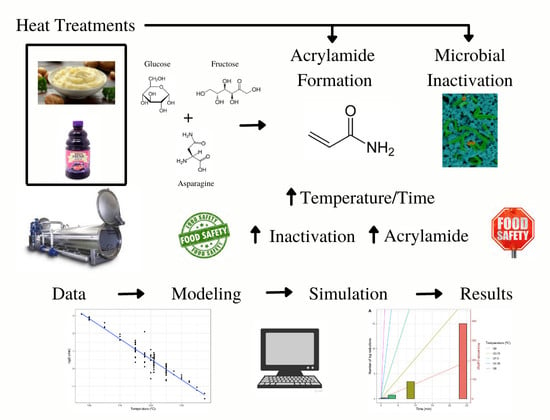
Dynamics of Microbial Inactivation and Acrylamide Production in High-Temperature Heat Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Study
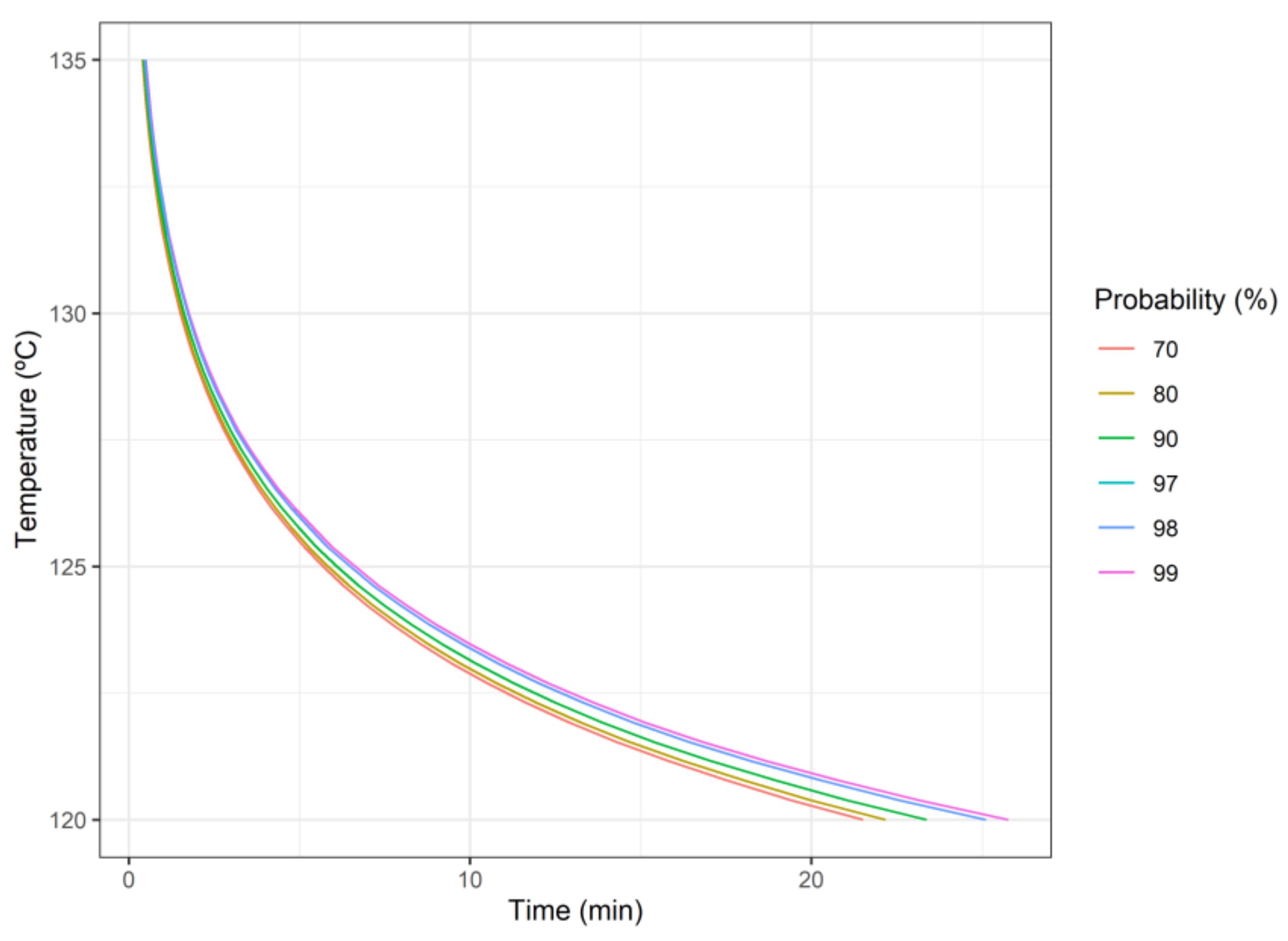
2.2. Microbial Inactivation Model and Parameter Estimation
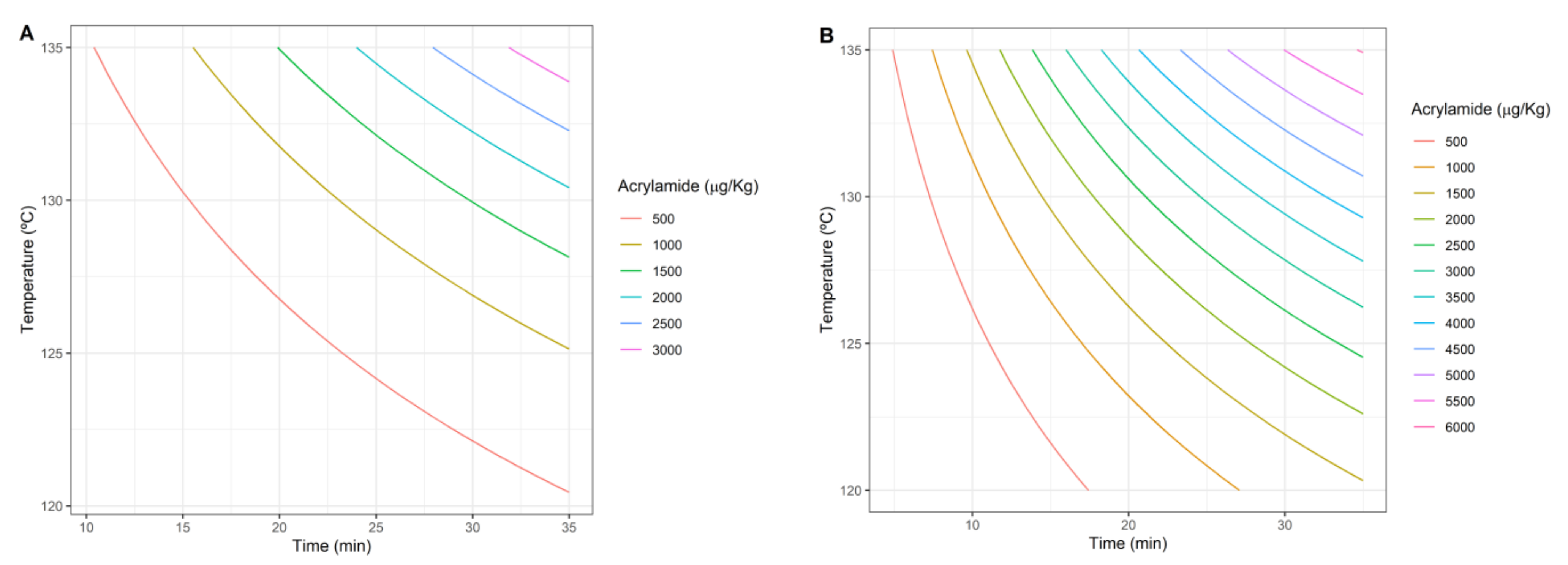
2.3. Acrylamide Production Objective
3. Results and Discussion
3.1. Single-Objective Analysis
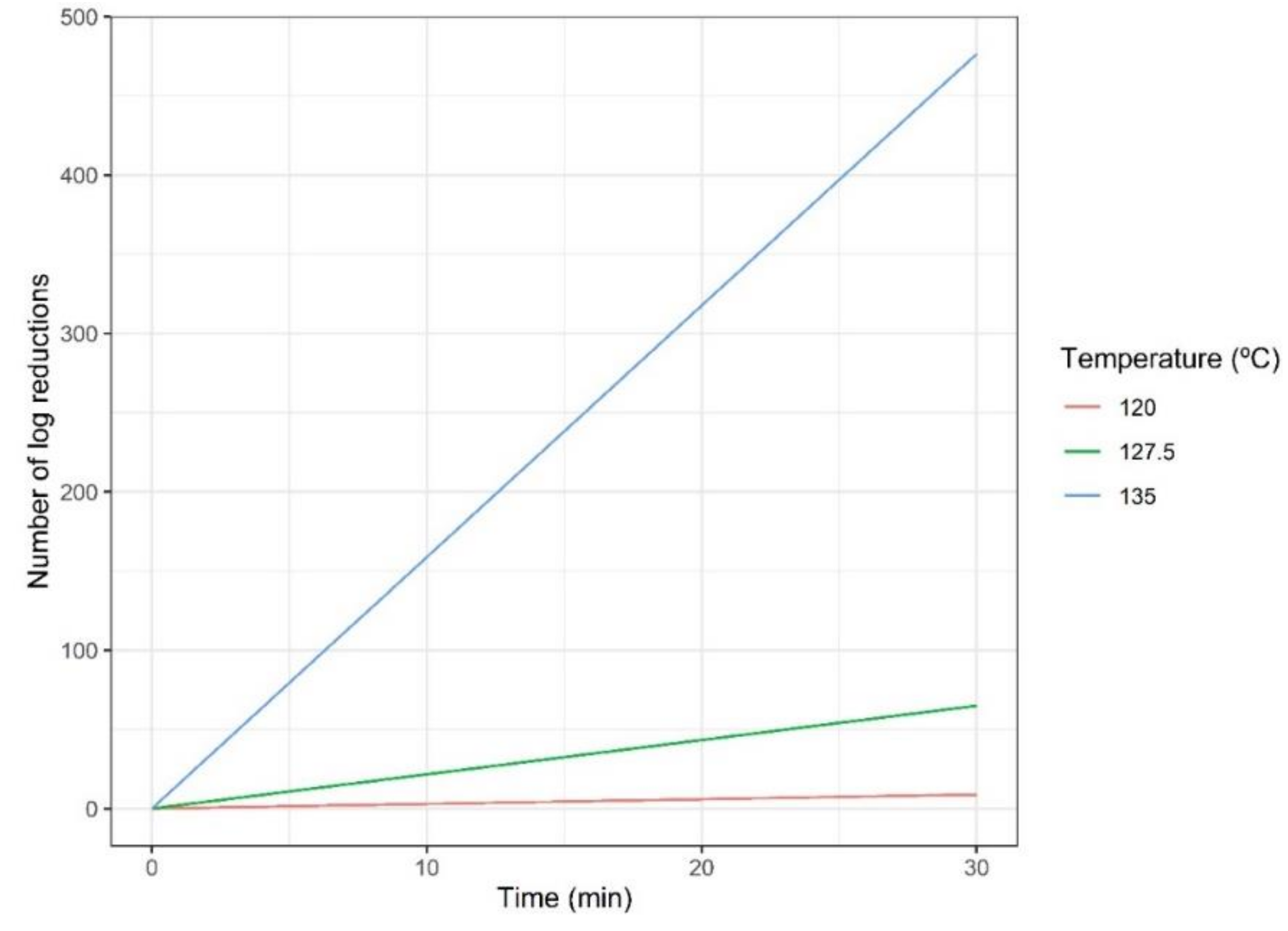
3.2. Dynamics of Geobacillus Inactivation and Acrylamide Formation
3.3. Multi-Objective Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Perrot, N.; Trelea, I.; Baudrit, C.; Trystram, G.; Bourgine, P. Modelling and analysis of complex food systems: State of the art and new trends. Trends Food Sci. Technol. 2011, 22, 304–314. [Google Scholar] [CrossRef]
- Trystram, G. Modelling of Food and Food Processes. J. Food Eng. 2012, 110, 269–277. [Google Scholar]
- Banga, J.R.; Balsa-Canto, E.; Moles, C.G.; Alonso, A. Improving food processing using modern optimization methods. Trends Food Sci. Technol. 2003, 14, 131–144. [Google Scholar] [CrossRef]
- Erdoğdu, F. Optimization in Food Engineering, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781420061420. [Google Scholar]
- Madoumier, M.; Trystram, G.; Sébastian, P.; Collignan, A. Towards a holistic approach for multi-objective optimization of food processes: A critical review. Trends Food Sci. Technol. 2019, 86, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gergely, S.; Békássy-Molnár, E.; Vatai, G. The use of multiobjective optimization to improve wine filtration. J. Food Eng. 2003, 58, 311–316. [Google Scholar] [CrossRef]
