Applicable Plant Proteins and Dietary Fibers for Simulate Plant-Based Yogurts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Protein Dispersions for Titration Capability
2.2.2. Protein Dispersions for Buffering Capacity
2.2.3. Fermentation of Vegetable Proteins
2.2.4. Dietary Fiber Dispersions
2.3. Buffering Capacity of Vegetable Proteins
2.3.1. Titration Capability
2.3.2. Buffering Capacity
2.4. Physical Properties
2.4.1. pH
2.4.2. Titratable Acidity (TA)
2.4.3. Coagulum Strength
2.4.4. Apparent Viscosity
2.4.5. Syneresis
2.5. Color and Soluble Solid Content
2.6. LAB Dertermination
2.7. Statistical Analysis
3. Results and Discussion
3.1. Buffering Capacity of the Vegetable Proteins
3.1.1. Titration Capability
3.1.2. Buffering Capability
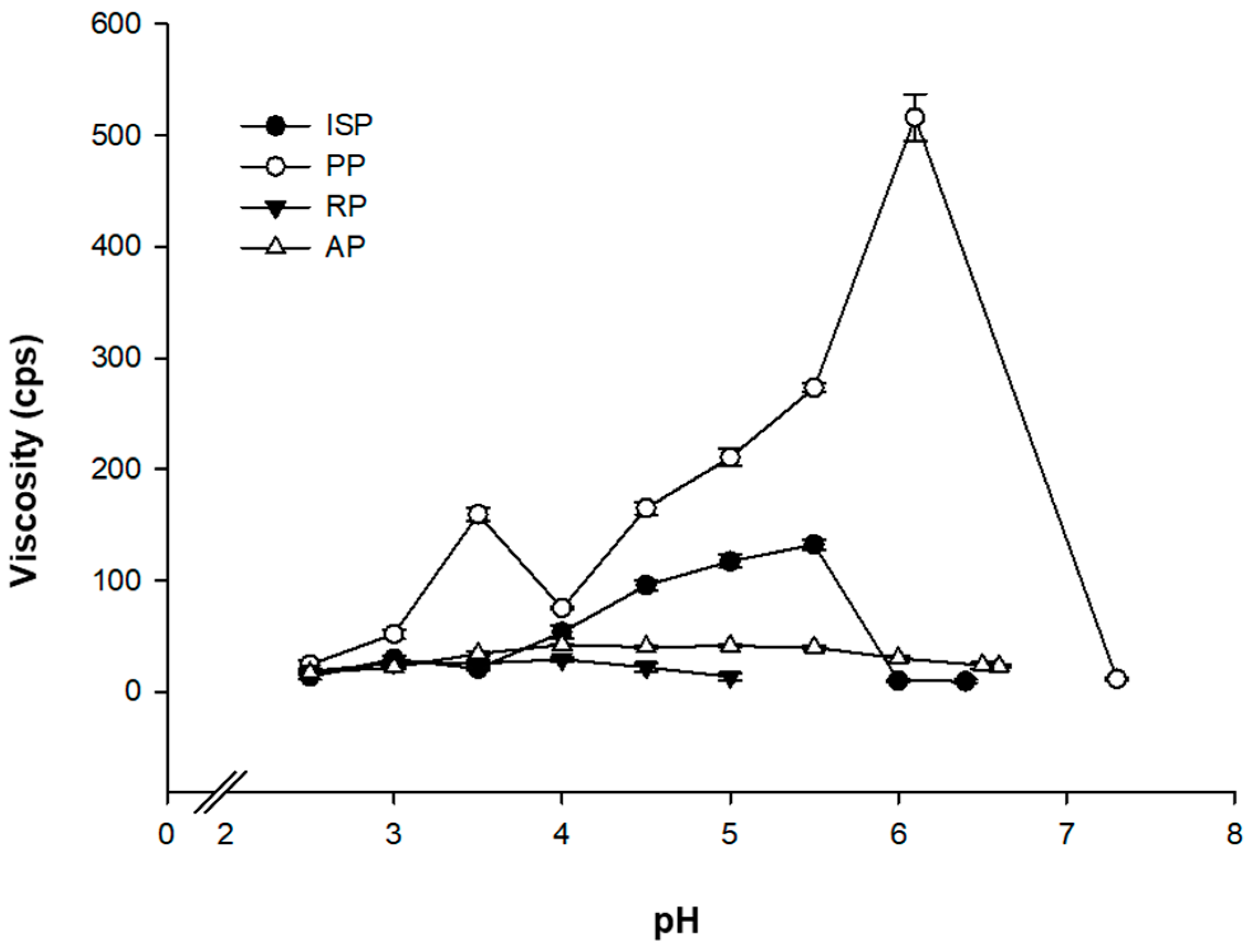
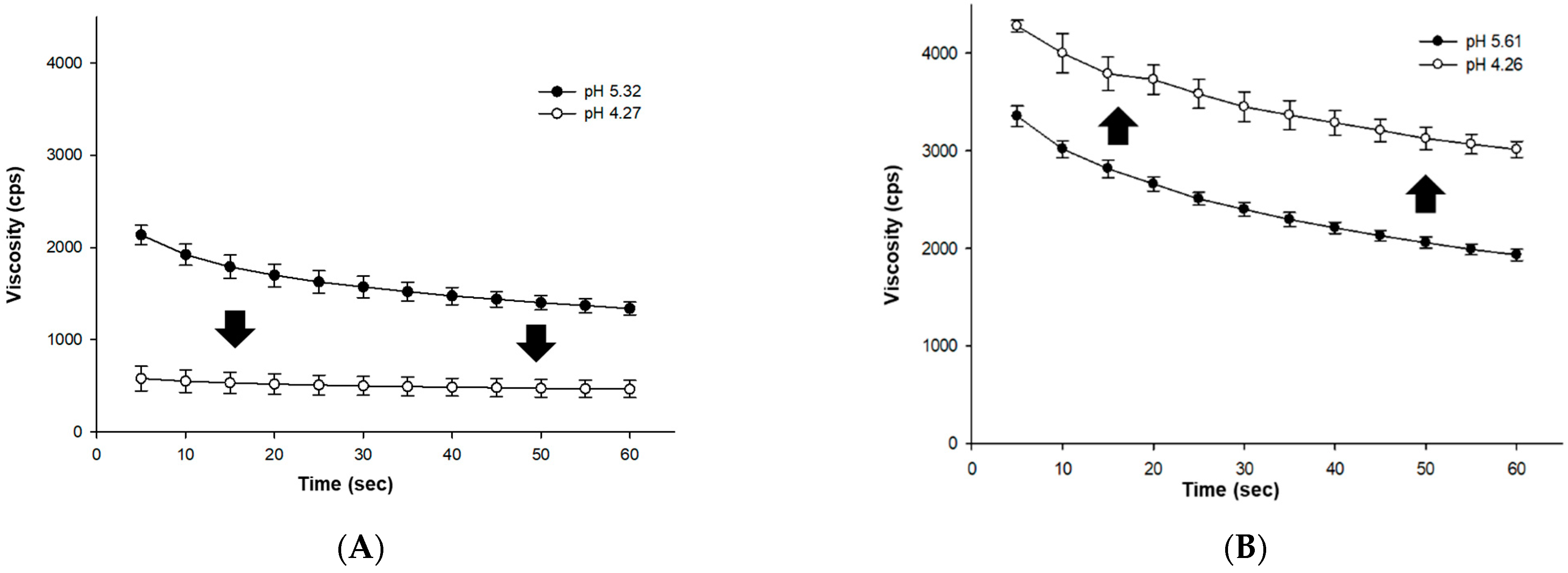
3.1.3. Apparent Viscosity Changes According to pH following the Physical Treatment
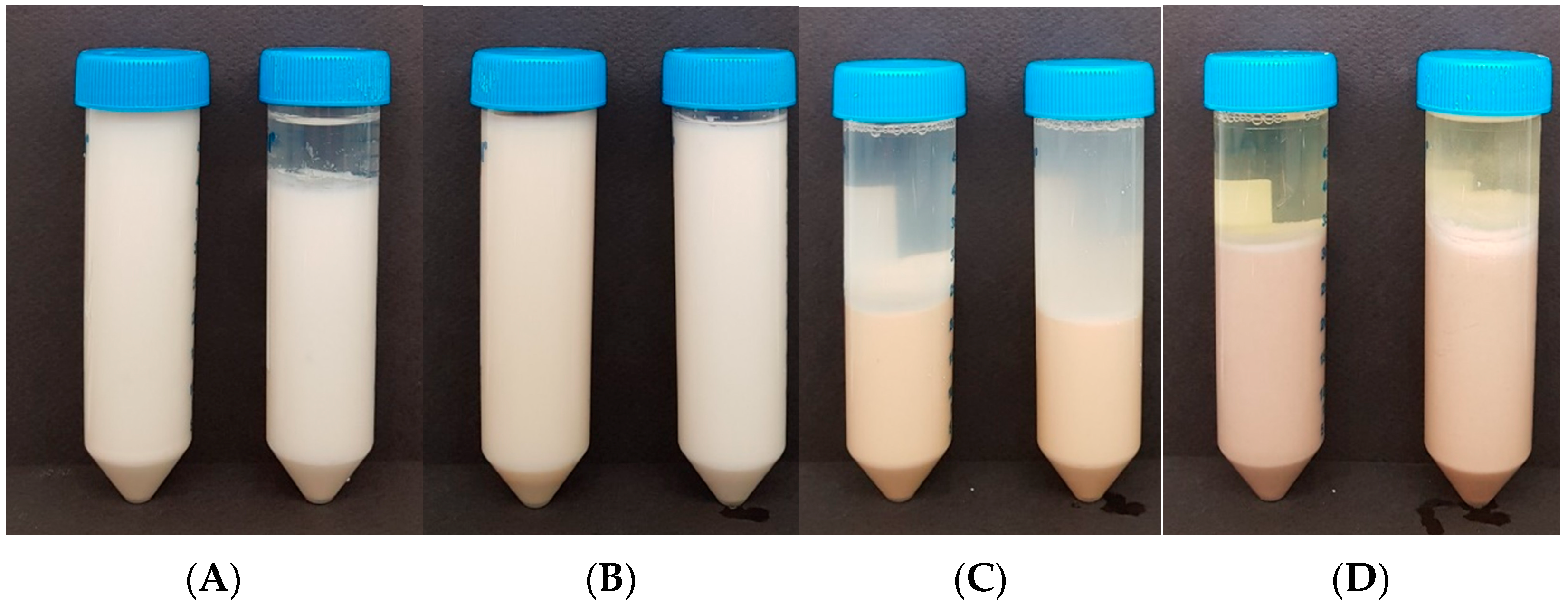
3.2. Fermentation Characteristics of Plant Proteins
3.2.1. pH, TA and LAB
3.2.2. Coagulum Strength and Viscosity
3.2.3. Syneresis
3.3. Color
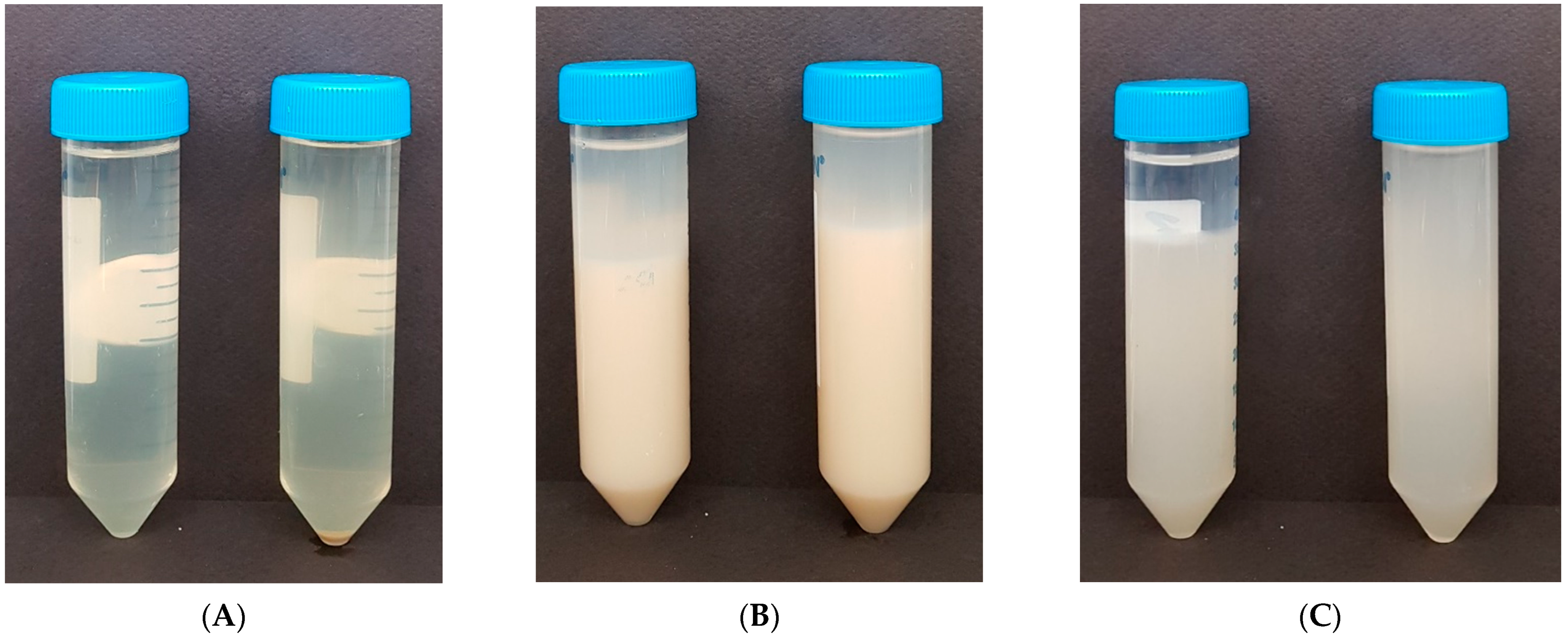
3.4. Physical Properties of Vegetable Dietary Fibers
3.4.1. Syneresis and Viscosity
3.4.2. Soluble Content
3.4.3. Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Incredible Increase in Yogurt Market by Changing from Normal Yogurt to Nutraceuticals. Available online: https://www.hankyung.com/economy/article/2021011706541 (accessed on 17 January 2021).
