Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food
Abstract
:1. Introduction
2. Microalgae as Food: Current Challenges and Opportunities
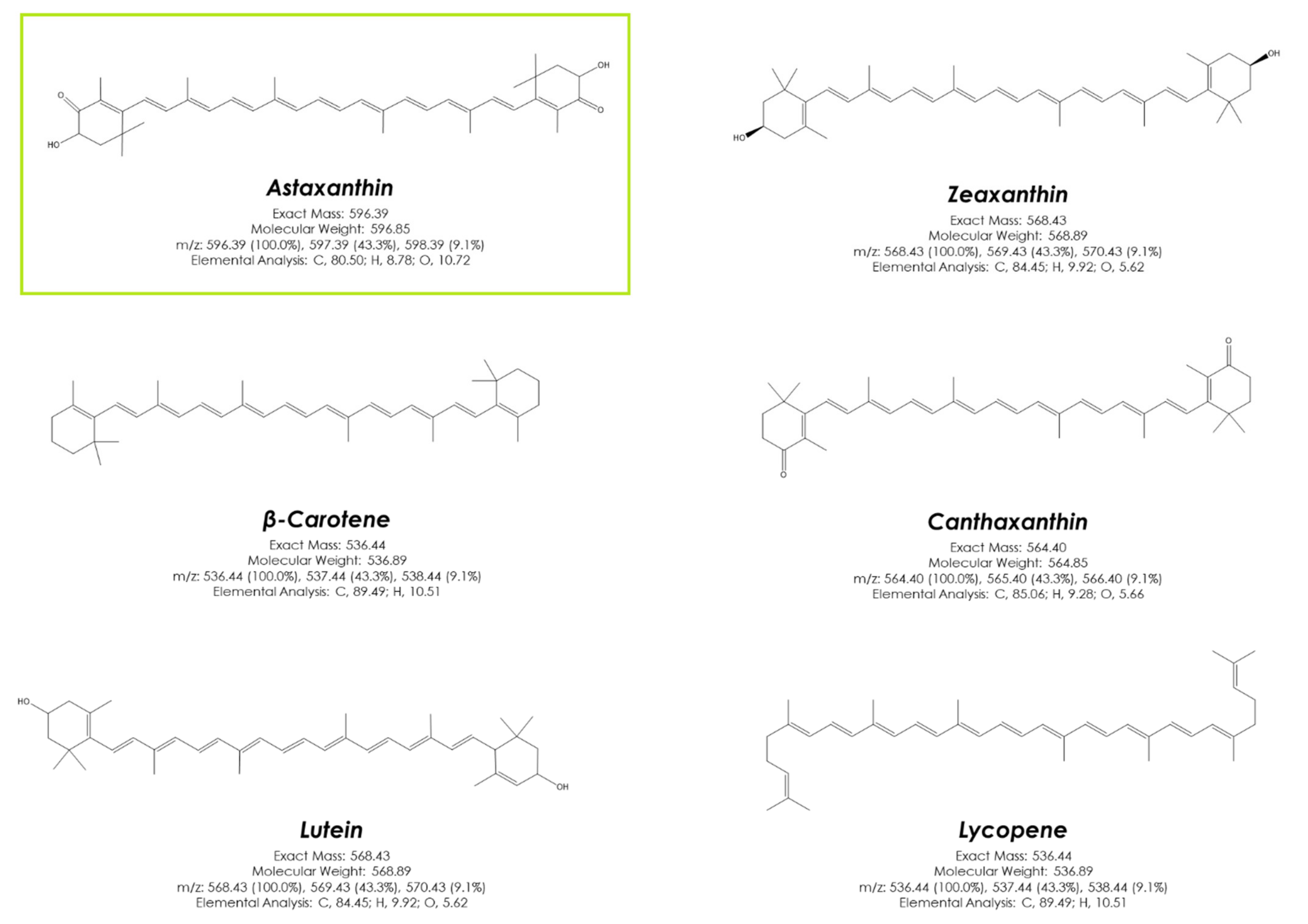
3. Astaxanthin, More than Just a Red Pigment
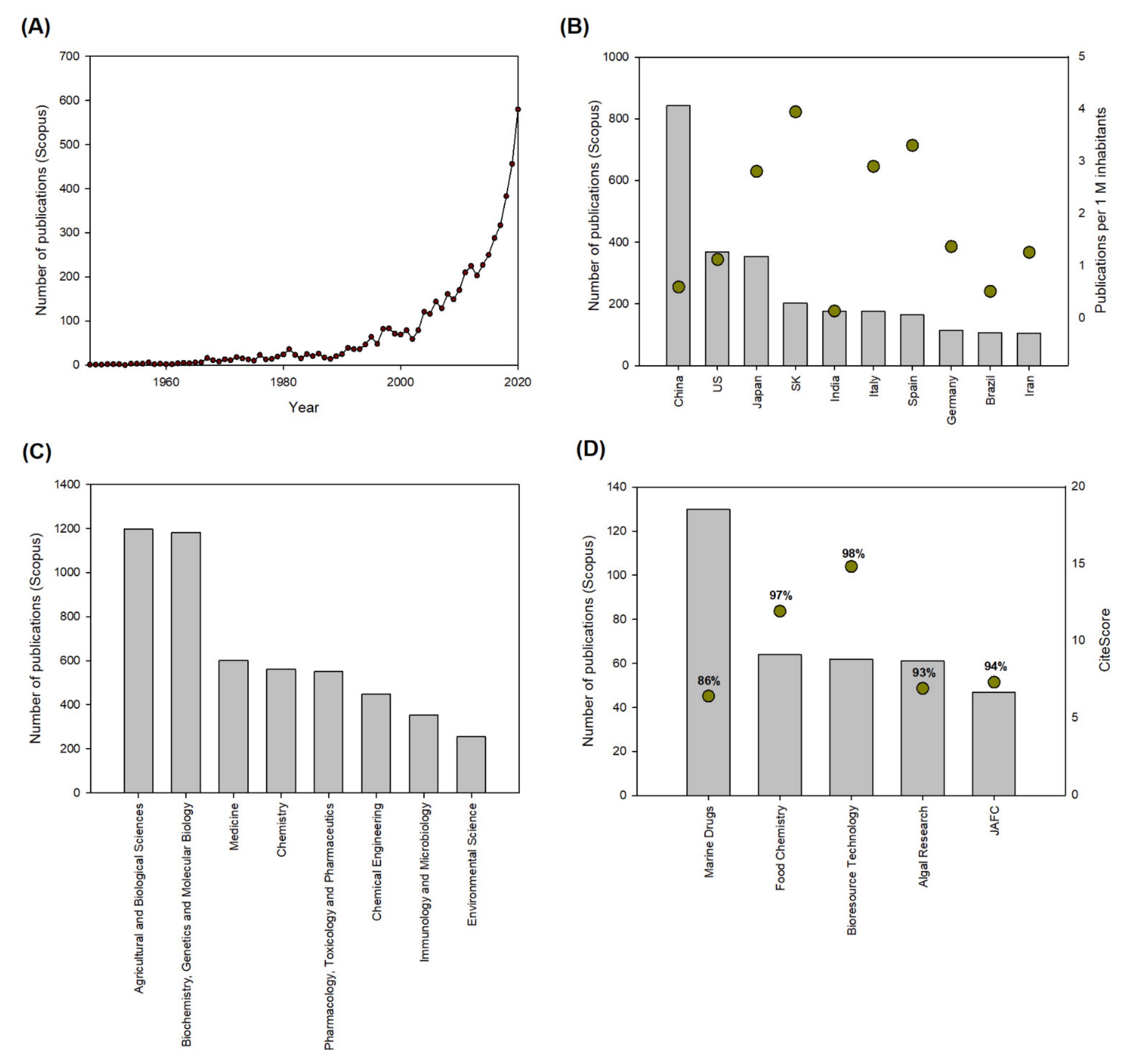
3.1. Astaxanthin Research 2010–2020
3.2. Industrial Use of Natural Astaxanthin
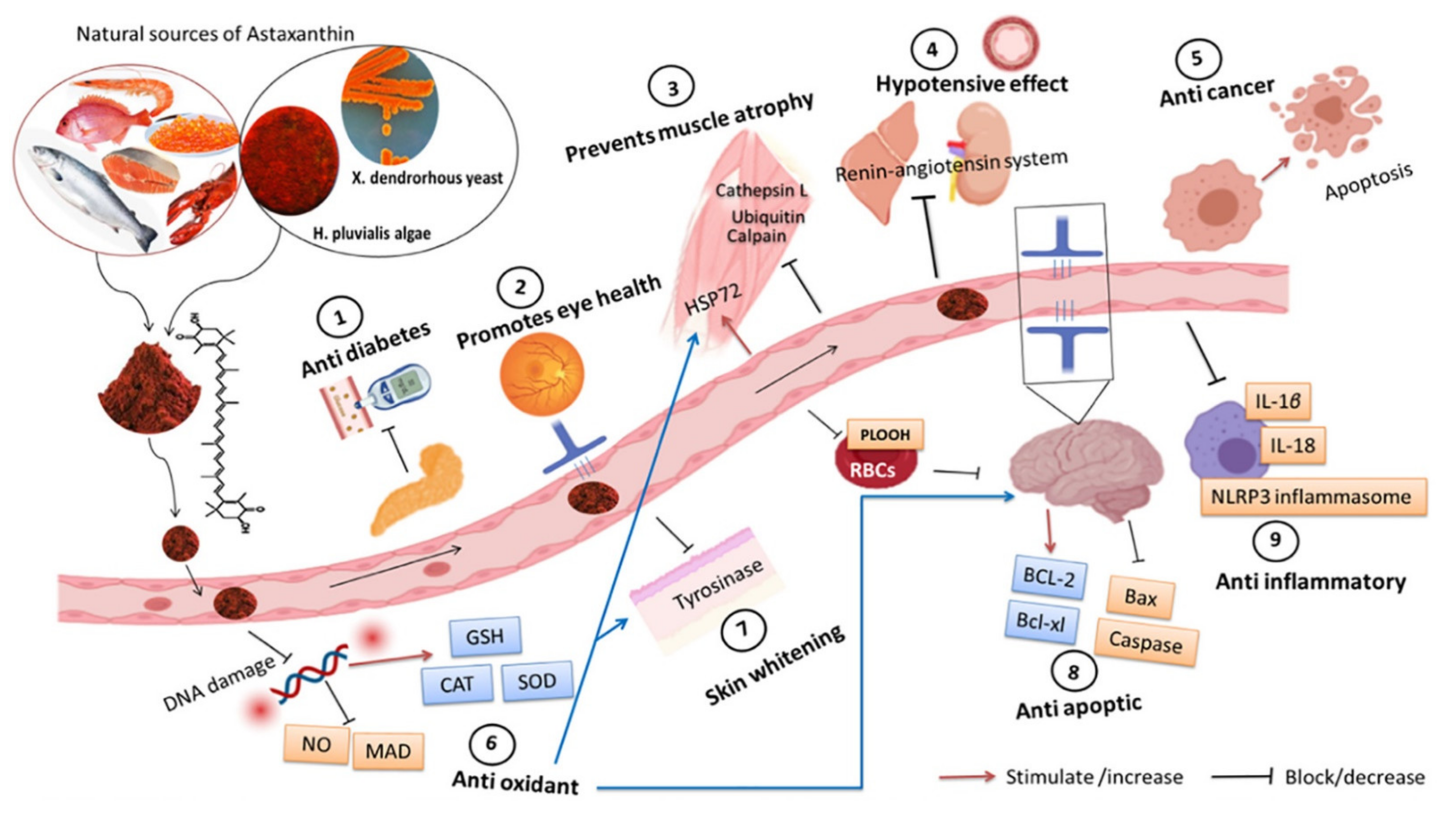
3.3. Health Benefits of Natural Astaxanthin
4. Astaxanthin Producing Microalgae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Smetana, S.; Sandmann, M.; Rohn, S.; Pleissner, D.; Heinz, V. Autotrophic and heterotrophic microalgae and cyanobacteria cultivation for food and feed: Life cycle assessment. Bioresour. Technol. 2017, 245, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.P.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- EFSA. Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, 5993. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Safety of Schizochytrium sp. oil as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, 6242. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Kathiresan, S.; Sarada, R. Towards genetic improvement of commercially important microalga Haematococcus pluvialis for biotech applications. J. Appl. Phycol. 2009, 21, 553–558. [Google Scholar] [CrossRef]
- Galarza, J.I.; Gimpel, J.A.; Rojas, V.; Arredondo-Vega, B.O.; Henríquez, V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res. 2018, 31, 291–297. [Google Scholar] [CrossRef]