- Sendín, J.O.H.; Alonso, A.; Banga, J.R. Efficient and robust multi-objective optimization of food processing: A novel approach with application to thermal sterilization. J. Food Eng. 2010, 98, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Kiranoudis, C.; Markatos, N. Pareto design of conveyor-belt dryers. J. Food Eng. 2000, 46, 145–155. [Google Scholar] [CrossRef]
- Holdsworth; Simpson, R. Multiobjective Optimization in Thermal Food Processing. In Thermal Processing of Packaged Foods; Springer International Publishing: Cham, Switzerland, 2016; pp. 415–424. ISBN1 978-3-319-24902-5. ISBN2 978-3-319-24904-9. [Google Scholar]
- Abakarov, A.; Sushkov, Y.; Mascheroni, R.H. A multi-criteria optimization and decision-making approach for improvement of food engineering processes. Int. J. Food Stud. 2013, 2, 1–21. [Google Scholar] [CrossRef]
- Winiczenko, R.; Górnicki, K.; Kaleta, A.; Martynenko, A.; Janaszek-Mańkowska, M.; Trajer, J. Multi-objective optimization of convective drying of apple cubes. Comput. Electron. Agric. 2018, 145, 341–348. [Google Scholar] [CrossRef]
- Krüger, C.; Castellani, F.; Geldermann, J.; Schöbel, A. Peat and pots: An application of robust multiobjective optimization to a mixing problem in agriculture. Comput. Electron. Agric. 2018, 154, 265–275. [Google Scholar] [CrossRef]
- Bortolini, M.; Faccio, M.; Ferrari, E.; Gamberi, M.; Pilati, F. Fresh food sustainable distribution: Cost, delivery time and carbon footprint three-objective optimization. J. Food Eng. 2016, 174, 56–67. [Google Scholar] [CrossRef]
