A Comprehensive Evaluation of Flavor Instability of Beer (Part 1): Influence of Release of Bound State Aldehydes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Malt, Wort, and Beer Production
2.3. Aging and Sample Treatment
2.4. pH, O2, and SO2
2.5. Sensory Analysis
2.6. Quantitation of Free Aldehydes via HS-SPME-GC-MS
2.7. Quantitation of Bound State Aldehydes after Release with 4VP vs. Acetaldehyde (ACA)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Brewing Trials
3.2. Sensory Analysis
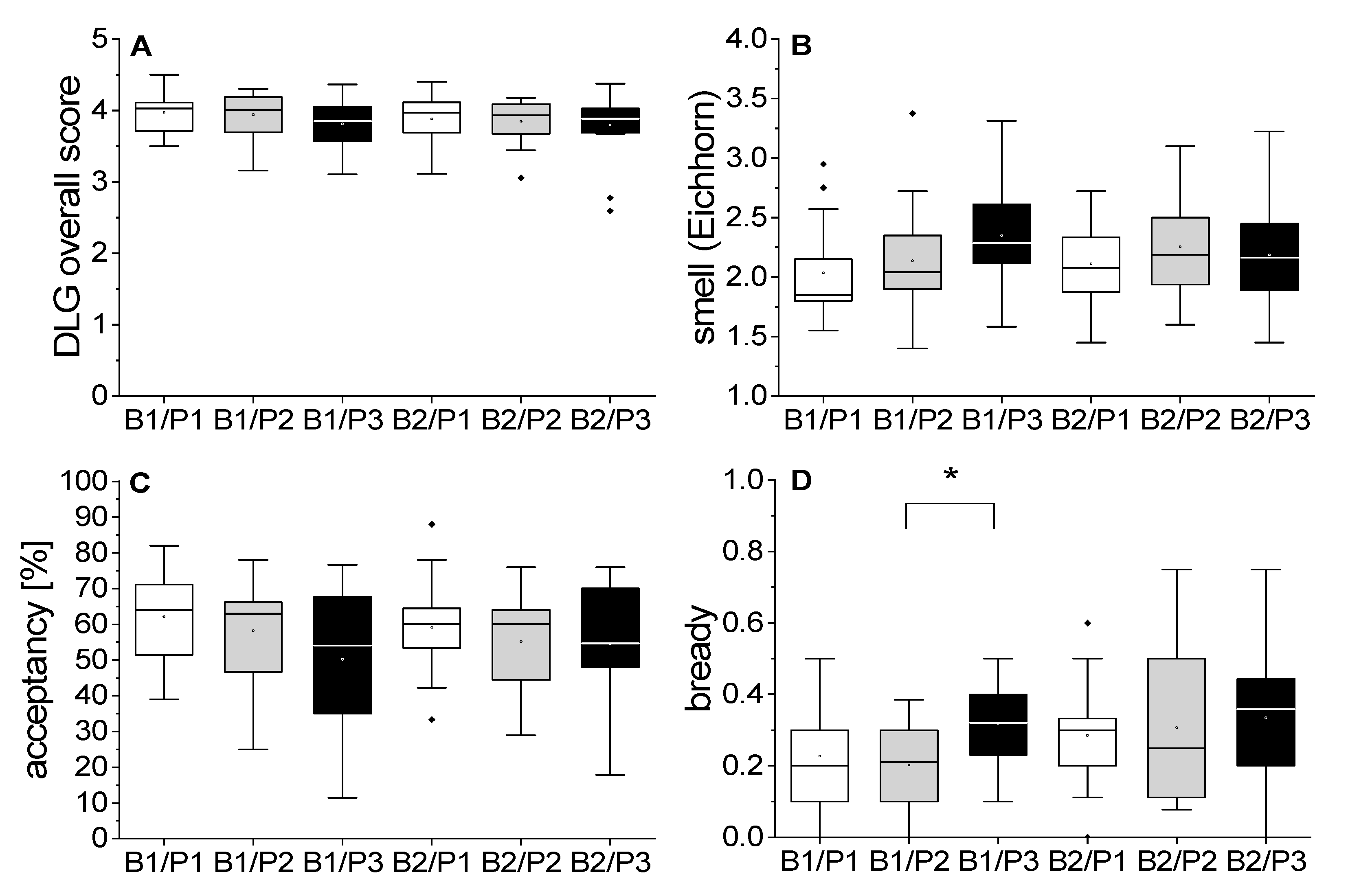
3.2.1. Quality Assessment and Descriptive Analysis by DLG, Eichhorn Scheme, and CATA
3.2.2. Triangle Tests
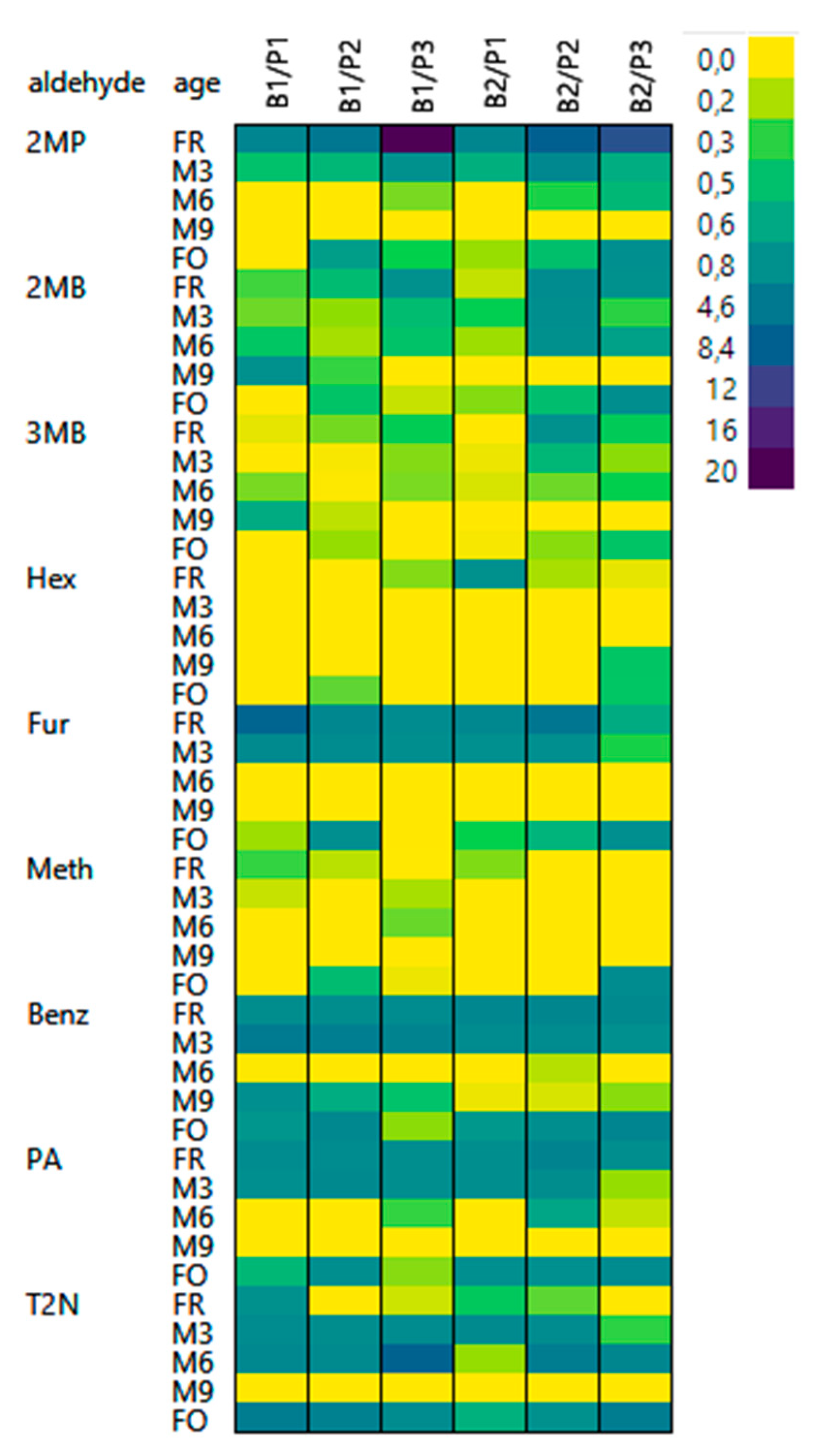
3.3. Behavior of Free Aldehydes
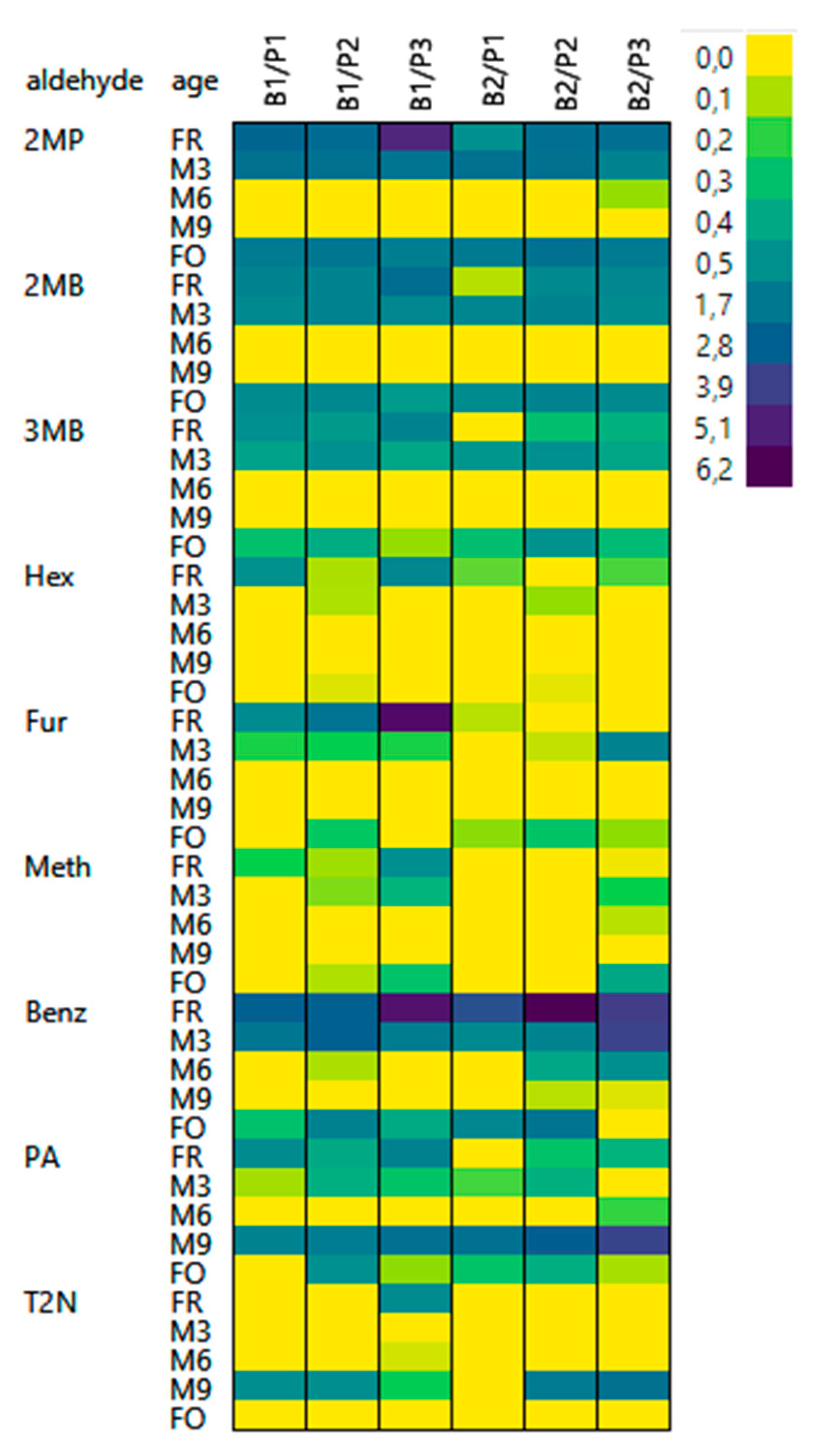
3.4. Release of Bound State Aldehydes
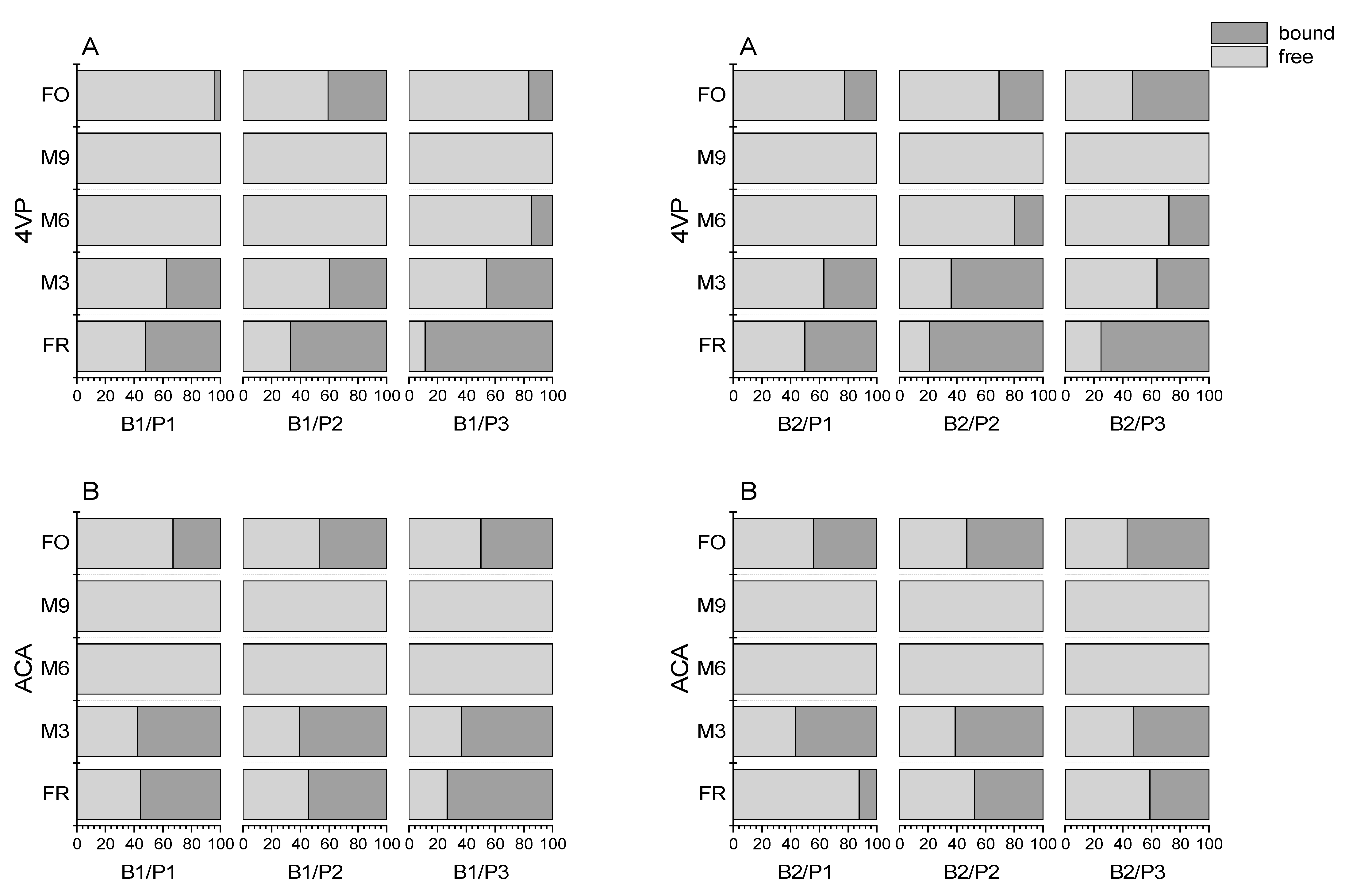
3.4.1. Release by 4VP
3.4.2. Release by ACA
3.5. Influence of Aldehyde Structure on Occurrence in a Bound State
