Variation in Volatile Flavor Compounds of Cooked Mutton Meatballs during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cooked Mutton Meatballs Samples
2.3. Sensory Evaluation
2.4. Solid-Phase Microextraction (SPME)
2.5. Solvent-Assisted Flavor Evaporation (SAFE)
2.6. Gas Chromatography−Olfactometry−Mass Spectrometry Analysis
2.7. Qualitative Analysis
2.8. Quantitative Analysis
2.9. Aroma Extraction Dilution Analysis
2.10. Odor Activity Value
2.11. Statistical Analysis
3. Results
3.1. Comparison of SPME and SAFE Extraction Methods
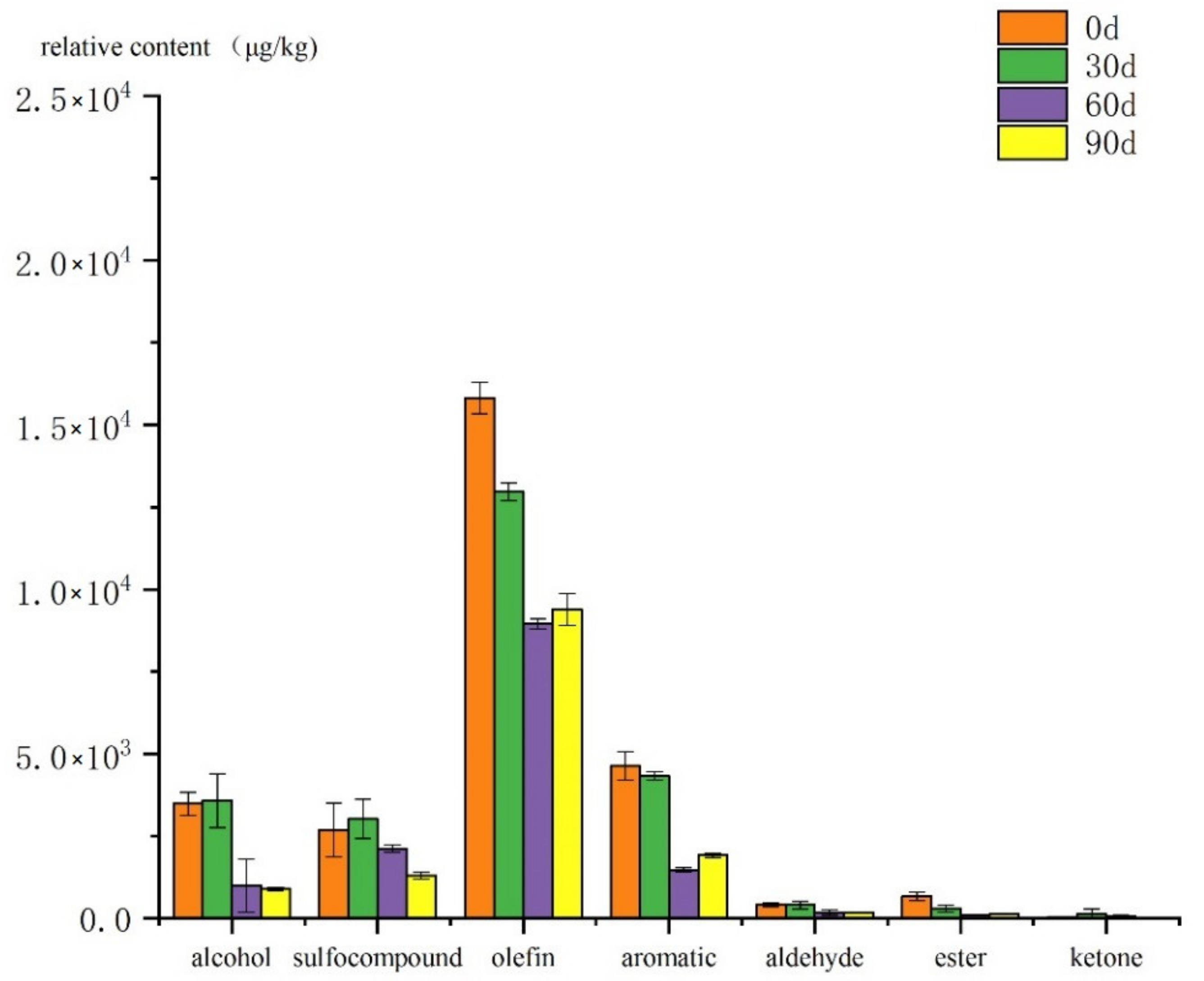
3.2. Analysis of Volatile Flavor Compounds in Cooked Mutton Meatballs during Storage
3.3. Sensory Evaluation
3.4. PLSR (Partial Least Squares Regression) Analysis of Different Storage Times
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Zhang, H.; Ma, L.; Tao, Y.; Liu, J.; Liu, D. Effects of ficin, high pressure and their combination on quality attributes of post-rigor tan mutton. LWT-Food Sci. Technol. 2020, 137, 110407. [Google Scholar] [CrossRef]
- Niu, J.; You, L.Q.; Bo, S.; Luo, R.M. Quality change of Tan sheep meat of aseptic packaging cold dishes at low temperature. Sci. Technol. Food Ind. 2017, 38, 220–225. [Google Scholar]
- Zhan, P.; Tian, H.L.; Sun, B.G.; Zhang, Y.; Chen, H. Quality Control of Mutton by Using Volatile Compound Fingerprinting Techniques and Chemometric Methods. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Whitfield, F.B. Volatiles from interactions of Maillard reactions and lipids. CRC Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Chunha, L.C.M.; Monteiroa, M.L.M.; Lorenzo, J.M.; Munekata, P.E.; Muchenje, V.; De Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar]
- Xiao, S.; Zhang, W.G.; Lee, J.E.; Ahn, D.U. Effects of diet, packaging and irradiation on protein oxidation, lipid oxidation of raw broiler thigh meat. Anim. Ind. Rep. 2013, 10. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, R.S.B.; Francisco, C.L.; Lino, D.M.; Borba, H. Meat quality of Santa Inês lamb chilled-then-frozen storage up to 12 months. Meat Sci. 2019, 148, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Um, K.M.W.; Bailey, M.E.; Clarke, A.D.; Chao, R.R. Concentration and identification of volatile compounds from heated beef fat using supercritical carbon dioxide extraction-gas liquid chromatography/ mass spectrometry. J. Agric. Food. Chem. 1999, 240, 1641–1646. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Liang, R.; Mao, Y.; Hopkins, D.L.; Li, K.; Dong, P.; Yang, X.; Niu, L.; Zhang, Y.; et al. Shelf-life and bacterial community dynamics of vacuum packaged beef during long-term super-chilled storage sourced from two Chinese abattoirs. Food Res. Int. 2020, 130, 108937. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, Q.; Zhao, H.; Zhao, M.; Yang, B. Volatile compounds of Cantonese sausage released at different stages of processing and storage. Food Chem. 2010, 121, 319–325. [Google Scholar] [CrossRef]
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016, 116, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Modi, V.K.; Yashoda, K.P.; Mahendrakar, N.S. Low-fat mutton kofta prepared by using carrageenan as fat replacer: Quality changes in cooked product during storage. J. Food Sci. Technol. 2009, 46, 316–319. [Google Scholar]
- Sun, Y.W.; Zhang, Y.; Song, H.L. Variation of aroma components during frozen storage of cooked beef balls by SPME and SAFE coupled with GC-O-MS. J. Food Process. Preserv. 2021, 45, e15036. [Google Scholar] [CrossRef]
- Parvin, R.; Zahid, M.A.; Seo, J.K.; Park, J.; Ko, J.; Yang, H.S. Influence of reheating methods and frozen storage on physicochemical characteristics and warmed-over flavor of nutmeg extract-enriched precooked beef meatballs. Antioxidants 2020, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Rubel, S.A.; Yu, Z.N.; Murshed, H.M.; Islam, S.M.; Sultana, D.; Rahman, S.M.E.; Wang, J. Addition of olive (olea europaea) leaf extract as a source of natural antioxidant in mutton meatball stored at refrigeration temperature. J. Food Sci. Technol. 2021, 58, 4002–4010. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, T.; Turhan, S. Physicochemical properties of pumpkin (Cucurbita pepo L.) seed kernel flour and its utilization in beef meatballs as a fat replacer and functional ingredient. J. Food Process. Preserv. 2020, 44, e14695. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wang, L.X.; Chen, D.Q.; Mao, J.L.; Wu, Y.H.C. Analysis of volatile flavor substances in Qianbei Ma goat meat cooked by different methods. Meat Res. 2020, 34, 78–83. [Google Scholar]
