Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review
Abstract
1. Introduction
2. Milk Protein Genetic Variants and Genotyping Frequency
2.1. Genotype Establishment and Protein Nomenclature
2.2. Genotype Frequency of β-CN
2.3. Genotype Frequency of αS1-CN
2.4. Genotype Frequency of κ-CN
2.5. Genotype Frequency of β-lg
2.6. Composite Genotype Frequencies
3. Impact of Protein Genotype on Milk Protein Structure
4. Milk Production and Milk Composition
4.1. The Effect of αS1-CN Variants on Milk Production and Composition
4.2. The Effect of β-CN Variants on Milk Production and Composition
4.3. The Effect of κ-CN Variants on Milk Production and Composition
4.4. The Effect of β-lg Variants on Milk Production and Composition
4.5. The Effect of Composite Genotypes on Milk Production and Composition
5. Milk Coagulation
5.1. Effect of αS1-CN Variants on Coagulation Properties
5.2. Effect of β-CN Genetic Variant on Coagulation Properties
5.3. Effect of κ-CN Genetic Variant on Coagulation Properties
5.4. Effect of β-lg Genetic Variant on Coagulation Properties
5.5. Effect of Composite Genotypes on Coagulation Properties
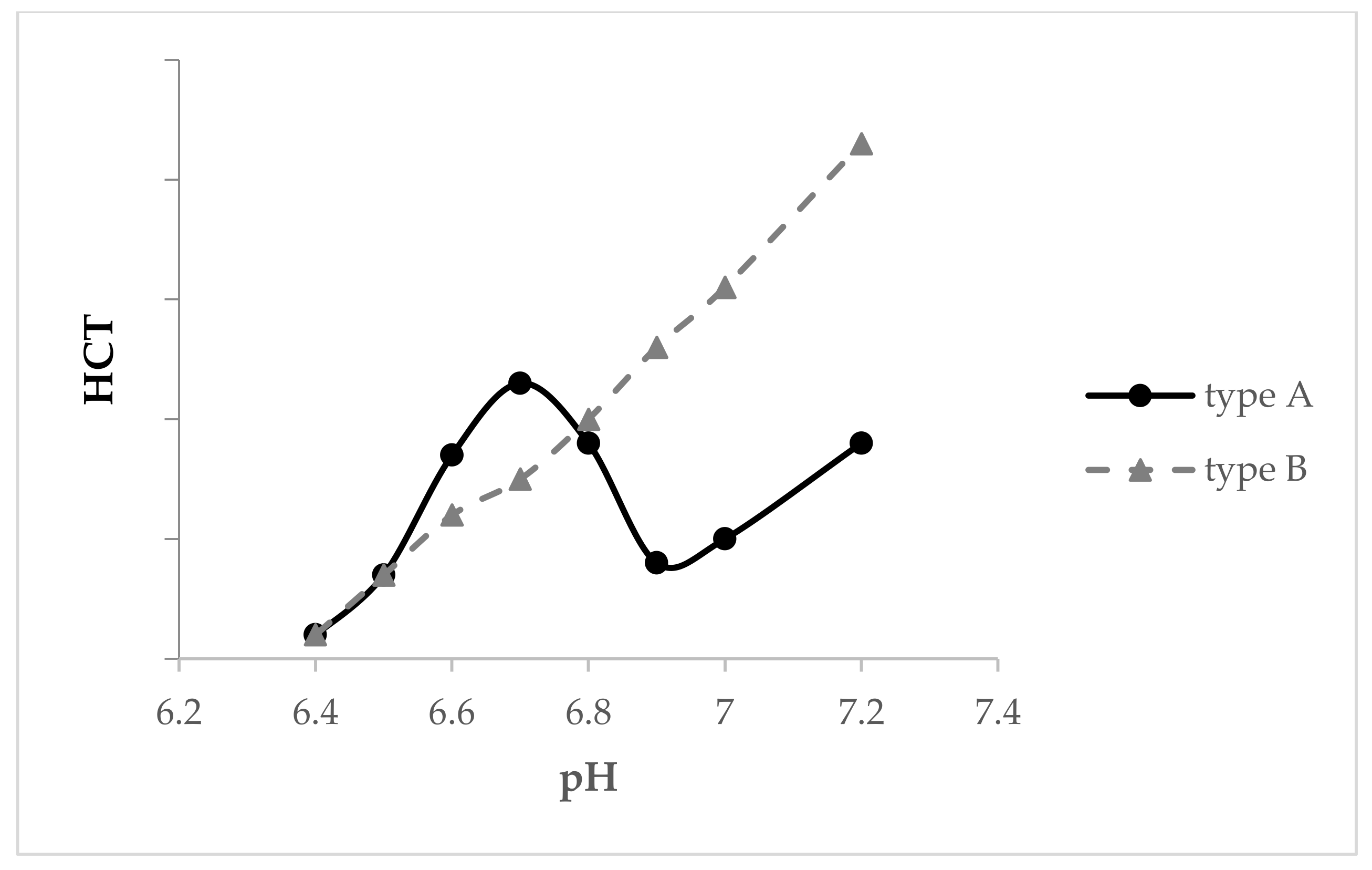
6. Heat Stability
7. Emulsifying and Foaming
7.1. Effects of Protein Genetic Variants on Emulsifying Properties
7.2. Effects of Protein Genetic Variants on Foaming Properties
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolenda, M.; Sitkowska, B. The Polymorphism in Various Milk Protein Genes in Polish Holstein-Friesian Dairy Cattle. Animals 2021, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Pellegrino, L.; Verduci, E.; Ghiselli, A.; Bernabei, R.; Calvani, R.; Cetin, I.; Giampietro, M.; Perticone, F.; Piretta, L. Cow’s Milk Consumption and Health: A Health Professional’s Guide. J. Am. Coll. Nutr. 2019, 38, 197–208. [Google Scholar] [CrossRef]
- Hayes, H.; Petit, E.; Bouniol, C.; Popescu, P. Localization of the αS2-Casein Gene (CASAS2) to the Homoeologous Cattle, Sheep, and Goat Chromosomes 4 by in Situ Hybridization. Cytogenet. Genome Res. 1993, 64, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.P.; Long, S.; Riggs, P.; Womack, J.; Schmutz, S.; Fries, R.; Gallagher, D.S. Standardization of Cattle Karyotype Nomenclature: Report of the Committee for the Standardization of the Cattle Karyotype. Cytogenet. Genome Res. 1996, 74, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, C.; Arcangeli, C.; Ciullo, M.; Torricelli, M.; Cinti, G.; Fisichella, S.; Biagetti, M. Frequencies Evaluation of β-Casein Gene Polymorphisms in Dairy Cows Reared in Central Italy. Animals 2020, 10, 252. [Google Scholar] [CrossRef]
- Caroli, A.M.; Chessa, S.; Erhardt, G.J. Invited Review: Milk Protein Polymorphisms in Cattle: Effect on Animal Breeding and Human Nutrition. J. Dairy Sci. 2009, 92, 5335–5352. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the Proteins of Cows’ Milk—Sixth Revision. J. Dairy Sci. 2004, 87, 1641–1674. [Google Scholar] [CrossRef]
- Hayes, H.C.; Petit, E.J. Mapping of the β-Lactoglobulin Gene and of an Immunoglobulin M Heavy Chain-like Sequence to Homoeologous Cattle, Sheep, and Goat Chromosomes. Mamm. Genome 1993, 4, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Grosclaude, F. Le Polymorphisme Génétique Des Principales Lactoprotéines Bovines. Relations Avec La Quantité, La Composition et Les Aptitudes Fromagères Du Lait. Prod. Anim. 1988, 1, 5–17. [Google Scholar] [CrossRef]
- Hallén, E.; Wedholm, A.; Andrén, A.; Lundén, A. Effect of β-casein, κ-casein and β-lactoglobulin Genotypes on Concentration of Milk Protein Variants. J. Anim. Breed. Genet. 2008, 125, 119–129. [Google Scholar] [CrossRef]
- Aschaffenburg, R.; Drewry, J. Genetics of the β-Lactoglobulins of Cow’s Milk. Nature 1957, 180, 376–378. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Chemistry of the caseins. In Advanced Dairy Chemistry—1 Proteins; Springer: Berlin/Heidelberg, Germany, 2003; pp. 139–201. [Google Scholar]
- Kamiński, S.; Cieślińska, A.; Kostyra, E. Polymorphism of Bovine Beta-Casein and Its Potential Effect on Human Health. J. Appl. Genet. 2007, 48, 189–198. [Google Scholar] [CrossRef]
- Lühken, G.; Caroli, A.; Ibeagha-Awemu, E.M.; Erhardt, G. Characterization and Genetic Analysis of Bovine αS1-casein I Variant. Anim. Genet. 2009, 40, 479–485. [Google Scholar] [CrossRef]
- Sulimova, G.E.; Sokolova, S.S.; Semikozova, O.P.; Nguet, L.M.; Berberov, E.M. Analysis of DNA Polymorphism of Cluster Genes in Cattle: Casein Genes and Major Histocompatibility Complex (MHC) Genes. TSitologiia I Genet. 1992, 26, 18–26. [Google Scholar]
- Prinzenberg, E.; Hiendleder, S.; Ikonen, T.; Erhardt, G.; Prinzenberg, E.; Hiendleder, S.; Erhardt, G.; Ikonen, T. Molecular Genetic Characterization of New Bovine Kappa-casein Alleles CSN3F and CSN3G and Genotyping by PCR-RFLP. Anim. Genet. 1996, 27, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, G. Detection of a New κ-casein Variant in Milk of Pinzgauer Cattle. Anim. Genet. 1996, 27, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of Milk Containing Only A2 Beta Casein versus Milk Containing Both A1 and A2 Beta Casein Proteins on Gastrointestinal Physiology, Symptoms of Discomfort, and Cognitive Behavior of People with Self-Reported Intolerance to Traditional Cows’ Milk. Nutr. J. 2015, 15, 35. [Google Scholar] [CrossRef]
- Brunner, J.R.; Ernstrom, C.A.; Hollis, R.A.; Larson, B.L.; Whitney, R.M.; Zittle, C.A. Nomenclature of the Proteins of Bovine Milk—First Revision: Report of the Committee on Milk Protein Nomenclature, Classification, and Methodology of the Manufacturing Section of A.D.S.A. for 1958–59. J. Dairy Sci. 1960, 43, 901–911. [Google Scholar] [CrossRef]
- Thompson, M.P.; Tarassuk, N.P.; Jenness, R.; Lillevik, H.A.; Ashworth, U.S.; Rose, D. Nomenclature of the Proteins of Cow’s Milk—Second Revision: Report of the Committee on Milk Protein Nomenclature, Classification, and Methodology of the Manufacturing Section of ADSA for 1963-64. J. Dairy Sci. 1965, 48, 159–169. [Google Scholar] [CrossRef]
- Rose, D.; Brunner, J.R.; Kalan, E.B.; Larson, B.L.; Melnychyn, P.; Swaisgood, H.E.; Waugh, D.F. Nomenclature of the Proteins of Cow’s Milk: Third Revision. J. Dairy Sci. 1970, 53, 1–17. [Google Scholar] [CrossRef]