- Vilas, C.; Mauricio-Iglesias, M.; García, M.R. Model-based design of smart active packaging systems with antimicrobial activity. Food Packag. Shelf Life 2020, 24, 100446. [Google Scholar] [CrossRef]
- Holdsworth, S. Optimisation of thermal processing—A review. J. Food Eng. 1985, 4, 89–116. [Google Scholar] [CrossRef]
- Mogol, B.A.; Gökmen, V. Thermal process contaminants: Acrylamide, chloropropanols and furan. Curr. Opin. Food Sci. 2016, 7, 86–92. [Google Scholar] [CrossRef]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Gorzinski, S.J.; Bodner, K.M.; Campbell, R.A.; Wolf, C.H.; Friedman, M.A.; Mast, R.W. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol. Appl. Pharmacol. 1986, 85, 154–168. [Google Scholar] [CrossRef]
- Rice, J.M. The carcinogenicity of acrylamide. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 580, 3–20. [Google Scholar] [CrossRef]
- European Food Safety Authority. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J. 2012, 10, 2938. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Maillard, L.C. Action Des Acides Aminés Sur Les Sucres: Formation Des Mélanoïdines Par Voie Méthodique. Comptes. R. Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Caixeta, A.T.; Moreira, R.; Castell-Perez, M.E. IMPINGEMENT DRYING OF POTATO CHIPS. J. Food Process. Eng. 2002, 25, 63–90. [Google Scholar] [CrossRef]
- Markowski, M.; Cenkowski, S.; Hatcher, D.W.; Dexter, J.E.; Edwards, N.M. The effect of superheated—Steam dehydration kinetics on textural properties of asian noodles. Trans. ASAE 2003, 46, 389–395. [Google Scholar] [CrossRef]