- Winning the Hearts of 1.5 Million Vegetarians, Food Industry Targets the Vegan Market. Available online: http://www.newscape.co.kr/news/articleView.html?idxno=71669 (accessed on 25 August 2021).
- Global Plant-Based Food and Beverage Market Size to Expand at Annual Rate of 8.71% over 2021–2028. Available online: https://www.globenewswire.com/news-release/2021/07/20/2265395/0/en/Global-plant-based-food-and-beverage-market-size-to-expand-at-annual-rate-of-8-71-over-2021-2028.html (accessed on 20 July 2021).
- Zhou, Y.; Li, X.; Hua, Y.; Kong, X.; Zhang, C.; Chen, Y.; Wang, S. The absence of lipoxygenase and 7S globulin of soybeans and heating temperatures on the properties of soymilks and soy yogurts. LWT-Food Sci. Technol. 2019, 115, 108431. [Google Scholar] [CrossRef]
- Li, H.; Yan, L.; Wang, J.; Zhang, Q.; Zhou, Q.; Sun, T.; Zhang, H. Fermentation characteristics of six probiotic strains in soymilk. Ann. Microbiol. 2012, 62, 1473–1483. [Google Scholar] [CrossRef]
- Subrota, H.; Shilpa, V.; Brij, S.; Vandna, K.; Surajit, M. Antioxidative activity and polyphenol content in fermented soy milk supplemented with WPC-70 by probiotic Lactobacilli. Int. Food Res. J. 2013, 20, 2125. [Google Scholar]