- Whitney, R.M.; Brunner, J.R.; Ebner, K.E.; Farrell, H.M., Jr.; Josephson, R.V.; Morr, C.V.; Swaisgood, H.E. Nomenclature of the Proteins of Cow’s Milk: Fourth Revision. J. Dairy Sci. 1976, 59, 795–815. [Google Scholar] [CrossRef]
- Eigel, W.N.; Butler, J.E.; Ernstrom, C.A.; Farrell, H.M., Jr.; Harwalkar, V.R.; Jenness, R.; Whitney, R.M. Nomenclature of Proteins of Cow’s Milk: Fifth Revision. J. Dairy Sci. 1984, 67, 1599–1631. [Google Scholar] [CrossRef]
- Thompson, M.P.; Zittle, C.A.; Pepper, L.; Kiddy, C.A. Casein Variants in Milk from Individual Cows. J. Dairy Sci. 1962, 45, 650. [Google Scholar]
- Thompson, M.P.; Kiddy, C.A.; Pepper, L.; Zittle, C.A. Variations in the α S-Casein Fraction of Individual Cow’s Milk. Nature 1962, 195, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Timasheff, S.N.; Townend, R. The Association Behaviour of β-lactoglobulins A and B. J. Am. Chem. Soc. 1958, 80, 4433–4434. [Google Scholar] [CrossRef]
- Timasheff, S.N. The Stoichiometry of β-Lactoglobulin Association. In Proceedings of the 135th Meeting of the American Chemical Society, Boston, MA, USA, 5–10 April 1959; American Chemical Society: Washington, DC, USA, 1959. Abstr. No. 34. [Google Scholar]
- Timasheff, S.N.; Townend, R. Molecular Interactions in β-Lactoglobulin. VI. Dissociation of the Genetic Species of β-Lactoglobulin at Acid pH’s. J. Am. Chem. Soc. 1961, 83, 470–473. [Google Scholar] [CrossRef]
- Conti, A.; Napolitano, L.; Maria Cantisani, A.; Davoli, R.; Dall’Olio, S. Bovine β-Lactoglobulin H: Isolation by Preparative Isoelectric Focusing in Immobilized PH Gradients and Preliminary Characterization. J. Biochem. Biophys. Methods 1988, 16, 205–214. [Google Scholar] [CrossRef]
- Davoli, R.; Dall’Olio, S.; Bigi, D. A New Beta-Lactoglobulin Variant in Bovine Milk. Sci. E Tec. Latt. -Casearia 1988, 39, 439–442. [Google Scholar]
- Chessa, S.; Chiatti, F.; Ceriotti, G.; Caroli, A.; Consolandi, C.; Pagnacco, G.; Castiglioni, B. Development of a Single Nucleotide Polymorphism Genotyping Microarray Platform for the Identification of Bovine Milk Protein Genetic Polymorphisms. J. Dairy Sci. 2007, 90, 451–464. [Google Scholar] [CrossRef]
- Damiani, G.; Ferretti, L.; Rognoni, G.; Sgaramella, V. Restriction Fragment Length Polymorphism Analysis of the Κ-casein Locus in Cattle. Anim. Genet. 1990, 21, 107–114. [Google Scholar] [CrossRef]
- Schlieben, S.; Erhardt, G.; Senft, B. Genotyping of Bovine κ-Casein (κ-CN A, κ-CN B, κ-CN C, κ-CN E) Following DNA Sequence Amplification and Direct Sequencing of κ-CN E PCR Product. Anim. Genet. 1991, 22, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Pilla, F.; Leone, P.; Caccio, S. Direct Sequencing and Bidirectional Allelle Specific Polymerase Chain Reaction of the Bovine Β-casein B Variant. Anim. Genet. 1992, 23, 561–566. [Google Scholar] [CrossRef]
- Barroso, A.; Dunner, S.; Canon, J. A Multiplex PCR-SSCP Test to Genotype Bovine Beta-Casein Alleles A1, A2, A3, B, and C. Anim. Genet. 1999, 30, 322–323. [Google Scholar] [CrossRef]
- Bonizzi, I.; Buffoni, J.N.; Feligini, M. Quantification of Bovine Casein Fractions by Direct Chromatographic Analysis of Milk. Approaching the Application to a Real Production Context. J. Chromatogr. A 2009, 1216, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Mollé, D.; Jardin, J.; Piot, M.; Pasco, M.; Léonil, J.; Gagnaire, V. Comparison of Electrospray and Matrix-Assisted Laser Desorption Ionization on the Same Hybrid Quadrupole Time-of-Flight Tandem Mass Spectrometer: Application to Bidimensional Liquid Chromatography of Proteins from Bovine Milk Fraction. J. Chromatogr. A 2009, 1216, 2424–2432. [Google Scholar] [CrossRef]
- Maurmayr, A.; Ribeca, C.; Cecchinato, A.; Penasa, M.; de Marchi, M.; Bittante, G. Effects of Stearoyl-CoA Desaturase 1 and Sterol Regulatory Element Binding Protein Gene Polymorphisms on Milk Production, Composition and Coagulation Properties of Individual Milk of Brown Swiss Cows. Agric. Conspec. Sci. 2011, 76, 235–237. [Google Scholar]
- Visser, S.; Slangen, C.J.; Lagerwerf, F.M.; van Dongen, W.D.; Haverkamp, J. Identification of a New Genetic Variant of Bovine β-Casein Using Reversed-Phase High-Performance Liquid Chromatography and Mass Spectrometric Analysis. J. Chromatogr. A 1995, 711, 141–150. [Google Scholar] [CrossRef]
- Dong, C.; Ng-Kwai-Hang, K.F. Characterization of a Non-Electrophoretic Genetic Variant of β-Casein by Peptide Mapping and Mass Spectrometric Analysis. Int. Dairy J. 1998, 8, 967–972. [Google Scholar] [CrossRef]
- Hacia, J.G. Resequencing and Mutational Analysis Using Oligonucleotide Microarrays. Nat. Genet. 1999, 21, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kurg, A.; Tõnisson, N.; Georgiou, I.; Shumaker, J.; Tollett, J.; Metspalu, A. Arrayed Primer Extension: Solid-Phase Four-Color DNA Resequencing and Mutation Detection Technology. Genet. Test. 2000, 4, 1–7. [Google Scholar] [CrossRef]
- Pastinen, T.; Raitio, M.; Lindroos, K.; Tainola, P.; Peltonen, L.; Syvänen, A.-C. A System for Specific, High-Throughput Genotyping by Allele-Specific Primer Extension on Microarrays. Genome Res. 2000, 10, 1031–1042. [Google Scholar] [CrossRef]
- Bell, K. One-Dimensional Starch-Gel Electrophoresis of Bovine Skim-Milk. Nature 1962, 195, 705–706. [Google Scholar] [CrossRef]
- Grosclancle, F.; Pujolle, J.; Garnier, J.; Ribadeau-Dumas, B. Evidence for Two Additional Variants in Proteins of Cow’s Milk: αS1-Casein D and β-Lactoglobulin, D. Ann. Biol. Anim. Biochim. Biophys. 1966, 6, 215. [Google Scholar]
- Larsen, B.; Thymann, M. Studies on Milk Protein Polymorphism in Danish Cattle and the Interaction of the Controlling Genes. Acta Vet. Scand. 1966, 7, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H. β-Lactoglobulin Polymorphism in German Cattle Breeds. Zuchthygiene 1966, 1, 49. [Google Scholar] [CrossRef]
- Michalak, W. Anomalous Electrophoretic Pattern of Milk Proteins. J. Dairy Sci. 1967, 50, 1319–1320. [Google Scholar] [CrossRef]
- Bell, K.; McKenzie, H.A.; Murphy, W.H.; Shaw, D.C. β-Lactoglobulin Droughtmaster: A Unique Protein Variant. Biochim. Biophys. Acta 1970, 214, 427–436. [Google Scholar] [CrossRef]