- Yang, P.; Liu, C.; Song, H.L.; Wang, L.J.; Wang, X.Q.; Hua, J.C. Sensory-directed flavor analysis of off-flavor compounds in infant formula with deeply hydrolyzed milk protein and their possible sources. LWT-Food Sci. Technol. 2018, 119, 108861. [Google Scholar] [CrossRef]
- Engel, W.; Bahr, W.; Schieberle, P. Solvent Assisted Flavour Evaporation–A New and Versatile Technique for the Careful and Direct Isolation of Aroma Compounds from Complex Food Matrices. Eur. Food Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; He, C.; Song, H.; Liu, Y.; Zhang, Y.; Wang, Y.; Guo, J.; Yang, H.; Su, X. Characterization and Comparison of Key Aroma-Active Compounds of Cocoa Liquors from Five Different Areas. Int. J. Food Proper. 2017, 20, 2396–2408. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, H.; Zhang, Y.; Tang, J.; Yu, D. Determination of Aroma Compounds in Pork Broth Produced by Different Processing Methods. Flavour Fragr. J. 2016, 31, 319–328. [Google Scholar] [CrossRef]
- Kim, T.H.; Sang, M.L.; Kim, Y.S.; Kim, K.H.; Oh, S.; Lee, H.J. Aroma Dilution Method Using Gc Injector Split Ratio for Volatile Compounds Extracted by Headspace Solid Phase Microextraction. Food Chem. 2003, 83, 151–158. [Google Scholar]
- Ferreira, V.; Aznar, M.; López, R.; Cacho, J. Quantitative Gas Chromatography−Olfactometry Carried Out at Different Dilutions of an Extract. Key Differences in the Odor Profiles of Four High-Quality Spanish Aged Red Wines. J. Agric. Food Chem. 2001, 49, 4818–4824. [Google Scholar] [CrossRef]
- Terry, A.; Heinrich, A. Flavornet and Human Odor Space. 2004. Available online: http://www.flavornet.org (accessed on 20 August 2021).
- Mottram, R. Flavour Research Group in the University of Reading, School of Food Biosciences The LRI and Odour Database. Available online: http://www.odour.org.uk/ (accessed on 20 August 2021).
- Béné, A.; Fornage, A.; Luisier, J.; Pichler, P.; Villettaz, J.C. A new method for the rapid determination of volatile substances: The SPME-direct method: Part I: Apparatus and working conditions. Sens. Actuators B. Chem. 2001, 72, 184–187. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.C.; Tao, N.P.; Ni, Y.Q.; Wu, R. Contribution of lipid Oxidation and Degradation to the Formation of Aroma Compounds in Animal-derived Foods. J. Chin. Inst. Food Sci. Technol. 2018, 16, 209–215. [Google Scholar]
- Sutherland, M.M.; Ames, J.M. The effect of castration on the headspace aroma components of cooked lamb. J. Sci. Food Agric. 1995, 69, 403–413. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavor formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]



| No | RI | CAS No | Compounds | Perception | Identification Method | Relative Content (μg/kg) | |||
|---|---|---|---|---|---|---|---|---|---|
| 0 d | 30 d | 60 d | 90 d | ||||||
| alcohol | |||||||||
| 1 | 1392 | 3391-86-4 | 1-Octen-3-ol | mushroom | RI/O/MS | 51.97 ± 12.88 a | 52.0 ± 17.94 a | 59.55 ± 52.29 ab | 37.19 ± 0.92 b |
| 2 | 1495 | 78-70-6 | Linalool | floral | RI/O/MS | 338.78 ± 17.45 a | 304.01 ± 58.97 b | 118.93 ± 59.61 c | 163.67 ± 11.19 d |
| 3 | 1542 | 619-62-5 | 1-methyl-4-(propan-2-yl)cyclohex-2-en-1-ol | herbal | RI/MS | 51.90 ± 12.90 a | 40.1 ± 4.10 b | 8.67 ± 0.61 c | ND d |
| 4 | 1544 | 15537-55-0 | unknown | balsam | RI/MS | 333.72 ± 72.77 a | 262.57 ± 24.10 a | 57.87 ± 47.30 b | 34.88 ± 11.70 c |
| 5 | 1592 | 562-74-3 | Terpinen-4-ol | pepper woody earth musty sweet | RI/MS | 2538.45 ± 201.46 a | 2310.3 ± 523.04 b | 676.25 ± 590.84 c | 1197.41 ± 12.38 d |
| 6 | 1612 | 586-81-2 | p-menth-4(8)-en-1-ol | terpineol lilac | RI/O/MS | 35.26 ± 3.22 a | 39.4 ± 15.32 a | 52.14 ± 52.21 b | 33.25 ± 2.19 a |