- Elias, A.; Roasto, M.; Reinik, M.; Nelis, K.; Nurk, E.; Elias, T. Acrylamide in commercial foods and intake by infants in Estonia. Food Addit. Contam. Part A 2017, 34, 1875–1884. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Fakhri, Y.; Nematollahi, A.; Seilani, F.; Vasseghian, Y. The Concentration of Acrylamide in Different Food Products: A Global Systematic Review, Meta-Analysis, and Meta-Regression. Food Rev. Int. 2020, 1–19. [Google Scholar] [CrossRef]
- Elmore, J.S.; Xu, F.; Maveddat, A.; Kapetanou, R.; Qi, H.; Oruna-Concha, M.J. Acrylamide content of vegetable chips. In Food-Borne Toxicants: Formation, Analysis, and Toxicology; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Volume 1306, pp. 15–26. ISBN 9780841233874. [Google Scholar]
- Becalski, A.; Brady, B.; Feng, S.; Gauthier, B.; Zhao, T. Formation of acrylamide at temperatures lower than 100 °C: The case of prunes and a model study. Food Addit. Contam. Part A 2011, 28, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Stumbo, C.R. Thermobacteriology in Food Processing; Elsevier: Amsterdam, The Netherlands, 1973; ISBN 978-0-12-675352-3. [Google Scholar]
- Postollec, F.; Mathot, A.-G.; Bernard, M.; Divanac’H, M.-L.; Pavan, S.; Sohier, D. Tracking spore-forming bacteria in food: From natural biodiversity to selection by processes. Int. J. Food Microbiol. 2012, 158, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brown, K. Spore resistance and ultra heat treatment processes. J. Appl. Bacteriol. 1994, 76, 67S–80S. [Google Scholar] [CrossRef] [PubMed]