3.6. Final Discussion of Release and Bound State Aldehydes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaskula-Goiris, B.; De Causmaecker, B.; De Rouck, G.; Aerts, G.; Paternoster, A.; Braet, J.; De Cooman, L. Influence of transport and storage conditions on beer quality and flavour stability. J. Inst. Brew. 2018, 125, 60–68. [Google Scholar] [CrossRef]
- Paternoster, A.; Jaskula-Goiris, B.; De Causmaecker, B.; Vanlanduit, S.; Springael, J.; Braet, J.; De Rouck, G.; De Cooman, L. The interaction effect between vibrations and temperature simulating truck transport on the flavor stability of beer. J. Sci. Food Agric. 2019, 99, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt, F.; Gastl, M.; Becker, T. Forced into aging: Analytical prediction of the flavor-stability of lager beer. A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Lehnhardt, F.; Steiner, J.; Gastl, M.; Becker, T. Prediction Power and Accuracy of Forced Ageing—Matching Sensory and Analytical Results for Lager Beer. Brew. Sci. 2018, 71, 39–48. [Google Scholar]
- Bueno, M.; Marrufo-Curtido, A.; Carrascón, V.; Fernández-Zurbano, P.; Escudero, A.; Ferreira, V. Formation and Accumulation of Acetaldehyde and Strecker Aldehydes during Red Wine Oxidation. Front. Chem. 2018, 6, 20. [Google Scholar] [CrossRef]