- Pandey, S.M.; Mishra, H. Optimization of the prebiotic & probiotic concentration and incubation temperature for the preparation of synbiotic soy yoghurt using response surface methodology. LWT-Food Sci. Technol. 2015, 62, 458–467. [Google Scholar]
- Ferragut, V.; Cruz, N.; Trujillo, A.; Guamis, B.; Capellas, M. Physical characteristics during storage of soy yogurt made from ultra-high pressure homogenized soymilk. J. Food Eng. 2009, 92, 63–69. [Google Scholar] [CrossRef]
- Cruz, N.; Capellas, M.; Jaramillo, D.; Trujillo, A.; Guamis, B.; Ferragut, V. Soymilk treated by ultra high-pressure homogenization: Acid coagulation properties and characteristics of a soy-yogurt product. Food Hydrocoll. 2009, 23, 490–496. [Google Scholar] [CrossRef]
- Van Slyke, D.D. On the measurement of buffer values and on the relationship of buffer value to the dissociation constant of the buffer and the concentration and reaction of the buffer solution. J. Biol. Chem. 1922, 52, 525–570. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering capacity of dairy products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Gallagher, D.P.; Lucey, J.A.; Mulvihill, D.M. Heat stability characteristics of porcine milk and mixed porcine-bovine milk systems. Int. Dairy J. 1996, 6, 597–611. [Google Scholar] [CrossRef]
- Ismail, A.; El Deeb, S.; El Difrawi, E. The buffering properties of cow and buffalo milks. Z. Für Lebensm. Unters. Forsch. 1973, 152, 25–31. [Google Scholar] [CrossRef]
