Maintenance of Postharvest Quality and Reactive Oxygen Species Homeostasis of Pitaya Fruit by Essential Oil p-Anisaldehyde Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
2.2. Determination of Fruit Physio-Chemical Quality
2.3. Measurement of Generation Rate of Superoxide Anion Radicals (O2•−) and Hydrogen Peroxide (H2O2) Concentration
2.4. Assessment of Activity of Superoxide Dismutase (SOD), Peroxidase (POD), and Catalase (CAT)
2.5. Determination of Components in Ascorbic Acid-Glutathione (ASA-GSH) Cycle
2.6. Determination of Content of Total Phenolics, Flavonoids, and Scavenging Rate of DPPH Radical
2.7. Gene Expression Analyses of Antioxidant Enzymes
2.8. Statistical Analysis
3. Results
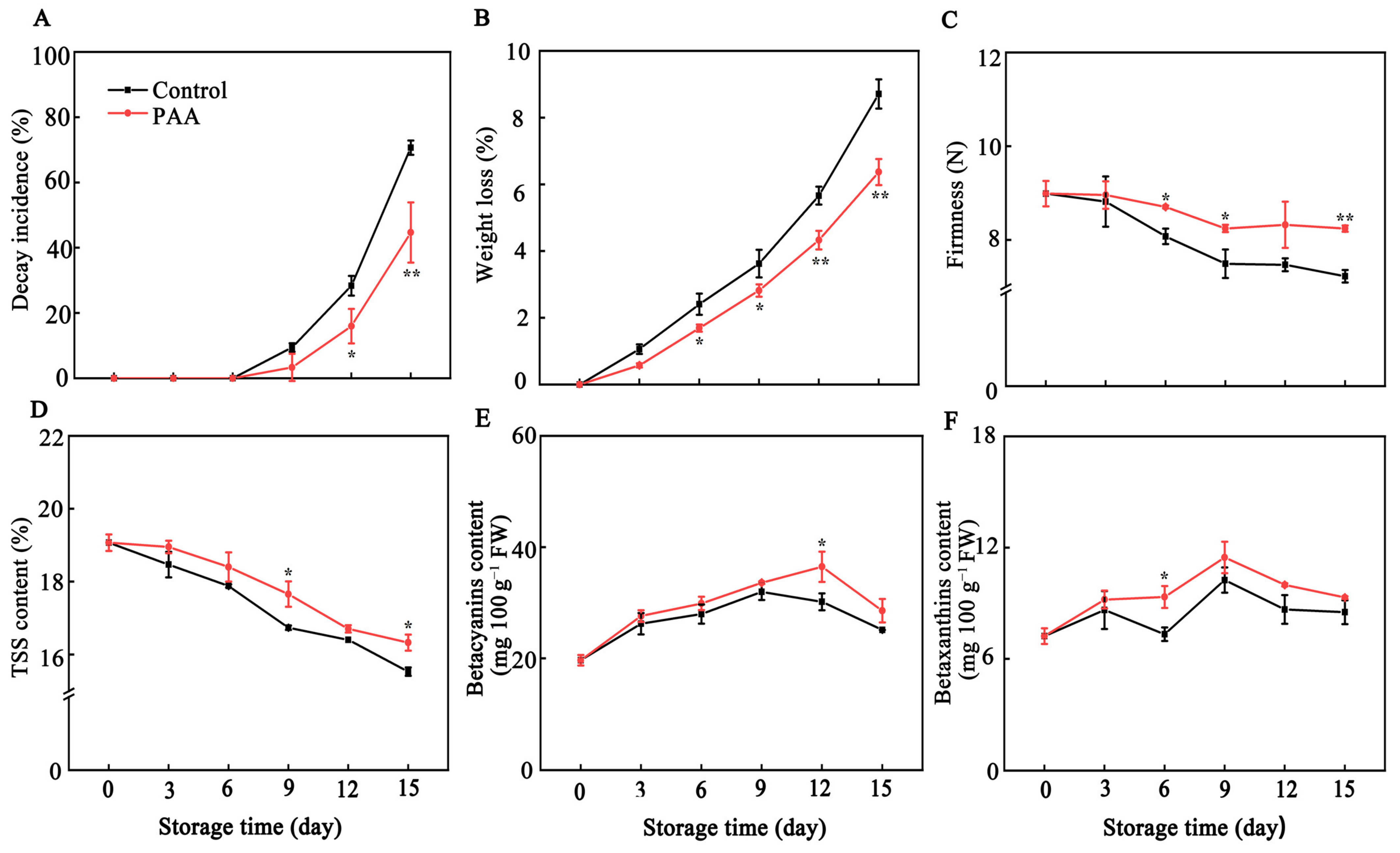
3.1. Effects of PAA on Visual Appearance and Physio-Chemical Quality Properties of Pitaya Fruits during Storage
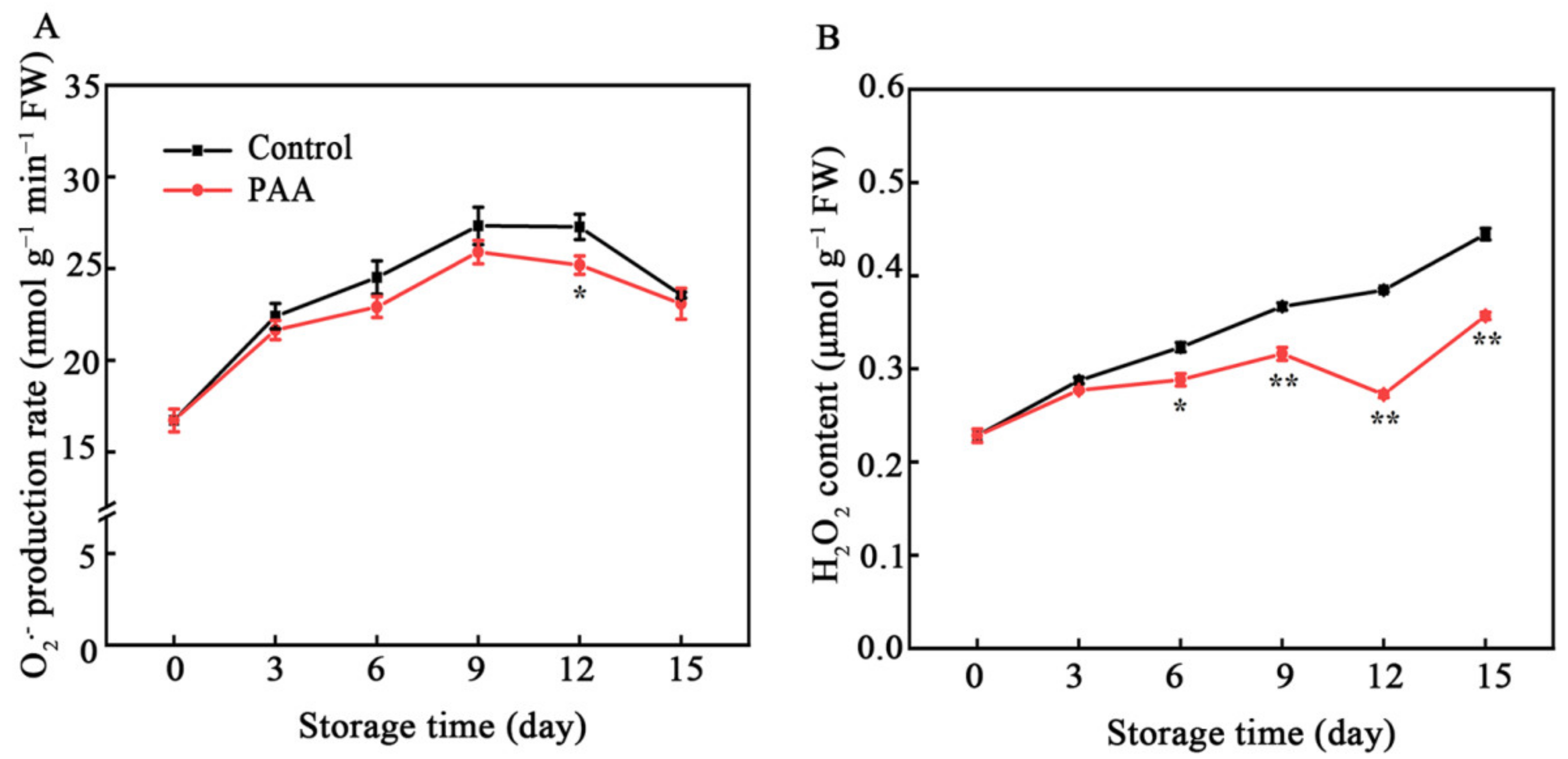
3.2. Effects of PAA on Generation Rate of O2•− and H2O2 Concentration of Pitaya Fruits during Storage
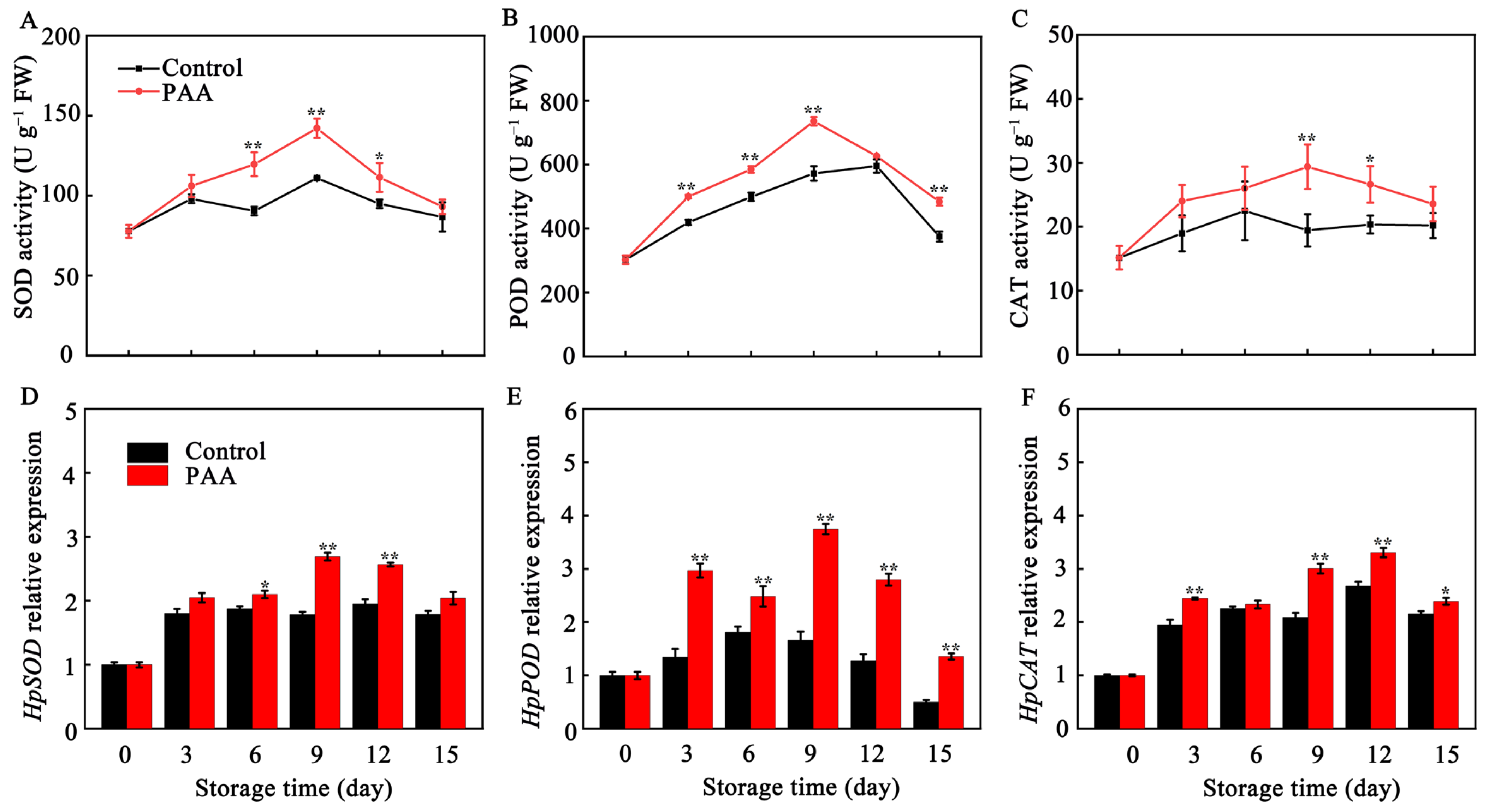
3.3. Effects of PAA on POD, SOD and CAT Enzymatic Activity and Gene Expression in Pitaya Fruits during Storage
3.4. Effects of PAA on Metabolite Content in ASA-GSH Cycle of Pitaya Fruits during Storage
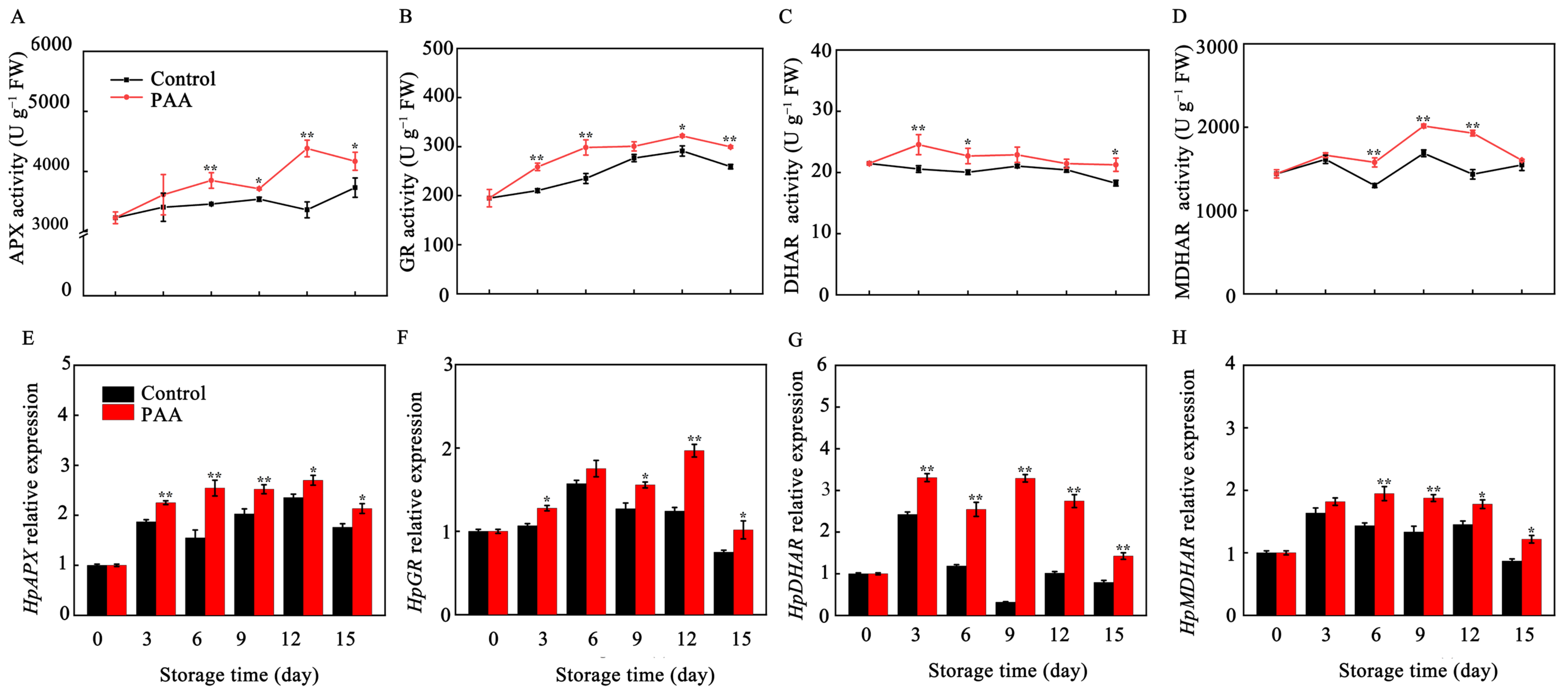
3.5. Effects of PAA on the Activity and Gene Expression of AsA-GSH Pathway Related Enzymes in Pitaya Fruits during Storage
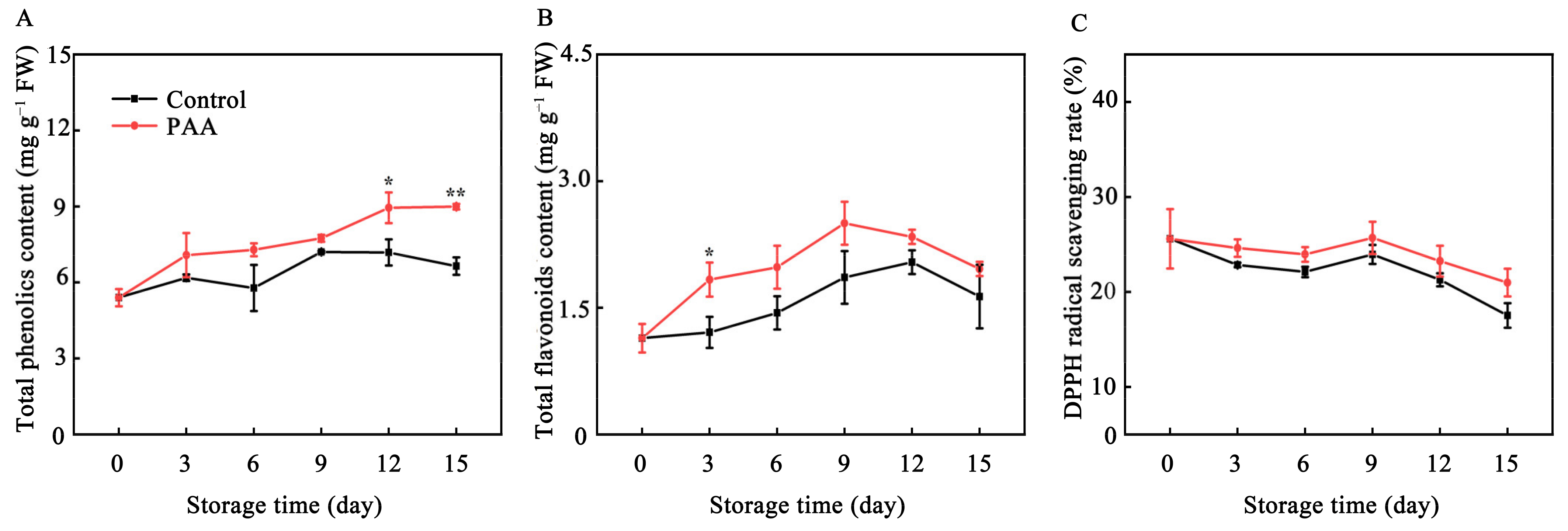
