The Effect of Arabinoxylan and Wheat Bran Incorporation on Dough Rheology and Thermal Processing of Rotary-Moulded Biscuits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Flour Blends
2.2. Proximate Composition Analysis
2.3. Water Retention Capacity of Flours
2.4. Biscuit Dough Preparation
2.5. Rheological Characterization
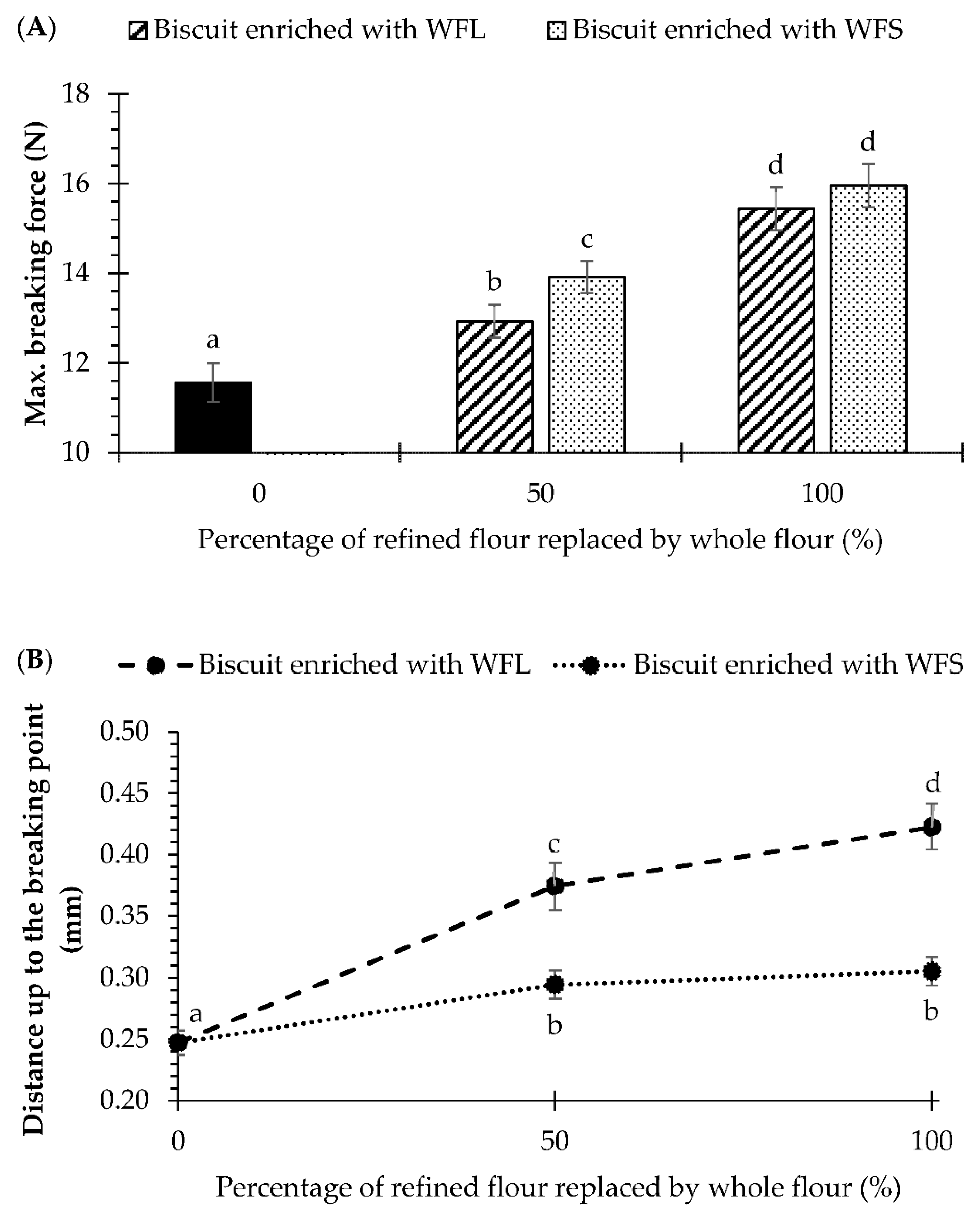
2.6. Texture Measurements
2.7. Differential Scanning Calorimetry (DSC)
2.8. Microstructural Analysis
2.8.1. Scanning Electron Microscopy (SEM)
2.8.2. X-ray Micro-Computed Tomography (X-ray Micro-CT)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Arabinoxylan Content and Water Retention Capacity of Flours
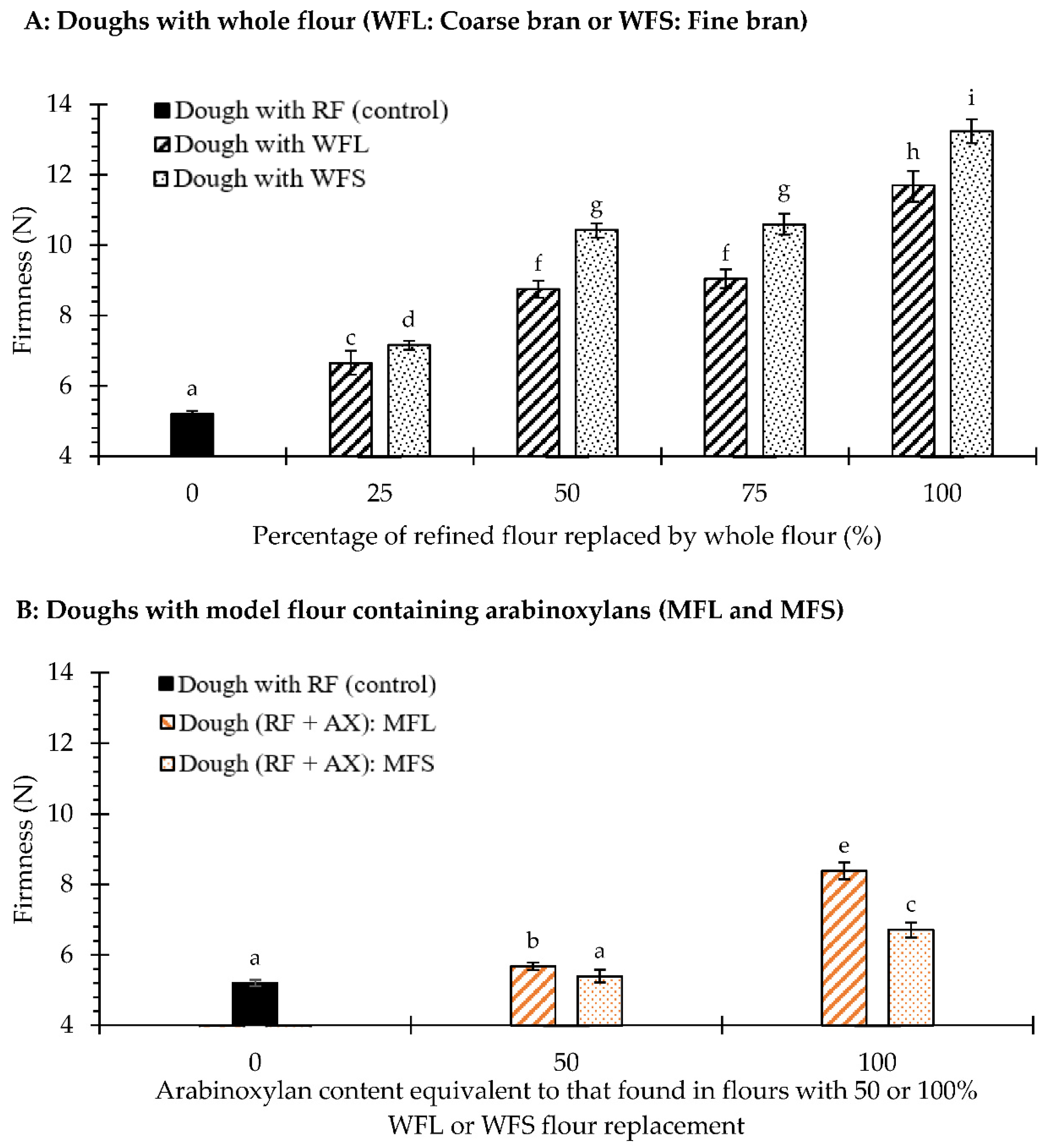
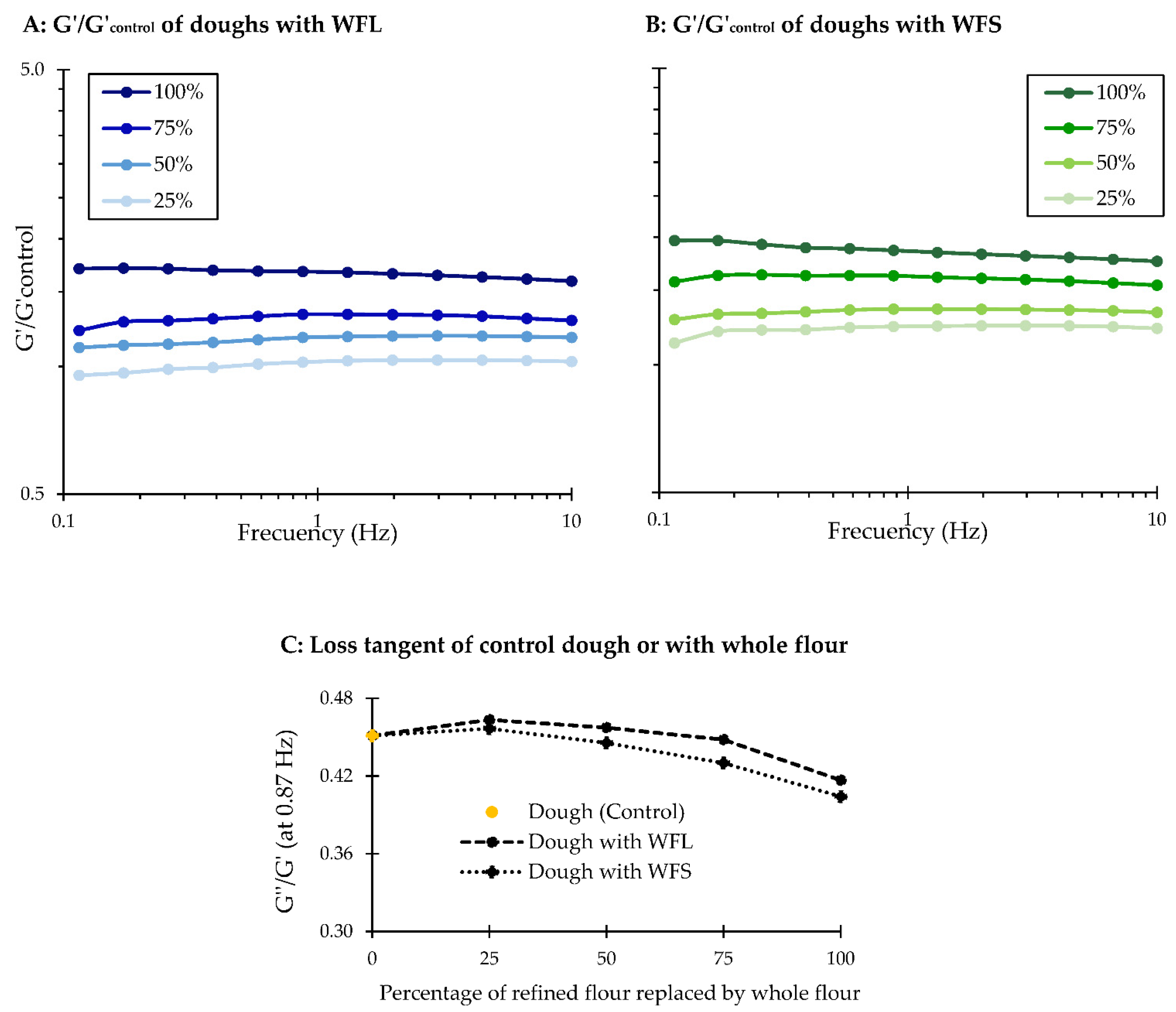
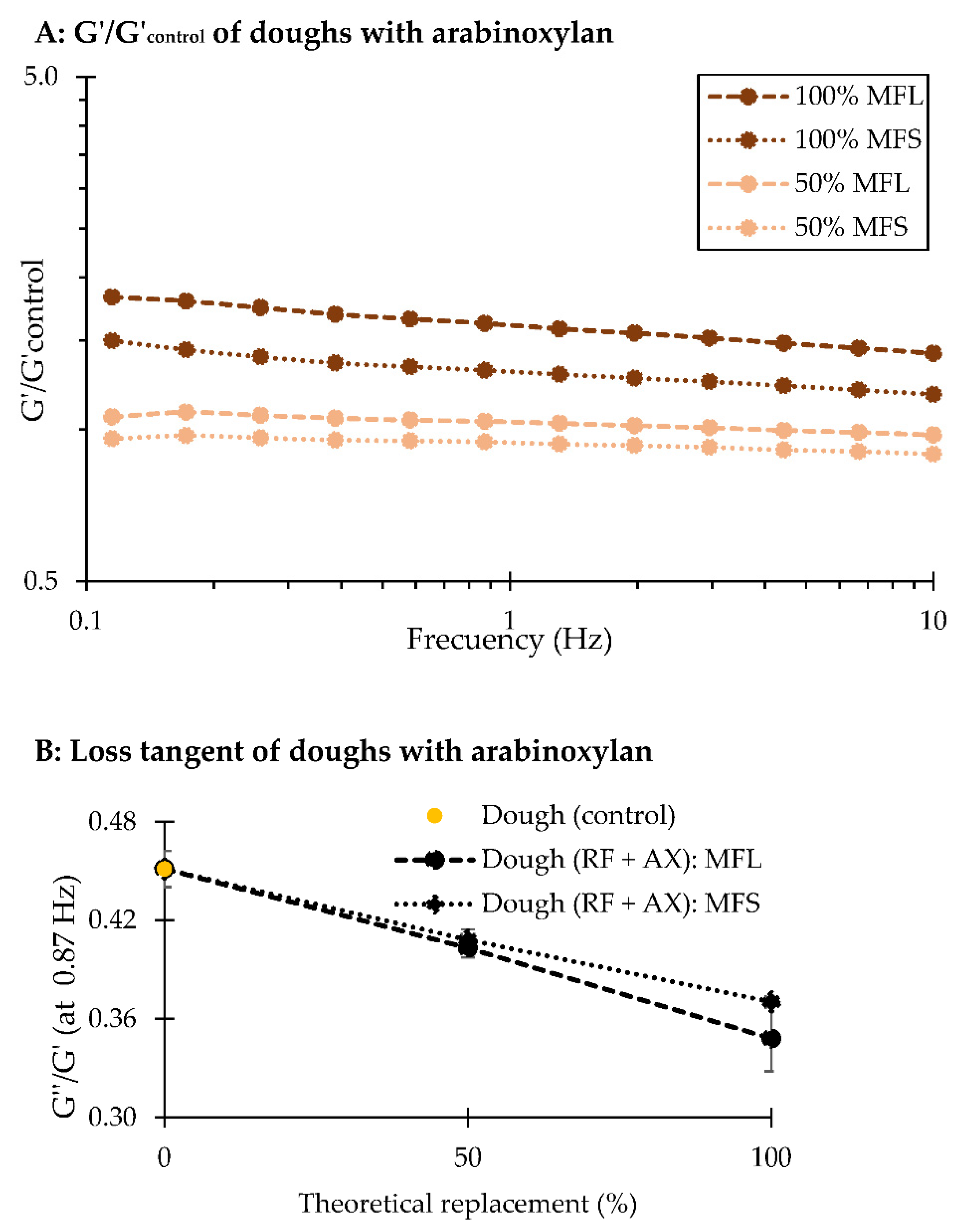
3.2. Firmness and Rheological Analyses of Biscuit Dough Enriched with Wheat Fibre
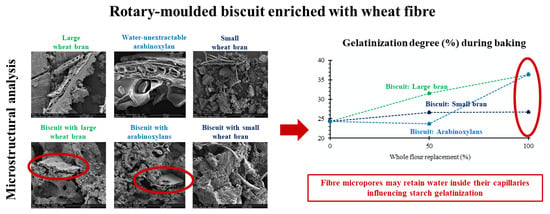
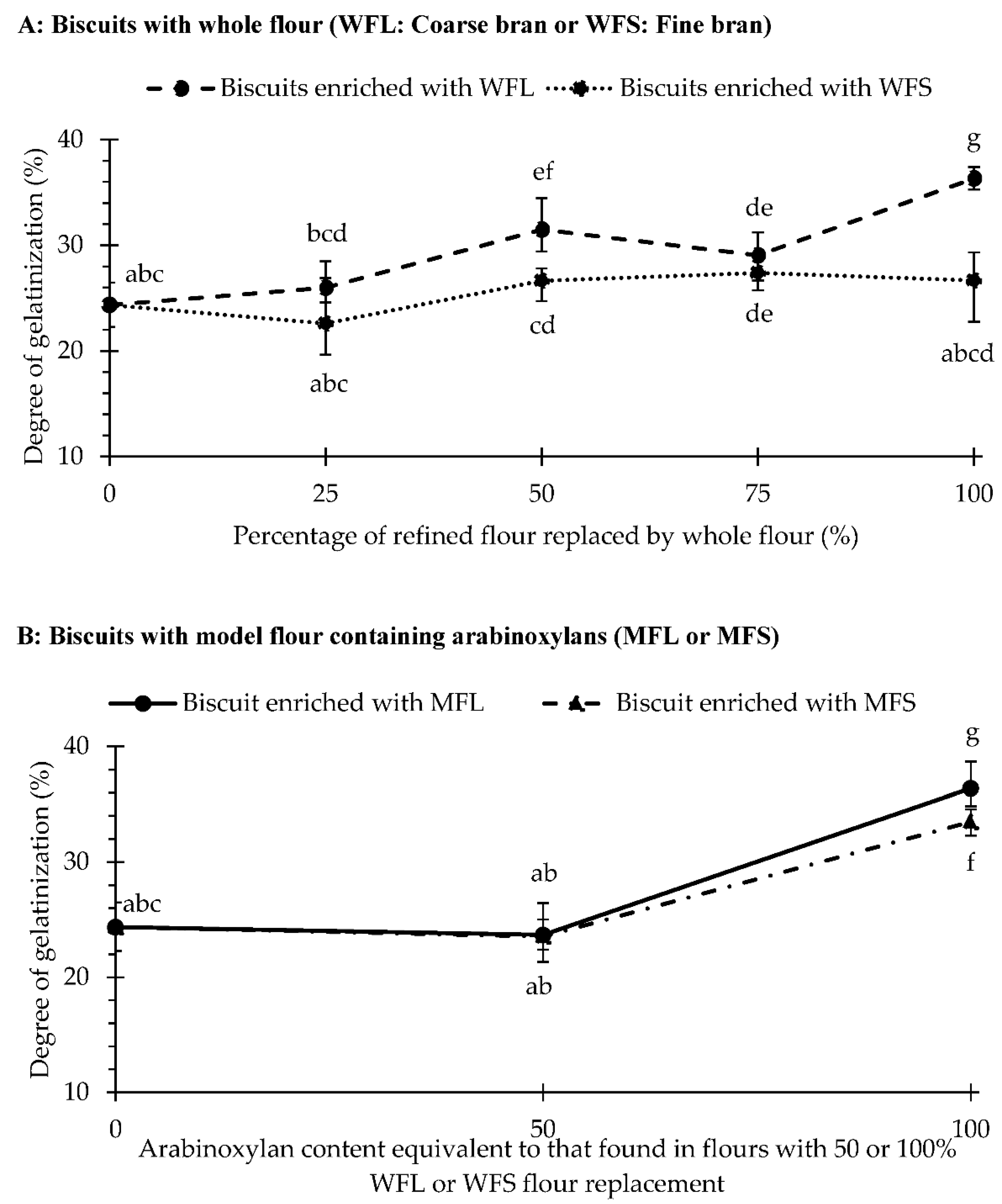
3.3. Starch Gelatinization during Baking and Microstructure of Fibre-Enriched Biscuits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onipe, O.O.; Jideani, A.I.O.; Beswa, D. Composition and functionality of wheat bran and its application in some cereal food products. Int. J. Food Sci. Technol. 2015, 50, 2509–2518. [Google Scholar] [CrossRef]