- Wietstock, P.C.; Kunz, T.; Methner, F.-J. The Relevance of Oxygen for the Formation of Strecker Aldehydes during Beer Production and Storage. J. Agric. Food Chem. 2016, 64, 8035–8044. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Baert, J.J.; De Clippeleer, J.; Hughes, P.S.; De Cooman, L.; Aerts, G. On the Origin of Free and Bound Staling Aldehydes in Beer. J. Agric. Food Chem. 2012, 60, 11449–11472. [Google Scholar] [CrossRef]
- Wietstock, P.C.; Methner, F.-J. Formation of Aldehydes by direct Oxidative Degradation of Amino Acids via Hydroxyl and Ethoxy Radical Attack in Buffered Model Solutions. Brew. Sci. 2013, 66, 104–113. [Google Scholar]
- Lermusieau, G.; Noël, S.; Collin, S. Nonoxidative Mechanism for Development of trans-2-Nonenal in Beer. J. Am. Soc. Brew. Chem. 1999, 57, 29–33. [Google Scholar] [CrossRef]
- Baert, J.J.; De Clippeleer, J.; Aerts, G. Exploring the Binding Behavior of Beer Staling Aldehydes in Model Systems. J. Am. Soc. Brew. Chem. 2015, 73, 100–108. [Google Scholar] [CrossRef]
