The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review
Abstract
1. Introduction
2. Decontamination Plans for Game Meat Animals during Slaughter
- Most decontamination strategies may change the appearance and texture of a product.
- Specific concentrations must be maintained to ensure that they do not alter the texture of meat products.
- There is a lack of sufficient data or information on the usage of different decontamination regiments on carcasses whilst still maintaining the quality of the product.
- There can be a large cost of implementing a decontamination plan on top of the general hygiene prescripts that must be followed during animal slaughter at an abattoir.
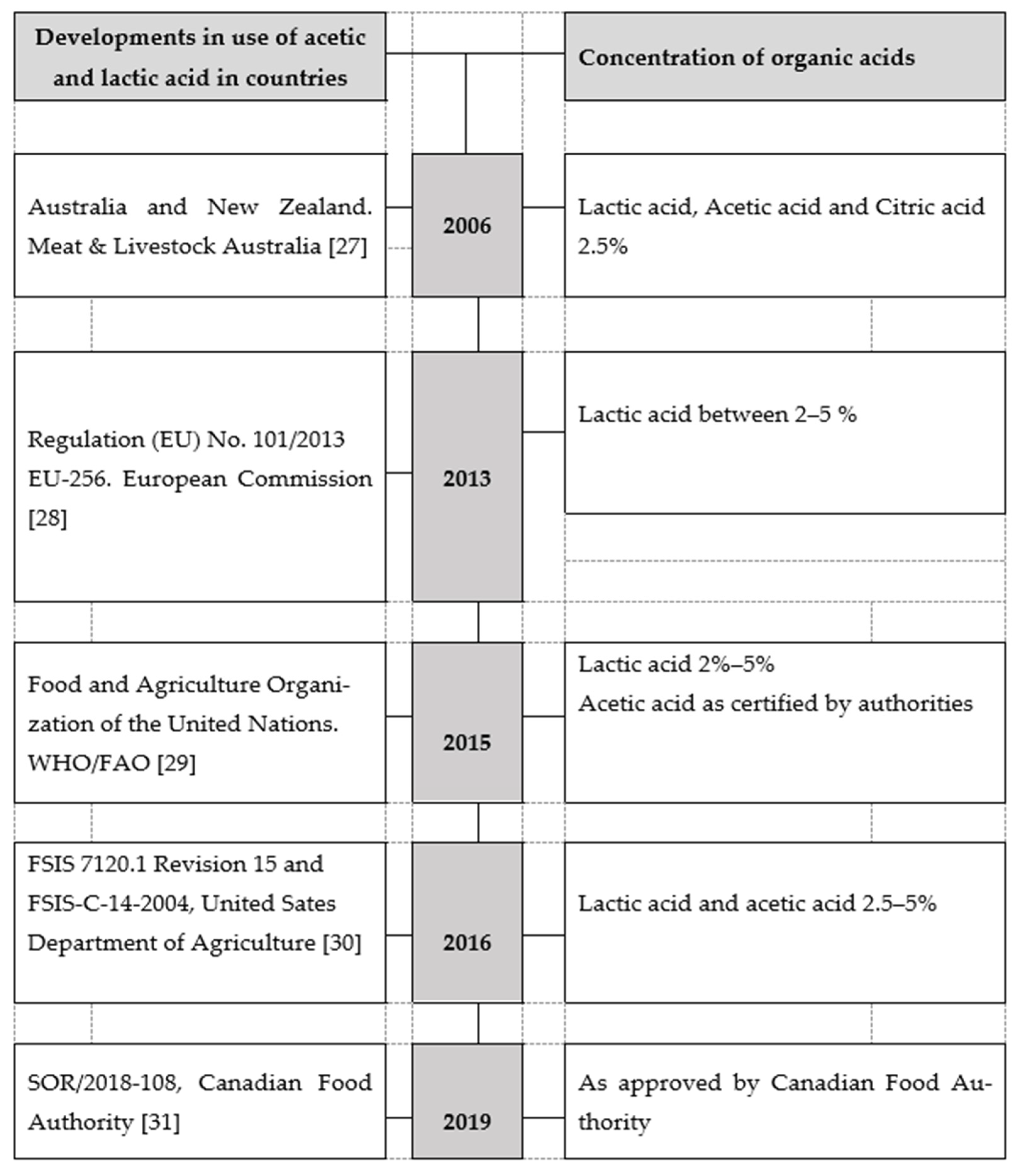
2.1. Organic Acid Usage
2.2. Lactic Acid Treatment
2.3. Acetic Acid Treatment
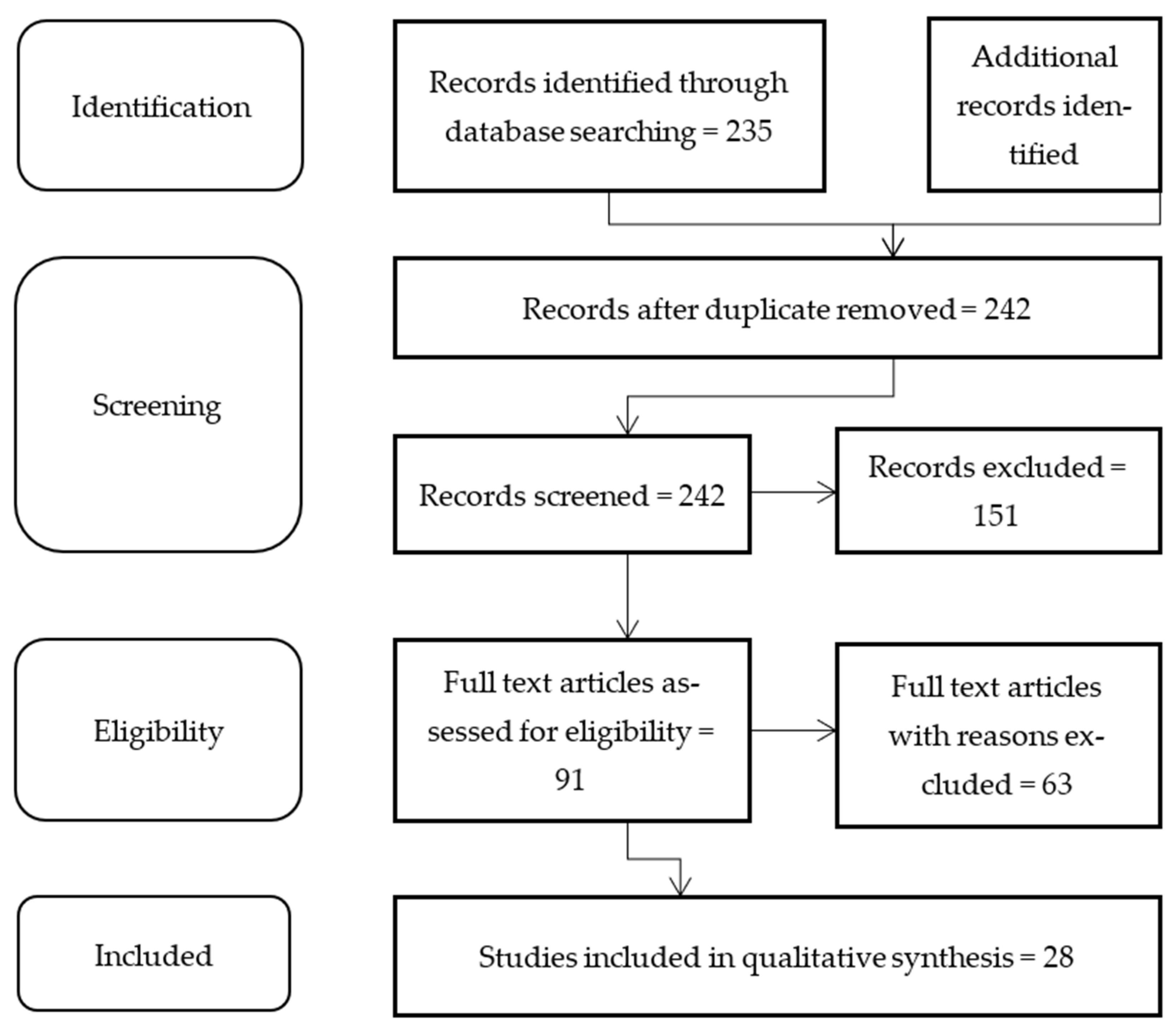
3. Materials and Methods
4. Results
| Country | Aim | Product Investigated | Experimental Conditions | Study Findings and Recommendations | Reference * |
|---|---|---|---|---|---|
| United States of America | To compare spray washing at 55.4 °C of a 2% levulinic acid with lactic or acetic acid for decontamination of pathogenic bacteria inoculated onto meat surfaces and their residual protection against later growth of pathogenic bacteria | Red meat and poultry |
|
| [33] |
| Switzerland | To examine antibacterial activity of LA, AA and steam as decontamination treatments for cattle hides and beef carcasses | Beef |
|
| [34,35,36] |
| United States of America | To examine mechanisms of reducing contamination by C. jejuni in broiler carcasses that were vaccinated with Lactobacilli as chicks. | Poultry |
|
| [37] |
| Serbia | To investigate possible interventions of controlling Salmonella contamination during poultry, beef and pig slaughter | Poultry, beef and pork |
|
| [38,39,40] |
| United States of America | To determine the effectiveness of eight antimicrobial compounds including LA and AA in a laboratory. | Beef surfaces |
|
| [41] |
| Turkey | To compare the inhibitory effects of various decontamination agents at different OA concentrations on Listeria monocytogenes contaminated raw beef samples. | Beef |
|
| [42] |
| Mexico | To investigate microbial adaptation to OA as antimicrobials to control Salmonella in meat and poultry products. | Poultry |