- Kathiresan, S.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Regulation of astaxanthin and its intermediates through cloning and genetic transformation of β-carotene ketolase in Haematococcus pluvialis. J. Biotechnol. 2015, 196, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Jaiswal, K.K.; Vlaskin, M.S.; Nanda, M.; Tripathi, M.K.; Kumar, S. Microalgae with a truncated light-harvesting antenna to maximize photosynthetic efficiency and biomass productivity: Recent advances and current challenges. Process Biochem. 2021, 104, 83–91. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Lafarga, T.; Sanchez-Zurano, A.; Morillas-España, A.; Acien-Fernandez, F.G. Extremophile microalgae as feedstock for high-value carotenoids: A review. Int. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Lafarga, T.; Rodríguez-Bermúdez, R.; Morillas-España, A.; Villaró, S.; García-Vaquero, M.; Morán, L.; Sánchez-Zurano, A.; González-López, C.V.; Acién-Fernández, F.G. Consumer knowledge and attitudes towards microalgae as food: The case of Spain. Algal Res. 2021, 54, 102174. [Google Scholar] [CrossRef]
- Lafarga, T.; Pieroni, C.; D’Imporzano, G.; Maggioni, L.; Adani, F.; Acién, G. Consumer Attitudes towards Microalgae Production and Microalgae-Based Agricultural Products: The Cases of Almería (Spain) and Livorno (Italy). ChemEngineering 2021, 5, 27. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, I.; Benavente Valdés, J.R.; Castellari, M.; Aguiló-Aguayo, I.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; Lafarga, T. Utilisation of the marine microalgae Nannochloropsis sp. and Tetraselmis sp. as innovative ingredients in the formulation of wheat tortillas. Algal Res. 2021, 58, 102361. [Google Scholar] [CrossRef]