- Spicher, G.; Peters, J.; Borchers, U. Microbiological efficacy of superheated steam. I. Communication: Results with spores of Bacillus subtilis and Bacillus stearothermophilus and with spore earth. Zentralblatt Hyg. Umweltmed. Int. J. Hyg. Environ. Med. 1999, 201, 541–553. [Google Scholar]
- Carlberg, D.M. 2005 Cleanroom Microbiology for the Non-Microbiologist, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-8493-1996-9. [Google Scholar]
- Fernández, P.; Ocio, M.; Rodrigo, F.; Martínez, A. Mathematical model for the combined effect of temperature and pH on the thermal resistance of Bacillus stearothermophilus and Clostridium sporogenes spores. Int. J. Food Microbiol. 1996, 32, 225–233. [Google Scholar] [CrossRef]
- Bigelow, W. The Logarithmic Nature of Thermal Death Time Curves. J. Infect. Dis. 1921, 29, 528–536. [Google Scholar] [CrossRef]
- Mann, A.; Kiefer, M.; Leuenberger, H. Thermal Sterilization of Heat-sensitive Products Using High-temperature Short-time Sterilization. J. Pharm. Sci. 2001, 90, 275–287. [Google Scholar] [CrossRef]
- Leguerinel, I.; Couvert, O.; Mafart, P. Modelling the influence of the incubation temperature upon the estimated heat resistance of heated bacillus spores. Lett. Appl. Microbiol. 2006, 43, 17–21. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Leguerinel, I.; Palop, A.; Desriac, N.; Planchon, S.; Mafart, P. Convergence of Bigelow and Arrhenius models over a wide range of heating temperatures. Int. J. Food Microbiol. 2018, 291, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Poschet, F.; Geeraerd, A.; Van Loey, A.; Hendrickx, M.; Van Impe, J. Assessing the optimal experiment setup for first order kinetic studies by Monte Carlo analysis. Food Control. 2005, 16, 873–882. [Google Scholar] [CrossRef]