- Maes, C.; Delcour, J. Structural Characterisation of Water-extractable and Water-unextractable Arabinoxylans in Wheat Bran. J. Cereal Sci. 2002, 35, 315–326. [Google Scholar] [CrossRef]
- McRorie, J.W., Jr.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [Green Version]
- McRorie, J.W., Jr. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 2: What to look for and how to recommend an effective fiber therapy. Nutr. Today 2015, 50, 90. [Google Scholar] [CrossRef] [Green Version]
- Bordenave, N.; Lamothe, L.M.; Kale, M.S. Dietary Fibers in Foods–Formulating and Processing for Nutritional Benefits. In Science and Technology of Fibers in Food Systems, 1st ed.; Welti-Chanes, J., Serna-Saldívar, S., Campanella, O., Tejada-Ortigoza, V., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 437–457. [Google Scholar]
- Lapčíková, B.; Burešová, I.; Lapčík, L.; Dabash, V.; Valenta, T. Impact of particle size on wheat dough and bread characteristics. Food Chem. 2019, 297, 124938. [Google Scholar] [CrossRef]
- Le Bleis, F.; Chaunier, L.; Chiron, H.; Della Valle, G.; Saulnier, L. Rheological properties of wheat flour dough and French bread enriched with wheat bran. J. Cereal Sci. 2015, 65, 167–174. [Google Scholar] [CrossRef]
- Wang, N.; Hou, G.G.; Kweon, M.; Lee, B. Effects of particle size on the properties of whole-grain soft wheat flour and its cracker baking performance. J. Cereal Sci. 2016, 69, 187–193. [Google Scholar] [CrossRef]
- Li, J.; Hou, G.G.; Chen, Z.; Chung, A.-L.; Gehring, K. Studying the effects of whole-wheat flour on the rheological properties and the quality attributes of whole-wheat saltine cracker using SRC, alveograph, rheometer, and NMR technique. LWT 2014, 55, 43–50. [Google Scholar] [CrossRef]
- Sozer, N.; Cicerelli, L.; Heiniö, R.-L.; Poutanen, K. Effect of wheat bran addition on in vitro starch digestibility, physico-mechanical and sensory properties of biscuits. J. Cereal Sci. 2014, 60, 105–113. [Google Scholar] [CrossRef]
- Nandeesh, K.; Jyotsna, R.; Rao, G.V. Effect of differently treated wheat bran on rheology, microstructure and quality characteristics of soft dough biscuits. J. Food Process. Preserv. 2011, 35, 179–200. [Google Scholar] [CrossRef]