- Lafarga, T.; Mayre, E.; Echeverria, G.; Viñas, I.; Villaró, S.; Acién-Fernández, F.G.; Castellari, M.; Aguiló-Aguayo, I. Potential of the microalgae Nannochloropsis and Tetraselmis for being used as innovative ingredients in baked goods. LWT 2019, 115, 108439. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- García-Segovia, P.; García Alcaraz, V.; Tárrega, A.; Martínez-Monzó, J. Consumer perception and acceptability of microalgae based breadstick. Food Sci. Technol. Int. 2020, 26, 493–502. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Uhlen, A.K.; Kousoulaki, K.; Haugen, J.E.; Rieder, A. Protein enrichment of wheat bread with the marine green microalgae Tetraselmis chuii–Impact on dough rheology and bread quality. LWT 2021, 143, 111115. [Google Scholar] [CrossRef]
- García-Segovia, P.; Pagán-Moreno, M.J.; Lara, I.F.; Martínez-Monzó, J. Effect of microalgae incorporation on physicochemical and textural properties in wheat bread formulation. Food Sci. Technol. Int. 2017, 23, 437–447. [Google Scholar] [CrossRef]
- Fradique, Ḿ.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef] [PubMed]
- Vakarelova, M.; Zanoni, F.; Lardo, P.; Rossin, G.; Mainente, F.; Chignola, R.; Menin, A.; Rizzi, C.; Zoccatelli, G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017, 221, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Alarcón, C.; Inostroza-Riquelme, M.; Torres-Gallegos, C.; Araya, C.; Miranda, M.; Sánchez-Caamaño, J.C.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. Protection of astaxanthin from photodegradation by its inclusion in hierarchically assembled nano and microstructures with potential as food. Food Hydrocoll. 2018, 83, 36–44. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci. Technol. 2021, 116, 176–185. [Google Scholar] [CrossRef]