3.6. Effects of PAA on Content of Total Phenolics, Total Flavonoids and DPPH Radical-Scavenging Rate of Pitaya Fruits during Storage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, P.H.; Huber, D.J.; Su, Z.H.; Hu, M.J.; Gao, Z.Y.; Li, M.; Shi, X.Q.; Zhang, Z.K. Effect ostharvest spray of apple polyphenols on the quality of fresh-cut red pitaya fruit during shelf life. Food Chem. 2018, 243, 19–25. [Google Scholar] [CrossRef]
- Grimaldo-Juárez, O.; Terrazas, T.; Garcia-Velásquez, A.; Cruz-Villagas, M.; Ponce-Medina, J.F. Morphometric analysis of 21 pitahaya (Hylocereus undatus) genotypes. J. Prof. Assoc. Cactus Dev. 2007, 9, 99–117. [Google Scholar] [CrossRef] [Green Version]
- Li, X.A.; Li, M.L.; Wang, L.; Wang, J.; Jin, P.; Zheng, Y.H. Methyl jasmonate primes defense responses against wounding stress and enhances phenolic accumulation in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2018, 145, 101–107. [Google Scholar] [CrossRef]
- Bellec, F.L.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Zahid, N.; Ali, A.; Siddiqui, Y.; Maqbool, M. Efficacy of ethanolic extract of propolis in maintaining postharvest quality of dragon fruit during storage. Postharvest Biol. Technol. 2013, 79, 69–72. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Mitcham, E.J. Quality of pitaya fruit (Hylocereus undatus) as influenced by storage temperature and packaging. Sci. Agric. 