- Bell, K.; McKenzie, H.A.; Shaw, D.C. Bovine beta-Lactoglobulin E, F and G of Bali (Banteng) Cattle, Bos (Bihos) Javanicus. Aust. J. Biol. Sci. 1981, 34, 133–148. [Google Scholar] [CrossRef]
- Brignon, G.; Dumas, B.R. Localisation Dans La Chaine Peptidique de La β Lactoglobuline Bovine de La Substitution Glu/Gln Differenciant Les Variants Genetiques B et D. FEBS Lett. 1973, 33, 73–76. [Google Scholar] [CrossRef]
- Grosclaude, F.; Marie-Françoise, M.; Mercier, J.C.; Bonnemaire, J.; Teissier, J.H. Polymorphisme des lactoprotéines de bovinés népalais. I.—Mise en evidence, chez le yak, et caractérisation biochimique de deux nouveaux variants: β-lactoglobuline Dyak et caséine αS1E. Ann. Genet. Sel. Anim. 1976, 8, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.F. High Resolution of Milk Proteins Obtained by Gel Electrophoresis. J. Dairy Sci. 1963, 46, 1136–1139. [Google Scholar] [CrossRef]
- Godovac-Zimmermann, J.; Krause, I.; Buchberger, J.; Weiss, G.; Klostermeyer, H. Genetic variants of bovine beta-lactoglobulin. A novel wild-type beta-lactoglobulin W and its primary sequence. Biol. Chem. Hoppe-Seyler 1990, 371, 255–260. [Google Scholar] [CrossRef]
- Godovac-Zimmermann, J.; Krause, I.; Baranyi, M.; Fischer-Frühholz, S.; Juszczak, J.; Erhardt, G.; Buchberger, J.; Klostermeyer, H. Isolation and Rapid Sequence Characterization of Two Novel Bovine β-Lactoglobulins I and J. J. Protein Chem. 1996, 15, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, U.; Ebner, K.E. Resolution of a Soluble Lactose Synthetase into Two Protein Components and Solubilization of Microsomal Lactose Synthetase. J. Biol. Chem. 1966, 241, 762–764. [Google Scholar] [CrossRef]
- Brodbeck, U.; Ebner, K.E. The Subcellular Distribution of the A and B Proteins of Lactose Synthetase in Bovine and Rat Mammary Tissue. J. Biol. Chem. 1966, 241, 5526–5532. [Google Scholar] [CrossRef]
- Aschaffenburg, R. Milk Protein Polymorphisms; Mourant, A.E., Zeuner, F.E., Eds.; Royal Anthropological Institute: London, UK, 1963; p. 18. [Google Scholar]
- Bhattacharya, S.D.; Roychoudhury, A.K.; Sinha, N.K.; Sen, A. Inherited α-Lactalbumin and β-Lactoglobulin Polymorphism in Indian Zebu Cattle. Comparison of Zebu and Buffalo α-Lactalbumins. Nature 1963, 197, 797–799. [Google Scholar] [CrossRef]
- Bell, K.; Hopper, K.E.; McKenzie, H.A. Bovine Alpha-Lactalbumin C and Alpha S1-, Beta-and Kappa-Caseins of Bali (Banteng) Cattle, Bos (Bibos) Javanicus. Aust. J. Biol. Sci. 1981, 34, 149–159. [Google Scholar] [CrossRef]
- Grosclaude, F.; Mahé, M.; Mercier, J.; Ribadeau-Dumas, B. Caractérisation Des Variants Génétiques Des Caséines Asl et β Bovines. Eur. J. Biochem. 1972, 26, 328–337. [Google Scholar] [CrossRef]
- Grosclaude, F.; Mercier, J.; Ribadeau-Dumas, B. Structure Primaire de La Caséine AS1 Bovine: Localisation Des Peptides Trypsiques Dans Les Fragments Obtenus Par Hydrolyse Trypsique de La Caséine Maléylée. Eur. J. Biochem. 1970, 14, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.-C.; Grosclaude, F.; Ribadeau-Dumas, B. Structure Primaire de La Caseine AlphaS1 Bovine. Enchainement Des Peptides Obtenus Par Action Du Bromure de Cyanogene et Des Peptides Resultant de l’hydrolyse Trypsique de La Caseine AlphaS1 Maleylee. Eur. J. Biochem. 1970, 16, 439–446. [Google Scholar] [CrossRef]
- Thompson, M.P. αs- and β-Caseins. In Milk Proteins: Chemistry and Molecular Biology; McKenzie, H.A., Ed.; Academic Press: Cambridge, MA, USA, 1971; Volume II, pp. 117–174. [Google Scholar]
- Erhardt, G. A New αS1-casein Allele in Bovine Milk and Its Occurrence in Different Breeds. Anim. Genet. 1993, 24, 65–66. [Google Scholar] [CrossRef]
- Rando, A.; Ramunno, L.; Di Gregorio, P.; Davoli, R.; Masina, P. A rare insertion in the bovine as l-casein gene. Anim. Genet. 1992, 23, 55. [Google Scholar]
- Rando, A.; Ramunno, L.; Di Gregorio, P.; Fiorella, A.; Davoli, R.; Masina, P. Localizzazione di siti polimorfi nella regione di DNA che contiene il gene della caseina αS1 di bovino. Proc. Assoc. Sci. Prod. Anim. Bologna Italy 1993, 10, 617–620. [Google Scholar]
- Ramunno, L.; Rando, A.; Pappalardo, M.; Fiorella, A.; Di Gregorio, P.; Capuano, M.; Masina, P. Molecular analyses on quantitative alleles at goat β-CN and cow αS1-CN loci. Proc. Soc. Ital. Per Il Prog. Della Zootec. Milano Italy 1994, 29, 233–240. [Google Scholar]
- Mahé, M.F.; Miranda, G.; Queval, R.; Bado, A.; Zafindrajaona, P.S.; Grosclaude, F. Genetic Polymorphism of Milk Proteins in African Bos Taurus and Bos Indicus Populations. Characterization of Variants αS1-Cn H and κ-Cn, J. Genet. Sel. Evol. GSE 1999, 31, 239. [Google Scholar] [CrossRef]
- Aschaffenburg, R. Inherited Casein Variants in Cow’s Milk. Nature 1961, 192, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Aschaffenburg, R. Inherited Casein Variants in Cow’s Milk: II. Breed Differences in the Occurrence of β-Casein Variants. J. Dairy Res. 1963, 30, 251–258. [Google Scholar] [CrossRef]
- Thompson, M.P.; Kiddy, C.A.; Johnston, J.O.; Weinberg, R.M. Genetic Polymorphism in Caseins of Cows’ Milk. II. Confirmation of the Genetic Control of β-Casein Variation. J. Dairy Sci. 1964, 47, 378–381. [Google Scholar] [CrossRef]
- Peterson, R.F.; Kopfler, F.C. Detection of New Types of β-Casein by Polyacrylamide Gel Electrophoresis at Acid PH: A Proposed Nomenclature. Biochem. Biophys. Res. Commun. 1966, 22, 388–392. [Google Scholar] [CrossRef]
- Kiddy, C.A.; Peterson, R.F.; Kopfler, F.C. Genetic control of variants of beta-casein A. J. Dairy Sci. 1966, 49, 742. [Google Scholar]
- Thompson, M.P.; Gordon, W.G.; Pepper, L.; Greenberg, R. Amino Acid Composition of β-Caseins from the Milks of Bos Indicus and Bos Taurus Cows: A Comparative Study. Comp. Biochem. Physiol. 1969, 30, 91–98. [Google Scholar] [CrossRef]
- Voglino, G.F. A New Β-casein Variant in Piedmont Cattle. Anim. Blood Groups Biochem. Genet. 1972, 3, 61–62. [Google Scholar] [CrossRef]
- Grosclaude, F.; Mahe, M.-F.; Voglino, G.-F. Le Variant ΒE et Le Code de Phosphorylation Des Caséines Bovines. FEBS Lett. 1974, 45, 3–5. [Google Scholar] [CrossRef]
- Kiddy, C.A. Gel Electrophoresis in Vertical Polyacrylamide Beds: Procedure III. Methods Gel Electrophor. Milk Proteins 1975, 18–19. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201303051988 (accessed on 22 May 2021).