- Peñalver-Soto, J.L.; Garre, A.; Esnoz, A.; Fernández, P.S.; Egea, J.A. Guidelines for the design of (optimal) isothermal inactivation experiments. Food Res. Int. 2019, 126, 108714. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, E.D.; Zwietering, M.H. A systematic approach to determine global thermal inactivation parameters for various food pathogens. Int. J. Food Microbiol. 2006, 107, 73–82. [Google Scholar] [CrossRef]
- Gibbiel, A.Y.; Al, A.T.H.A.-E. Measurement of Heat Resistance Parameters for Spores Isolated from Canned Products. J. Appl. Bacteriol. 1973, 36, 321–327. [Google Scholar] [CrossRef]
- Hassan, H.F.; Ramaswamy, H.S. Heat resistance of g. stearothermophilus and c. sporogenes in carrot and meat alginate purees. J. Food Process. Preserv. 2011, 35, 376–385. [Google Scholar] [CrossRef]
- Fernandez, P.S.; Ocio, M.J.; Sanchez, T.; Martinez, A. Thermal Resistance of Bacillus stearothermophilus Spores Heated in Acidified Mushroom Extract. J. Food Prot. 1994, 57, 37–41. [Google Scholar] [CrossRef]
- Fernandez, P.S.; Gómez, F.J.; Ocio, M.J.; Rodrigo, M.; Sánchez, T.; Martínez, A. D Values of Bacillus stearothermophilus Spores as a Function of pH and Recovery Medium Acidulant. J. Food Prot. 1995, 58, 628–632. [Google Scholar] [CrossRef]
- Ocio, M.; Fernández, P.; Rodrigo, F.; Martínez, A. Heat resistance of Bacillus stearothermophilus spores in alginate-mushroom puree mixture. Int. J. Food Microbiol. 1996, 29, 391–395. [Google Scholar] [CrossRef]