|
| [43,44] |
| Canada | To investigate microbial decontamination of raw and ready-to-eat meats using OA | Raw and ready to eat meat |
|
| [44,45] |
| Singapore | To establish different intervention technologies ensuring microbial safety of meat | Raw meat |
|
| [46,47,48] |
| Spain | To investigate effective control and treatment plans for Campylobacter in abattoirs. | Poultry |
|
| [49,50] |
| United States of America | To establish the efficiency and effect of different concentrations of LA, AA, citric and propionic acid dipping solutions on bacterial contamination of raw chicken skin | Poultry |
|
| [51] |
| United States of America | To investigate the survival and adaptation of Salmonella spp. when subjected to acidic conditions on carcass surfaces. | Beef and porcine |
|
| [52] |
| Greece | To analyse carcass decontamination strategies employable in slaughterhouses: a review | Meat animal carcass |
|
| [35,53] |
| Sri Lanka | To investigate the effect of natural compounds and acids on Salmonella typhimurium in broiler chicken meat | Poultry |
|
| [54] |
| United states of America | To determine the bactericidal activity of lactic acid (LA), levulinic acid (LV) and sodium dodecyl sulfate (SDS) applied individually and in combination with Shiga toxin-producing Escherichia coli (STEC) under laboratory conditions | Beef cuts-experiments on trimmings |
|
| [55] |
| France | To investigate lactic acid bacteria (LAB) and their controversial role in fresh meat spoilage | Raw meat |
|
| [56,57] |
| United States of America | To investigate antimicrobial formulations and sanitation methods for meat and poultry product processing. | Poultry |
|
| [58,59] |
| Pakistan | Postharvest intervention technologies for safety enhancement of meat and meat-based products; a critical review | Beef |
|
| [4,60] |
| United States of America | To evaluate the ability of a bromine-based antimicrobial lactic acid (LA) and peroxyacetic acid (PAA) applied in a final carcass wash to reduce non-pathogenic Escherichia coli. | Bovine |
|
| [61] |
| Spain | To test the efficiency of lactic acid concentrations on the reduction of microbial load yet minimally impact the colour and sensory characteristics of beef | Beef |
|
| [62] |