| 7 | 1690 | 98-55-5 | p-menth-1-en-8-ol | pine, citrus, woody, floral | RI/MS | ND a | 441.54 ± 147.51 b | ND a | 218.86 ± 3.66 c |
| 8 | 1695 | 507-70-0 | ((1S)-endo)-(-)-borneol | pine, woody, camphor, balsamic | RI/MS | ND a | ND a | ND a | 43.72 ± 1.50 b |
| 9 | 1698 | 464-45-9 | L(-)-Borneol | pine, woody, camphor | RI/MS | 72.59 ± 8.79 a | 68.6 ± 15.78 b | ND a | ND a |
| 10 | 1753 | 106-22-9 | (-)-β-citronellol | floral, leather, waxy, rose, bud citrus | RI/MS | 26.83 ± 6.89 a | 27.9 ± 6.09 a | 6.05 ± 3.10 b | 11.22 ± 4.54 b |
| 11 | 1834 | 106-24-1 | Geraniol | waxy | RI/O/MS | 24.25 ± 7.75 a | 31.9 ± 9.48 b | 4.07 ± 1.93 c | ND c |
| 12 | 2073 | 639-99-6 | (1S,2S,4R)-(-)-α,α-dimethyl-1-vinyl-o-menth-8-ene-4-methanol | green, woody, spi-cy, rose | RI/MS | 10.08 ± 4.60 a | ND b | ND b | 2.49 ± 0.22 a |
| Sulfur compound | |||||||||
| 13 | 871 | 870-23-5 | Allyl mercaptan | garlic, onion | RI/O/MS | 1307.61 ± 116.28 a | 1048.8 ± 109.27 a | 683.03 ± 171.19 b | 435.02 ± 37.73 c |
| 14 | 944 | 10152-76-8 | Allyl methyl sulfide | garlic, onion | RI/O/MS | 122.05 ± 11.25 a | 67.4 ± 8.54 b | 38.57 ± 11.95 c | 38.12 ± 6.46 c |
| 15 | 1126 | 592-88-1 | diallyl sulfide | sulfurous onion garlic horseradish metallic | RI/MS | 103.42 ± 42.97 a | ND b | ND b | ND b |
| 16 | 1262 | 2179-58-0 | allyl methyl disulphide | garlic, onion | RI/O/MS | ND a | 75.9 ± 65.85 b | 315.58 ± 238.35 c | ND a |
| 17 | 1454 | 2179-57-9 | Diallyl disulfide | garlic, onion | RI/O/MS | 1115.71 ± 642.81 a | 1794.1 ± 397.58 b | 1069.36 ± 749.64 a | 824.12 ± 56.83 c |
| 18 | 1754 | 2050-87-5 | Diallyl trisulfide | garlic green onion metallic | RI/O/MS | 30.55 ± 8.51 a | 37.0 ± 10.14 a | 13.27 ± 3.79 b | ND c |
| olefin | |||||||||
| 19 | 1013 | 80-56-8 | α-Pinene | pine | RI/O/MS | 1660 ± 46.03 a | 1040.2 ± 269.08 b | 764.83 ± 153.34 b | 842.45 ± 73.18 c |
| 20 | 1019 | 2867/5/2 | 3-Thujene | woody, green, herb | RI/MS | 2623.09 ± 449.39 a | 2321.4 ± 607.24 a | 1873.77 ± 263.03 b | 1103.67 ± 110.19 c |
| 21 | 1047 | 471-84-1 | unknown | camphor | RI/MS | 9 ± 0.42 a | 7.0 ± 3.53 b | 7.48 ± 1.42 b | 4.69 ± 0.40 a |
| 22 | 1101 | 3387-41-5 | Sabinene | woody, terpene, citrus, pine, spice | RI/MS | 4032.92 ± 3660.72 a | 3705.8 ± 232.28 b | 2189.24 ± 537.18 c | 3273.35 ± 39.60 b |
| 23 | 1141 | 123-35-3 | Myrcene | peppery terpene spicy balsam plastic | RI/MS | 2397.03 ± 134.08 a | 1704.6 ± 510.91 b | 1382.91 ± 214.23 c | 1485.10 ± 95.96 c |
| 24 | 1158 | 99-86-5 | p-mentha-1,3-diene | lemon | RI/O/MS | 1418.14 ± 256.37 a | 1131.9 ± 371.75 b | 1111.79 ± 152.95 b | 760.87 ± 40.85 c |
| 25 | 1188 | 555-10-2 | p-mentha-1(7),2-diene | terpentine | RI/O/MS | 2087.99 ± 118.32 a | 1667.5 ± 297.74 b | 1309.34 ± 185.19 c | 1293.51 ± 36.65 c |
| 26 | 1214 | 3779-61-1 | trans-Ocimene | sweet, herbal | RI/O/MS | 14.61 ± 4.02 a | 10.6 ± 7.94 a | 8.05 ± 2.22 b | ND c |
| 27 | 1233 | 3338-55-4 | (Z)-β-Ocimene | warm floral herb flower sweet | RI/MS | 64.4 ± 2.06 a | ND b | ND b | ND b |
| 28 | 1244 | 100-42-5 | Styrene | sweet balsam floral plastic | RI/MS | 26.35 ± 10.64 a | 24.3 ± 6.64 a | 32.18 ± 8.15 b | 30.76 ± 9.50 b |
| 29 | 1418 | 18368-95-1 | p-menthatriene, p-mentha-1,3,8-triene | turpentine | RI/O/MS | 27.79 ± 8.32 a | ND b | ND b | ND b |
| 30 | 1428 | 1195-32-0 | α,para-Dimethylstyrene | clove | RI/O/MS | 143.6 ± 21.5 a | 116.79 ± 32.31 b | 63.24 ± 36.96 c | 66.96 ± 2.25 c |
| 31 | 1540 | 13744-15-5 | cubebene | citrus, fruity | RI/MS | 5.52 ± 4.80 a | ND b | ND b | ND b |
| 32 | 1553 | 469-61-4 | (-)-α-cedrene | woody | RI/O/MS | ND a | 8.7 ± 0.96 b | ND a | ND a |
| 33 | 1585 | 13474-59-4 | (E)-α-bergamotene | warm | RI/O/MS | 54.61 ± 2.72 a | 44.9 ± 11.66 a | 11.81 ± 1.08 b | 23.42 ± 3.84 c |
| 34 | 1591 | 515-13-9 | (-)-β-Elemene | sweet | RI/O/MS | 56.50 ± 3.18 a | 50.9 ± 6.94 a | 15.99 ± 0.55 b | 13.05 ± 3.57 b |
| 35 | 1638 | unknown | RI/MS | 17.21 ± 5.56 a | ND b | ND b | ND b | ||
| 36 | 1645 | 30021-74-0 | γ-muurolene | herbal | RI/O/MS | 8.30 ± 7.98 a | ND b | 6.27 ± 4.45 a | 26.15 ± 0.87 c |