2013, 70, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.L.; Tran, D.T.; Hertog, M.; Nicola, B.M. Effect of controlled atmosphere storage on the quality attributes and volatile organic compounds profile of dragon fruit (Hylocereus undatus). Postharvest Biol. Technol. 2020, 173, 111406. [Google Scholar] [CrossRef]
- Li, X.A.; Li, M.L.; Wang, J.; Wang, L.; Han, C.; Jin, P.; Zheng, Y.H. Methyl jasmonate enhances wound-induced phenolic accumulation in pitaya fruit by regulating sugar content and energy status. Postharvest Biol. Technol. 2018, 137, 106–112. [Google Scholar] [CrossRef]
- Wall, M.M.; Khan, S.A. Postharvest quality of dragon fruit (Hylocereus spp.) after X-ray irradiation quarantine treatment. HortScience 2008, 43, 2115–2119. [Google Scholar] [CrossRef]
- Liu, R.L.; Gao, H.Y.; Chen, H.J.; Fang, X.J.; Wu, W.J. Synergistic effect of 1-methylcyclopropene and carvacrol on preservation of red pitaya (Hylocereus polyrhizus). Food Chem. 2019, 283, 588–595. [Google Scholar] [CrossRef]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Basir, S.F.; Manzoor, N.; Khan, L.A. Exposure of Candida to p-anisaldehyde inhibits its growth and ergosterol biosynthesis. J. Gen. Appl. Microbiol. 2011, 57, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhao, X.C.; Meng, R.Z.; Liu, Z.J.; Zhang, G.N.; Guo, N. Synergistic antimicrobial effects of nisin and p-Anisaldehyde on Staphylococcus aureus in pasteurized milk. LWT-Food Sci. Technol. 2017, 84, 222–230. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, R.; Sun, X.X.; Yu, X.; Xiao, Y.; Wang, L.; Hu, W.Z.; Zhong, T. The p-Anisaldehyde/β-cyclodextrin inclusion complexes as fumigation agent for control of postharvest decay and quality of strawberry. Food Control 2021, 130, 108346. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chen, M.Y.; Lin, H.T.; Hung, Y.-C.; Lin, Y.X.; Chen, Y.H.; Wang, H.; Shi, J. DNP and ATP induced alteration in disease development of phomopsis longanae Chi-inoculated longan fruit by acting on energy status and reactive oxygen species production-scavenging system. Food Chem. 2017, 228, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.; Kumar, A.; Kaur, A. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Huang, D.D.; Chen, C.B.; Zhu, S.H.; Gao, J.G. Regulation of ascorbate-glutathione cycle in peaches via nitric oxide treatment during cold storage. Sci. Hortic. 2019, 247, 400–406. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Chen, Y.; Wei, J.; Wu, B. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 2019, 152, 9–18. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Wang, X.Y.; Li, X.H. Postharvest nitric oxide treatment delays the senescence of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage by regulating reactive oxygen species metabolism. Sci. Hortic. 2020, 261, 109009. [Google Scholar] [CrossRef]
- Xu, F.X.; Wang, S.H.; Xu, J.; Liu, S.Y.; Li, G.D. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol. Technol. 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Wannabussapawich, B.; Seraypheap, K. Effects of putrescine treatment on the quality attributes and antioxidant activities of ‘Nam Dok Mai No.4’ mango fruit during storage. Sci. Hortic. 2018, 233, 22–28. [Google Scholar] [CrossRef]
- Zhao, H.D.; Liu, B.D.; Zhang, W.L.; Cao, J.K.; Jiang, W.B. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hung, Y.-C.; Chen, M.Y.; Lin, M.S.; Lin, H.T. Enhanced storability of blueberries by acidic electrolyzed oxidizing water application may be mediated by regulating ROS metabolism. Food Chem. 2019, 270, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Behnamian, M.; Dezhsetan, S.; Karimirad, R. Shelf life extension of bell pepper by application of chitosan nanoparticles containing Heracleum persicum fruit essential oil. Postharvest Biol. Technol. 2020, 170, 111313. [Google Scholar] [CrossRef]
- Xie, F.F.; Hua, Q.Z.; Chen, C.B.; Zhang, L.L.; Chen, J.Y.; Zhang, R.; Zhao, J.S.; Hu, G.B.; Zhao, J.T.; Qin, Y.H. Transcriptomics-based identification and characterization of glucosyltransferases involved in betalain biosynthesis in Hylocereus megalanthus. Plant Physiol. Bioch. 2020, 152, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; Zhao, Y.T.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Tao, N.G.; Lakshmanan, P.; Chen, J.Y. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Gao, H.J.; Chen, X.; Chen, Z.S.Z.; Zhang, Z.Z.K.; Li, T.T.; Qu, H.X.; Jiang, Y.M. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2021, 336, 127618. [Google Scholar] [CrossRef]