| Canada | To investigate possible pathogens reduction strategies employable at the primary production level especially in relation to multi drug-resistant strains | Raw meat |
|
| [63] |
| Romania | To assess the efficiency of organic acids LA, AA and citric acid in different concentrations on pathogens such as Salmonella, Listeria and Escherichia on beef. | Beef |
|
| [27] |
| Japan | To evaluate the effect of LA with and without organic material at various post-treatment recovery times on the heat resistance of Listeria monocytogenes. | Bovine products |
|
| [18] |
| Egypt | To test the antibacterial effect of lactic acid (LA) and acetic acid (AA) on sheep carcass surface after 20 min of spraying. | Sheep carcasses |
|
| [64] |
| United States of America | To investigate the effectiveness of organic acids (LA) on Salmonella ssp. reduction on ground beef. | Beef |
|
| [65] |
| United States of America | To establish the interactions of organic acids (LA and AA) with Campylobacter coli from swine | Red meat |
|
| [66] |
| Australia | To investigate meat safety risks for the Australian red meat market | Red meat |
|
| [67,68] |
| Egypt | To investigate the effect of LA, AA and trisodium phosphate (TSP) spray on the microbiological population. | Beef carcasses |
|
| [69] |
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Matthews, K.R.; Kniel, K.E.; Montville, T.J. Food Microbiology: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Santos, E.C.C.d.; Castro, V.S.; Cunha-Neto, A.; Santos, L.F.d.; Vallim, D.C.; Lisbôa, R.d.C.; Carvalho, R.C.T.; Junior, C.A.C.; Figueiredo, E.E.d.S. Escherichia coli O26 and O113: H21 on carcasses and beef from a slaughterhouse located in Mato Grosso, Brazil. Foodborne Pathog. Dis. 2018, 15, 653–659. [Google Scholar] [CrossRef]
- Mallhi, I.Y.; Sohaib, M.; Khan, A.U.; Nawaz, M. Evaluating food safety knowledge, practices, and microbial profile of meat in abattoirs and butchery shops in Lahore, Pakistan. J. Food Saf. 2019, 39, e12612. [Google Scholar] [CrossRef]
- Sohaib, M.; Anjum, F.M.; Arshad, M.S.; Rahman, U.U. Postharvest intervention technologies for safety enhancement of meat and meat based products; a critical review. J. Food Sci. Technol. 2016, 53, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Wardhana, D.K. Risk Factors for Bacterial Contamination of Bovine Meat during Slaughter in Ten Indonesian Abattoirs. Vet. Med. Int. 2019, 2019, 2707064. [Google Scholar]