- Ribadeau-Dumas, B.; Brignon, G.; Grosclaude, F.; Mercier, J.C. Structure Primaire de La Caséine β Bovine. Séquence Complète. Eur. J. Biochem. 1972, 25, 505–514. [Google Scholar] [CrossRef]
- Han, S.K.; Shin, Y.C.; Byun, H.D. Biochemical, Molecular and Physiological Characterization of a New Β-casein Variant Detected in Korean Cattle. Anim. Genet. 2000, 31, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Senocq, D.; Mollé, D.; Pochet, S.; Léonil, J.; Dupont, D.; Levieux, D. A New Bovine β-Casein Genetic Variant Characterized by a Met93→ Leu 93 Substitution in the Sequence A2. Le Lait 2002, 82, 171–180. [Google Scholar] [CrossRef]
- Chung, E.R.; Han, S.K.; Rhim, T.J. Milk Protein Polymorphisms as Genetic Marker in Korean Native Cattle. Asian-Australas. J. Anim. Sci. 1995, 8, 187–194. [Google Scholar] [CrossRef]
- Jann, O.; Ceriotti, G.; Caroli, A.; Erhardt, G. A New Variant in Exon VII of Bovine β-casein Gene (CSN2) and Its Distribution among European Cattle Breeds. J. Anim. Breed. Genet. 2002, 119, 65–68. [Google Scholar] [CrossRef]
- Jensen, H.B.; Poulsen, N.A.; Andersen, K.K.; Hammershøj, M.; Poulsen, H.D.; Larsen, L.B. Distinct Composition of Bovine Milk from Jersey and Holstein-Friesian Cows with Good, Poor, or Noncoagulation Properties as Reflected in Protein Genetic Variants and Isoforms. J. Dairy Sci. 2012, 95, 6905–6917. [Google Scholar] [CrossRef]
- Woychik, J.H. Polymorphism in κ-Casein of Cow’s Milk. Biochem. Biophys. Res. Commun. 1964, 16, 267–271. [Google Scholar] [CrossRef]
- Mackinlay, A.G.; Hill, R.J.; Wake, R.G. The Action of Rennin on χ-Casein the Heterogeneity and Origin of the Insoluble Products. Biochim. Biophys. Acta Gen. Subj. 1966, 115, 103–112. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Methods of Gel Electrophoresis of Milk Proteins; American Dairy Science Association: Champaign, IL, USA, 1975; p. 33. [Google Scholar]
- Jolles, J.; Schoentgen, F.; Alais, C.; Jolles, P. Studies on Primary Structure of Cow Kappa-Casein-Primary Sequence of Cow Para-Kappa-Casein. Chimia 1972, 26, 645–646. [Google Scholar]
- Mercier, J.; Brignon, G.; Ribadeau-dumas, B. Structure Primaire de La Caséine ΚB Bovine: Séquence Complète. Eur. J. Biochem. 1973, 35, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Gorodetskiĭ, S.I.; Kaledin, A.S. Nucleotide Sequence of the CDNA of Kappa Casein in Cows. Genetika 1987, 23, 596–604. [Google Scholar] [PubMed]
- Miranda, G.; Anglade, P.; Mahé, M.F.; Erhardt, G. Biochemical Characterization of the Bovine Genetic κ-casein C and E Variants. Anim. Genet. 1993, 24, 27–31. [Google Scholar] [CrossRef]
- Sulimova, G.E.; IuN, B.; Udina, I.G. Polymorphism of the Kappa-Casein Gene in Populations of the Subfamily Bovinae. Genetika 1996, 32, 1576–1582. [Google Scholar]
- Prinzenberg, E.; Krause, I.; Erhardt, G. SSCP Analysis at the Bovine CSN3 Locus Discriminates Six Alleles Corresponding to Known Protein Variants (A, B, C, E, F, G) and Three New DNA Polymorphisms (H, I, A1). Anim. Biotechnol. 1999, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Zwierzchowski, L. Cattle Genomics-Functional Polymorphism in Milk Protein Genes and Other Genes Related to Milk and Meat Production. In Proceedings of the Workshop on Genomics and Bioinformatics in Animal Biotechnology, Jastrzebiec, Poland, 31 January–4 February 2005. [Google Scholar]
- Caroli, A.M.; Savino, S.; Bulgari, O.; Monti, E. Detecting β-Casein Variation in Bovine Milk. Molecules 2016, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Brooke-Taylor, S.; Dwyer, K.; Woodford, K.; Kost, N. Systematic Review of the Gastrointestinal Effects of A1 Compared with A2 β-Casein. Adv. Nutr. 2017, 8, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.H.; Ong, L.; Lopez, C.; Kentish, S.E.; Gras, S.L. Microstructure and Physicochemical Properties Reveal Differences between High Moisture Buffalo and Bovine Mozzarella Cheeses. Food Res. Int. 2017, 102, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.H.; Schwendel, H.; Harland, D.; Day, L. Differences in the Yoghurt Gel Microstructure and Physicochemical Properties of Bovine Milk Containing A1A1 and A2A2 β-Casein Phenotypes. Food Res. Int. 2018, 112, 217–224. [Google Scholar] [CrossRef]
- Lien, S.; Kantanen, J.; Olsaker, I.; Holm, L.; Eythorsdottir, E.; Sandberg, K.; Dalsgard, B.; Adalsteinsson, S. Comparison of Milk Protein Allele Frequencies in Nordic Cattle Breeds. Anim. Genet. 1999, 30, 85–91. [Google Scholar] [CrossRef]
- Ketto, I.A.; Knutsen, T.M.; Øyaas, J.; Heringstad, B.; Ådnøy, T.; Devold, T.G.; Skeie, S.B. Effects of Milk Protein Polymorphism and Composition, Casein Micelle Size and Salt Distribution on the Milk Coagulation Properties in Norwegian Red Cattle. Int. Dairy J. 2017, 70, 55–64. [Google Scholar] [CrossRef]
- Heck, J.M.L.; Schennink, A.; van Valenberg, H.J.F.; Bovenhuis, H.; Visker, M.; van Arendonk, J.A.M.; van Hooijdonk, A.C.M. Effects of Milk Protein Variants on the Protein Composition of Bovine Milk. J. Dairy Sci. 2009, 92, 1192–1202. [Google Scholar] [CrossRef]
- Jõudu, I.; Henno, M.; Värv, S. Milk Protein Genotypes and Milk Coagulation Properties of Estonian Native Cattle. Agric. Food Sci. 2007, 16, 222–231. [Google Scholar] [CrossRef]
- Poulsen, N.A.; Bertelsen, H.P.; Jensen, H.B.; Gustavsson, F.; Glantz, M.; Månsson, H.L.; Andrén, A.; Paulsson, M.; Bendixen, C.; Buitenhuis, A.J. The Occurrence of Noncoagulating Milk and the Association of Bovine Milk Coagulation Properties with Genetic Variants of the Caseins in 3 Scandinavian Dairy Breeds. J. Dairy Sci. 2013, 96, 4830–4842. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, F.; Buitenhuis, A.J.; Johansson, M.; Bertelsen, H.P.; Glantz, M.; Poulsen, N.A.; Månsson, H.L.; Stålhammar, H.; Larsen, L.B.; Bendixen, C. Effects of Breed and Casein Genetic Variants on Protein Profile in Milk from Swedish Red, Danish Holstein, and Danish Jersey Cows. J. Dairy Sci. 2014, 97, 3866–3877. [Google Scholar] [CrossRef]
- Comin, A.; Cassandro, M.; Chessa, S.; Ojala, M.; Dal Zotto, R.; de Marchi, M.; Carnier, P.; Gallo, L.; Pagnacco, G.; Bittante, G. Effects of Composite β- and κ-Casein Genotypes on Milk Coagulation, Quality, and Yield Traits in Italian Holstein Cows. J. Dairy Sci. 2008, 91, 4022–4027. [Google Scholar] [CrossRef]
- Bobe, G.; Beitz, D.C.; Freeman, A.E.; Lindberg, G.L. Effect of Milk Protein Genotypes on Milk Protein Composition and Its Genetic Parameter Estimates. J. Dairy Sci. 1999, 82, 2797–2804. [Google Scholar] [CrossRef]
- Boettcher, P.J.; Caroli, A.; Stella, A.; Chessa, S.; Budelli, E.; Canavesi, F.; Ghiroldi, S.; Pagnacco, G. Effects of Casein Haplotypes on Milk Production Traits in Italian Holstein and Brown Swiss Cattle. J. Dairy Sci. 2004, 87, 4311–4317. [Google Scholar] [CrossRef]
- Farrell, H.M., Jr.; Thompson, M.P.; Larsen, B. Verification of the Occurrence of the αS1-Casein a Allele in Red Danish Cattle. J. Dairy Sci. 1971, 54, 423–425. [Google Scholar] [CrossRef]
- Kučerova, J.; Matejicek, A.; Jandurová, O.M.; Sorensen, P.; Nemcova, E.; Stipkova, M.; Kott, T.; Bouska, J.; Frelich, J. Milk Protein Genes CSN1S1, CSN2, CSN3, LGB and Their Relation to Genetic Values of Milk Production Parameters in Czech Fleckvieh. Czech J. Anim. Sci. 2006, 51, 241. [Google Scholar] [CrossRef]
- Havlíček, Z. Polymorfismus Mléčných Proteinů ve Vztahu k Jejich Produkci a Kvalitě. Dizertační Práce, Mendel University in Brno, Brno, Czech Republic, 1996. [Google Scholar]
- Jakob, E. Frequencies of Casein Phenotypes and Haplotypes in Different Breeds in Switzerland and the Effect of K-Casein C and E on Renneting Properties of Milk. In Proceedings of the Specialists Meeting of the International Circle of Dairy Research Leaders on Genetic Polymorphism of Milk Proteins, Zürich, Switzerland, 11–12 April 1991. [Google Scholar]
- Van Eenennaam, A.; Medrano, J.F. Milk Protein Polymorphisms in California Dairy Cattle. J. Dairy Sci. 1991, 74, 1730–1742. [Google Scholar] [CrossRef]
- Velmala, R.; Vilkki, J.; Elo, K.; Mäki-Tanila, A. Casein Haplotypes and Their Association with Milk Production Traits in the Finnish Ayrshire Cattle. Anim. Genet. 1995, 26, 419–425. [Google Scholar] [CrossRef]
- Ikonen, T.; Ojala, M.; Ruottinen, O. Associations between Milk Protein Polymorphism and First Lactation Milk Production Traits in Finnish Ayrshire Cows. J. Dairy Sci. 1999, 82, 1026–1033. [Google Scholar] [CrossRef]
- Jensen, H.B.; Holland, J.W.; Poulsen, N.A.; Larsen, L.B. Milk Protein Genetic Variants and Isoforms Identified in Bovine Milk Representing Extremes in Coagulation Properties. J. Dairy Sci. 2012, 95, 2891–2903. [Google Scholar] [CrossRef]
- Visker, M.; Dibbits, B.W.; Kinders, S.M.; van Valenberg, H.J.F.; van Arendonk, J.A.M.; Bovenhuis, H. Association of Bovine β-casein Protein Variant I with Milk Production and Milk Protein Composition. Anim. Genet. 2011, 42, 212–218. [Google Scholar] [CrossRef]
- Bech, A.-M.; Kristiansen, K.R. Milk Protein Polymorphism in Danish Dairy Cattle and the Influence of Genetic Variants on Milk Yield. J. Dairy Res. 1990, 57, 53–62. [Google Scholar] [CrossRef]
- Lundén, A.; Nilsson, M.; Janson, L. Marked Effect of β-Lactoglobulin Polymorphism on the Ratio of Casein to Total Protein in Milk. J. Dairy Sci. 1997, 80, 2996–3005. [Google Scholar] [CrossRef]
- Horne, D.S. Casein Structure, Self-Assembly and Gelation. Curr. Opin. Colloid Interface Sci. 2002, 7, 456–461. [Google Scholar] [CrossRef]
- Smith, Y. Protein Structure and Function, News-Medical, 23 August 2018. Available online: https://www.news-medical.net/life-sciences/Protein-Structure-and-Function.aspx (accessed on 30 September 2021).