- Han, X.Y.; Mao, L.C.; Wei, X.P.; Lu, W.J. Stimulatory involvement of abscisic acid in wound suberization of postharvest kiwifruit. Sci. Hortic. 2017, 224, 244–250. [Google Scholar] [CrossRef]
- Chen, C.B.; Wu, J.Y.; Hua, Q.Z.; Tel-Zur, N.; Xie, F.F.; Zhang, Z.K.; Chen, J.Y.; Zhang, R.; Hu, G.B.; Zhao, J.T.; et al. Identification of reliable reference genes for quantitative real-time PCR normalization in pitaya. Plant Methods 2019, 15, 70. [Google Scholar] [CrossRef] [Green Version]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Che, J.X.; Chen, X.M.; Ouyang, Q.L.; Tao, N.G. p-Anisaldehyde exerts its antifungal activity against Penicillium digitatum and Penicillium italicum by disrupting the cell wall integrity and membrane permeability. J. Microbiol. Biotechnol. 2020, 30, 878–884. [Google Scholar] [CrossRef]
- Chaemsanit, S.; Matan, N.; Matan, N. Effect of peppermint oil on the shelf-life of dragon fruit during storage. Food Control 2018, 90, 172–179. [Google Scholar] [CrossRef]
- Jiang, H.T.; Zhang, W.; Li, X.X.; Shu, C.; Jiang, W.B.; Cao, J.K. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Tian, S.P.; Qin, G.Z.; Li, B.Q. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, H.T.; Shi, J.; Zhang, S.; Lin, Y.F.; Lin, T. Effects of a feasible 1-methylcyclopropene postharvest treatment on senescence and quality maintenance of harvested Huanghua pears during storage at ambient temperature. LWT-Food Sci. Technol. 