- Hedman, H.D.; Varga, C.; Duquette, J.; Novakofski, J.; Mateus-Pinilla, N.E. Food Safety Considerations Related to the Consumption and Handling of Game Meat in North America. Vet. Sci. 2020, 7, 188. [Google Scholar] [CrossRef]
- Gouws, P.A.; Shange, N.; Hoffman, L.C. Microbial quality of springbok (Antidorcas marsupialis) meat in relation to harvesting and production process. In Game Meat Hygiene: Food Safety and Security; Wageningen Academic Publishers: Wageningen, The Nearthelands, 2017; pp. 67–92. [Google Scholar]
- Kautto, A.H.; Vågsholm, I.; Niskanen, R. Reindeer–wild game ante and post mortem. In Game Meat Hygiene–Food Safety and Security; Wageningen Academic Publishers: Wageningen, The Nearthelands, 2017; pp. 141–152. [Google Scholar]
- Yang, X.; Tran, F.; Wolters, T. Microbial ecology of decontaminated and not decontaminated beef carcasses. J. Food Res. 2017, 6, 85–91. [Google Scholar] [CrossRef]
- Hochreutener, M.; Zweifel, C.; Corti, S.; Stephan, R. Effect of a commercial steam-vacuuming treatment implemented after slaughtering for the decontamination of cattle carcasses. Ital. J. Food Saf. 2017, 16, 6864. [Google Scholar] [CrossRef][Green Version]
- Van Ba, H.; Seo, H.-W.; Pil-Nam, S.; Kim, Y.-S.; Park, B.Y.; Moon, S.-S.; Kang, S.-J.; Choi, Y.-M.; Kim, J.-H. The effects of pre-and post-slaughter spray application with organic acids on microbial population reductions on beef carcasses. Meat Sci. 2018, 137, 16–23. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Da Costa, R.J.; Voloski, F.L.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef]
- Hilbig, J.; Loeffler, M.; Herrmann, K.; Weiss, J. Application of exopolysaccharide-forming lactic acid bacteria in cooked ham model systems. Food Res. Int. 2019, 119, 761–768. [Google Scholar] [CrossRef]
- Doyle, N.; Mbandlwa, P.; Kelly, W.J.; Attwood, G.; Li, Y.; Ross, R.P.; Stanton, C.; Leahy, S. Use of lactic acid bacteria to reduce methane production in ruminants, a critical review. Front. Microbiol. 2019, 10, 2207. [Google Scholar] [CrossRef]