- Sawyer, L.; Barlow, P.N.; Boland, M.J.; Creamer, L.K.; Denton, H.; Edwards, P.J.B.; Holt, C.; Jameson, G.B.; Kontopidis, G.; Norris, G.E. Milk Protein Structure—What Can It Tell the Dairy Industry? Int. Dairy J. 2002, 12, 299–310. [Google Scholar] [CrossRef]
- Caroli, A.; Rizzi, R.; Lühken, G.; Erhardt, G. Milk Protein Genetic Variation and Casein Haplotype Structure in the Original Pinzgauer Cattle. J. Dairy Sci. 2010, 93, 1260–1265. [Google Scholar] [CrossRef]
- Schmidt, D.G. Differences between the Association of the Genetic Variants B, C and D of αS1-Casein. Biochim. Biophys. Acta 1970, 221, 140–142. [Google Scholar] [CrossRef]
- Fox, P.F. Milk proteins: General and historical aspects. In Advanced Dairy Chemistry—1 Proteins; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–48. [Google Scholar]
- Sadler, A.M.; Kiddy, C.A.; McCann, R.E.; Mattingly, W.A. Acid Production and Curd Toughness in Milks of Different αS1-Casein Types. J. Dairy Sci. 1968, 51, 28–30. [Google Scholar] [CrossRef]
- Creamer, L.K.; Zoerb, H.F.; Olson, N.F.; Richardson, T. Surface Hydrophobicity of αS1-I, αS1-Casein A and B and Its Implications in Cheese Structure. J. Dairy Sci. 1982, 65, 902–906. [Google Scholar] [CrossRef]
- Bouniol, C.; Printz, C.; Mercier, J.-C. Bovine αS2-Casein D Is Generated by Exon VIII Skipping. Gene 1993, 128, 289–293. [Google Scholar] [CrossRef]
- Kontopidis, G.; Holt, C.; Sawyer, L. Invited Review: β-Lactoglobulin: Binding Properties, Structure, and Function. J. Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef]
- Qin, B.Y.; Bewley, M.C.; Creamer, L.K.; Baker, E.N.; Jameson, G.B. Functional Implications of Structural Differences between Variants A and B of Bovine β-Lactoglobulin. Protein Sci. 1999, 8, 75–83. [Google Scholar] [CrossRef]
- Brownlow, S.; Cabral, J.H.M.; Cooper, R.; Flower, D.R.; Yewdall, S.J.; Polikarpov, I.; North, A.C.T.; Sawyer, L. Bovine β-Lactoglobulin at 1.8 Å Resolution—Still an Enigmatic Lipocalin. Structure 1997, 5, 481–495. [Google Scholar] [CrossRef]
- Qin, B.Y.; Bewley, M.C.; Creamer, L.K.; Baker, H.M.; Baker, E.N.; Jameson, G.B. Structural Basis of the Tanford Transition of Bovine β-Lactoglobulin. Biochemistry 1998, 37, 14014–14023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, X.; Zhang, H.; Liu, C.; Jiao, W.; Chang, Z. Chaperone-like Activity of β-Casein. Int. J. Biochem. Cell Biol. 2005, 37, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.E.; Treweek, T.M.; Lindner, R.A.; Price, W.E.; Carver, J.A. Casein Proteins as Molecular Chaperones. J. Agric. Food Chem. 2005, 53, 2670–2683. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited Review: Caseins and the Casein Micelle: Their Biological Functions, Structures, and Behavior in Foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Zondlo, N.J. A Propensity Scale for Type II Polyproline Helices (PPII): Aromatic Amino Acids in Proline-Rich Sequences Strongly Disfavor PPII Due to Proline—Aromatic Interactions. Biochemistry 2012, 51, 5041–5051. [Google Scholar] [CrossRef]
- Raynes, J.K.; Day, L.; Augustin, M.A.; Carver, J.A. Structural Differences between Bovine A1 and A2 β-Casein Alter Micelle Self-Assembly and Influence Molecular Chaperone Activity. J. Dairy Sci. 2015, 98, 2172–2182. [Google Scholar] [CrossRef]
- Kaminogawa, S.; Mizobuchi, H.; Yamauchi, K. Comparison of Bovine Milk Protease with Plasmin. Agric. Biol. Chem. 1972, 36, 2163–2167. [Google Scholar] [CrossRef]
- Eigel, W.N.; Hofmann, C.J.; Chibber, B.A.; Tomich, J.M.; Keenan, T.W.; Mertz, E.T. Plasmin-Mediated Proteolysis of Casein in Bovine Milk. Proc. Natl. Acad. Sci. USA 1979, 76, 2244–2248. [Google Scholar] [CrossRef]
- Eigel, W.N. Formation of γ1 -A2, γ2 -A2 and γ3 -A Caseins by in Vitro Proteolysis of β-Casein A2 with Bovine Plasmin. Int. J. Biochem. 1977, 8, 187–192. [Google Scholar] [CrossRef]
- Brantl, V. Novel Opioid Peptides Derived from Human β-Casein: Human β-Casomorphins. Eur. J. Pharmacol. 1984, 106, 213–214. [Google Scholar] [CrossRef]
- Henschen, A.; Lottspeich, F.; Brantl, V.; Teschemacher, H. Novel Opioid Peptides Derived from Casein (Beta-Casomorphins). II. Structure of Active Components from Bovine Casein Peptone. Hoppe-Seyler’s Z. Fur Physiol. Chem. 1979, 360, 1217–1224. [Google Scholar]
- Parashar, A.; Saini, R.K. A1 Milk and Its Controversy-a Review. Int. J. Bioassays 2015, 4, 4611–4619. [Google Scholar]
- Asledottir, T.; Le, T.T.; Poulsen, N.A.; Devold, T.G.; Larsen, L.B.; Vegarud, G.E. Release of β-Casomorphin-7 from Bovine Milk of Different β-Casein Variants after Ex Vivo Gastrointestinal Digestion. Int. Dairy J. 2018, 81, 8–11. [Google Scholar] [CrossRef]
- Lambers, T.T.; Broeren, S.; Heck, J.; Bragt, M.; Huppertz, T. Processing Affects Beta-Casomorphin Peptide Formation during Simulated Gastrointestinal Digestion in Both A1 and A2 Milk. Int. Dairy J. 2021, 121, 105099. [Google Scholar] [CrossRef]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, Beta-Casein and Lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef]
- McLachlan, C.N.S. β-Casein A1, Ischaemic Heart Disease Mortality, and Other Illnesses. Med. Hypotheses 2001, 56, 262–272. [Google Scholar] [CrossRef]
- Cieśińska, A.; Sienkiewicz-Szłapka, E.; Wasilewska, J.; Fiedorowicz, E.; Chwała, B.; Moszyńska-Dumara, M.; Cieśiński, T.; Bukało, M.; Kostyra, E. Influence of Candidate Polymorphisms on the Dipeptidyl Peptidase IV and μ-Opioid Receptor Genes Expression in Aspect of the β-Casomorphin-7 Modulation Functions in Autism. Peptides 2015, 65, 6–11. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Z.; Wang, X.; Cade, R.; Elmir, Z.; Fregly, M. Relation of β-Casomorphin to Apnea in Sudden Infant Death Syndrome. Peptides 2003, 24, 937–943. [Google Scholar] [CrossRef]
- Deth, R.; Clarke, A.; Ni, J.; Trivedi, M. Clinical Evaluation of Glutathione Concentrations after Consumption of Milk Containing Different Subtypes of β-Casein: Results from a Randomized, Cross-over Clinical Trial. Nutr. J. 2015, 15, 1–6. [Google Scholar] [CrossRef]
- De Noni, I.; FitzGerald, R.J.; Korhonen, H.J.T.; le Roux, Y.; Livesey, C.T.; Thorsdottir, I.; Tomé, D.; Witkamp, R. Review of the Potential Health Impact of β-Casomorphins and Related Peptides. EFSA Sci. Rep. 2009, 231, 1–107. [Google Scholar]
- Küllenberg de Gaudry, D.; Lohner, S.; Schmucker, C.; Kapp, P.; Motschall, E.; Hörrlein, S.; Röger, C.; Meerpohl, J.J. Milk A1 β-Casein and Health-Related Outcomes in Humans: A Systematic Review. Nutr. Rev. 2019, 77, 278–306. [Google Scholar] [CrossRef]
- Darewicz, M.; Dziuba, J. Formation and Stabilization of Emulsion with A1, A2 and B β-Casein Genetic Variants. Eur. Food Res. Technol. 2007, 226, 147–152. [Google Scholar] [CrossRef]
- Wedholm, A.; Larsen, L.B.; Lindmark-Månsson, H.; Karlsson, A.H.; Andrén, A. Effect of Protein Composition on the Cheese-Making Properties of Milk from Individual Dairy Cows. J. Dairy Sci. 2006, 89, 3296–3305. [Google Scholar] [CrossRef]
- Ng-Kwai-Hang, K.F.; Hayes, J.F.; Moxley, J.E.; Monardes, H.G. Relationships between Milk Protein Polymorphisms and Major Milk Constituents in Holstein-Friesian Cows. J. Dairy Sci. 1986, 69, 22–26. [Google Scholar] [CrossRef]
- Aleandri, R.; Buttazzoni, L.G.; Schneider, J.C.; Caroli, A.; Davoli, R. The Effects of Milk Protein Polymorphisms on Milk Components and Cheese-Producing Ability. J. Dairy Sci. 1990, 73, 241–255. [Google Scholar] [CrossRef]