- Molina, E.; Fernández, J.; Acién, F.G.; Chisti, Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- AECOSAN. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition on a request for initial assessment for marketing of the marine microalgae Tetraselmis chuii under Regulation (EC) No 258/97 on novel foods and novel food ingredients. Rev. Com. Cient. 2017, 25, 1–10. [Google Scholar]
- Sharon-Gojman, R.; Maimon, E.; Leu, S.; Zarka, A.; Boussiba, S. Advanced methods for genetic engineering of Haematococcus pluvialis (Chlorophyceae, Volvocales). Algal Res. 2015, 10, 8–15. [Google Scholar] [CrossRef]
- Fujii, K.; Nakashima, H.; Hashidzume, Y.; Uchiyama, T.; Mishiro, K.; Kadota, Y. Potential use of the astaxanthin-producing microalga, Monoraphidium sp. GK12, as a functional aquafeed for prawns. J. Appl. Phycol. 2010, 22, 363–369. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, Y.; Yu, Y.; Xiang, J.; Li, F. Effects of natural astaxanthin from microalgae and chemically synthetic astaxanthin supplementation on two different varieties of the ridgetail white prawn (Exopalaemon carinicauda). Algal Res. 2021, 57, 102347. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel Insights into the Biotechnological Production of Haematococcus pluvialis-Derived Astaxanthin: Advances and Key Challenges to Allow Its Industrial Use as Novel Food Ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: A Comparative Case of Synthetic vs. Natural Production; Faculty Publications and Other Works—Chemical and Biomolecular Engineering; The University of Tennessee: Knoxville, TN, USA, 2013; Available online: https://trace.tennessee.edu/cgi/viewcontent.cgi?article=1094&context=utk_chembiopubs (accessed on 15 June 2021).
