Nutritional Metabolites of Red Pigmented Lettuce (Lactuca sativa) Germplasm and Correlations with Selected Phenotypic Characters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Standards, and Chemicals
2.2. Plant Materials
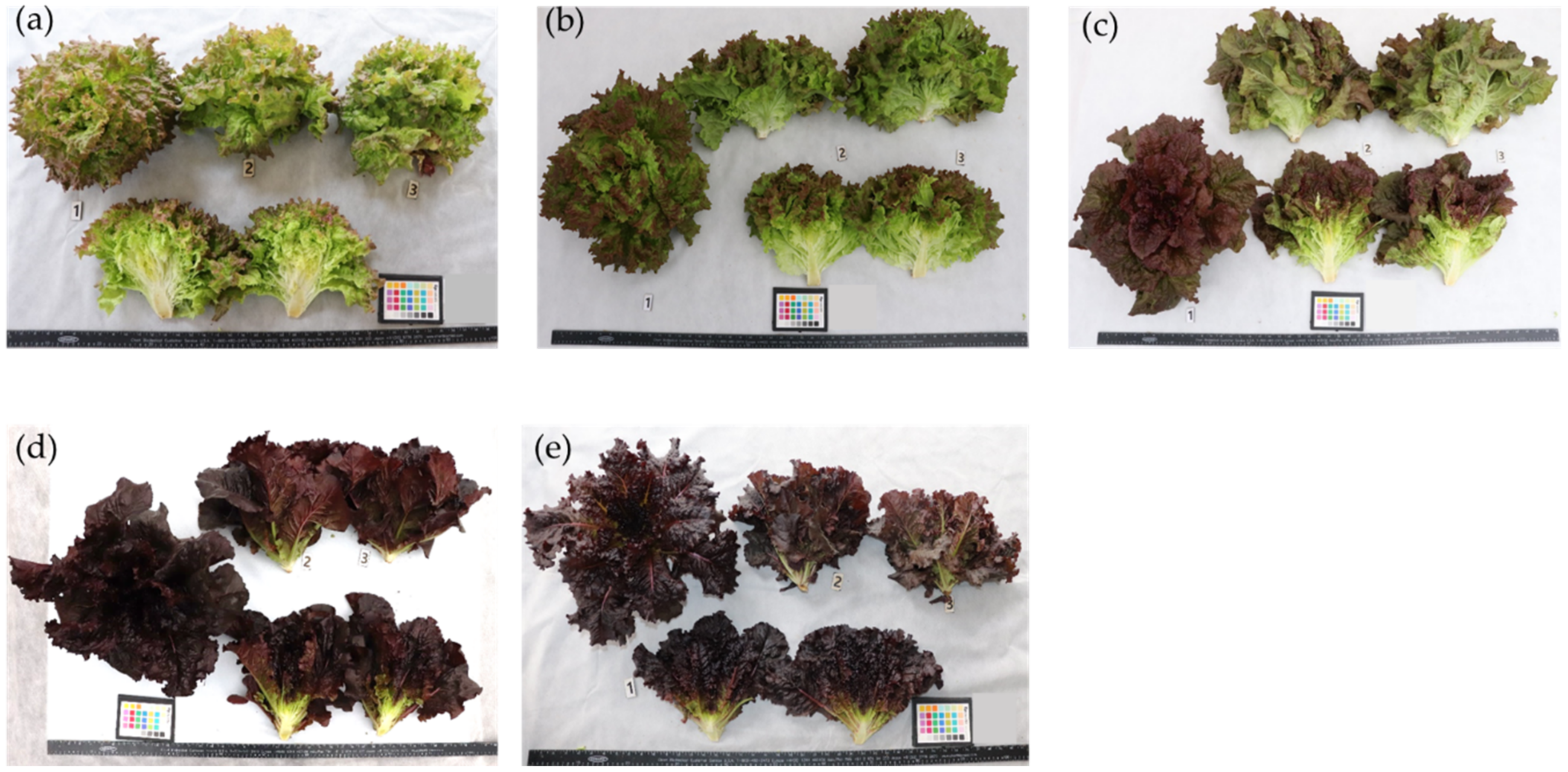
2.3. Description of the Morphological Characters of Lettuce Samples
2.4. Extraction and Analysis of Phenolic Compounds and Antioxidants
2.4.1. Extraction
2.4.2. Determination of Total Phenolic Content (TPC)
2.4.3. 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical Scavenging Activity
2.4.4. Analysis of Hydroxycinnamoyl Derivatives and Flavonoids
2.5. Extraction and Analysis of Anthocyanins
2.6. Statistical Analysis
3. Results and Discussions
3.1. Phenotypic Characters
3.2. Total Phenolic Content Antioxidant Activity