- Bustillo Trueba, P.; Jaskula-Goiris, B.; Ditrych, M.; Filipowska, W.; De Brabanter, J.; De Rouck, G. Monitoring the evolution of free and cysteinylated aldehydes from malt to fresh and forced aged beer. Food Res. Int. 2021, 140, 110049. [Google Scholar] [CrossRef]
- Nobis, A.; Lehnhardt, F.; Gebauer, M.; Becker, T.; Gastl, M. The influence of proteolytic malt modification on the aging potential of final wort. Foods 2021, 10, 2320. [Google Scholar] [CrossRef]
- Dugulin, A.C.; Clegg, S.C.; De Rouck, G.; Cook, D.J. Overcoming technical barriers to brewing with green (non-kilned) malt: A feasibility study. J. Inst. Brew. 2020, 126, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Drost, B.W.; van den Berg, R.; Freijee, F.J.M.; van der Velde, E.G.; Hollemans, M. Flavor Stability. J. Am. Soc. Brew. Chem. 1990, 48, 124–131. [Google Scholar] [CrossRef]
- Baert, J.J.; De Clippeleer, J.; Jaskula-Goiris, B.; van Opstaele, F.; De Rouck, G.; Aerts, G. Further Elucidation of Beer Flavor Instability: The Potential Role of Cysteine-Bound Aldehydes. J. Am. Soc. Brew. Chem. 2015, 73, 243–252. [Google Scholar] [CrossRef]