- Jakob, E. Genetic Polymorphism of Milk Proteins. Mljekarstvo Časopis Za Unaprjeđenje Proizvodnje i Prerade Mlijeka 1994, 44, 197–217. [Google Scholar]
- Lodes, A.; Buchberger, J.; Krause, J.; Aumann, J.; Klostermeyer, H. The Influence of Genetic Variants of Milk Proteins on the Compositional and Technological Properties of Milk. 3. Content of Protein, Casein, Whey Protein, and Casein Number. Milchwissenschaft 1997, 52, 3–8. [Google Scholar]
- Puhan, Z. Session I: Introduction to the subject. In Milk Protein Polymorphism; Hill, J.P., Boland, M., Eds.; International Dairy Federation: Brussels, Belgium, 1997; pp. 12–21. [Google Scholar]
- Devold, T.G.; Brovold, M.J.; Langsrud, T.; Vegarud, G.E. Size of Native and Heated Casein Micelles, Content of Protein and Minerals in Milk from Norwegian Red Cattle—Effect of Milk Protein Polymorphism and Different Feeding Regimes. Int. Dairy J. 2000, 10, 313–323. [Google Scholar] [CrossRef]
- McLean, D.M.; Graham, E.R.B.; Ponzoni, R.W.; McKenzie, H.A. Effects of Milk Protein Genetic Variants on Milk Yield and Composition. J. Dairy Res. 1984, 51, 531–546. [Google Scholar] [CrossRef]
- Gonyon, D.S.; Mather, R.E.; Hines, H.C.; Haenlein, G.F.W.; Arave, C.W.; Gaunt, S.N. Associations of Bovine Blood and Milk Polymorphisms with Lactation Traits: Holsteins. J. Dairy Sci. 1987, 70, 2585–2598. [Google Scholar] [CrossRef]
- Ng-Kwai-Hang, K.F.; Monardes, H.G.; Hayes, J.F. Association between Genetic Polymorphism of Milk Proteins and Production Traits during Three Lactations. J. Dairy Sci. 1990, 73, 3414–3420. [Google Scholar] [CrossRef]
- Graml, R.; Buchberger, J.; Klostermeyer, H.; Pirchner, F. Pleiotropic Effects of β-Lactoglobulin and Casein Genotypes on Milk Composition of Simmentals and German Browns in Bavaria. Z. Tierzücht. Züchtgsbiol. 1985, 102, 355–370. [Google Scholar]
- Bovenhuis, H.; van Arendonk, J.A.M.; Korver, S. Associations between Milk Protein Polymorphisms and Milk Production Traits. J. Dairy Sci. 1992, 75, 2549–2559. [Google Scholar] [CrossRef]
- Von Oloffs, K.; Schulte-Coerne, H.; Pabst, K.; Gravert, H.O. Die Bedeutung Der Proteinvarianten Für Genetische Unterschiede in Der Käsereitauglichkeit Der Milch. Züchtungskunde 1992, 64, 20–26. [Google Scholar]
- Famula, T.R.; Medrano, J.F. Estimation of Genotype Effects for Milk Proteins with Animal and Sire Transmitting Ability Models. J. Dairy Sci. 1994, 77, 3153–3162. [Google Scholar] [CrossRef]
- Hristov, P.; Neov, B.; Sbirkova, H.; Teofanova, D.; Radoslavov, G.; Shivachev, B. Genetic Polymorphism of Kappa Casein and Casein Micelle Size in the Bulgarian Rhodopean Cattle Breed. Biotechnol. Anim. Husb. 2014, 30, 561–570. [Google Scholar] [CrossRef]
- Mao, I.L.; Buttazzoni, L.G.; Aleandri, R. Effects of Polymorphic Milk Protein Genes on Milk Yield and Composition Traits in Holstein Cattle. Acta Agric. Scand. A-Anim. Sci. 1992, 42, 1–7. [Google Scholar] [CrossRef]
- Rahali, V.; Ménard, J.L. Influence Des Variants Génétiques de La β-Lactoglobuline et de La κ-Caséine Sur La Composition Du Lait et Son Aptitude Fromagère. Le Lait 1991, 71, 275–297. [Google Scholar] [CrossRef][Green Version]
- Ikonen, T.; Bovenhuis, H.; Ojala, M.; Ruottinen, O.; Georges, M. Associations between Casein Haplotypes and First Lactation Milk Production Traits in Finnish Ayrshire Cows. J. Dairy Sci. 2001, 84, 507–514. [Google Scholar] [CrossRef]
- Ojala, M.; Famula, T.R.; Medrano, J.F. Effects of Milk Protein Genotypes on the Variation for Milk Production Traits of Holstein and Jersey Cows in California. J. Dairy Sci. 1997, 80, 1776–1785. [Google Scholar] [CrossRef]
- Visker, M.; Bovenhuis, H.; van Arendonk, J.A.M.; Schopen, G.C.B. Genome Wide Association for Casein Index in Milk of Dairy Cattle. Presented at 9th World Congress on Genetic Applied to Livestock Production (WCGALP), Leipzig, Germany, 1–6 August 2010. [Google Scholar]
- Caroli, A.; Chessa, S.; Bolla, P.; Budelli, E.; Gandini, G.C. Genetic Structure of Milk Protein Polymorphisms and Effects on Milk Production Traits in a Local Dairy Cattle. J. Anim. Breed. Genet. 2004, 121, 119–127. [Google Scholar] [CrossRef]
- Glantz, M.; Devold, T.G.; Vegarud, G.E.; Månsson, H.L.; Stålhammar, H.; Paulsson, M. Importance of Casein Micelle Size and Milk Composition for Milk Gelation. J. Dairy Sci. 2010, 93, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, F.; Glantz, M.; Buitenhuis, A.J.; Lindmark-Månsson, H.; Stålhammar, H.; Andrén, A.; Paulsson, M. Factors Influencing Chymosin-Induced Gelation of Milk from Individual Dairy Cows: Major Effects of Casein Micelle Size and Calcium. Int. Dairy J. 2014, 39, 201–208. [Google Scholar] [CrossRef]
- Jõudu, I.; Henno, M.; Kaart, T.; Püssa, T.; Kärt, O. The Effect of Milk Protein Contents on the Rennet Coagulation Properties of Milk from Individual Dairy Cows. Int. Dairy J. 2008, 18, 964–967. [Google Scholar] [CrossRef]
- Tyrisevä, A.-M.; Ikonen, T.; Ojala, M. Repeatability Estimates for Milk Coagulation Traits and Non-Coagulation of Milk in Finnish Ayrshire Cows. J. Dairy Res. 2003, 70, 91. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, T.; Morri, S.; Tyrisevä, A.-M.; Ruottinen, O.; Ojala, M. Genetic and Phenotypic Correlations between Milk Coagulation Properties, Milk Production Traits, Somatic Cell Count, Casein Content, and PH of Milk. J. Dairy Sci. 2004, 87, 458–467. [Google Scholar] [CrossRef]
- Auldist, M.J.; Coats, S.J.; Sutherland, B.J.; Hardham, J.F.; McDowell, G.H.; Rogers, G.L. Effect of Somatic Cell Count and Stage of Lactation on the Quality and Storage Life of Ultra High Temperature Milk. J. Dairy Res. 1996, 63, 377–386. [Google Scholar] [CrossRef]
- Auldist, M.J.; Mullins, C.; O’brien, B.; O’kennedy, B.T.; Guinee, T. Effect of Cow Breed on Milk Coagulation Properties. Milchwissenschaft 2002, 57, 140–143. [Google Scholar]
- O’brien, B.; Dillon, P.; Murphy, J.J.; Mehra, R.A.J.K.; Guinee, T.P.; Connolly, J.F.; Kelly, A.; Joyce, P. Effects of Stocking Density and Concentrate Supplementation of Grazing Dairy Cows on Milk Production, Composition and Processing Characteristics. J. Dairy Res. 1999, 66, 165–176. [Google Scholar] [CrossRef]
- Verdier-Metz, I.; Coulon, J.-B.; Pradel, P.; Viallon, C.; Berdagué, J.-L. Effect of Forage Conservation (Hay or Silage) and Cow Breed on the Coagulation Properties of Milks and on the Characteristics of Ripened Cheeses. J. Dairy Res. 1998, 65, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, B.; Green, M.L. Determination of the Proportion of κ-Casein Hydrolysed by Rennet on Coagulation of Skim-Milk. J. Dairy Res. 1980, 47, 351–358. [Google Scholar] [CrossRef]
- Sandra, S.; Alexander, M.; Dalgleish, D.G. The Rennet Coagulation Mechanism of Skim Milk as Observed by Transmission Diffusing Wave Spectroscopy. J. Colloid Interface Sci. 2007, 308, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Chemistry and Biochemistry of Cheese. In Dairy Chemistry and Biochemistry; Springer: Berlin/Heidelberg, Germany, 2015; p. 504. [Google Scholar]
- Inglingstad, R.A.; Steinshamn, H.; Dagnachew, B.S.; Valenti, B.; Criscione, A.; Rukke, E.O.; Devold, T.G.; Skeie, S.B.; Vegarud, G.E. Grazing Season and Forage Type Influence Goat Milk Composition and Rennet Coagulation Properties. J. Dairy Sci. 2014, 97, 3800–3814. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.; Månsson, H.L.; Stålhammar, H.; Paulsson, M. Effect of Polymorphisms in the Leptin, Leptin Receptor, and Acyl-Coenzyme A: Diacylglycerol Acyltransferase 1 (DGAT1) Genes and Genetic Polymorphism of Milk Proteins on Cheese Characteristics. J. Dairy Sci. 2011, 94, 3295–3304. [Google Scholar] [CrossRef]