3.3. Individual Phenolic Compounds
3.3.1. Anthocyanins
3.3.2. Hydroxycinnamoyl Derivatives
3.3.3. Flavone and Flavonols
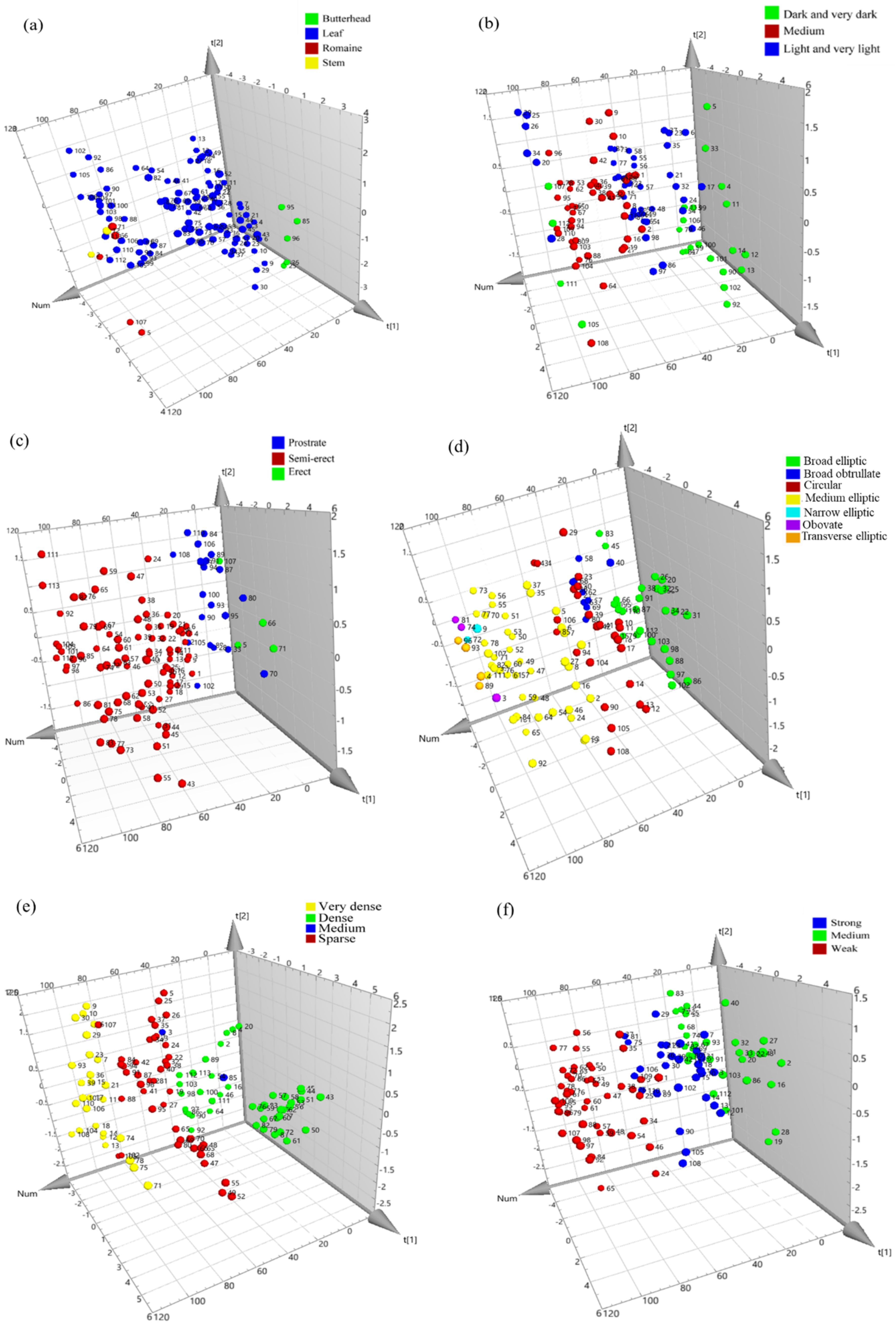
3.4. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, D.-E.; Shang, X.; Assefa, A.D.; Keum, Y.-S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.D.; Choi, S.; Lee, J.-E.; Sung, J.-S.; Hur, O.-S.; Ro, N.-Y.; Lee, H.-S.; Jang, S.-W.; Rhee, J.-H. Identification and quantification of selected metabolites in differently pigmented leaves of lettuce (Lactuca sativa L.) cultivars harvested at mature and bolting stages. BMC Chem. 2019, 13, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Food and Agriculture Organization of the United Nations. Available online: http://fao.org/faostat/en/#data (accessed on 1 July 2021).