- Casas, D.E.; Vargas, D.A.; Randazzo, E.; Lynn, D.; Echeverry, A.; Brashears, M.M.; Sanchez-Plata, M.X.; Miller, M.F. In-Plant Validation of Novel On-Site Ozone Generation Technology (Bio-Safe) Compared to Lactic Acid Beef Carcasses and Trim Using Natural Microbiota and Salmonella and E. coli O157: H7 Surrogate Enumeration. Foods 2021, 10, 1002. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E.; Ergin, F.; Küçükçetin, A. Reduction of Salmonella enterica in Turkey breast slices kept under aerobic and vacuum conditions by application of lactic acid, a bacteriophage, and ultrasound. J. Food Saf. 2021, e12923. [Google Scholar]
- Omori, Y.; Miake, K.; Nakamura, H.; Kage-Nakadai, E.; Nishikawa, Y. Influence of lactic acid and post-treatment recovery time on the heat resistance of Listeria monocytogenes. Int. J. Food Microbiol. 2017, 257, 10–18. [Google Scholar] [CrossRef]
- Han, J.; Luo, X.; Zhang, Y.; Zhu, L.; Mao, Y.; Dong, P.; Yang, X.; Liang, R.; Hopkins, D.L.; Zhang, Y. Effects of spraying lactic acid and peroxyacetic acid on the bacterial decontamination and bacterial composition of beef carcasses. Meat Sci. 2020, 164, 108104. [Google Scholar] [CrossRef] [PubMed]
- Dan, S.D.; Mihaiu, M.; Reget, O.; Oltean, D.; Tabaran, A. Influence on week organic acids on pathogens on swine carcasses. In Lucrari Stiintifice-Medicina Veterinara; Universitatea de Stiinte Agricole si Medicina Veterinara “Ion. Ionescu de la Brad” Iasi: Iași, Romania, 2017; Volume 60, pp. 265–273. [Google Scholar]
- Kure, C.F.; Axelsson, L.; Carlehög, M.; Måge, I.; Jensen, M.R.; Holck, A. The effects of a pilot-scale steam decontamination system on the hygiene and sensory quality of chicken carcasses. Food Control 2020, 109, 106948. [Google Scholar] [CrossRef]
- Shange, N.; Gouws, P.; Hoffman, L.C. Campylobacter and Arcobacter species in food-producing animals: Prevalence at primary production and during slaughter. World J. Microbiol. Biotechnol. 2019, 35, 1–16. [Google Scholar] [CrossRef]
- Pohlman, F.; Dias-Morse, P.; Pinidiya, D. Product safety and color characteristics of ground beef processed from beef trimmings treated with peroxyacetic acid alone or followed by novel organic acids. J. Microbiol. Biotechnol. Food Sci. 2019, 2019, 93–101. [Google Scholar] [CrossRef]
- South Africa. Meat Safety Act. Act 40 of 2000; No. 1106; Agriculture, D.O., Ed.; Goverment Gazzet: Pretoria, South Africa, 2000.