- Devnani, B.; Ong, L.; Kentish, S.; Gras, S. Heat induced denaturation, aggregation and gelation of almond proteins in skim and full fat almond milk. Food Chem. 2020, 325, 126901. [Google Scholar] [CrossRef] [PubMed]
- Bernat, N.; Cháfer, M.; Rodríguez-García, J.; Chiralt, A.; González-Martínez, C. Effect of high pressure homogenisation and heat treatment on physical properties and stability of almond and hazelnut milks. LWT-Food Sci. Technol. 2015, 62, 488–496. [Google Scholar] [CrossRef]
- Nakai, S. Structure-function relationships of food proteins: With an emphasis on the importance of protein hydrophobicity. J. Agric. Food Chem. 1983, 31, 676–683. [Google Scholar] [CrossRef]
- Klost, M.; Brzeski, C.; Drusch, S. Effect of protein aggregation on rheological properties of pea protein gels. Food Hydrocoll. 2020, 108, 106036. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Structure formation and rheological properties of pea protein-based gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Mietton, B.; Gaucheron, F.; Salaün-Michel, F. Minéraux et transformations fromagères. In Minéraux et Produits Laitiers; Gaucheron, F., Ed.; Lavoisier Tec & Doc: Paris, France, 2004; pp. 472–563. [Google Scholar]
- Srilaorkul, S.; Ozimek, L.; Wolfe, F.; Dziuba, J. The effect of ultrafiltration on physicochemical properties of retentate. Can. Inst. Food Sci. Technol. J. 1989, 22, 56–62. [Google Scholar] [CrossRef]