- Still, D.W. Lettuce. In Vegetables. Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5, pp. 127–140. ISBN 9783540345367. [Google Scholar]
- Yang, X.; Wei, S.; Liu, B.; Guo, D.; Zheng, B.; Feng, L.; Liu, Y.; Tomás-Barberán, F.A.; Luo, L.; Huang, D. A novel integrated non-targeted metabolomic analysis reveals significant metabolite variations between different lettuce (Lactuca sativa L.) varieties. Hortic. Res. 2018, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Mou, B. Genetic variation of beta-carotene and lutein contents in lettuce. J. Am. Soc. Hortic. Sci. 2005, 130, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-W.; Lee, S.-H.; Asamenew, G.; Lee, M.; Lee, S.; Park, J.J.; Choi, Y.; Lee, S.H. Study on phenolic compounds in lettuce samples cultivated from Korea using UPLC-DAD-QToF/MS. Korean J. Food Nutr. 2019, 32, 717–729. [Google Scholar]
- Viacava, G.E.; Roura, S.I.; Berrueta, L.A.; Iriondo, C.; Gallo, B.; Alonso-Salces, R.M. Characterization of phenolic compounds in green and red oak-leaf lettuce cultivars by UHPLC-DAD-ESI-QToF/MS using MSE scan mode. J. Mass Spectrom. 2017, 52, 873–902. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2008, 7, 281–299. [Google Scholar] [CrossRef]
- Khoo, H.E. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef]
- Güder, A. Influence of total anthocyanins from bitter melon (Momordica charantia Linn.) as antidiabetic and radical scavenging agents. Iran. J. Pharm. Res. 2016, 15, 301–309. [Google Scholar]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fimognari, C.; Berti, F.; Nüsse, M.; Forti, G.C.; Hrelia, P. In vitro antitumor activity of Cyanidin-3-O-β-glucopyranoside. Chemotherapy 2005, 51, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, M.; Okuno, S.; Yamaguchi, M.; Yamakawa, O. Antimutagenicity of deacylated anthocyanins in purple-fleshed sweet potato. Biosci. Biotechnol. Biochem. 2001, 65, 1652–1655. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Giacometti, J.; Russo, G.L. Antiobesity effects of anthocyanins in preclinical and clinical studies. Oxid. Med. Cell. Longev. 2017, 2017, 2740364. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. Hydroxycinnamic acids and their derivatives: Future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Meyer, A.S.; Donovan, J.L.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Fruit hydroxycinnamic acids inhibit human low-density lipoprotein oxidation in vitro. J. Agric. Food Chem. 1998, 46, 1783–1787. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [Green Version]
- Magana, A.A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021. [Google Scholar] [CrossRef]
- Qin, X.-X.; Zhang, M.-Y.; Han, Y.-Y.; Hao, J.-H.; Liu, C.-J.; Fan, S.-X. Beneficial phytochemicals with anti-tumor potential revealed through metabolic profiling of new red pigmented lettuces (Lactuca sativa L.). Int. J. Mol. Sci. 2018, 19, 1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- International Union for the Protection of New Varieties of Plants (UPOV). Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability of Lactuca sativa L. Available online: www.upov.int (accessed on 30 July 2021).