- Sudha, M.; Vetrimani, R.; Leelavathi, K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007, 100, 1365–1370. [Google Scholar] [CrossRef]
- Manley, D. (Ed.) Classification of biscuits. In Manley’s Technology of Biscuits, Crackers and Cookies, 4th ed.; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 221–228. [Google Scholar]
- Pareyt, B.; Delcour, J.A. The role of wheat flour constituents, sugar, and fat in low moisture cereal-based products: A review on sugar-snap cookies. Crit. Rev. Food Sci. Nutr. 2008, 48, 824–839. [Google Scholar] [CrossRef]
- Kweon, M.; Slade, L.; Levine, H. Solvent Retention Capacity (SRC) Testing of Wheat Flour: Principles and Value in Predicting Flour Functionality in Different Wheat-Based Food Processes and in Wheat Breeding—A Review. Cereal Chem. J. 2011, 88, 537–552. [Google Scholar] [CrossRef]
- Duyvejonck, A.E.; Lagrain, B.; Pareyt, B.; Courtin, C.M.; Delcour, J.A. Relative contribution of wheat flour constituents to Solvent Retention Capacity profiles of European wheats. J. Cereal Sci. 2011, 53, 312–318. [Google Scholar] [CrossRef]
- Ram, S.; Singh, R.P. Solvent Retention Capacities of Indian Wheats and Their Relationship with Cookie-Making Quality. Cereal Chem. J. 2004, 81, 128–133. [Google Scholar] [CrossRef]
- Pareyt, B.; Goovaerts, M.; Broekaert, W.F.; Delcour, J. Arabinoxylan oligosaccharides (AXOS) as a potential sucrose replacer in sugar-snap cookies. LWT 2011, 44, 725–728. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of Analysis, 11th ed.; AACC International: St. Paul, MN, USA, 1999. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Ramseyer, D.D.; Bettge, A.D.; Morris, C.F. Distribution of Total, Water-Unextractable, and Water-Extractable Arabinoxylans in Wheat Flour Mill Streams. Cereal Chem. J. 2011, 88, 209–216. [Google Scholar] [CrossRef]
- Kiszonas, A.; Courtin, C.; Morris, C. A Critical Assessment of the Quantification of Wheat Grain Arabinoxylans Using a Phloroglucinol Colorimetric Assay. Cereal Chem. J. 2012, 89, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Hemdane, S.; Langenaeken, N.; Jacobs, P.; Verspreet, J.; Delcour, J.; Courtin, C. Study of the role of bran water binding and the steric hindrance by bran in straight dough bread making. Food Chem. 2018, 253, 262–268. [Google Scholar] [CrossRef]