- South Africa. Standard for Microbiological Monitoring of Meat Process Hygiene and Cleaning; VPN/15/2010/01; Government Gazette: Pretoria, South Africa, 2010.
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Dan, S.D.; Mihaiu, M.; Reget, O.; Oltean, D.; Tăbăran, A. Pathogens Contamination Level Reduction on Beef Using Organic Acids Decontamination Methods. Bull. UASVM Vet. Med. 2017, 74, 2. [Google Scholar] [CrossRef][Green Version]
- European Commission. Commission Regulation (EU) No 101/2013 of 4 February 2013 concerning the use of lactic acid to reduce microbiological surface contamination on bovine carcasses. Off. J. Eur. Union L 2013, 34, 1–3. [Google Scholar]
- WHO; FAO. Interventions for the control of non-typhoidal Salmonella spp. in beef and pork. In Microbiological Risk Assessment Series 30; FAO: Rome, Italy, 2016. [Google Scholar]
- United States Department of Agriculture (USDAS). Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products; Directive, F., Ed.; 7120.1 Rev. 55; USDA ERS: Washington, DC, USA, 2020.
- Signorini, M.; Costa, M.; Teitelbaum, D.; Restovich, V.; Brasesco, H.; García, D.; Superno, V.; Petroli, S.; Bruzzone, M.; Arduini, V. Evaluation of decontamination efficacy of commonly used antimicrobial interventions for beef carcasses against Shiga toxin-producing Escherichia coli. Meat Sci. 2018, 142, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Agency, C.F.I. Safe Food for Canadians Regulations. In SOR/2018-108; Minister of Justice: Ottawa, ON, Canada, 2021. [Google Scholar]
- Carpenter, C.; Smith, J.; Broadbent, J. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 88, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Loretz, M.; Stephan, R.; Zweifel, C. Antibacterial activity of decontamination treatments for cattle hides and beef carcasses. Food Control. 2011, 22, 347–359. [Google Scholar] [CrossRef]
- Algino, R.; Ingham, S.; Zhu, J. Survey of antimicrobial effects of beef carcass intervention treatments in very small state-inspected slaughter plants. J. Food Sci. 2007, 72, M173–M179. [Google Scholar] [CrossRef]
- Bell, K.Y.; Cutter, C.N.; Sumner, S.S. Reduction of foodborne micro-organisms on beef carcass tissue using acetic acid, sodium bicarbonate, and hydrogen peroxide spray washes. Food Microbiol. 1997, 14, 439–448. [Google Scholar] [CrossRef]
- Neal-McKinney, J.M.; Lu, X.; Duong, T.; Larson, C.L.; Call, D.R.; Shah, D.H.; Konkel, M.E. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE 2012, 7, e43928. [Google Scholar] [CrossRef]
- Buncic, S.; Sofos, J. Interventions to control Salmonella contamination during poultry, cattle and pig slaughter. Food Res. Int. 2012, 45, 641–655. [Google Scholar] [CrossRef]
- Carlson, B.A.; Geornaras, I.; Yoon, Y.; Scanga, J.A.; Sofos, J.N.; Smith, G.C.; Belk, K.E. Studies to evaluate chemicals and conditions with low-pressure applications for reducing microbial counts on cattle hides. J. Food Prot. 2008, 71, 1343–1348. [Google Scholar] [CrossRef]
- Castillo, A.; Lucia, L.; Goodson, K.; Savell, J.; Acuff, G. Decontamination of beef carcass surface tissue by steam vacuuming alone and combined with hot water and lactic acid sprays. J. Food Prot. 1999, 62, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Yoder, S.F.; Henning, W.R.; Mills, E.W.; Doores, S.; Ostiguy, N.; Cutter, C.N. Investigation of chemical rinses suitable for very small meat plants to reduce pathogens on beef surfaces. J. Food Prot. 2012, 75, 14–21. [Google Scholar] [CrossRef]