- Waterhouse, A.L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley and Sons: New York, NY, USA, 2002; Volume 6, pp. 11.1.1–11.1.8. [Google Scholar]
- Assefa, A.D.; Jeong, Y.-J.; Kim, D.-J.; Jeon, Y.-A.; Ok, H.-C.; Baek, H.-J.; Sung, J.-S. Characterization, identification, and quantification of phenolic compounds using UPLC-Q-TOF-MS and evaluation of antioxidant activity of 73 Perilla frutescens accessions. Food Res. Int. 2018, 111, 153–167. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S.; Nakajima, J.; Tanaka, I.; Seo, S.; Yamazaki, M.; Saito, K. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 5, 7846–7856. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Bunning, M.L.; Kendall, P.A.; Stone, M.B.; Stonaker, F.H.; Stushnoff, C. Effects of seasonal variation on sensory properties and total phenolic content of 5 lettuce cultivars. J. Food Sci. 2010, 75, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Iwamoto, T.; Carey, E.E. Antioxidant capacity of leafy vegetables as affected by high tunnel environment, fertilisation and growth stage. J. Sci. Agric. 2007, 87, 2692–2699. [Google Scholar] [CrossRef]
- Lopez, A.; Javier, G.-A.; Fenoll, J.; Hellin, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (romaine) and baby-sized (little gem and mini romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Viacava, G.E.; Gonzalez-Aguilar, G.; Roura, S.I. Determination of Phytochemicals and Antioxidant Activity in Butterhead Lettuce Related to Leaf Age and Position. J. Food Biochem. 2014, 38, 352–362. [Google Scholar] [CrossRef]
- Donner, H.; Gao, L.; Mazza, G. Separation and characterization of simple and malonylated anthocyanins in red onions, Allium cepa L. Food Res. Int. 1997, 30, 637–643. [Google Scholar] [CrossRef]
- Kohyama, N.; Ono, H.; Yanagisawa, T. Changes in anthocyanins in the grains of purple waxy hull-less barley during seed maturation and after harvest. J. Agric. Food Chem. 2008, 56, 5770–5774. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Ngouajio, M.; Nair, A.; Zhang, Y.; Gottumukkala, A.L.; Nair, M.G. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food Chem. 2010, 118, 300–306. [Google Scholar] [CrossRef]
- Ferreres, F.; Gil, I.; Castan, M.; Toma, F.A. Phenolic metabolites in red pigmented lettuce (Lactuca sativa). Changes with minimal processing and cold storage. J. Agric. Food Chem. 1997, 8561, 4249–4254. [Google Scholar] [CrossRef]
- Ferioli, F.; Manco, M.A.; Antuono, L.F.D. Variation of sesquiterpene lactones and phenolics in chicory and endive germplasm. J. Food Compos. Anal. 2015, 39, 77–86. [Google Scholar] [CrossRef]
- Gazula, A.; Kleinhenz, M.D.; Scheerens, J.C.; Ling, P.P. Anthocyanin levels in nine lettuce (Lactuca sativa) cultivars: Influence of planting date and relations among analytic, instrumented, and visual assessments of color. HortScience 2007, 42, 232–238. [Google Scholar] [CrossRef]
- Pérez-López, U.; Pinzino, C.; Quartacci, M.F.; Ranieri, A.; Sgherri, C. Phenolic composition and related antioxidant properties in differently colored lettuces: A study by electron paramagnetic resonance (EPR) kinetics. J. Agric. Food Chem. 2014, 62, 12001–12007. [Google Scholar] [CrossRef]