| 37 | 1657 | 18794-84-8 | trans-β-Farnesene | woody, citrus, herbal, sweet | RI/MS | 21.60 ± 2.55 a | 23.4 ± 7.00 a | ND b | ND b |
| 38 | 1664 | unknown | RI/MS | ND a | 22.7 ± 9.95 b | ND a | ND a | ||
| 39 | 1718 | 495-60-3 | zingiberene | spice fresh sharp | RI/O/MS | 298.12 ± 37.39 a | 302.21 ± 64.91 a | 42.35 ± 8.71 b | 125.85 ± 28.22 c |
| 40 | 1726 | 495-61-4 | unknown | balsamic woody | RI/MS | 232.12 ± 20.91 a | 111.86 ± 96.97 b | 17.01 ± 2.20 c | 55.14 ± 3.50 d |
| 41 | 1739 | unknown | RI/MS | ND a | 65.3 ± 20.46 b | ND a | ND a | ||
| 42 | 1771 | 644-30-4 | unknown | herbal | RI/MS | 564.20 ± 14.56 a | 565.82 ± 137.26 a | 104.65 ± 12.08 b | 251.63 ± 22.28 c |
| 43 | 1837 | 483-77-2 | unknown | herb, spice | RI/MS | 59.89 ± 2.5 a | 32.3 ± 9.08 b | 9.49 ± 2.93 a | 24.29 ± 9.29 b |
| aromatic | |||||||||
| 44 | 1029 | 108-88-3 | Toluene | sweet | RI/O/MS | 32.58 ± 8.15 a | 30.0 ± 3.47 a | 16.30 ± 3.34 b | 23.83 ± 1.23 c |
| 45 | 1207 | 95-63-6 | 1,2,4-Trimethylbenzene | plastic | RI/MS | 4.37 ± 2.07 a | ND b | ND b | ND b |
| 46 | 1125 | 108-38-3 | m-Xylene | plastic | RI/O/MS | ND a | 8.4 ± 0.85 b | 6.37 ± 1.38 b | ND a |
| 47 | 1166 | 95-47-6 | o-Xylene | geranium | RI/O/MS | 3 ± 0.33 a | ND b | ND b | 8.80 ± 4.19 c |
| 48 | 1256 | 99-87-6 | p-Cymene | citrus | RI/O/MS | 1212.91 ± 176.4 a | 908.03 ± 224.20 b | 725.57 ± 507.91 c | 731.00 ± 3.31 d |
| 49 | 1358 | 95-93-2 | 1,2,4,5-Tetramethylbenzene | rancid sweet | RI/MS | 10.93 ± 3.16 a | ND b | ND b | ND b |
| 50 | 1780 | 104-46-1 | Anethole | sweet anise licorice medicinal | RI/MS | 5.57 ± 1.93 a | 9.1 ± 2.38 b | 7.07 ± 2.75 a | ND c |
| 51 | 1701 | 90-12-0 | 1-Methylnaphthalene | naphthyl chemical medicinal camphor | RI/MS | ND a | 15.5 ± 5.39 b | ND a | 3.69 ± 0.89 a |
| 52 | 1846 | 91-57-6 | 2-Methylnaphthalene | sweet floral woody | RI/MS | 15.19 ± 4.93 a | 10.2 ± 5.30 a | 140.80 ± 18.86 b | ND c |
| 53 | 1870 | 94-59-7 | Safrole | spicy | RI/O/MS | 1183.47 ± 114.81 a | 1100.6 ± 363.06 a | 256.62 ± 40.63 b | 329.51 ± 2.18 b |
| 54 | 1944 | 120-58-1 | Isosafrole (Controlled Chemical) | sweet sassafrass spicy | RI/MS | 5.43 ± 2.67 a | ND b | ND b | ND b |
| 55 | 2001 | 93-15-2 | 1,2-Dimethoxy-4-allylbenzene | sweet fresh warm spicy clove carnation cinnamon | RI/MS | 629.17 ± 45.82 a | 661.22 ± 183.00 a | ND b | 248.16 ± 9.78 c |
| 56 | 2146 | 607-91-0 | Myristicin | spicy warm balsamic woody | RI/MS | 927.43 ± 54.89 a | 973.65 ± 277.95 a | 185.01 ± 21.45 b | 328.50 ± 19.14 d |
| 57 | 2156 | 97-53-0 | Eugenol | sweet, clove | RI/O/MS | 51.19 ± 3.45 a | ND b | 14.63 ± 2.79 c | ND b |
| 58 | 2213 | 487-11-6 | 5-allyl-1,2,3-trimethoxybenzene | spice, flower | RI/MS | 553.06 ± 16.46 a | 607.01 ± 193.07 a | 118.76 ± 17.70 b | 242.12 ± 22.34 c |
| aldehyde | |||||||||
| 59 | 964 | 110-62-3 | Valeraldehyde | fermented bready fruity nutty berry | RI/MS | ND a | ND a | ND a | 0.44 ± 0.10 b |
| 60 | 1071 | 66-25-1 | Hexanal | grass | RI/O/MS | 22.83 ± 4.33 a | 41.9 ± 5.29 b | 27.57 ± 3.41 a | 35.30 ± 5.15 c |
| 61 | 1172 | 111-71-7 | Heptanal | fresh, fatty, green, herbal | RI/MS | 7.85 ± 1.58 a | 39.4 ± 54.44 b | ND c | ND c |
| 62 | 1277 | 124-13-0 | Octanal | fatty | RI/O/MS | 25.12 ± 3.62 a | ND b | ND b | ND b |
| 63 | 1384 | 124-19-6 | Nonanal | fresh | RI/O/MS | 298.49 ± 35.41 a | 274.60 ± 55.28 a | 104.22 ± 24.45 b | 121.14 ± 11.17 b |
| 64 | 1517 | 100-52-7 | Benzaldehyde | sharp, sweet, bitter, almond, cherry | RI/MS | 47.37 ± 6.55 a | 42.8 ± 2.00 a | 51.21 ± 60.18 b | 25.41 ± 0.87 c |
| 65 | 1769 | 122-03-2 | p-Isopropylbenzaldehyde | spicy, green, herbal | RI/MS | 2.98 ± 0.37 a | 2.6 ± 0.82 a | ND b | ND b |
| ester | |||||||||
| 66 | 1345 | 103-09-3 | 2-Ethylhexyl acetate | earthy, herbal, undergrowth | RI/MS | 37.44 ± 6.73 a | ND b | ND b | ND b |
| 67 | 1572 | 76-49-3 | Bornyl acetate | woody, pine, herbal, spice | RI/MS | 154.48 ± 10.13 a | 129.59 ± 37.71 a | 51.54 ± 3.52 b | 63.45 ± 4.45 b |
| 68 | 1619 | 110-38-3 | ethyl caprate | sweet, waxy, fruity, apple grape, oily, brandy | RI/MS | 19.08 ± 3.32 a | 32.3 ± 15.78 b | 8.53 ± 0.46 c | 9.12 ± 2.28 c |