- López, M. Effect of pH heating medium on the thermal resistance of Bacillus stearothermophilus spores. Int. J. Food Microbiol. 1996, 28, 405–410. [Google Scholar] [CrossRef]
- Molin, N.; Snygg, B.G. Effect of Lipid Materials on Heat Resistance of Bacterial Spores. Appl. Microbiol. 1967, 15, 1422–1426. [Google Scholar] [CrossRef]
- Yildiz, F.; Westhoff, D. Sporulation and thermal resistance of Bacillus stearothermophilus spores in milk. Food Microbiol. 1989, 6, 245–250. [Google Scholar] [CrossRef]
- Etoa, F.-X.; Michiels, L. Heat-induced resistance of Bacillus stearothermophilus spores. Lett. Appl. Microbiol. 1988, 6, 43–45. [Google Scholar] [CrossRef]
- Knol, J.J.; van Loon, W.A.M.; Linssen, J.P.H.; Ruck, A.-L.; van Boekel, M.A.J.S.; Voragen, A.G.J. Toward a Kinetic Model for Acrylamide Formation in a Glucose−Asparagine Reaction System. J. Agric. Food Chem. 2005, 53, 6133–6139. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, V.; Finotti, E.; Friedman, M. Level of Acrylamide Precursors Asparagine, Fructose, Glucose, and Sucrose in Potatoes Sold at Retail in Italy and in the United States. J. Food Sci. 2006, 71, C81–C85. [Google Scholar] [CrossRef]
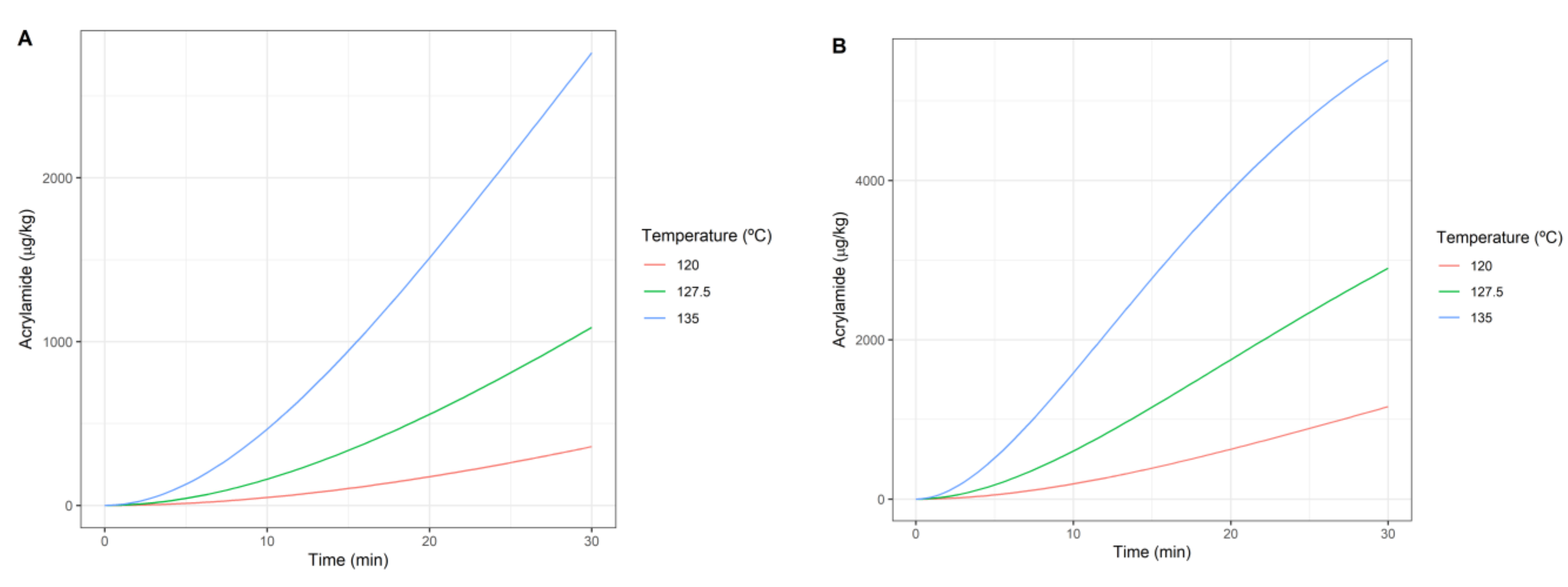
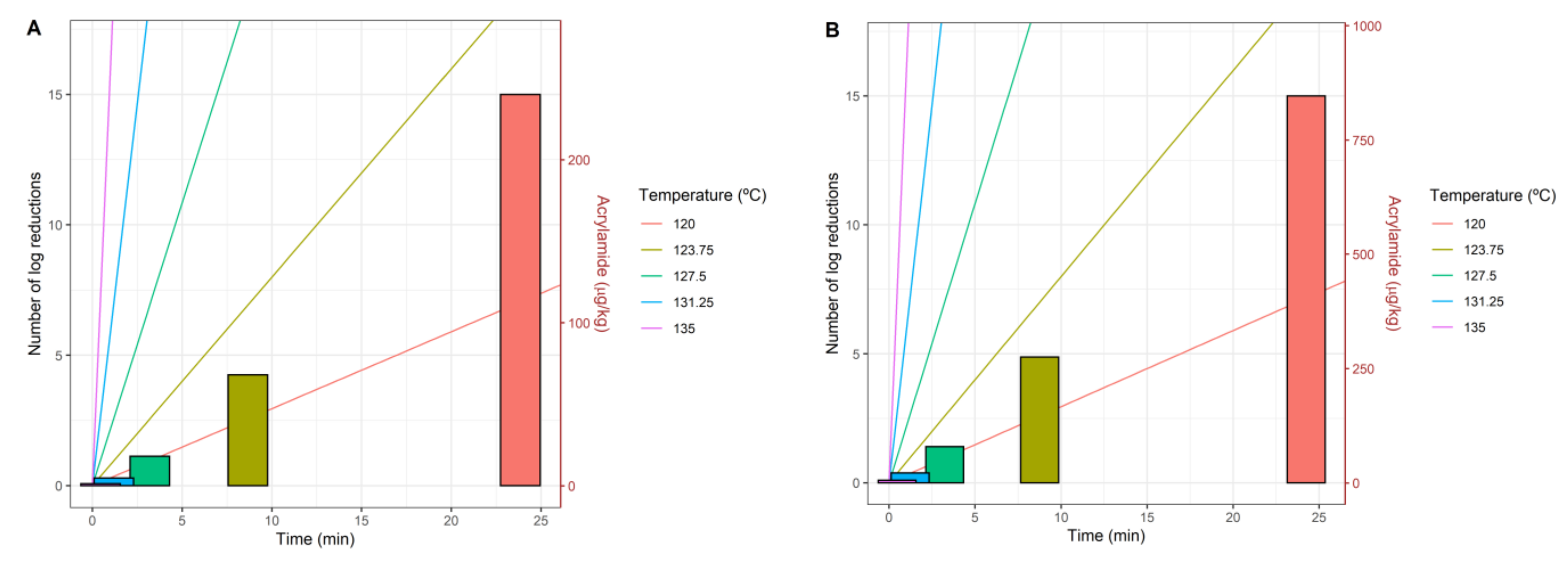
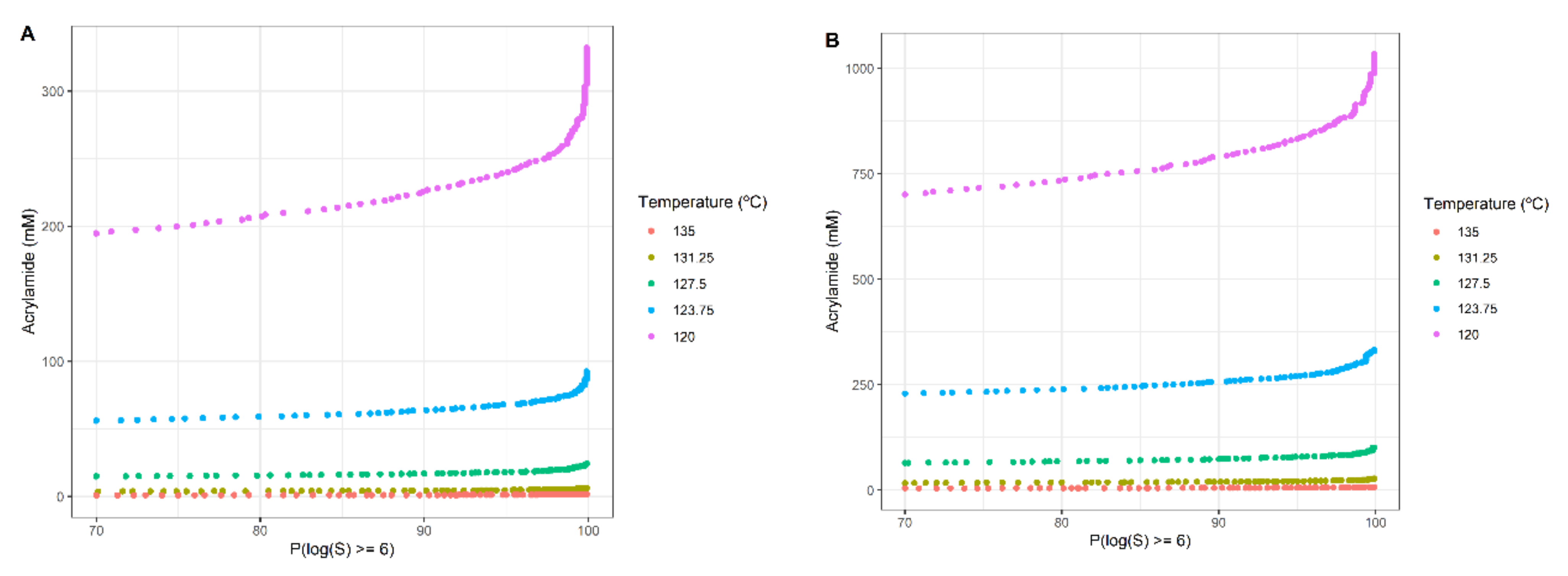

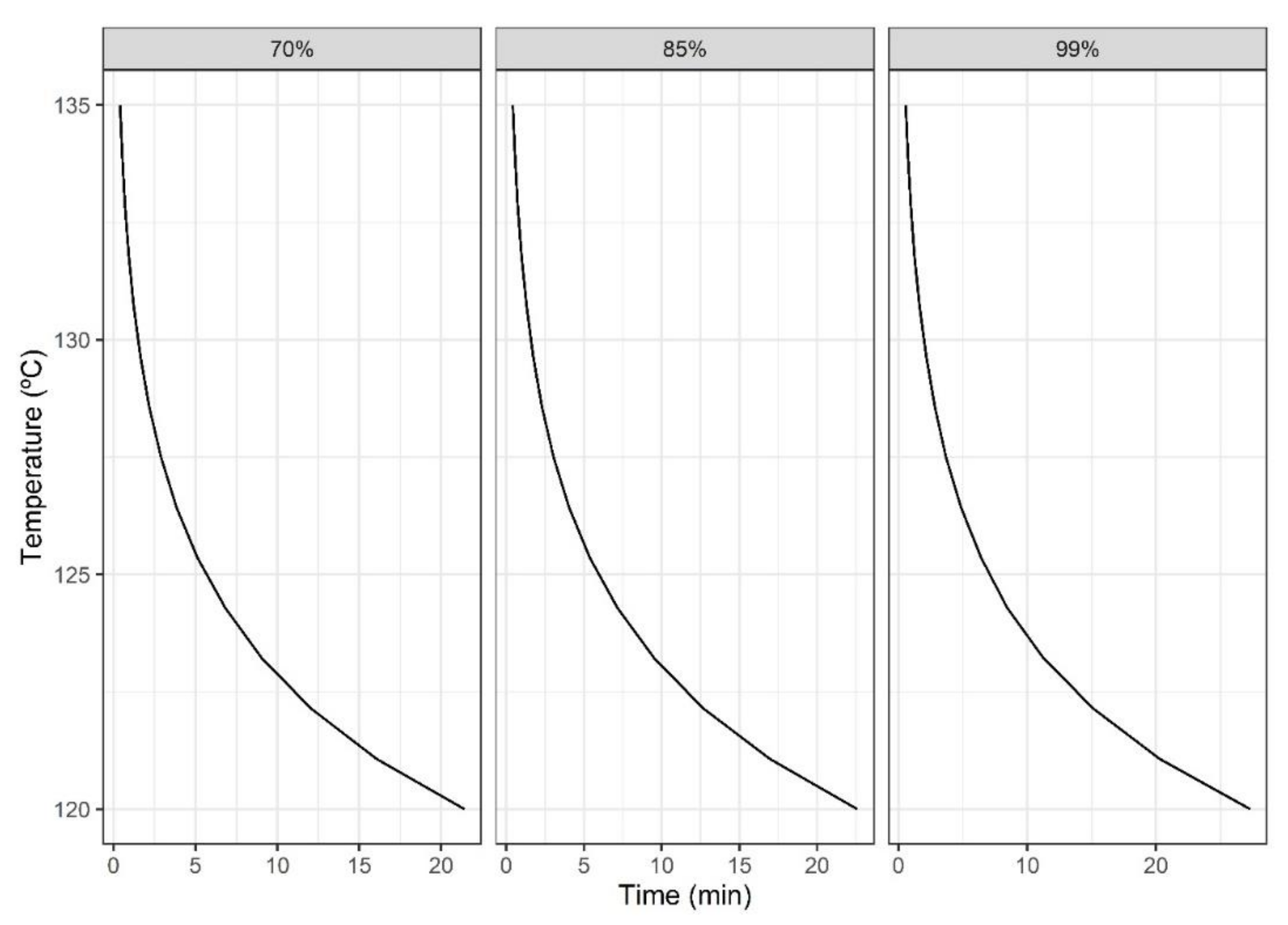
| Concentration (mM) | Pureed Potato | Prune Juice |
|---|---|---|
| Glucose | 10.740 | 213.426 |
| Fructose | 7.190 | 8.731 |
| Asparagine | 36.551 | 213.421 |
| Temperature (°C) | 90% | 95% | 99% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Acrylamide (μg/kg) | Acrylamide (μg/kg) | Acrylamide (μg/kg) | |||||||