- Elmali, M.; Yaman, H.; Tekinsen, K.K.; Öner, S.; Çekin, E. Inhibitory Effects of Different Decontamination Agents on the Levels of Listeria monocytogenes in the Experimentally Inoculated Raw Beef Samples in the Laboratory Conditions. J. Fac. Vet. Med. 2012, 18, 763–768. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- De Martinez, Y.B.; Ferrer, K.; Salas, E.M. Combined effects of lactic acid and nisin solution in reducing levels of microbiological contamination in red meat carcasses. J. Food Prot. 2002, 65, 1780–1783. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.; Gill, C. Microbial decontamination of raw and ready-to-eat meats. In Microbial Decontamination in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 30–59. [Google Scholar]
- Chen, J.; Ren, Y.; Seow, J.; Liu, T.; Bang, W.; Yuk, H. Intervention technologies for ensuring microbiological safety of meat: Current and future trends. Compr. Rev. Food Sci. Food Saf. 2012, 11, 119–132. [Google Scholar] [CrossRef]
- Avens, J.S.; Albright, S.N.; Morton, A.S.; Prewitt, B.E.; Kendall, P.A.; Sofos, J.N. Destruction of microorganisms on chicken carcasses by steam and boiling water immersion. Food Control. 2002, 13, 445–450. [Google Scholar] [CrossRef]
- Stopforth, J.D.; Yoon, Y.; Belk, K.; Scanga, J.; Kendall, P.; Smith, G.; Sofos, J. Effect of simulated spray chilling with chemical solutions on acid-habituated and non–acid-habituated Escherichia coli O157: H7 cells attached to beef carcass tissue. J. Food Prot. 2004, 67, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Rovira, R.F.; Bermudo, F.M.; Cameán, A.M.; Fernández, A.C.S.; Álvarez, M.D.; Marteache, A.H.; Toledano, F.L.; de Santos, M.R.M.; de Victoria Muñoz, E.M.; Larrañaga, M.R.M. Report of the Scientific Committee of the Spanish Agency for Food Safety and Nutrition (AESAN) on the Control Strategies to Reduce the Burden of Campylobacter spp. in Fresh Poultry Meat (Broiler); AESAN: Madrid, Spain, 2012; Report number: 2012-005.
- Koolman, L.; Whyte, P.; Meade, J.; Lyng, J.; Bolton, D. Use of chemical treatments applied alone and in combination to reduce Campylobacter on raw poultry. Food Control. 2014, 46, 299–303. [Google Scholar] [CrossRef]
- Menconi, A.; Shivaramaiah, S.; Huff, G.; Prado, O.; Morales, J.; Pumford, N.; Morgan, M.; Wolfenden, A.; Bielke, L.; Hargis, B. Effect of different concentrations of acetic, citric, and propionic acid dipping solutions on bacterial contamination of raw chicken skin. Poult. Sci. 2013, 92, 2216–2220. [Google Scholar] [CrossRef]
- Burin, R.C.K.; Silva, A., Jr.; Nero, L.A. Influence of lactic acid and acetic acid on Salmonella spp. growth and expression of acid tolerance-related genes. Food Res. Int. 2014, 64, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Milios, K.; Drosinos, E.; Zoiopoulos, P. Carcass decontamination methods in slaughterhouses: A review. J. Hell. Vet. Med Soc. 2014, 65, 65–78. [Google Scholar] [CrossRef][Green Version]
- Madushanka, D.; Jayaweera, T.; Jayasinghe, J.; Ruwandeepika, H. Effect of organic acids (citric, acitic, lactic) and natural compounds (nutmeg, mace, cardemom) on Salmonella typhimurium in broiler chicken meat. In Proceedings of the ISAE 2014—International Symposium on Agriculture and Environment 2014, Ruhuna, Sri Lanka, 27 November 2014; pp. 273–277. [Google Scholar]