2015, 64, 6–13. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.Q.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.Y.; Cheng, Y.; Hou, J.B.; Zhu, J.H.; Ge, Y.H. Postharvest application of acibenzolar-S-methyl delays the senescence of pear fruit by regulating reactive oxygen species and fatty acid metabolism. J. Agric. Food Chem. 2020, 68, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges-a little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 161, 217037. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, K.; Xiao, X.; Cao, S.F.; Chen, W.; Yang, Z.F.; Shi, L.Y. Effect of 1-MCP on the regulation processes involved in ascorbate metabolism in kiwifruit. Postharvest Biol. Technol. 2021, 179, 111563. [Google Scholar] [CrossRef]
- Li, C.Y.; Wei, M.L.; Ge, Y.H.; Zhao, J.R.; Chen, Y.R.; Hou, J.B.; Chen, Y.; Cheng, J.X.; Li, J.R. The role of glucose-6-phosphate dehydrogenase in reactive oxygen species metabolism in apple exocarp induced by acibenzolar-s-methyl. Food Chem. 2019, 308, 125663. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.Y.; Ge, W.Y.; Zhou, Q.; Zhou, X.; Luo, M.L.; Zhao, Y.B.; Wei, B.D.; Ji, S.J. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Xu, X.F.; Zhang, Z.K.; Jiang, G.X.; Feng, L.Y.; Duan, X.W.; Jiang, Y.M. 6-Benzylaminopurine improves the quality of harvested litchi fruit. Postharvest Biol. Technol. 2018, 143, 137–142. [Google Scholar] [CrossRef]
