.02 ± 0.64 | 8.36 ± 0.57 | 8.07 ± 0.37 | 7.74 ± 0.32 | 7.31 ± 0.19 ** | 7.96 ± 0.13 |
| HGB (g/dL) | 14.78 ± 0.98 | 15.30 ± 1.08 | 14.96 ± 0.77 | 14.52 ± 0.56 | 13.43 ± 0.43 ** | 14.73 ± 0.33 |
| HCT (%) | 45.52 ± 4.06 | 46.97 ± 4.05 | 45.66 ± 4.31 | 44.45 ± 1.68 | 43.33 ± 1.07 | 44.43 ± 0.92 |
| MCV (fL) | 56.73 ± 1.71 | 56.15 ± 2.32 | 56.52 ± 3.14 | 57.42 ± 1.50 | 59.27 ± 1.40 | 55.83 ± 1.09 |
| MCH (pg) | 18.45 ± 0.30 | 18.30 ± 0.30 | 18.56 ± 0.36 | 18.73 ± 0.35 | 18.37 ± 0.23 | 18.52 ± 0.39 |
| MCHC (g/dL) | 32.53 ± 0.97 | 32.62 ± 0.92 | 32.86 ± 1.45 | 32.65 ± 0.33 | 30.98 ± 0.77 ** | 33.18 ± 0.29 |
| PLT (103/µL) | 754.33 ± 104.40 | 796.67 ± 217.62 | 841.80 ± 100.6 | 845.17 ± 418.56 | 1061.17 ± 123.6 | 899.67 ± 281.1 |
| RDW-SD (fL) | 27.83 ± 2.08 | 27.83 ± 1.15 | 27.68 ± 2.41 | 34.27 ± 1.68 ### | 37.68 ± 3.68 | 32.97 ± 1.52 |
| RDW-CV (%) | 15.60 ± 1.60 | 16.17 ± 1.21 | 15.70 ± 1.71 | 19.25 ± 0.69 ### | 20.15 ± 1.32 | 19.28 ± 0.84 |
| PDW (fL) | 7.28 ± 0.87 | 7.95 ± 0.71 | 7.46 ± 0.55 | 8.60 ± 0.29 ## | 7.43 ± 0.33 ** | 8.28 ± 0.41 |
| MPV (fL) | 7.43 ± 0.58 | 7.70 ± 0.39 | 7.40 ± 0.34 | 8.22 ± 0.18 ## | 7.60 ± 0.23 * | 8.03 ± 0.022 |
| P-LCR (%) | 6.63 ± 3.39 | 8.25 ± 2.34 | 6.24 ± 1.74 | 11.42 ± 1.35## | 8.38 ± 1.35 ** | 10.25 ± 1.69 |
| PCT (%) | 0.57 ± 0.12 | 0.61 ± 0.17 | 0.63 ± 0.09 | 0.83 ± 0.11 # | 0.80 ± 0.08 | 0.72 ± 0.23 |
| NEU (103/µL) | 0.57 ± 0.32 | 0.74 ± 0.21 | 0.83 ± 0.39 | 0.28 ± 0.04 | 0.55 ± 0.10 * | 0.75 ± 0.21 ** |
| LYM (103/µL) | 4.40 ± 0.57 | 4.58 ± 0.48 | 4.37 ± 1.08 | 2.65 ± 0.63 ## | 3.12 ± 0.61 | 2.94 ± 0.63 |
| MONO (103/µL) | 0.13 ± 0.11 | 0.24 ± 0.09 | 0.20 ± 0.09 | 0.09 ± 0.04 | 0.29 ± 0.07 *** | 0.12 ± 0.09 |
| EO (103/µL) | 0.12 ± 0.14 | 0.12 ± 0.04 | 0.13 ± 0.04 | 0.09 ± 0.02 | 0.25 ± 0.12 * | 0.23 ± 0.10 * |
| BASO (103/µL) | 0.09 ± 0.22 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.38 ± 0.12 # | 0.00 ± 0.00 ** | 0.09 ± 0.21 * |
| NEU (%) | 10.43 ± 4.97 | 13.02 ± 3.13 | 14.16 ± 4.34 | 8.33 ± 2.55 | 13.30 ± 1.91 | 19.38 ± 4.51 ** |
| LYM (%) | 83.28 ± 5.59 | 80.85 ± 4.02 | 79.86 ± 4.92 | 74.83 ± 4.73 | 74.28 ± 1.74 | 71.92 ± 4.39 |
| MONO (%) | 2.35 ± 1.85 | 4.10 ± 1.29 | 3.54 ± 0.96 | 2.85 ± 1.53 | 6.73 ± 1.10 *** | 2.72 ± 1.68 |
| EO (%) | 2.40 ± 3.04 | 2.03 ± 0.62 | 2.44 ± 0.27 | 2.73 ± 0.96 | 5.68 ± 2.15 * | 5.72 ± 2.00 * |
| BASO (%) | 1.53 ± 3.76 | 0.00 ± 0.00 | 0.00 ± 0.00 | 10.97 ± 1.88 ### | 0.00 ± 0.00 *** | 1.77 ± 4.33 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utaiwat, S.; Senawong, G.; Khongsukwiwat, K.; Woranam, K.; Sattayasai, J.; Senawong, T. Immunomodulatory Potential of the Industrialized Houttuynia cordata Fermentation Product In Vitro and in Wistar Rats. Foods 2021, 10, 2582. https://doi.org/10.3390/foods10112582
Utaiwat S, Senawong G, Khongsukwiwat K, Woranam K, Sattayasai J, Senawong T. Immunomodulatory Potential of the Industrialized Houttuynia cordata Fermentation Product In Vitro and in Wistar Rats. Foods. 2021; 10(11):2582. https://doi.org/10.3390/foods10112582
Chicago/Turabian StyleUtaiwat, Suppawit, Gulsiri Senawong, Kanoknan Khongsukwiwat, Khanutsanan Woranam, Jintana Sattayasai, and Thanaset Senawong. 2021. "Immunomodulatory Potential of the Industrialized Houttuynia cordata Fermentation Product In Vitro and in Wistar Rats" Foods 10, no. 11: 2582. https://doi.org/10.3390/foods10112582
APA StyleUtaiwat, S., Senawong, G., Khongsukwiwat, K., Woranam, K., Sattayasai, J., & Senawong, T. (2021). Immunomodulatory Potential of the Industrialized Houttuynia cordata Fermentation Product In Vitro and in Wistar Rats. Foods, 10(11), 2582. https://doi.org/10.3390/foods10112582