| 69 | 1629 | 150-84-5 | Citronellyl acetate | green, rose, fruity, citrus, woody, fruit | RI/MS | 73.87 ± 3.22 a | 72.0 ± 28.66 a | 25.68 ± 2.39 b | 35.58 ± 6.76 c |
| 70 | 1665 | 80-26-2 | ( + /-)-α-terpinyl acetate | herbal, lavender, citrus | RI/MS | 310.29 ± 61.43 a | ND b | ND b | ND b |
| 71 | 1711 | 141-12-8 | Neryl acetate | rose, soapy, citrus, pear | RI/MS | 64.97 ± 36.40 a | 60.4 ± 19.49 a | 10.03 ± 3.75 b | 20.19 ± 5.79 c |
| ketone | |||||||||
| 72 | 856 | 67-64-1 | Acetone | solvent, ethereal, apple, pear | RI/MS | ND a | ND a | 5.10 ± 1.89 b | ND a |
| 73 | 1309 | 110-93-0 | 6-Methyl-5-hepten-2-one | citrus, musty, | RI/O/MS | 36.76 ± 3.11 a | 27.9 ± 6.06 b | 44.99 ± 41.95 c | 16.56 ± 2.43 d |
| 74 | 1639 | 89-81-6 | Piperiton | herbal, minty, camphor, medicinal | RI/MS | ND a | 99.7 ± 151.32 b | ND c | ND c |
| terpene | |||||||||
| 75 | 1048 | 79-92-5 | Camphene | camphor | RI/MS | ND a | ND a | 16.07 ± 13.69 b | 17.48 ± 0.12 b |
| 76 | 1088 | 127-91-3 | β-pinene | camphor | RI/O/MS | 179.37 ± 207.01 a | 1217.9 ± 169.21 b | 935.41 ± 166.65 c | 919.37 ± 57.82 c |
| 77 | 1124 | 13466-78-9 | 3-carene | pine | RI/O/MS | 337.9 ± 72.66 a | 284.22 ± 42.93 b | 294.13 ± 32.84 b | 213.76 ± 34.51 b |
| 78 | 1158 | 99-86-5 | p-mentha-1,3-diene | lemon | RI/O/MS | 1418.14 ± 256.37 a | 1131.9 ± 371.75 b | 1111.79 ± 152.95 b | 760.87 ± 40.85 c |
| 79 | 1178 | 5989-27-5 | ( + )-Limonene | citrus, orange, fresh, sweet | RI/MS | 212.14 ± 139.31 a | ND b | ND b | ND b |
| 80 | 1186 | 138-86-3 | DL-Limonene | citrus, herbal, terpene, camphor | RI/MS | 158.36 ± 106.1 a | 1415.9 ± 1281.47 b | 1632.77 ± 233.11 b | 1644.62 ± 93.95 b |
| 81 | 1187 | 470-82-6 | Cineole | herbal | RI/O/MS | 127.86 ± 17.99 a | 540.02 ± 748.14 b | 123.76 ± 22.81 a | 65.99 ± 5.19 c |
| 82 | 1229 | 99-85-4 | p-mentha-1,4-diene | oily, woody, terpene, lemo, herbal | RI/MS | 2360.05 ± 356.15 a | 2033.6 ± 590.91 a | 1796.05 ± 251.18 b | 1161.38 ± 52.07 c |
| 83 | 1268 | 586-62-9 | terpinolene | camphor | RI/O/MS | 1084.81 ± 127.42 a | 209 ± 64.16 b | 433.93 ± 372.24 ab | 510.30 ± 29.11 c |
| 84 | 1459 | 17699-16-0 | (1α,2α,5α)-2-methyl-5-(1-methylethyl)bicyclo [3.1.0]hexan-2-ol | woody, balsam | RI/MS | 146.83 ± 20 a | 383.09 ± 32.25 b | 512.91 ± 583.52 c | NDd |
| 85 | 1495 | 3856-25-5 | (-)-α-copaene | woody, spicy, honey | RI/MS | 317.19 ± 29.09 a | 233.11 ± 68.25 a | 68.22 ± 48.39 b | NDc |
| 86 | 1604 | 87-44-5 | Caryophyllene | spice | RI/O/MS | 190.16 ± 20.13 a | 191.55 ± 56.34 a | 461.25 ± 545.27 b | 140.08 ± 2.22 c |
| 87 | 1648 | 118-65-0 | (-)-isocaryophyllene | woody, spicy | RI/MS | 4.31 ± 1.90 a | ND b | ND b | ND b |
| 88 | 1677 | 6753-98-6 | α-humulene | woody | RI/MS | 59.70 ± 6.75 a | 50.2 ± 16.84 a | ND b | 9.18 ± 6.36 c |
| 89 | 1761 | 483-76-1 | unknown | thyme, woody, dry | RI/O/MS | 34.28 ± 1.42 a | 33.9 ± 9.08 a | 8.73 ± 1.25 b | 21.17 ± 4.87 c |
| No | RI | CAS No | Compounds | Perception | Identification | Relative Content (μg/g) | |||
|---|---|---|---|---|---|---|---|---|---|
| 0 d | 30 d | 60 d | 90 d | ||||||
| alcohol | |||||||||
| 1 | 1462 | 3391-86-4 | 1-Octen-3-ol | mushroom | MS/RI | ND b | ND b | 0.37 ± 0.16 a | ND b |
| 2 | 1554 | 89-79-2 | Isopulegol | minty, cooling | MS/RI | ND c | 0.24 ± 0.09 ab | 0.07 ± 0.02 a | 0.33 ± 0.13 b |
| 3 | 1559 | 78-70-6 | Linalool | citrus floral, rose | MS/RI/O/ | 0.40 ± 0.02 a | 1.52 ± 0.19 b | 1.22 ± 0.31 a | 3.81 ± 0.28 c |
| 4 | 1629 | 562-74-3 | Terpinen-4-ol | pepper, woody, earth, musty, sweet | MS/RI | 3.13 ± 0.12 a | 2.53 ± 0.40 b | 8.93 ± 1.97 c | 15.11 ± 1.19 d |
| 5 | 1721 | 98-55-5 | p-menth-1-en-8-ol | citrus | MS/RI/O/ | 0.33 ± 0.02 a | 1.61 ± 0.38 | 1.61 ± 0.38 b | 13.26 ± 0.39 c |
| 6 | 1729 | 507-70-0 | ((1S)-endo)-(-)-borneol | camphor | MS/RI/O/ | 0.10 ± 0.06 a | 3.54 ± 0.46 b | 0.55 ± 0.15 a | 3.23 ± 0.12 b |
| 7 | 1778 | 617-94-7 | 2-Phenyl-2-propanol | green, earthy | MS/RI/O/ | 0.70 ± 0.06 c | 7.13 ± 4.24 a | 1.48 ± 1.05 b | 11.32 ± 1.26 a |
| 8 | 1864 | 106-24-1 | Geraniol | rose, waxy, citrus | MS/RI/O/ | 0.91 ± 0.32 a | 1.03 ± 0.84 a | 0.20 ± 0.04 a | 1.23 ± 0.06 a |