- Li, X.A.; Li, M.L.; Han, C.; Jin, P.; Zheng, Y.H. Increased temperature elicits higher phenolic accumulation in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017, 129, 90–96. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Cai, Z.; Ba, L.; Qin, Y.; Su, X.; Luo, D.; Shan, W.; Kuang, J.; Lu, W.; Li, L.; et al. Maintenance of Postharvest Quality and Reactive Oxygen Species Homeostasis of Pitaya Fruit by Essential Oil p-Anisaldehyde Treatment. Foods 2021, 10, 2434. https://doi.org/10.3390/foods10102434
Xu Y, Cai Z, Ba L, Qin Y, Su X, Luo D, Shan W, Kuang J, Lu W, Li L, et al. Maintenance of Postharvest Quality and Reactive Oxygen Species Homeostasis of Pitaya Fruit by Essential Oil p-Anisaldehyde Treatment. Foods. 2021; 10(10):2434. https://doi.org/10.3390/foods10102434
Chicago/Turabian StyleXu, Yanmei, Zhijun Cai, Liangjie Ba, Yonghua Qin, Xinguo Su, Donglan Luo, Wei Shan, Jianfei Kuang, Wangjin Lu, Liling Li, and et al. 2021. "Maintenance of Postharvest Quality and Reactive Oxygen Species Homeostasis of Pitaya Fruit by Essential Oil p-Anisaldehyde Treatment" Foods 10, no. 10: 2434. https://doi.org/10.3390/foods10102434
APA StyleXu, Y., Cai, Z., Ba, L., Qin, Y., Su, X., Luo, D., Shan, W., Kuang, J., Lu, W., Li, L., Chen, J., & Zhao, Y. (2021). Maintenance of Postharvest Quality and Reactive Oxygen Species Homeostasis of Pitaya Fruit by Essential Oil p-Anisaldehyde Treatment. Foods, 10(10), 2434. https://doi.org/10.3390/foods10102434