- Malacarne, M.; Franceschi, P.; Formaggioni, P.; Sandri, S.; Mariani, P.; Summer, A. Influence of Micellar Calcium and Phosphorus on Rennet Coagulation Properties of Cows Milk. J. Dairy Res. 2014, 81, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Battistotti, B. Milk Quality for Cheesemaking. In Proceedings of the ASPA Congress-Recent Progress in Animal Production Science, Piacenza, Italy, 21–24 June 1999. [Google Scholar]
- Ji, Y.D.; Lee, S.K.; Anema, S.G. Effect of Heat Treatments and Homogenisation Pressure on the Acid Gelation Properties of Recombined Whole Milk. Food Chem. 2011, 129, 463–471. [Google Scholar] [CrossRef]
- Hallén, E.; Allmere, T.; Näslund, J.; Andrén, A.; Lundén, A. Effect of Genetic Polymorphism of Milk Proteins on Rheology of Chymosin-Induced Milk Gels. Int. Dairy J. 2007, 17, 791–799. [Google Scholar] [CrossRef]
- Meza-Nieto, M.A.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Félix, L.; Goycoolea, F.M. Effect of β-Lactoglobulin A and B Whey Protein Variants on the Rennet-Induced Gelation of Skim Milk Gels in a Model Reconstituted Skim Milk System. J. Dairy Sci. 2007, 90, 582–593. [Google Scholar] [CrossRef]
- Ikonen, T.; Ahlfors, K.; Kempe, R.; Ojala, M.; Ruottinen, O. Genetic Parameters for the Milk Coagulation Properties and Prevalence of Noncoagulating Milk in Finnish Dairy Cows. J. Dairy Sci. 1999, 82, 205–214. [Google Scholar] [CrossRef]
- Yun, S.-E.; Ohmiya, K.; Shimizu, S. Role of β-Casein in Milk Curdling. Agric. Biol. Chem. 1982, 46, 443–449. [Google Scholar] [CrossRef][Green Version]
- Poulsen, N.A.; Rosengaard, A.K.; Szekeres, B.D.; Gregersen, V.R.; Jensen, H.B.; Larsen, L.B. Protein Heterogeneity of Bovine β-Casein in Danish Dairy Breeds and Association of Rare β-Casein F with Milk Coagulation Properties. Acta Agric. Scand. Sect. A—Anim. Sci. 2016, 66, 190–198. [Google Scholar] [CrossRef]
- Day, L.; Williams, R.P.W.; Otter, D.; Augustin, M.A. Casein Polymorphism Heterogeneity Influences Casein Micelle Size in Milk of Individual Cows. J. Dairy Sci. 2015, 98, 3633–3644. [Google Scholar] [CrossRef]
- Niki, R.; Arima, S. Effects of Size of Casein Micelle on Firmness of Rennet Curd. Jpn. J. Zootech. Sci. 1984, 55, 409–415. [Google Scholar]
- Ford, G.D.; Grandison, A.S. Effect of Size of Casein Micelles on Coagulation Properties of Skim Milk. J. Dairy Res. 1986, 53, 129–133. [Google Scholar] [CrossRef]
- Frederiksen, P.D.; Andersen, K.K.; Hammershøj, M.; Poulsen, H.D.; Sørensen, J.; Bakman, M.; Qvist, K.B.; Larsen, L.B. Composition and Effect of Blending of Noncoagulating, Poorly Coagulating, and Well-Coagulating Bovine Milk from Individual Danish Holstein Cows. J. Dairy Sci. 2011, 94, 4787–4799. [Google Scholar] [CrossRef] [PubMed]
- Hallén, E.; Lundén, A.; Tyrisevä, A.-M.; Westerlind, M.; Andrén, A. Composition of Poorly and Non-Coagulating Bovine Milk and Effect of Calcium Addition. J. Dairy Res. 2010, 77, 398. [Google Scholar] [CrossRef]
- Martin, P.; Szymanowska, M.; Zwierzchowski, L.; Leroux, C. The Impact of Genetic Polymorphisms on the Protein Composition of Ruminant Milks. Reprod. Nutr. Dev. 2002, 42, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, L.; Mariani, P. The Role of Protein Polymorphism in the Genetic Improvement of Milk Production. Zootec. E Nutr. Anim. 2000, 26, 69–90. [Google Scholar]
- Holland, J.W. Post-translational modifications of caseins. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2008; pp. 107–132. [Google Scholar]
- Mollé, D.; Léonil, J. Heterogeneity of the Bovine κ-Casein Caseinomacropeptide, Resolved by Liquid Chromatography on-Line with Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 1995, 708, 223–230. [Google Scholar] [CrossRef]
- Coolbear, K.P.; Elgar, D.F.; Ayers, J.S. Profiling of Genetic Variants of Bovine κ-Casein Macropeptide by Electrophoretic and Chromatographic Techniques. Int. Dairy J. 1996, 6, 1055–1068. [Google Scholar] [CrossRef]
- Bittante, G.; Penasa, M.; Cecchinato, A. Invited Review: Genetics and Modeling of Milk Coagulation Properties. J. Dairy Sci. 2012, 95, 6843–6870. [Google Scholar] [CrossRef] [PubMed]
- Jõudu, I.; Henno, M.; Värv, S.; Viinalass, H.; Püssa, T.; Kaart, T.; Arney, D.; Kärt, O. The Effect of Milk Proteins on Milk Coagulation Properties in Estonian Dairy Breeds. Vet. ir Zootech. 2009, 46, 14–19. [Google Scholar]
- Choi, J.W.; Ng-Kwai-Hang, K.F. Effects of Genetic Variants of κ-Casein and β-Lactoglobulin and Heat Treatment of Milk on Cheese and Whey Compositions. Asian-Australas. J. Anim. Sci. 2002, 15, 732–739. [Google Scholar] [CrossRef]
- Di Gregorio, P.; di Grigoli, A.; di Trana, A.; Alabiso, M.; Maniaci, G.; Rando, A.; Valluzzi, C.; Finizio, D.; Bonanno, A. Effects of Different Genotypes at the CSN3 and LGB Loci on Milk and Cheese-Making Characteristics of the Bovine Cinisara Breed. Int. Dairy J. 2017, 71, 1–5. [Google Scholar] [CrossRef]
- Bonfatti, V.; di Martino, G.; Cecchinato, A.; Degano, L.; Carnier, P. Effects of β-κ-Casein (CSN2-CSN3) Haplotypes, β-Lactoglobulin (BLG) Genotypes, and Detailed Protein Composition on Coagulation Properties of Individual Milk of Simmental Cows. J. Dairy Sci. 2010, 93, 3809–3817. [Google Scholar] [CrossRef]
- Vallas, M.; Kaart, T.; Värv, S.; Pärna, K.; Jõudu, I.; Viinalass, H.; Pärna, E. Composite β-κ-Casein Genotypes and Their Effect on Composition and Coagulation of Milk from Estonian Holstein Cows. J. Dairy Sci. 2012, 95, 6760–6769. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Heat Stability of Milk. Int. J. Dairy Technol. 2004, 57, 111–119. [Google Scholar] [CrossRef]
- O’connell, J.E.; Fox, P.F. Heat-induced coagulation of milk. In Advanced Dairy Chemistry—1 Proteins; Springer: Berlin/Heidelberg, Germany, 2003; pp. 879–945. [Google Scholar]
- Singh, H.; Fox, P.F. Heat Stability of Milk: Role of β-Lactoglobulin in the PH-Dependent Dissociation of Micellar κ-Casein. J. Dairy Res. 1987, 54, 509–521. [Google Scholar] [CrossRef]
- Rose, D. Factors Affecting the PH-Sensitivity of the Heat Stability of Milk from Individual Cows. J. Dairy Sci. 1961, 44, 1405–1413. [Google Scholar] [CrossRef]
- Robitaille, G. Influence of κ-Casein and β-Lactoglobulin Genetic Variants on the Heat Stability of Milk. J. Dairy Res. 1995, 62, 593–600. [Google Scholar] [CrossRef]
- McLean, D.M.; Graham, E.R.B.; Ponzoni, R.W.; Mckenzie, H.A. Effects of Milk Protein Genetic Variants and Composition on Heat Stability of Milk. J. Dairy Res. 1987, 54, 219–235. [Google Scholar] [CrossRef]
- Keppler, J.K.; Sönnichsen, F.D.; Lorenzen, P.-C.; Schwarz, K. Differences in Heat Stability and Ligand Binding among β-Lactoglobulin Genetic Variants A, B and C Using 1H NMR and Fluorescence Quenching. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 1083–1093. [Google Scholar] [CrossRef]
- Thresher, W.C.; Knighton, D.R.; Otter, D.E.; Hill, J.P. Subunit interactions in β-lactoglobulin: Effects of ligand binding and pH. In Brief Communications: 24th International Dairy Congress; Australian National Committee of the International Dairy Federation (ANCIDF): Melbourne, Australia, 1994; p. 101-undefined. [Google Scholar]