- Baert, J.J.; De Clippeleer, J.; Bustillo Trueba, P.; Jaskula-Goiris, B.; De Rouck, G.; Aerts, G. Exploring Aldehyde Release in Beer by 4-Vinylpyridine and the Effect of Cysteine Addition on the Beer’s Pool of Bound Aldehydes. J. Am. Soc. Brew. Chem. 2018, 76, 257–271. [Google Scholar] [CrossRef]
- Barker, R.L.; Gracey, D.E.F.; Irwin, A.J.; Pipasts, P.; Leiska, E. Liberation of staling aldehydes during storage of beer. J. Inst. Brew. 1983, 89, 411–415. [Google Scholar] [CrossRef]
- Dufour, J.-P.; Leus, M.; Baxter, A.J.; Hayman, A.R. Characterization of the Reaction of Bisulfite with Unsaturated Aldehydes in a Beer Model System Using Nuclear Magnetic Resonance Spectroscopy. J. Am. Soc. Brew. Chem. 1999, 57, 138–144. [Google Scholar] [CrossRef]
- Filipowska, W.; Jaskula-Goiris, B.; Ditrych, M.; Bustillo Trueba, P.; De Rouck, G.; Aerts, G. On the contribution of malt quality and the malting process to the formation of beer staling aldehydes: A review. Inst. Brew. Distill. 2021, 127, 107–126. [Google Scholar] [CrossRef]
- Back, W.; Gastl, M.; Krottenhaler, M.; Narziß, L.; Zarnkow, M. Brewing Techniques in Practice: An In-Depth Review of Beer Production with Problem Solving Strategies; Fachverlag Hans Carl: Nürnberg, Germany, 2020. [Google Scholar]
- Hill, A.; Stewart, G.G. Free Amino Nitrogen in Brewing. Fermentation 2019, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Gastl, M.; Kupetz, M.; Becker, T. Determination of Cytolytic Malt Modification—Part I: Influence of Variety Characteristics. J. Am. Soc. Brew. Chem. 2021, 79, 53–65. [Google Scholar]
- Stephan, A.; Kusche, M.; Stettner, G. Influence of malt quality on the generation of odour-active Strecker aldehydes during beer aging. In Proceedings of the 31st EBC Congress, Venice, Italy, 6–10 May 2007. [Google Scholar]
- Gastl, M.; Spieleder, E.; Hermann, M.; Thiele, F.; Burberg, F.; Kogin, A. The influence of malt quality and malting technology on the flavour stability of beer. Mon. Brauwiss. 2006, 72, 163–175. [Google Scholar]
- Malt Analysis: Industry-Wide Change in Analysis Approach for Barley Malt 5 EBC. In BRAUWELT International 2020; B. Deutscher Brauer-Bund e.V.: Berlin, Germany; Deutscher Mälzerbund e.V.: Frankfort on the Main, Germany; Braugersten-Gemeinschaft e.V.: Munich, Germany, 2020.