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.Y.; Deng, D.F.; Dominy, W.G.; Forster, I.P. Pigmentation of Pacific White Shrimp, Litopenaeus vannamei, by Dietary Astaxanthin Extracted from Haematococcus pluvialis. J. World Aquac. Soc. 2011, 42, 633–644. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, G.; Mangott, A.; Kent, M.; Pirozzi, I. Nutrient efficacy of microalgae as aquafeed additives for the adult black tiger prawn, Penaeus monodon. Aquac. Res. 2016, 47, 3625–3635. [Google Scholar] [CrossRef]
- Barclay, M.C.; Irvin, S.J.; Williams, K.C.; Smith, D.M. Comparison of diets for the tropical spiny lobster Panulirus ornatus: Astaxanthin-supplemented feeds and mussel flesh. Aquac. Nutr. 2006, 12, 117–125. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Dietary astaxanthin augments disease resistance of Asian seabass, Lates calcarifer (Bloch, 1790), against Vibrio alginolyticus infection. Fish Shellfish Immunol. 2021, 114, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Perenlei, G.; Tojo, H.; Okada, T.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary astaxanthin rich yeast, Phaffia rhodozyma, on meat quality of broiler chickens. Anim. Sci. J. 2014, 85, 895–903. [Google Scholar] [CrossRef]
- Somagond, Y.M.; Singh, S.V.; Deshpande, A. Effect of dietary supplementation of astaxanthin, prill fat and combination on stress indicators, milk yield and composition during heat stress in buffaloes. Biol. Rhythm Res. 2019. [Google Scholar] [CrossRef]
- Tolba, S.A.; Magnuson, A.D.; Sun, T.; Lei, X.G. Dietary supplemental microalgal astaxanthin modulates molecular profiles of stress, inflammation, and lipid metabolism in broiler chickens and laying hens under high ambient temperatures. Poult. Sci. 2020, 99, 4853–4860. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.V. Influence of astaxanthin supplementation on attainment of puberty and lipid peroxidation in Sahiwal and Karan Fries (Holstein × Tharparkar) heifers during summer season. Biol. Rhythm Res. 2020, 51, 15–28. [Google Scholar] [CrossRef]
- Carballo, D.E.; Caro, I.; Andrés, S.; Giráldez, F.J.; Mateo, J. Assessment of the antioxidant effect of astaxanthin in fresh, frozen and cooked lamb patties. Food Res. Int. 2018, 111, 342–350. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Ghlissi, Z.; Taktak, M.A.; Ayadi, M.A.; Bougatef, A. The Influence of Natural Astaxanthin on the Formulation and Storage of Marinated Chicken Steaks. J. Food Biochem. 2016, 40, 393–403. [Google Scholar] [CrossRef]
- Pogorzelska, E.; Godziszewska, J.; Brodowska, M.; Wierzbicka, A. Antioxidant potential of Haematococcus pluvialis extract rich in astaxanthin on colour and oxidative stability of raw ground pork meat during refrigerated storage. Meat Sci. 2018, 135, 54–61. [Google Scholar] [CrossRef]
- Jiang, G.L.; Zhu, M.J. Preparation of astaxanthin-encapsulated complex with zein and oligochitosan and its application in food processing. LWT 2019, 106, 179–185. [Google Scholar] [CrossRef]
- Lafarga, T.; Gallagher, E.; Walsh, D.; Valverde, J.; Hayes, M. Chitosan-containing bread made using marine shellfishery byproducts: Functional, bioactive, and quality assessment of the end product. J. Agric. Food Chem. 2013, 61, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.Y.; Show, P.L.; Lim, M.H.; Ooi, C.W.; Ling, T.C. Natural red pigments from plants and their health benefits: A review. Food Rev. Int. 2018, 34, 463–482. [Google Scholar] [CrossRef]
- Lafarga, T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Xiong, Y.J.; Yang, M.M.; Zhu, M.J. Preparation of astaxanthin mask from Phaffia rhodozyma and its evaluation. Process Biochem. 2019, 79, 195–202. [Google Scholar] [CrossRef]