- Molina, M.; Vaz, S.; Bouchon, P. The Creaming of Short Doughs and Its Impact on the Quality Attributes of Rotary-Molded Biscuits. Foods 2021, 10, 621. [Google Scholar] [CrossRef]
- Ahmed, J.; Almusallam, A.S.; Al-Salman, F.; AbdulRahman, M.H.; Al-Salem, E. Rheological properties of water insoluble date fibre incorporated wheat flour dough. LWT-Food Sci. Technol. 2013, 51, 409–416. [Google Scholar] [CrossRef]
- Colakoglu, A.S.; Özkaya, H. Potential use of exogenous lipases for DATEM replacement to modify the rheological and thermal properties of wheat flour dough. J. Cereal Sci. 2012, 55, 397–404. [Google Scholar] [CrossRef]
- Jambrec, D.; Pestorić, M.; Sakač, M.; Nedeljković, N.; Hadnađev, M.; Filipčev, B.; Šimurina, O. Sensory and instrumental properties of novel gluten-free products. J. Process. Energy Agric. 2013, 17, 86–88. [Google Scholar]
- Molina, M.T.; Vaz, S.M.; Leiva, Á.; Bouchon, P. Rotary-moulded biscuits: Dough expansion, microstructure and sweetness perception as affected by sucrose: Flour ratio and sucrose particle size. Food Struct. 2021, 29, 100199. [Google Scholar] [CrossRef]