- Chon, S.U.; Boo, H.O.; Heo, B.G.; Gorinstein, S. Anthocyanin content and the activities of polyphenol oxidase, peroxidase and phenylalanine ammonia-lyase in lettuce cultivars. Int. J. Food Sci. Nutr. 2012, 63, 45–48. [Google Scholar] [CrossRef]
- Gazula, A.; Kleinhenz, M.D.; Streeter, J.G.; Miller, A.R. Temperature and cultivar effects on anthocyanin and chlorophyll b concentrations in three related lollo rosso lettuce cultivars. HortScience 2005, 40, 1731–1733. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Mori, T.; Nakabayashi, R.; Kobayashi, M.; Saito, K.; Okazaki, S.; Wang, N.; Kusano, M. Metabolomic evaluation of the quality of leaf lettuce grown in practical plant factory to capture metabolite signature. Front. Plant Sci. 2018, 9, 665. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Neill, S.O.; Gould, K.S. Anthocyanins in leaves: Light attenuators or antioxidants? Funct. Plant Biol. 2003, 30, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Gould, K.S.; Markham, K.R.; Smith, R.H.; Goris, J.J. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 2000, 51, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Kwak, H.-K.; Hwang, K.T. Antioxidant and antiinflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide. Food Sci. Biotechnol. 2014, 23, 2053–2062. [Google Scholar] [CrossRef]
- Asamenew, G.; Kim, H.; Lee, M.; Lee, S.; Lee, S.; Cha, Y.; Hoon, S.; Mi, S.; Kim, J. Comprehensive characterization of hydroxycinnamoyl derivatives in green and roasted coffee beans: A new group of methyl hydroxycinnamoyl quinate. Food Chem. X 2019, 2, 100033. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-W.; Bai, J.-P.; Zhang, Q.; Hu, X.-L.; Tian, X.; Zhu, J.; Liu, J.; Meng, W.-H.; Zhao, Q.-C. Caffeoylquinic acid derivatives protect SH-SY5Y neuroblastoma cells from hydrogen peroxide-induced injury through modulating oxidative status. Cell. Mol. Neurobiol. 2017, 37, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of caffeoylquinic acids in coffee brews by HPLC-DAD. J. Anal. Methods Chem. 2014, 2014, 965353. [Google Scholar] [CrossRef]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.; Rock, E.; Michel, H.; Amouroux, P.; Remesy, C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J. Sci. Food Agric. 2004, 84, 2061–2069. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Krueger, C.G.; Johnson, W.D.; Raskin, I. Development and phytochemical characterization of high polyphenol red lettuce with anti-diabetic properties. PLoS ONE 2014, 9, e91571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, M.S.; Mondin, Z.; Williamson, G.; Price, K.R. Effect of variety, processing, and storage on the flavonoid glycoside Ccontent and composition of lettuce and endive. J. Agric. Food Chem. 2000, 48, 3957–3964. [Google Scholar] [CrossRef] [PubMed]



| The Intensity of Red Color of the Outer Leaves | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hydroxycinnamoyl derivatives (μg/g DW) | Flavone and flavonols (μg/g DW) | ||||||||
| 3-CQA | 5-CQA | 2,3-DCTA | 3,5-DCQA | Total | Q3-G | L7-G | Q3-6″MG | Total | |