- Lucey, J.; Gorry, C.; Fox, P. Acid-base buffering properties of heated milk. Milk Sci. Int. Milchwiss. 1993, 48, 438–441. [Google Scholar]
- Famelart, M.-H.; Gaucheron, F.; Mariette, F.; Le Graet, Y.; Raulot, K.; Boyaval, E. Acidification of pressure-treated milk. Int. Dairy J. 1997, 7, 325–330. [Google Scholar] [CrossRef]
- García-Pérez, F.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.; Sendra, E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Rackis, J.J.; Smith, A.; Babcock, G.; Sasame, H. An ultracentrifugal study on the association-dissociation of glycinin in acid solution. J. Am. Chem. Soc. 1957, 79, 4655–4658. [Google Scholar] [CrossRef]
- Wolf, W.; Babcock, G.; Smith, A. Purification and stability studies of the 11 S component of soybean proteins. Arch. Biochem. Biophys. 1962, 99, 265–274. [Google Scholar] [PubMed]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re) formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, C.; Wu, Z.; Zhang, Z.; Xu, C. Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J. Food Biochem. 2020, 44, e13157. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Bhowal, J. Optimization of ingredient and processing parameter for the production of Spirulina platensis incorporated soy yogurt using response surface methodology. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1081–1085. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Zhu, X.; Wang, Y.; Li, D.; Wang, L. Effects of high-pressure homogenization on physical and thermal properties of citrus fiber. LWT-Food Sci. Technol. 2019, 116, 108573. [Google Scholar] [CrossRef]
- Su, D.; Zhu, X.; Adhikari, B.; Li, D.; Wang, L. Effect of high-pressure homogenization on the rheology, microstructure and fractal dimension of citrus fiber-oil dispersions. J. Food Eng. 2020, 277, 109899. [Google Scholar] [CrossRef]
- Heo, Y.-D.; Heo, K.-T. Wine & Coffee Glossary, 1st ed.; Baeksan Publishing Co: Seoul, Korea, 2009; pp. 55–106. [Google Scholar]
- Sandra, S.; Dalgleish, D. Effects of ultra-high-pressure homogenization and heating on structural properties of casein micelles in reconstituted skim milk powder. Int. Dairy J. 2005, 15, 1095–1104. [Google Scholar] [CrossRef]
- Serra, M.; Trujillo, A.; Quevedo, J.; Guamis, B.; Ferragut, V. Acid coagulation properties and suitability for yogurt production of cows’ milk treated by high-pressure homogenisation. Int. Dairy J. 2007, 17, 782–790. [Google Scholar] [CrossRef]
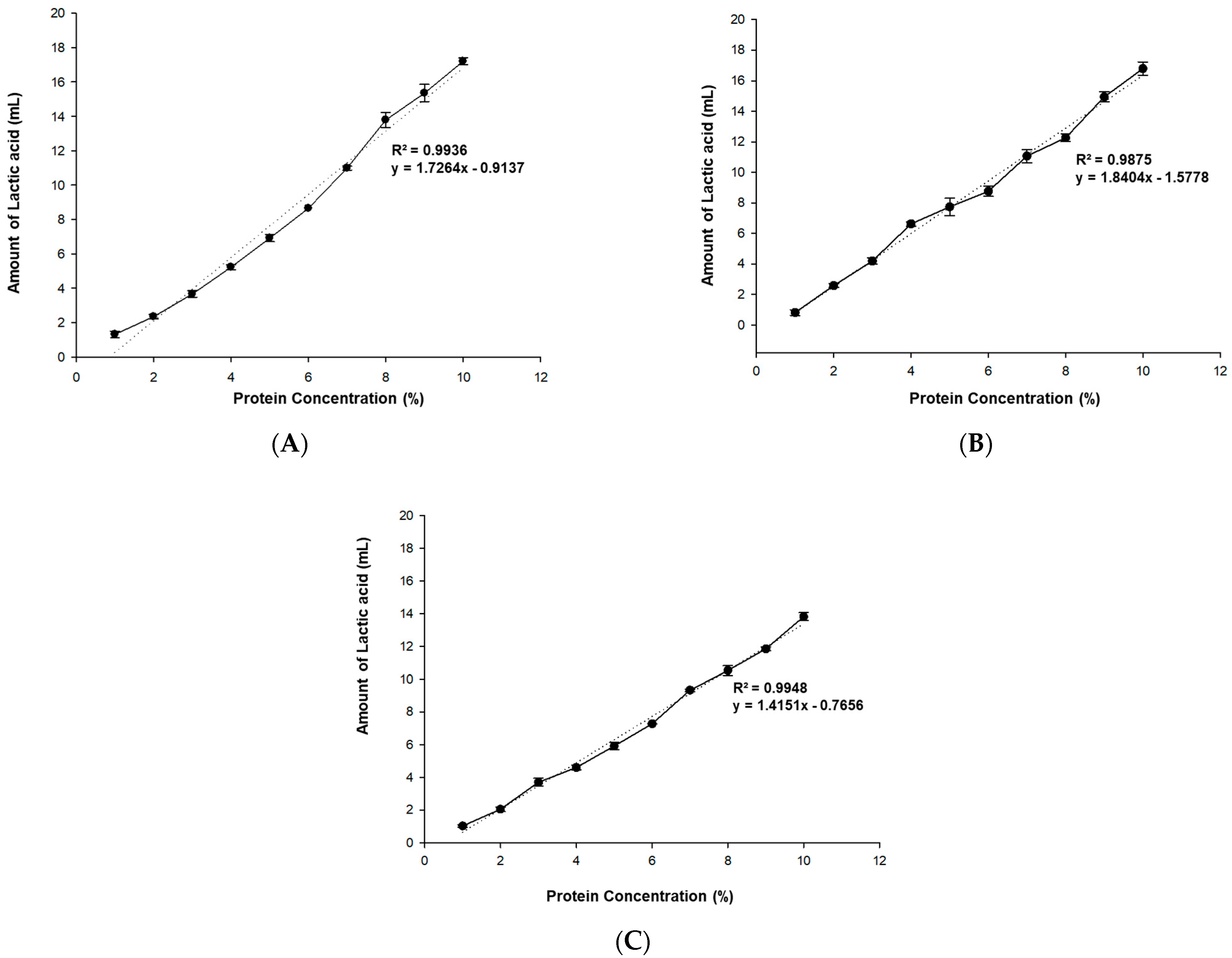
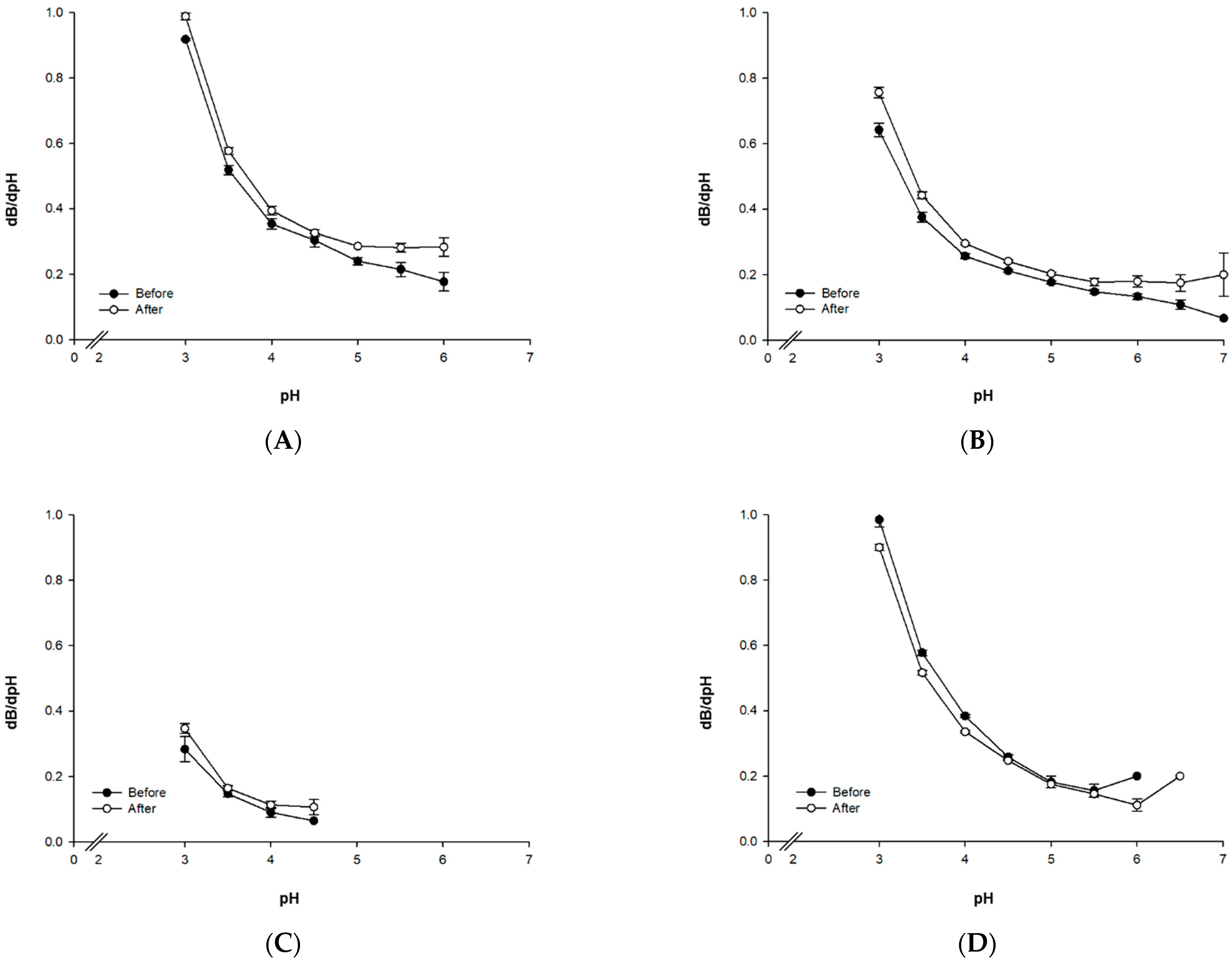
| Protein | Before Physical Treatment | After Physical Treatment | Lactic Acid Ratio (% Increase) | ||
|---|---|---|---|---|---|
| Initial pH | Lactic Acid (mL) | Initial pH | Lactic Acid (mL) | ||