- Hill, J.P.; Boland, M.J.; Creamer, L.K.; Anema, S.G.; Otter, D.E.; Paterson, G.R.; Lowe, R.; Motion, R.L.; Thresher, W.C. Effect of the Bovine β-Lactoglobulin Phenotype on the Properties of β-Lactoglobulin, Milk Composition, and Dairy Products. In Macromolecular Interactions in Food Technology; American Chemical Society: Washington, DC, USA, 1996; Volume 650, pp. 281–294. [Google Scholar]
- Thresher, W.; Hill, J. Thermodynamic Characterization of Beta-Lactoglobulin A, B and C Subunit Interactions Using Analytical Affinity Chromatography. In Milk Protein Polymorphism; International Dairy Federation: Palmerston North, New Zealand, 1997. [Google Scholar]
- Timasheff, S.N. The Nature of Interactions in Proteins Derived from Milk. In Symposium on Foods: Proteins and Their Reactions; Avi Publishing Company (AVI): Westport, CT, USA, 1964; pp. 179–208. [Google Scholar]
- Pessen, H.; Purcell, J.M.; Farrell, H.M., Jr. Proton Relaxation Rates of Water in Dilute Solutions of β-Lactoglobulin. Determination of Cross Relaxation and Correlation with Structural Changes by the Use of Two Genetic Variants of a Self-Associating Globular Protein. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1985, 828, 1–12. [Google Scholar] [CrossRef]
- Manderson, C.G.; Hardman, M.J.; Creamer, L.K. Thermal Denaturation of Bovine β-Lactoglobulin A, B and C. J. Dairy Sci. 1995, 78 (Suppl. S1), 132-undefined. [Google Scholar]
- Tessier, H.; Rose, D. Influence of κ-Casein and β-Lactoglobulin on the Heat Stability of Skimmilk. J. Dairy Sci. 1964, 47, 1047–1051. [Google Scholar] [CrossRef]
- Jenness, R.; Parkash, S. Heat Stability of Milks Containing Different Casein Polymorphs. J. Dairy Sci. 1967, 50, 952. [Google Scholar]
- Feagan, J.T.; Bailey, L.F.; Hehir, A.F.; McLean, D.M.; Ellis, N.J.S. Coagulation of Milk Proteins 1. Effect of Genetic Variants of Milk Proteins on Rennet Coagulation and Heat Stability of Normal Milk. Aust. J. Dairy Technol. 1972, 27, 129. [Google Scholar]
- Hailing, P.J.; Walstra, P. Protein-stabilized Foams and Emulsions. Crit. Rev. Food Sci. Nutr. 1981, 15, 155–203. [Google Scholar] [CrossRef]
- Cabra, V.; Arreguín, R.; Farres, A. Emulsifying Properties of Proteins. Boletín de la Sociedad Química de México 2008, 2, 80–89. [Google Scholar]
- Dalgleish, D.G. Food Emulsions—Their Structures and Structure-Forming Properties. In Proceedings of the Food Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2006; Volume 20, pp. 415–422. [Google Scholar]
- Kumosinski, T.F.; Brown, E.M.; Farrell, H.M., Jr. Three-Dimensional Molecular Modeling of Bovine Caseins: An Energy-Minimized β-Casein Structure. J. Dairy Sci. 1993, 76, 931–945. [Google Scholar] [CrossRef]
- Dickinson, E. Caseins in Emulsions: Interfacial Properties and Interactions. Int. Dairy J. 1999, 9, 305–312. [Google Scholar] [CrossRef]
- Darewicz, M.; Dziuba, J.; Caessens, P.W.J.R.; Gruppen, H. Dephosphorylation-Induced Structural Changes in β-Casein and Its Amphiphilic Fragment in Relation to Emulsion Properties. Biochimie 2000, 82, 191–195. [Google Scholar] [CrossRef]
- Dziuba, J.; Darewicz, M.; Mioduszewska, H. Physico-Chemical Characteristics of Different Genetic Variants of Bovine β-Casein, Modified Covalently by Glucose, Galactose and Lactose. Pol. J. Food Nutr. Sci. 1998, 7, 166–171. [Google Scholar]
- Maldonado-Valderrama, J.; Martín-Rodriguez, A.; Gálvez-Ruiz, M.J.; Miller, R.; Langevin, D.; Cabrerizo-Vílchez, M.A. Foams and Emulsions of β-Casein Examined by Interfacial Rheology. Colloids Surf. A Physicochem. Eng. Asp. 2008, 323, 116–122. [Google Scholar] [CrossRef]
- Ipsen, R.; Otte, J. The Relation between Protein Structure, Interfacial Rheology and Foam Formation for Various Milk Proteins. Annu. Trans. Nord. Rheol. Soc. 2004, 21, 143–178. [Google Scholar]
| Method | Description | Example |
|---|---|---|
| Electrophoresis | Distinguishing variants from protein level depending on their electrophoretic mobility [6]. | Isolation of αS1-CN variants [20,24,25], β-lg variants [26,27,28]. |
| IEF (isoelectric focusing) | Separating β-lg H variant from B [29,30]. | |
| DNA sequencing | PCR-RFLP [32] and direct sequencing for κ-CN [33], allele-specific-PCR [34] and PCR-single-strand conformation polymorphism for β-CN [35]. | |
| HPLC chromatography with mass spectrometry | Identify and quantify genetic variants [36,37,38]. | Identification of β-CN F and G alleles [39,40]. |
| Microarray technology |
| Distinguishing κ-CN variants [31]. |
| Protein | Genotype | Methodology | Date |
|---|---|---|---|
| β-lg | Variant A, variant B | Electrophoresis | 1958 [26], 1959 [27], 1961 [28] |
| Variant C | Electrophoresis | 1962 [44] | |
| Variant D | - | 1966 [45] | |
| Variant E, variant F, variant G | Electrophoresis | 1957 [11], 1963 [53] 1970 [49], 1973 [51], 1976 [52], 1981 [50] | |
| Variant H | IEF-IPG | 1988 [29,30] | |
| Variant W | chromatofocusing | 1990 [54] | |
| Variant I, variant J | Ion-exchange chromatography | 1996 [55] | |
| α-lac | Variant A, variant B | Electrophoresis | 1963 [58,59] |
| Variant C | Electrophoresis | 1981 [60] | |
| αS1-CN | Variant A, variant B, variant C | Electrophoresis | 1962 [24,25] |
| Variant D | Electrophoresis | 1965 [20] | |
| Vaiant E | Electrophoresis | 1963 [53], 1971 [64], 1976 [52] | |
| Variant F | pI | 1993 [65] | |
| Variant G | Endonucleases | 1992–1994 [66,67,68] | |
| Variant H | pI | 1999 [69] | |
| Variant I | IEF, PCR | 2009 [14] | |
| αS2-CN | Variant A, variant B, variant C, variant D | Electrophoresis | 1984 [23] |
| β-CN | Variant A, variant B, variant C | Electrophoresis | 1961 [70], 1963 [71], 1964 [72] |
| Variant A1, variant A2, variant A3 | Electrophoresis | 1966 [73,74] | |
| Variant D | Amino acid composition | 1969 [75] | |
| Variant E | - | 1972 [76], 1974 [77] | |
| Variant A4 | Electrophoresis | 1981 [60], 1995 [82] | |
| Variant BZ (special case) | Peptide profiling | 1970 [21] | |
| Variant F, variant G | RP-HPLC | 1995 [39], 1998 [40] | |
| Variant H1 | Electrophoresis, PCR | 2000 [80] | |
| Variant H2 | LC-MS | 2002 [81] | |
| Variant I | PCR | 2002 [83] | |
| κ-CN | Variant A, variant B | Electrophoresis | 1966 [86], 1975 [87] |
| Variant J | RP-HPLC | 1999 [69] | |
| Variant B2 | Nucleotide sequencing | 1987 [90] | |
| Variant C, variant E | RP-HPLC | 1993 [91] | |
| Variant F1 | PCR | 1992 [15] | |
| Variant F2 | PCR | 1996 [16] | |
| Variant G1 | IEF | 1996 [17] | |
| Variant G2 | PCR | 1996 [92] | |
| Variant H, Variant I | DNA sequencing | 1999 [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gai, N.; Uniacke-Lowe, T.; O’Regan, J.; Faulkner, H.; Kelly, A.L. Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods 2021, 10, 2409. https://doi.org/10.3390/foods10102409
Gai N, Uniacke-Lowe T, O’Regan J, Faulkner H, Kelly AL. Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods. 2021; 10(10):2409. https://doi.org/10.3390/foods10102409
Chicago/Turabian StyleGai, Nan, Therese Uniacke-Lowe, Jonathan O’Regan, Hope Faulkner, and Alan L. Kelly. 2021. "Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review" Foods 10, no. 10: 2409. https://doi.org/10.3390/foods10102409
APA StyleGai, N., Uniacke-Lowe, T., O’Regan, J., Faulkner, H., & Kelly, A. L. (2021). Effect of Protein Genotypes on Physicochemical Properties and Protein Functionality of Bovine Milk: A Review. Foods, 10(10), 2409. https://doi.org/10.3390/foods10102409