| Very light + light (n = 48) | 163.6 a | 2251.7 a | 4464.1 a | 355 a | 7234.4 a | 1979.9 a | 246.3 a | 2289.4 a | 4515.6 a |
| Medium (n = 42) | 199.9 a | 5069.5 b | 6983.3 b | 695.1 b | 12,947.7 b | 3092.7 a | 305.5 a | 4636.7 b | 8034.9 b |
| Dark + very dark (n = 23) | 217.4 a | 4675.6 b | 6740.9 ab | 635.5 b | 12,269.5 b | 3265.1 a | 488.9 b | 6029.7 b | 9783.6 b |
| Anthocyanins (μg/g DW) | ABTS | TPC | Leaf length | Leaf width | Plant weight | ||||
| C3-G | C3-3″MG | C3-6″MG | Total | ||||||
| Very light + light (n = 48) | 24.2 a | 5.5 a | 291.4 a | 321.1 a | 41,942.6 a | 44,529.4 a | 25.0 a | 19.0 a | 299.9 a |
| Medium (n = 42) | 38.5 ab | 11.7 a | 630.5 a | 680.8 a | 58,459.9 b | 57,758.4 b | 26.7 a | 20.5 a | 319.1 a |
| Dark + very dark (n = 23) | 65.0 b | 36.1 b | 1224 b | 1325.1 b | 61,652.6 b | 57,428.5 b | 25.5 a | 19.2 a | 322.3 a |
| Leaf Shape | |||||||||
| Hydroxycinnamoyl derivatives (μg/g DW) | Flavone and flavonols (μg/g DW) | ||||||||
| 3-CQA | 5-CQA | 2,3-DCTA | 3,5-DCQA | Total | Q3-G | L7-G | Q3-6″MG | Total | |
| Medium elliptic (n = 43) | 182.6 b | 3139.9 b | 5145.2 b | 503.8 b | 8971.5 b | 1927.5 ab | 369.4 ab | 3278.7 ab | 5575.6 ab |
| Broad elliptic (n = 27) | 207.9 b | 4120.0 ab | 6562.2 bc | 597.6 b | 11,487.7 bc | 3228.7 bc | 272.6 ab | 3864.4 b | 7365.7 bc |
| Circular (n = 25) | 228.1 b | 5840.3 c | 8432.1 c | 723.4 b | 15,223.9 c | 4429.1 c | 407.9 b | 6846.5 c | 11,683.5 c |
| Broad obtrullate (n = 9) | 53.9 a | 594.9 a | 947.9 a | 92.4 a | 1689.1 a | 628.0 a | 117.2 a | 739.0 a | 1484.2 a |
| Anthocyanins (μg/g DW) | ABTS | TPC | Leaf length | Leaf width | Plant weight | ||||
| C3-G | C3-3″MG | C3-6″MG | Total | ||||||
| Medium elliptic (n = 43) | 34.6 ab | 8.3 ab | 453.4 ab | 496.3 ab | 47,204.0 b | 50,143.3 b | 27.3 b | 17.3 a | 295.6 a |
| Broad elliptic (n = 27) | 45.0 ab | 18.9 ab | 739.2 b | 803.0 b | 58,892.8 bc | 54,236.4 b | 27.7 b | 19.1 a | 330.8 a |
| Circular (n = 25) | 61.7 b | 27.8 b | 998.1 b | 1087.6 b | 64,500.1 c | 62,395.0 b | 21.7 a | 22.7 b | 297.7 a |
| Broad obtrullate (n = 9) | 3.6 a | 0.0 a | 36.9 a | 40.5 a | 22,470.2 a | 29,089.1 a | 23.7 ab | 22.8 b | 307.3 a |
| Leaf-Blade: Degree of Undulation of Margin | |||||||||
| Hydroxycinnamoyl derivatives (μg/g DW) | Flavone and flavonols (μg/g DW) | ||||||||
| 3-CQA | 5-CQA | 2,3-DCTA | 3,5-DCQA | Total | Q3-G | L7-G | Q3-6″MG | Total | |
| Weak (n = 48) | 175.5 a | 2078.6 a | 4374.6 a | 344.7 a | 6973.4 a | 1750.6 a | 316.7 a | 2515.2 a | 4582.4 a |
| Medium (n = 34) | 169.3 a | 3999.4 b | 5594.5 a | 586.5 b | 10,349.6 a | 2575.6 a | 259.7 a | 3547.5 a | 6382.8 a |
| Strong (n= 31) | 228.0 a | 6218.9 c | 8465.3 b | 785.9 b | 15,698.2 b | 4143.0 b | 382.8 a | 6515.2 b | 11,041.0 b |
| Anthocyanins (μg/g DW) | ABTS | TPC | Leaf length | Leaf width | Plant weight | ||||
| C3-G | C3-3″MG | C3-6″MG | Total | ||||||
| Weak (n = 48) | 21.9 a | 6.0 a | 325.4 a | 353.3 a | 42,626.3 a | 43,499.4 a | 27.5 b | 17.4 a | 344.6 b |
| Medium (n = 34) | 39.1 ab | 11.9 a | 598.5 a | 649.5 a | 53,055.6 a | 52,732.3 a | 27.1 b | 19.9 b | 301.1 ab |
| Strong (n= 31) | 61.2 b | 28.9 b | 1053.3 b | 1143.3 b | 65,697.3 b | 64,621.0 b | 21.6 a | 22.7 c | 275.6 a |