- Pareyt, B.; Van Steertegem, B.; Brijs, K.; Lagrain, B.; Delcour, J. The impact of redox agents on sugar-snap cookie making. J. Cereal Sci. 2010, 52, 192–199. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Efron, B. Bootstrap methods: Another look at the jackknife. In Breakthroughs in Statistics, 1st ed.; Kotz, S., Johnson, N.L., Eds.; Springer International Publishing: New York, NY, USA, 1992; pp. 569–593. [Google Scholar]
- Dornez, E.; Holopainen, U.; Cuyvers, S.; Poutanen, K.; Delcour, J.A.; Courtin, C.M.; Nordlund, E. Study of grain cell wall structures by microscopic analysis with four different staining techniques. J. Cereal Sci. 2011, 54, 363–373. [Google Scholar] [CrossRef]
- Saulnier, L.; Sado, P.-E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281. [Google Scholar] [CrossRef]
- Courtin, C.; Delcour, J. Arabinoxylans and Endoxylanases in Wheat Flour Bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, S.; Gu, X.; Zhang, J. Isolation and structural characterization of water unextractable arabinoxylans from Chinese black-grained wheat bran. Carbohydr. Polym. 2011, 85, 615–621. [Google Scholar] [CrossRef]
- Megazyme. Available online https://www.megazyme.com/arabinoxylan-wheat-flour-insoluble?sSearch=wheat (accessed on 10 September 2021).