- Jiang, G.L.; Zhou, L.Y.; Wang, Y.T.; Zhu, M.J. Astaxanthin from Jerusalem artichoke: Production by fed-batch fermentation using Phaffia rhodozyma and application in cosmetics. Process Biochem. 2017, 63, 16–25. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1050–1064. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Naqvi, S.R.Z.; Abdelnour, S.A.; Schreurs, N.; Mohammedsaleh, Z.M.; Khan, I.; Shater, A.F.; Abd El-Hack, M.E.; Khafaga, A.F.; Quan, G.; et al. Beneficial effects and health benefits of Astaxanthin molecules on animal production: A review. Res. Vet. Sci. 2021, 138, 69–78. [Google Scholar] [CrossRef]
- Ahmed, F.; Li, Y.; Fanning, K.; Netzel, M.; Schenk, P.M. Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 2015, 74, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Karppi; Rissanen; Nyyssönen; Kaikkonen; Olsson; Voutilainen; Salonen. Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2013, 77, 3–11. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Sokri, N.; Phd, M.; Zakerkish, M.; Mohammadiasl Phd, J.; Phd, M.Z.; Mohammadshahi, M.; Hossein, M.; Msc, H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of Dietary Supplementation of Astaxanthin and Sesamin on Daily Fatigue: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Sun, Z.; Chen, A.; Guo, X.; Wang, J. Effect of astaxanthin and exercise on antioxidant capacity of human body, blood lactic acid and blood uric acid metabolism. Sci. Sports 2019, 34, 348–352. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Turrin, N.P.; Rivest, S. Molecular and cellular immune mediators of neuroprotection. Mol. Neurobiol. 2006, 34, 221–242. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the neuroprotective role of astaxanthin: New perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef] [Green Version]
- Grimmig, B.; Kim, S.-H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. GeroScience 2017, 39, 19–32. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Arunkumar, E.; Viswanathan, P.; Anuradha, C.V. Astaxanthin restricts weight gain, promotes insulin sensitivity and curtails fatty liver disease in mice fed a obesity-promoting diet. Process Biochem. 2010, 45, 1406–1414. [Google Scholar] [CrossRef]
- Fakhri, S.; Aneva, I.Y.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective effects of astaxanthin: Therapeutic targets and clinical perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef] [Green Version]
- Bolin, A.P.; Macedo, R.C.; Marin, D.P.; Barros, M.P.; Otton, R. Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol. Toxicol. 2010, 26, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, W.; Gao, Y.; Yang, R.; Zhang, X.; Xu, J.; Tang, Q. Astaxanthin (ATX) enhances the intestinal mucosal functions in immunodeficient mice. Food Funct. 2020, 11, 3371–3381. [Google Scholar] [CrossRef]
- Lin, K.H.; Lin, K.C.; Lu, W.J.; Thomas, P.A.; Jayakumar, T.; Sheu, J.R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and Il-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int. J. Mol. Sci. 2015, 17, 44. [Google Scholar] [CrossRef] [Green Version]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 2000, 70, 185–189. [Google Scholar] [CrossRef]
- Wang, X.; Willen, R.; Wadstrom, T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob. Agents Chemother. 2000, 44, 2452–2457. [Google Scholar] [CrossRef] [Green Version]
- Mageswari, A.; Subramanian, P.; Srinivasan, R.; Karthikeyan, S.; Gothandam, K.M. Astaxanthin from psychrotrophic Sphingomonas faeni exhibits antagonism against food-spoilage bacteria at low temperatures. Microbiol. Res. 2015, 179, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Shpigel, T.; Harris, L.G.; Schuster, R.; Lewis, E.C.; Lewitus, D.Y. Astaxanthin-based polymers as new antimicrobial compounds. Polym. Chem. 2017, 8, 4182–4189. [Google Scholar] [CrossRef]
- Santoyo, S.; Jaime, L.; Plaza, M.; Herrero, M.; Rodriguez-Meizoso, I.; Ibañez, E.; Reglero, G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2011, 24, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-N.; Kapupara, K.; Wen, Y.-T.; Chen, Y.-H.; Pan, I.-H.; Tsai, R.-K. Haematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia. Mar. Drugs 2020, 18, 85. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Fang, H.-H.; Liu, Z.-Z.; Huang, M.-Q.; Su, M.; Zhang, C.-W.; Gao, B.-Y.; Niu, J. A newly isolated strain of Haematococcus pluvialis JNU35 improves the growth, antioxidation, immunity and liver function of golden pompano (Trachinotus ovatus). Aquac. Nutr. 2021, 27, 342–354. [Google Scholar] [CrossRef]