- Lund, M.N.; Petersen, M.A.; Andersen, M.L.; Lunde, C. Effect of Protease Treatment during Mashing on Protein-Derived Thiol Content and Flavor Stability of Beer during Storage. Am. Soc. Brew. Chem. 2015, 73, 287–295. [Google Scholar] [CrossRef]
- Jacob, F. (Ed.) MEBAK Brautechnische Analysemethoden—Würze, Bier, Biermischgetränke: Methodensammlung der Mitteleuropäischen Brautechnischen Analysenkommission; MEBAK: Freising-Weihenstephan, Germany, 2012. [Google Scholar]
- Jacob, F. (Ed.) MEBAK Brautechnische Analysenmethoden—Sensorik: Methodensammlung der Mitteleuropäischen Brautechnischen Analysenkommission; MEBAK: Freising-Weihenstephan, Germany, 2013. [Google Scholar]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Optimisation of a complete method for the analysis of volatiles involved in the flavour stability of beer by solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1190, 342–349. [Google Scholar] [CrossRef]
- Lehnhardt, F.; Becker, T.; Gastl, M. Flavor stability assessment of lager beer: What we can learn by comparing established methods. Eur. Food Res. Technol. 2020, 246, 1105–1118. [Google Scholar] [CrossRef] [Green Version]
- Saison, D.; De Schutter, D.P.; Uyttenhove, B.; Delvaux, F.; Delvaux, F.R. Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem. 2009, 114, 1206–1215. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gavin, T. Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef]
- Schmid, M.B.; Zeitler, K.; Gschwind, R.M. The Elusive Enamine Intermediate in Proline-Catalyzed Aldol Reactions: NMR Detection, Formation Pathway, and Stabilization Trends. Angew. Chem. Int. Ed. 2010, 49, 4997–5003. [Google Scholar] [CrossRef] [PubMed]
- Zameo, S.; Vauzeilles, B.; Beau, J.-M. Direct Composition Analysis of a Dynamic Library of Imines in an Aqueous Medium. Eur. J. Org. Chem. 2006, 24, 5441–5444. [Google Scholar] [CrossRef]

| Sample | Steeping Degree (%) | Target Soluble N (ISO 65 °C (mg/100 g Malt d.m.) | Soluble N (mg/100 g Malt d.m.) | Total Amino Acids in Fresh Beer (mg/L) | pH (Final Beer) | O2 (mg/L) | Bound SO2 (mg/L) |
|---|---|---|---|---|---|---|---|
| B1/P1 | 38 | 550 ± 25 | 573 ± 10 | 909 | 4.57 ± 0.02 | 0.07 ± 0.04 | 0.84 ± 0.33 |
| B1/P2 | 41 | 625 ± 25 | 601 ± 1 | 753 | 4.44 ± 0.05 | 0.01 ± 0.00 | 0.52 ± 0.20 |
| B1/P3 | 44 | 700 ± 25 | 660 ± 1 | 980 | 4.52 ± 0.07 | 0.02 ± 0.00 | 0 ± 0 |
| B2/P1 | 39 | 550 ± 25 | 569 ± 3 | 666 | 4.45 ± 0.07 | 0.07 ± 0.05 | 0 ± 0 |
| B2/P2 | 43 | 625 ± 25 | 620 ± 14 | 784 | 4.39 ± 0.05 | 0.08 ± 0.01 | 0.16 ± 0.18 |
| B2/P3 | 47 | 700 ± 25 | 731 ± 1 | 1121 | 4.55 ± 0.02 | 0.02 ± 0.01 | 3.16 ± 1.43 |
| Tested Pair | Correct Answers | Wrong Answers | p-Value |
|---|---|---|---|
| B1/P1–B1/P2 | 8 | 8 | >0.05 |
| B1/P1–B1/P3 | 12 | 4 | <0.001 |
| B1/P2–B1/P3 | 13 | 3 | <0.001 |
| B2/P1–B2/P2 | 10 | 6 | <0.01 |
| B2/P1–B2/P3 | 14 | 2 | <0.001 |
| B2/P2–B2/P3 | 14 | 2 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehnhardt, F.; Nobis, A.; Skornia, A.; Becker, T.; Gastl, M. A Comprehensive Evaluation of Flavor Instability of Beer (Part 1): Influence of Release of Bound State Aldehydes. Foods 2021, 10, 2432. https://doi.org/10.3390/foods10102432
Lehnhardt F, Nobis A, Skornia A, Becker T, Gastl M. A Comprehensive Evaluation of Flavor Instability of Beer (Part 1): Influence of Release of Bound State Aldehydes. Foods. 2021; 10(10):2432. https://doi.org/10.3390/foods10102432
Chicago/Turabian StyleLehnhardt, Florian, Arndt Nobis, Andreas Skornia, Thomas Becker, and Martina Gastl. 2021. "A Comprehensive Evaluation of Flavor Instability of Beer (Part 1): Influence of Release of Bound State Aldehydes" Foods 10, no. 10: 2432. https://doi.org/10.3390/foods10102432
APA StyleLehnhardt, F., Nobis, A., Skornia, A., Becker, T., & Gastl, M. (2021). A Comprehensive Evaluation of Flavor Instability of Beer (Part 1): Influence of Release of Bound State Aldehydes. Foods, 10(10), 2432. https://doi.org/10.3390/foods10102432