| Pureed Potato | Prune Juice | Pureed Potato | Prune Juice | Pureed Potato | Prune Juice | ||||
| 120.00 | 23.15 | 226.52 | 789.23 | 23.92 | 241.29 | 829.20 | 25.66 | 275.45 | 913.46 |
| 123.75 | 8.43 | 64.94 | 257.49 | 8.71 | 68.14 | 269.85 | 9.28 | 77.56 | 300.61 |
| 127.50 | 3.11 | 17.43 | 73.78 | 3.22 | 18.15 | 78.79 | 3.47 | 21.06 | 87.71 |
| 131.25 | 1.16 | 4.50 | 19.70 | 1.21 | 4.74 | 21.38 | 1.31 | 5.55 | 23.79 |
| 135.00 | 0.44 | 1.14 | 5.18 | 0.46 | 1.21 | 5.61 | 0.49 | 1.40 | 6.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñalver-Soto, J.L.; Garre, A.; Aznar, A.; Fernández, P.S.; Egea, J.A. Dynamics of Microbial Inactivation and Acrylamide Production in High-Temperature Heat Treatments. Foods 2021, 10, 2535. https://doi.org/10.3390/foods10112535
Peñalver-Soto JL, Garre A, Aznar A, Fernández PS, Egea JA. Dynamics of Microbial Inactivation and Acrylamide Production in High-Temperature Heat Treatments. Foods. 2021; 10(11):2535. https://doi.org/10.3390/foods10112535
Chicago/Turabian StylePeñalver-Soto, Jose Lucas, Alberto Garre, Arantxa Aznar, Pablo S. Fernández, and Jose A. Egea. 2021. "Dynamics of Microbial Inactivation and Acrylamide Production in High-Temperature Heat Treatments" Foods 10, no. 11: 2535. https://doi.org/10.3390/foods10112535
APA StylePeñalver-Soto, J. L., Garre, A., Aznar, A., Fernández, P. S., & Egea, J. A. (2021). Dynamics of Microbial Inactivation and Acrylamide Production in High-Temperature Heat Treatments. Foods, 10(11), 2535. https://doi.org/10.3390/foods10112535