| 9 | 1869 | 1197-01-9 | 2-p-Tolylpropan-2-ol | sweet, fruity, cherry, camphor | MS/RI | ND c | 3.81 ± 1.24 a | 0.83 ± 0.17 b | 0.29 ± 0.29 a |
| Sulfur compound | |||||||||
| 10 | 1162 | 592-88-1 | Diallyl sulfide | sulfurous, onion, garlic | MS/RI | 1.24 ± 0.12 a | 0.08 ± 0.12 b | ND c | ND c |
| 11 | 1297 | 2179-58-0 | Allyl methyl disulphide | garlic, onion | MS/RI/O/ | 2.95 ± 0.03 a | 3.09 ± 0.54 a | 1.17 ± 0.25 b | 0.25 ± 0.08 a |
| 12 | 1503 | 2179-57-9 | Diallyl disulfide | onion, garlic | MS/RI/O/ | 9.67 ± 0.50 a | 7.09 ± 0.92 b | 7.22 ± 1.65 a | 1.60 ± 0.09 c |
| olefin | |||||||||
| 13 | 1226 | 555-10-2 | p-mentha-1(7),2-diene | mint, terpentine | MS/RI | 2.53 ± 0.18 a | 9.65 ± 1.18 b | 7.71 ± 1.76 c | ND d |
| 14 | 1272 | 100-42-5 | Styrene | sweet, balsam, floral, plastic | MS/RI | 8.83 ± 0.56 a | 3.53 ± 0.72 b | 3.45 ± 0.74 c | 1.49 ± 0.19 d |
| 15 | 1458 | 1195-32-0 | Alpha, para-Dimethylstyrene | phenolic | MS/RI/O/ | ND c | 0.72 ± 0.26 a | 0.22 ± 0.05 b | ND c |
| 16 | 1582 | 13474-59-4 | (E)-α-bergamotene | woody, warm, tea | MS/RI | ND d | 0.18 ± 0.08 a | 0.70 ± 0.18 b | 2.22 ± 0.16 c |
| 17 | 1750 | 495-60-3 | Zingiberene | spice, sharp | MS/RI/O/ | 0.08 ± 0.03 a | 3.38 ± 0.37 a | 1.19 ± 0.22 b | 17.02 ± 1.08 c |
| 18 | 1757 | 495-61-4 | Unknown | balsamic, woody | MS/RI | ND d | 0.94 ± 0.14 b | 0.41 ± 0.08 a | 5.17 ± 0.60 c |
| 19 | 1054 | 108-88-3 | Toluene | paint | MS/RI | 44.31 ± 2.00 a | 15.57 ± 2.47 b | 39.11 ± 7.42 a | 1.01 ± 0.08 c |
| 20 | 1198 | 95-47-6 | o-Xylene | geranium | MS/RI | 1.91 ± 0.05 a | ND c | 0.85 ± 0.19 b | ND c |
| 21 | 1287 | 99-87-6 | p-Cymene | fresh, citrus, terpene, woody, spice | MS/RI | 0.83 ± 0.08 a | 2.15 ± 1.52 b | 2.44 ± 0.57 c | ND d |
| 22 | 1802 | 644-30-4 | 1-methyl-4-(6-methylhept-5-en-2-yl)benzene | herbal | MS/RI | 0.42 ± 0.02 a | 3.58 ± 0.41 b | 1.37 ± 0.26 a | 13.57 ± 9.15 b |
| 23 | 1855 | 104-46-1 | Anethole | sweet, anise, licorice, medicinal | MS/RI | ND d | 0.54 ± 0.05 a | 0.08 ± 0.02 b | 0.70 ± 0.07 c |
| 24 | 1892 | 91-57-6 | 2-Methylnaphthalene | sweet, floral | MS/RI/O/ | ND d | 0.71 ± 0.27 a | 0.12 ± 0.04 b | 1.20 ± 0.16 c |
| 25 | 1906 | 94-59-7 | Safrole | sweet, warm, spicy, woody, floral | MS/RI | 1.36 ± 0.16 a | 7.37 ± 0.65 b | 4.87 ± 0.97 c | 11.68 ± 1.49 d |
| 26 | 2046 | 581-42-0 | 2,6-Dimethylnaphthalene | grass | MS/RI | ND c | 0.71 ± 0.43 a | ND c | 0.42 ± 0.10 b |
| 27 | 2250 | 487-11-6 | 5-allyl-1,2,3-trimethoxybenzene | spice, flower | MS/RI/O/ | 0.41 ± 0.03 a | 5.20 ± 0.43 a | 2.19 ± 0.39 a | 10.06 ± 1.36 b |
| 28 | 2298 | 607-91-0 | myristicin | spicy, warm, balsamic, woody | MS/RI/O/ | 0.74 ± 0.05 a | 7.39 ± 0.61 b | 3.33 ± 0.56 a | 18.46 ± 2.39 c |
| aldehyde | |||||||||
| 29 | 1095 | 66-25-1 | Hexanal | fresh, green, fatty, aldehydic, grass | MS/RI/O/ | 4.68 ± 0.20 a | 1.38 ± 0.23 b | 0.11 ± 0.02 c | 0.42 ± 0.12 c |
| 30 | 1411 | 124-19-6 | Nonanal | fresh | MS/RI/O/ | 0.95 ± 0.05 a | 0.61 ± 0.08 b | 0.39 ± 0.09 c | 2.37 ± 0.12 b |
| 31 | 1548 | 100-52-7 | Benzaldehyde | almond, cherry | MS/RI | 0.32 ± 0.07 a | 0.26 ± 0.05 b | 0.25 ± 0.08 a | ND c |
| ester | |||||||||
| 32 | 912 | 141-78-6 | Ethyl acetate | fruity, sweet, green | MS/RI | 0.25 ± 0.19 c | 0.52 ± 0.15 a | 0.36 ± 0.09 b | ND d |
| 33 | 1044 | 2867-05--2 | Alpha-thujene | woody, green, herb | MS/RI | 1.75 ± 0.07 a | 1.28 ± 0.18 b | 0.92 ± 0.19 c | ND d |
| 34 | 1610 | 76-49-3 | Bornyl acetate | woody, pine, herbal, spice | MS/RI | 0.13 ± 0.02 a | 0.79 ± 0.08 b | 0.42 ± 0.10 c | 4.07 ± 0.15 d |
| 35 | 1659 | 110-38-3 | Ethyl caprate | sweet, waxy, fruity | MS/RI | ND d | 0.63 ± 0.17 a | 0.10 ± 0.04 b | 0.90 ± 0.03 c |
| 36 | 1725 | 80-26-2 | (+/-)-α-terpinyl acetate | herbal, bergamot, lavender | MS/RI | 0.30 ± 0.09 a | 0.74 ± 0.12 b | 0.41 ± 0.09 a | 4.91 ± 0.23 c |
| ketone | |||||||||
| 37 | 1679 | 98-86-2 | Acetophenone | sweet, pungent | MS/RI/O/ | 2.69 ± 0.16 a | 5.49 ± 0.72 b | 5.48 ± 1.22 c | 8.43 ± 0.16 ab |
| terpene | |||||||||
| 38 | 1040 | 80-56-8 | α-pinene | pine, earthy, woody | MS/RI/O/ | 0.39 ± 0.01 a | 17.61 ± 2.89 b | 10.81 ± 2.35 c | ND a |
| 39 | 1082 | 79-92-5 | Camphene | woody, camphor, terpenic | MS/RI | ND c | 0.68 ± 0.03 a | 0.11 ± 0.03 b | ND c |