- Filipčev, B.; Nedeljković, N.; Šimurina, O.; Sakač, M.; Pestorić, M.; Jambrec, D.; Šarić, B.; Jovanov, P. Partial replacement of fat with wheat bran in formulation of biscuits enriched with herbal blend. Hem. Ind. 2017, 71, 61–67. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Xu, F.; Liu, Z.; Zhang, L.; Zhang, H. Effects of aleurone-rich fraction on the hydration and rheological properties attributes of wheat dough. Int. J. Food Sci. Technol. 2018, 54, 1777–1786. [Google Scholar] [CrossRef]
- Hemdane, S.; Jacobs, P.; Bosmans, G.; Verspreet, J.; Delcour, J.; Courtin, C. Study on the effects of wheat bran incorporation on water mobility and biopolymer behavior during bread making and storage using time-domain 1 H NMR relaxometry. Food Chem. 2017, 236, 76–86. [Google Scholar] [CrossRef]
- Li, J.; Kang, J.; Wang, L.; Li, Z.; Wang, R.; Chen, Z.X.; Hou, G.G. Effect of Water Migration between Arabinoxylans and Gluten on Baking Quality of Whole Wheat Bread Detected by Magnetic Resonance Imaging (MRI). J. Agric. Food Chem. 2012, 60, 6507–6514. [Google Scholar] [CrossRef]
- Roozendaal, H.; Abu-Hardan, M.; Frazier, R. Thermogravimetric analysis of water release from wheat flour and wheat bran suspensions. J. Food Eng. 2012, 111, 606–611. [Google Scholar] [CrossRef]
- Sabanis, D.; Lebesi, D.; Tzia, C. Effect of dietary fibre enrichment on selected properties of gluten-free bread. LWT 2009, 42, 1380–1389. [Google Scholar] [CrossRef]
- Holopainen-Mantila, U.; Raulio, M. Cereal Grain Structure by Microscopic Analysis. In Imaging Technologies and Data Processing for Food Engineers, 1st ed.; Sozer, N., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–39. [Google Scholar]
- Jacobs, P.J.; Hemdane, S.; Dornez, E.; Delcour, J.; Courtin, C.M. Study of hydration properties of wheat bran as a function of particle size. Food Chem. 2015, 179, 296–304. [Google Scholar] [CrossRef]
- Canalis, M.S.B.; Valentinuzzi, M.C.; Acosta, R.; León, A.E.; Ribotta, P.D. Effects of Fat and Sugar on Dough and Biscuit Behaviours and their Relationship to Proton Mobility Characterized by TD-NMR. Food Bioprocess Technol. 2018, 11, 953–965. [Google Scholar] [CrossRef]
- Jribi, S.; Sahagún, M.; Belorio, M.; Debbabi, H.; Gomez, M. Effect of sprouting time on dough and cookies properties. J. Food Meas. Charact. 2020, 14, 1595–1600. [Google Scholar] [CrossRef]

| Enrichment with Whole Flour | Flour Nomenclature | Water Retention Capacity (g H2O/g Dry Sample × 100) | C.I. at 95% |
|---|---|---|---|
| 0% | RF-Control | 64.1 a | (60.6, 66.1) |
| 25% | WFL | 65.3 a | (63.4, 66.5) |
| WFS | 66.4 a | (66.4, 66.5) | |
| 50% | WFL | 69.6 b | (68.2, 70.7) |
| WFS | 71.3 c | (70.9, 71.4) | |
| 75% | WFL | 74.4 d | (71.4, 76.0) |
| WFS | 75.4 d | (73.5, 77.0) | |
| 100% | WFL | 79.4 e | (77.7, 82.0) |
| WFS | 81.9 f | (80.8, 83.0) | |
| Enrichment with Arabinoxylans | Flour nomenclature | Water retention capacity (g H2O/g dry sample × 100) | C.I. at 95% |
| 50% | MFL | 73.7 d | (72.6, 74.3) |
| MFS | 72.2 bcd | (70.4, 75.9) | |
| 100% | MFL | 92.7 h | (90.8, 95.4) |
| MFS | 86.5 g | (86.2, 87.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, M.T.; Lamothe, L.; Gunes, D.Z.; Vaz, S.M.; Bouchon, P. The Effect of Arabinoxylan and Wheat Bran Incorporation on Dough Rheology and Thermal Processing of Rotary-Moulded Biscuits. Foods 2021, 10, 2335. https://doi.org/10.3390/foods10102335
Molina MT, Lamothe L, Gunes DZ, Vaz SM, Bouchon P. The Effect of Arabinoxylan and Wheat Bran Incorporation on Dough Rheology and Thermal Processing of Rotary-Moulded Biscuits. Foods. 2021; 10(10):2335. https://doi.org/10.3390/foods10102335
Chicago/Turabian StyleMolina, María Teresa, Lisa Lamothe, Deniz Z. Gunes, Sandra M. Vaz, and Pedro Bouchon. 2021. "The Effect of Arabinoxylan and Wheat Bran Incorporation on Dough Rheology and Thermal Processing of Rotary-Moulded Biscuits" Foods 10, no. 10: 2335. https://doi.org/10.3390/foods10102335
APA StyleMolina, M. T., Lamothe, L., Gunes, D. Z., Vaz, S. M., & Bouchon, P. (2021). The Effect of Arabinoxylan and Wheat Bran Incorporation on Dough Rheology and Thermal Processing of Rotary-Moulded Biscuits. Foods, 10(10), 2335. https://doi.org/10.3390/foods10102335