| Leaf-Blade: Density of Incisions on the Margin on the Apical Part | |||||||||
| Hydroxycinnamoyl derivatives (μg/g DW) | Flavone and flavonols (μg/g DW) | ||||||||
| 3-CQA | 5-CQA | 2,3-DCTA | 3,5-DCQA | Total | Q3-G | L7-G | Q3-6″MG | Total | |
| Sparse (n = 42) | 222.8 b | 3344.0 a | 6389.5 ab | 601.9 b | 10,558.2 ab | 2199.0 a | 305.1 a | 3459.5 a | 5963.6 a |
| Dense (n = 42) | 144.1 a | 3046.6 a | 4285.4 ab | 362.8 a | 7838.9 a | 2488.2 ab | 332.4 a | 3439.1 a | 6259.7 ab |
| Very dense (n = 27) | 200.6 ab | 5520.4 b | 7388.2 b | 696.2 b | 13,805.4 b | 3727.2 b | 327.4 a | 5508.1 a | 9562.7 b |
| Anthocyanins (μg/g DW) | ABTS | TPC | Leaf length | Leaf width | Plant weight | ||||
| C3-G | C3-3″MG | C3-6″MG | Total | ||||||
| Sparse (n = 42) | 46.1 a | 13.7 a | 546.1 a | 605.9 a | 54,766.2 ab | 52,468.3 ab | 27.6 b | 18.1 a | 306.6 a |
| Dense (n = 42) | 35.1 a | 12.4 a | 586.2 a | 633.6 a | 44,272.3 a | 47,073.9 ab | 26.5 b | 19.3 a | 335.3 a |
| Very dense (n = 27) | 32.2 a | 17.7 a | 735.9 a | 785.8 a | 60,146.6 b | 58,655.7 b | 21.9 a | 22.2 b | 282.3 a |
| 3-CQA | 5-CQA | 2,3-DCTA | 3,5-DCQA | Q3-G | L7-G | Q3-6″MG | C3-G | C3-3″MG | C3-6″MG | ABTS | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-CQA | 0.488 | ||||||||||
| 2,3-DCTA | 0.881 | 0.786 | |||||||||
| 3,5-DCQA | 0.604 | 0.839 | 0.812 | ||||||||
| Q3-G | 0.544 | 0.551 | 0.649 | 0.431 | |||||||
| L7-G | 0.334 | 0.416 | 0.392 | 0.369 | 0.434 | ||||||
| Q3-6″MG | 0.499 | 0.699 | 0.686 | 0.596 | 0.707 | 0.798 | |||||
| C3-G | 0.380 | 0.518 | 0.526 | 0.384 | 0.503 | 0.366 | 0.549 | ||||
| C3-3″MG | 0.340 | 0.591 | 0.538 | 0.415 | 0.431 | 0.437 | 0.646 | 0.753 | |||
| C3-6″MG | 0.447 | 0.748 | 0.668 | 0.571 | 0.539 | 0.526 | 0.770 | 0.700 | 0.946 | ||
| ABTS | 0.760 | 0.777 | 0.876 | 0.770 | 0.665 | 0.400 | 0.663 | 0.486 | 0.441 | 0.601 | |
| TPC | 0.741 | 0.893 | 0.926 | 0.822 | 0.645 | 0.425 | 0.688 | 0.574 | 0.599 | 0.751 | 0.892 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assefa, A.D.; Hur, O.-S.; Hahn, B.-S.; Kim, B.; Ro, N.-Y.; Rhee, J.-H. Nutritional Metabolites of Red Pigmented Lettuce (Lactuca sativa) Germplasm and Correlations with Selected Phenotypic Characters. Foods 2021, 10, 2504. https://doi.org/10.3390/foods10102504
Assefa AD, Hur O-S, Hahn B-S, Kim B, Ro N-Y, Rhee J-H. Nutritional Metabolites of Red Pigmented Lettuce (Lactuca sativa) Germplasm and Correlations with Selected Phenotypic Characters. Foods. 2021; 10(10):2504. https://doi.org/10.3390/foods10102504
Chicago/Turabian StyleAssefa, Awraris Derbie, On-Sook Hur, Bum-Soo Hahn, Bichsaem Kim, Na-Young Ro, and Ju-Hee Rhee. 2021. "Nutritional Metabolites of Red Pigmented Lettuce (Lactuca sativa) Germplasm and Correlations with Selected Phenotypic Characters" Foods 10, no. 10: 2504. https://doi.org/10.3390/foods10102504
APA StyleAssefa, A. D., Hur, O.-S., Hahn, B.-S., Kim, B., Ro, N.-Y., & Rhee, J.-H. (2021). Nutritional Metabolites of Red Pigmented Lettuce (Lactuca sativa) Germplasm and Correlations with Selected Phenotypic Characters. Foods, 10(10), 2504. https://doi.org/10.3390/foods10102504