| 40 | 1124 | 127-91-3 | β-pinene | pine, green | MS/RI/O/ | 10.22 ± 0.48 a | 17.46 ± 2.51 b | 14.47 ± 3.30 a | ND c |
| 41 | 1166 | 13466-78-9 | 3-carene | pine, woody | MS/RI/O/ | ND c | 2.52 ± 0.56 a | 2.03 ± 0.43 b | ND c |
| 42 | 1196 | 99-86-5 | p-mentha-1,3-diene | woody, lemon, herbal | MS/RI/O/ | 0.92 ± 0.08 a | 3.78 ± 0.82 b | 1.98 ± 0.43 a | ND a |
| 43 | 1216 | 5989-27-5 | (+)-Limonene | citrus, orange | MS/RI | 3.56 ± 0.14 a | 11.73 ± 1.66 b | 9.62 ± 2.27 c | ND d |
| 44 | 1264 | 99-85-4 | p-mentha-1,4-diene | woody, terpene | MS/RI/O/ | 3.70 ± 0.28 a | 6.89 ± 0.95 b | 5.06 ± 1.19 a | ND c |
| 45 | 1302 | 586-62-9 | terpinolene | pine, citrus | MS/RI/O/ | 0.94 ± 0.09 a | 3.21 ± 0.61 b | 2.35 ± 0.53 c | 0.41 ± 0.19 a |
| 46 | 1527 | 3856-25-5 | (-)-α-copaene | woody, spicy, honey | MS/RI | 0.28 ± 0.00 a | 0.82 ± 0.17 b | 0.82 ± 0.17 c | 10.31 ± 0.32 d |
| 47 | 1638 | 87-44-5 | Caryophyllene | sweet, woody, spice | MS/RI/O/ | 0.20 ± 0.01 a | 5.60 ± 0.19 b | 0.62 ± 0.14 c | 8.49 ± 0.24 d |
| 48 | 1710 | 6753-98-6 | α-Humulene | woody | MS/RI | ND b | 0.70 ± 0.24 a | 0.08 ± 0.04 b | 1.10 ± 0.28 a |
| 49 | 1763 | - | unknown | herbal, woody | MS/RI | ND c | 0.51 ± 0.13 a | 0.19 ± 0.04 b | 2.53 ± 0.57 a |
| 50 | 1935 | 128-37-0 | 2,6-Di-tert-butyl-4-methylphenol | camphor | MS/RI/O/ | 5.26 ± 0.31 a | 9.81 ± 13.80 a | 3.19 ± 0.60 a | 18.12 ± 2.56 b |
| 51 | 2025 | 108-95-2 | Phenol | plastic, rubber | MS/RI | 0.13 ± 0.00 a | 0.12 ± 0.02 b | 0.09 ± 0.03 a | ND c |
| 52 | 2034 | 93-15-2 | 1,2-Dimethoxy-4-allylbenzene | sweet, fresh, | MS/RI | 0.55 ± 0.03 a | 7.72 ± 10.64 a | 2.90 ± 0.56 a | 11.01 ± 1.35 b |
| 53 | 2195 | 97-53-0 | Eugenol | sweet, spicy | MS/RI | 0.17 ± 0.06 a | 0.79 ± 0.10 b | 0.42 ± 0.09 a | 5.54 ± 0.25 c |
| 54 | 2325 | 96-76-4 | 2,4-Ditert-butylphenol | phenolic | MS/RI | 0.93 ± 0.06 b | 2.07 ± 0.10 c | 0.24 ± 0.09 a | 0.62 ± 0.34 ab |
| other | |||||||||
| 55 | 1440 | 104-90-5 | 5-Ethyl-2-methylpyridine | nutty, strong | MS/RI | 0.03 ± 0.01 a | ND b | ND b | ND b |
| 56 | 1484 | 17699-16-0 | unknown | woody, balsam | MS/RI/O/ | 1.46 ± 0.11 a | 4.97 ± 0.7 b | 3.95 ± 0.96 c | 5.71 ± 0.38 c |
| 57 | 1647 | 107-92-6 | n-Butyric acid | cheesy | MS/RI/O/ | 0.92 ± 0.35 a | 1.43 ± 0.58 b | 0.10 ± 0.04 b | 0.90 ± 0.03 c |
| 58 | 1679 | 98-86-2 | Acetophenone | sweet, pungent | MS/RI/O/ | 2.69 ± 0.16 a | 5.49 ± 0.72 b | 5.48 ± 1.22 c | 8.43 ± 0.16 ab |
| NO. | RI | CAS No | Compound | Odor | FD Factor | |||
|---|---|---|---|---|---|---|---|---|
| 0 d | 30 d | 60 d | 90 d | |||||
| A1 | 871 | 870-23-5 | Allyl mercaptan | garlic | 243 | 81 | 9 | 9 |
| A2 | 1187 | 470-82-6 | Cineole | mint | 3 | 9 | - | - |
| A3 | 1392 | 3391-86-4 | 1-Octen-3-ol | mushroom | 9 | 9 | 9 | - |
| A4 | 1495 | 78-70-6 | Linalool | floral | 27 | 3 | 3 | 1 |
| A5 | 1834 | 106-24-1 | Geraniol | waxy | 27 | - | - | - |
| A6 | 1262 | 2179-58-0 | allyl methyl disulphide | garlic, onion | - | 9 | - | - |
| A7 | 1454 | 2179-57-9 | Diallyl Disulfide | onion garlic | 3 | 9 | 9 | - |
| A8 | 1754 | 2050-87-5 | Diallyl trisulfide | garlic onion | 243 | 27 | 9 | 27 |
| A9 | 1013 | 80-56-8 | α-pinene | pine | 27 | 9 | 1 | 1 |
| A10 | 1088 | 127-91-3 | β-pinene | resin | 3 | 1 | 3 | - |
| A11 | 1124 | 13466-78-9 | 3-carene | pine resin | 3 | 3 | 3 | 3 |
| A12 | 1188 | 555-10-2 | p-mentha-1(7),2-diene | terpentine | 9 | - | - | - |
| A13 | 1214 | 3779-61-1 | trans-Ocimene | sweet | - | 1 | 3 | 1 |
| A14 | 1268 | 586-62-9 | terpinolene | pine | 243 | - | - | - |
| A15 | 1418 | 18368-95-1 | p-menthatriene, p-mentha-1,3,8-triene | turpentine | 9 | - | - | - |
| A16 | 1428 | 1195-32-0 | α,para-Dimethylstyrene | citrus | 3 | 1 | 3 | 1 |
| A17 | 1455 | 3856-25-5 | (-)-α-copaene | spicy | 27 | - | - | 9 |
| A18 | 1591 | 515-13-9 | (-)-β-Elemene | sweet | 9 | 3 | 1 | - |
| A19 | 1604 | 87-44-5 | Caryophyllene | spice | 3 | - | - | - |
| A20 | 1761 | 483-76-1 | unknown | medicine | 9 | 9 | 1 | 9 |
| A21 | 1256 | 99-87-6 | p-Cymene | citrus | - | - | 9 | 27 |
| A22 | 1701 | 90-12-0 | 1-Methylnaphthalene | camphor | - | - | 3 | - |