- Ng, Q.X.; de Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of Astaxanthin Supplementation on Skin Health: A Systematic Review of Clinical Studies. J. Diet. Suppl. 2020, 18, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Z.; Gerken, H.; Liu, Z.; Jiang, Y.; Chen, F. Chlorella zofingiensis as an Alternative Microalgal Producer of Astaxanthin: Biology and Industrial Potential. Mar. Drugs 2014, 12, 3487–3515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, L.; Liu, J. Exogenous sodium acetate enhances astaxanthin accumulation and photoprotection in Haematococcus pluvialis at the non-motile stage. J. Appl. Phycol. 2018, 31, 1001–1008. [Google Scholar] [CrossRef]
- Katsuda, T.; Shimahara, K.; Shiraishi, H.; Yamagami, K.; Ranjbar, R.; Katoh, S. Effect of flashing light from blue light emitting diodes on cell growth and astaxanthin production of Haematococcus pluvialis. J. Biosci. Bioeng. 2006, 102, 442–446. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J. Growth-associated biosynthesis of astaxanthin in heterotrophic Chlorella zofingiensis (Chlorophyta). World J. Microbiol. Biotechnol. 2008, 24, 1915–1922. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.J.; Sun, D.; Lee, Y.; Chen, F. Two-step cultivation for production of astaxanthin in Chlorella zofingiensis using a patented energy-free rotating floating photobioreactor (RFP). Bioresour. Technol. 2017, 224, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, B.; Duan, R.; Jia, J.; Li, J.; Xiong, W.; Ling, X.; Chen, C.; Huang, X.; Zhang, G.; et al. Enhancing astaxanthin accumulation in Xanthophyllomyces dendrorhous by a phytohormone: Metabolomic and gene expression profiles. Microb. Biotechnol. 2020, 13, 1446–1460. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Wang, H.; Xiao, S.; Li, C.; Li, Y.; Liu, B. Xanthophyllomyces dendrorhous-Derived Astaxanthin Regulates Lipid Metabolism and Gut Microbiota in Obese Mice Induced by A High-Fat Diet. Mar. Drugs 2019, 17, 337. [Google Scholar] [CrossRef] [Green Version]
- Gervasi, T.; Santini, A.; Daliu, P.; Salem, A.Z.M.; Gervasi, C.; Pellizzeri, V.; Barrega, L.; De Pasquale, P.; Dugo, G.; Cicero, N. Astaxanthin production by Xanthophyllomyces dendrorhous growing on a low cost substrate. Agrofor. Syst. 2019, 94, 1229–1234. [Google Scholar] [CrossRef]
- Hara, K.Y.; Kageyama, Y.; Tanzawa, N.; Hirono-Hara, Y.; Kikukawa, H.; Wakabayashi, K. Development of astaxanthin production from citrus peel extract using Xanthophyllomyces dendrorhous. Environ. Sci. Pollut. Res. 2020, 28, 12640–12647. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Benameur, Q.; Gervasi, C.; Santini, A.; Cicero, N.; Dugo, G. Valorization of raw materials from agricultural industry for astaxanthin and β-carotene production by Xanthophyllomyces dendrorhous. Nat. Prod. Res. 2017, 32, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Lee, J.-H.; Chon, J.-W.; Seo, K.-H.; Kim, S.-K. Improved astaxanthin production by Xanthophyllomyces dendrorhous SK984 with oak leaf extract and inorganic phosphate supplementation. Food Sci. Biotechnol. 2019, 28, 1171–1176. [Google Scholar] [CrossRef] [PubMed]

| Brand | Company | Description | Astaxanthin Content |
|---|---|---|---|
| Better Foods Astaxanthin | Better Foods GmbH, Göttingen, Germany | Food supplement rich in astaxanthin extracted from H. pluvialis. Contains 80 capsules, and the recommended daily intake is two capsules. | 4 mg per capsule |
| Time Health Astaxanthin | Time Health Ltd., Uckfield, UK | Capsules containing 350 mg of powdered H. pluvialis. Commercialized as super antioxidant, GMO free, gluten free, and 100% vegan. The recommended daily intake is one capsule. | 7 mg per capsule |