| ISP ** | 6.4 | 33.7 ± 0.8 b,* | 6.4 | 36.8 ± 0.6 a | 9.2 |
| PP | 7.3 | 32.4 ± 1.0 b | 7.3 | 38.0 ± 0.6 a | 17.3 |
| RP | 4.8 | 4.0 ± 0.5 d | 5.0 | 5.5 ± 0.2 d | 37.5 |
| AP | 6.1 | 30.4 ± 0.6 c | 6.6 | 33.8 ± 0.3 b | 11.2 |
| ISP ** | PP | RP | AP | ||
|---|---|---|---|---|---|
| pH | 5.29 ± 0.03 b,* | 5.61 ± 0.04 a | 4.95 ± 0.03 c | 4.30 ± 0.04 d | |
| Titratable Acidity (%) | 0.27 ± 0.02 a | 0.28 ± 0.03 a | 0.09 ± 0.03 b | 0.29 ± 0.04 a | |
| LAB *** (10 8 cfu/g) | 0.91 ± 0.12 b | 1.68 ± 0.11 a | - | 1.86 ± 0.20 a | |
| Coagulum strength (gf) | 13.0 ± 1.91 b | 47.5 ± 2.41 a | - | - | |
| Viscosity (cp) | 1337 ± 71 b | 1932 ± 59 a | 16 ± 2 c | 87 ± 1 c | |
| Syneresis (%) | Before fermentation | 0.0 | 0.0 | 45.2 ± 0.4 a | 35.0 ± 0.6 b |
| After fermentation | 10.0 ± 0.6 d | 0.0 | 45.0 ± 0.2 a | 30.0 ± 0.8 c | |
| Properties | Soy Milk | ISP ** | PP | RP | AP |
|---|---|---|---|---|---|
| L | 84.80 | 78.24 ± 0.01 a,* | 71.45 ± 0.01 b | 64.31 ± 0.02 c | 55.17 ± 0.02 d |
| a | 0.23 | 0.07 ± 0.01 d | 1.77 ± 0.01 c | 8.15 ± 0.02 b | 9.65 ± 0.01 a |
| b | 16.68 | 14.25 ± 0.03 d | 17.52 ± 0.02 c | 28.74 ± 0.03 a | 22.23 ± 0.02 b |
| E *** | 7.00 ± 0.00 d | 13.48 ± 0.01 c | 25.06 ± 0.01 b | 31.59 ± 0.01 a | |
| WI *** | 77.43 | 73.99 ± 0.02 a | 66.44 ± 0.01 b | 53.46 ± 0.00 c | 49.04 ± 0.01 d |
| Properties | SF ** | AF | CF |