| A23 | 1944 | 120-58-1 | Isosafrole (Controlled Chemical) | sweet | 3 | 3 | 9 | 3 |
| A24 | 2001 | 93-15-2 | 1,2-Dimethoxy-4-allylbenzene | clove | - | 9 | - | - |
| A25 | 1619 | 110-38-3 | ethyl caprate | grape | 3 | 1 | 3 | 3 |
| A26 | 1639 | 110-93-0 | 6-Methyl-5-hepten-2-one | mushroom | 3 | 1 | 3 | 3 |
| No. | RI | CAS No | Compounds | Odor | FD Factor | |||
|---|---|---|---|---|---|---|---|---|
| 0 d | 30 d | 60 d | 90 d | |||||
| B1 | 1040 | 80-56-8 | α-pinene | pine | - | 3 | 3 | - |
| B2 | 1166 | 13466-78-9 | 3-carene | resin | - | 3 | 1 | - |
| B3 | 1196 | 99-86-5 | p-mentha-1,3-diene | lemon | - | 1 | 3 | - |
| B4 | 1458 | 1195-32-0 | α,para-Dimethylstyrene | pine | - | 3 | 9 | - |
| B5 | 1638 | 87-44-5 | Caryophyllene | spice | 3 | 27 | 9 | 1 |
| B6 | 1750 | 495-60-3 | zingiberene | sharp | 1 | 3 | 1 | - |
| B7 | 1095 | 66-25-1 | Hexanal | fat | 9 | 1 | 1 | 1 |
| B8 | 1297 | 2179-58-0 | allyl methyl disulphide | garlic | 9 | 3 | 81 | 3 |
| B9 | 1503 | 2179-57-9 | Diallyl Disulfide | onion | 27 | 81 | 9 | 3 |
| B10 | 2298 | 607-91-0 | myristicin | spicy | 1 | 3 | - | - |
| B11 | 1559 | 78-70-6 | Linalool | floral | 3 | 3 | 9 | 3 |
| B12 | 1721 | 98-55-5 | p-menth-1-en-8-ol | lilac | 1 | 1 | 1 | 3 |
| B13 | 1729 | 507-70-0 | ((1S)-endo)-(-)-borneol | camphor | - | 3 | - | - |
| B14 | 1778 | 617-94-7 | 2-Phenyl-2-propanol | green | - | 3 | 1 | 1 |
| B15 | 1864 | 106-24-1 | Geraniol | sweet | 9 | 9 | 9 | 3 |
| B16 | 1484 | 17699-16-0 | unknown | balsam | 3 | 3 | 9 | 3 |
| B17 | 1647 | 107-92-6 | n-Butyric acid | sharp | 3 | 3 | 3 | 3 |
| B18 | 1679 | 98-86-2 | Acetophenone | almond | - | 3 | 1 | - |
| B19 | 1892 | 91-57-6 | 2-Methylnaphthalene | floral | - | - | 9 | 1 |
| B20 | 2250 | 487-11-6 | 5-allyl-1,2,3-trimethoxybenzene | spice | - | 3 | - | 9 |
| No. | CAS No | Compounds | Odor | OAV | Threshold Value (μg/kg) | |||
|---|---|---|---|---|---|---|---|---|
| 0 d | 30 d | 60 d | 90 d | |||||
| 1 | 470-82-6 | Cineole | Herbal, medicinal | 98.35 | 415.4 | 95.2 | 50.76 | 1.3 |
| 2 | 3391-86-4 | 1-Octen-3-ol | mushroom | 25.99 | 26 | 29.78 | 18.6 | 2 |
| 3 | 78-70-6 | Linalool | floral | 56.46 | 50.67 | 19.82 | 27.28 | 6 |
| 4 | 10152-76-8 | Allyl methyl sulfide | Garlic, onion | 244.1 | 134.8 | 77.14 | 76.24 | 0.5 |
| 5 | 2179-57-9 | Diallyl Disulfide | garlic | 858.24 | 1380.08 | 822.58 | 633.94 | 1.3 |
| 6 | 80-56-8 | α-pinene | Pine, woody | 16.6 | 10.4 | 7.65 | 8.42 | 100 |
| 7 | 127-91-3 | β-pinene | Woody, pine | 1.28 | 8.7 | 6.68 | 6.57 | 140 |
| 8 | 3856-25-5 | (-)-α-copaene | woody spicy honey | 6.53 | - | - | 24.63 | 6 |
| 9 | 93-15-2 | 1,2-Dimethoxy-4-allylbenzene | sweet | 9.25 | 9.72 | 0 | 3.65 | 68 |
| 10 | 66-25-1 | Hexanal | grassy | 104 | 30.67 | 2.44 | 9.33 | 45 |
| 11 | 124-19-6 | Nonanal | Waxy, aldehydic | 27.14 | 17.43 | 11.14 | 67.71 | 35 |
| 12 | 607-91-0 | myristicin | spicy | 24.67 | 246.33 | 111 | 615.33 | 30 |
| 13 | 507-70-0 | ((1S)-endo)-(-)-borneol | Pine, woody | 1.92 | 68.08 | 10.58 | 62.12 | 52 |
| 14 | 128-37-0 | 2,6-Di-tert-butyl-4-methylphenol | mild phenolic camphor | 5.26 | 9.81 | 3.19 | 18.12 | 100 |
| 15 | 107-92-6 | n-Butyric acid | sweaty | 920 | 1430 | 100 | 900 | 1 |
| 16 | 98-86-2 | Acetophenone | pungent | 15.82 | 32.29 | 32.24 | 49.59 | 170 |
| 17 | 91-57-6 | 2-Methylnaphthalene | sweet | - | 177.5 | 30 | 300 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Sun, Y.; Song, H. Variation in Volatile Flavor Compounds of Cooked Mutton Meatballs during Storage. Foods 2021, 10, 2430. https://doi.org/10.3390/foods10102430
Zhang Y, Sun Y, Song H. Variation in Volatile Flavor Compounds of Cooked Mutton Meatballs during Storage. Foods. 2021; 10(10):2430. https://doi.org/10.3390/foods10102430
Chicago/Turabian StyleZhang, Yu, Yuwei Sun, and Huanlu Song. 2021. "Variation in Volatile Flavor Compounds of Cooked Mutton Meatballs during Storage" Foods 10, no. 10: 2430. https://doi.org/10.3390/foods10102430
APA StyleZhang, Y., Sun, Y., & Song, H. (2021). Variation in Volatile Flavor Compounds of Cooked Mutton Meatballs during Storage. Foods, 10(10), 2430. https://doi.org/10.3390/foods10102430