| Natural Astaxanthin | Nutravita Ltd., Maidenhead, UK | Food supplement containing astaxanthin-rich oleoresin from H. pluvialis. Softgels commercialized as free from artificial colors and flavors, GMO free, and easy to swallow | 0.9 mg per softgel |
| Astaxanthin 10 mg | Solgar Inc., New Jersey, USA | Softgels containing natural astaxanthin labelled as non-GMO; glute, wheat, and dairy free. Product commercialized as an antioxidant support and a support for a healthy skin | 10 mg per softgel |
| Sila Astaxanthin | Sila, Hsinchu, Taiwan | Food supplement containing astaxanthin extracted from H. pluvialis plus vitamins and Omega-3. | 12 mg per capsule |
| Vivanaturals Astaxanthin from microalgae | Viva Naturals Inc., North York, Canada | Softgels sold as GMO free, dairy free, and gluten free that are good for inspiring firm and healthy skin, supporting immune health, and providing antioxidant protection. | 4 mg per softgel |
| Ox Nature Natural Hawaiian Astaxanthin | Ox Nature, Lisbon, Portugal | Softgels commercialized as 100% pure, vegan, GMO free, and wheat free. | 4 mg per softgel |
| Synthetic Astaxanthin | Natural Astaxanthin | Reference | |
|---|---|---|---|
| Stereochemistry | (3R,3′R), (3R,3′S), (3S,3′S) optical isomers-1:2:1 | (3R,3′R), (3R,3′S), (3S,3′S) optical isomers-1:2:22 | [40] |
| Structure | Non-esterified | More than 95% of the natural astaxanthin molecules are esterified | [40,41] |
| Industrial uses | Aquaculture feed | Food and dietary supplements, cosmetics, nutraceuticals, aquaculture feed | [41,42] |
| Market Price | USD 2000 per kg | USD 7000 per kg | [43] |
| Production |
|
| [44] |
| Production Price * | 800–900 €·kg−1 | 1000–7000 €·kg−1 | [43] |
| Health Benefit | Conditions Studied | Comments | Reference |
|---|---|---|---|
| Protecting against lipid peroxidation | Two 4-mg astaxanthin (Astaxin®) capsules daily for 3 months | Astaxanthin consumption decreased the plasma levels of 12- and 15-fatty acids of healthy non-smoking men | [69] |
| Decreasing TG levels and increasing HDL-cholesterol | 12 mg·day−1 astaxanthin for 12 weeks | Non-obese subjects with fasting serum triglyceride of 120–200 mg/dL and without diabetes and hypertension, aged 25–60 years. | [70] |
| Reducing oxidative stress and reversing age-related morphological changes of skin | 4 mg·day−1 astaxanthin for 4 weeks | The study included 31 volunteers (17 men and 14 women) over the age of 40. | [71] |
| Improving glucose metabolism and reducing blood pressure | 8 mg·day−1 astaxanthin for 8 weeks | The study enrolled 44 participants with type 2 diabetes. | [72] |
| Recovering from mental fatigue | 3 mg·day−1 and 5 mg·day−1 of sesamin for 4 weeks | The study included 24 healthy volunteers. | [73] |
| Protecting against UV-induced skin deterioration | 4 mg·day−1 astaxanthin for 10 weeks | The study included 23 healthy participants. | [74] |
| Maintaining the body’s antioxidant capacity after high-intensity exercise | 12 mg·day−1 astaxanthin for 4 weeks | The study enrolled 16 male students. | [75] |
| Decreasing a DNA damage biomarker and enhancing immune response | 2–8 mg·day−1 astaxanthin for 8 weeks | The study included 42 young healthy females. | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. https://doi.org/10.3390/foods10102303
Villaró S, Ciardi M, Morillas-España A, Sánchez-Zurano A, Acién-Fernández G, Lafarga T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods. 2021; 10(10):2303. https://doi.org/10.3390/foods10102303
Chicago/Turabian StyleVillaró, Silvia, Martina Ciardi, Ainoa Morillas-España, Ana Sánchez-Zurano, Gabriel Acién-Fernández, and Tomas Lafarga. 2021. "Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food" Foods 10, no. 10: 2303. https://doi.org/10.3390/foods10102303
APA StyleVillaró, S., Ciardi, M., Morillas-España, A., Sánchez-Zurano, A., Acién-Fernández, G., & Lafarga, T. (2021). Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods, 10(10), 2303. https://doi.org/10.3390/foods10102303