|---|---|---|---|
| Syneresis (%) | 20.0 ± 1.2 | - *** | 0 |
| Viscosity (cp) | 85 ± 4 b,* | 14 ± 6 c | 2873 ± 8 a |
| Soluble content (Brix) | 0.52 ± 0.01 c | 4.70 ± 0.07 a | 2.35 ± 0.03 b |
| Properties | Soy Milk | AF ** | SF | CF |
|---|---|---|---|---|
| L | 84.80 | 10.95 ± 0.04 c,* | 65.25 ± 0.04 a | 31.26 ± 0.03 b |
| a | 0.23 | −1.18 ± 0.01 c | 0.08 ± 0.02 a | −1.07 ± 0.03 b |
| b | 16.68 | −3.40 ± 0.04 c | 15.15 ± 0.03 a | −1.15 ± 0.02 b |
| E *** | 76.55 ± 0.03 a | 19.61 ± 0.03 c | 56.45 ± 0.04 b | |
| WI *** | 62.09 | 10.87 ± 0.04 c | 62.09 ± 0.04 a | 31.24 ± 0.03 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-S.; Kim, B.-H.; Baik, M.-Y. Applicable Plant Proteins and Dietary Fibers for Simulate Plant-Based Yogurts. Foods 2021, 10, 2305. https://doi.org/10.3390/foods10102305
Shin J-S, Kim B-H, Baik M-Y. Applicable Plant Proteins and Dietary Fibers for Simulate Plant-Based Yogurts. Foods. 2021; 10(10):2305. https://doi.org/10.3390/foods10102305
Chicago/Turabian StyleShin, Jae-Sung, Beom-Hee Kim, and Moo-Yeol Baik. 2021. "Applicable Plant Proteins and Dietary Fibers for Simulate Plant-Based Yogurts" Foods 10, no. 10: 2305. https://doi.org/10.3390/foods10102305
APA StyleShin, J.-S., Kim, B.-H., & Baik, M.-Y. (2021). Applicable Plant Proteins and Dietary Fibers for Simulate Plant-Based Yogurts. Foods, 10(10), 2305. https://doi.org/10.3390/foods10102305