- Zhao, T.; Zhao, P.; Chen, D.; Jadeja, R.; Hung, Y.-C.; Doyle, M.P. Reductions of Shiga toxin–producing Escherichia coli and Salmonella Typhimurium on beef trim by lactic acid, levulinic acid, and sodium dodecyl sulfate treatments. J. Food Prot. 2014, 77, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef]
- Jones, R.J.; Hussein, H.M.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Isolation of lactic acid bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008, 25, 228–234. [Google Scholar] [CrossRef]
- Nesbakken, T. Update on Yersinia as a foodborne pathogen: Analysis and control. In Advances in Microbial Food Safety; Elsevier: Amsterdam, The Netherlands, 2015; pp. 33–58. [Google Scholar]
- Delmore, L.G.; Sofos, J.; Schmidt, G.; Smith, G. Decontamination of inoculated beef with sequential spraying treatments. J. Food Sci. 1998, 63, 890–900. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Fernández, A.; Bernardo, A.; López, M. Acid tolerance in Salmonella typhimurium induced by culturing in the presence of organic acids at different growth temperatures. Food Microbiol. 2010, 27, 44–49. [Google Scholar] [CrossRef]
- Bullard, B.; Geornaras, I.; Delmore, R.; Woerner, D.; Martin, J.; Belk, K. Validation of Antimicrobial Interventions Including the Use of 1, 3-Dibromo-5, 5-Dimethylhydantoin Applied in a Final Carcass Wash in a Commercial Beef Harvest Operation. Meat Muscle Biol. 2017, 1, 121–122. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Lactic acid concentrations that reduce microbial load yet minimally impact colour and sensory characteristics of beef. Meat Sci. 2017, 129, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Warriner, K.; Namvar, A. Current Challenges in Enhancing the Microbiological Safety of Raw Meat. In New Aspects of Meat Quality; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–222. [Google Scholar]
- Saad, S.M.; Hassanin, F.S.; Salem, A.M.; Saleh, E.A.E. Efficiency of some organic acids as decontaminants in sheep carcasses. Benha Vet. Med J. 2020, 38, 116–119. [Google Scholar]
- Yeh, Y.; De Moura, F.; Van Den Broek, K.; De Mello, A. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef]
- Beier, R.C.; Harvey, R.B.; Hernandez, C.A.; Hume, M.E.; Andrews, K.; Droleskey, R.E.; Davidson, M.K.; Bodeis-Jones, S.; Young, S.; Duke, S.E. Interactions of organic acids with Campylobacter coli from swine. PLoS ONE 2018, 13, e0202100. [Google Scholar] [CrossRef]
- Sumner, J.; Kiermeier, A.; Jolley, J. Microbiological Food Safety and Storage Life of Australian Red Meat; AMPC: Sidney, Australia, 2018; Report number 2018-1086. [Google Scholar]
- Barlow, R.S.; Mellor, G.E. Prevalence of enterohemorrhagic Escherichia coli serotypes in Australian beef cattle. Foodborne Pathog. Dis. 2010, 7, 1239–1245. [Google Scholar] [CrossRef]
- Sallam, K.I.; Abd-Elghany, S.M.; Hussein, M.A.; Imre, K.; Morar, A.; Morshdy, A.E.; Sayed-Ahmed, M.Z. Microbial decontamination of beef carcass surfaces by lactic acid, acetic acid, and trisodium phosphate sprays. Bio. Med. Res. Int. 2020, 2020, 2324358. [Google Scholar] [CrossRef] [PubMed]
- Van den Honert, M.; Gouws, P.; Hoffman, L. Importance and implications of antibiotic resistance development in livestock and wildlife farming in South Africa: A Review. S. Af. J. Anim. Sci. 2018, 48, 401–412. [Google Scholar] [CrossRef]
- Van den Honert, M.S.; Gouws, P.A.; Hoffman, L.C. Escherichia coli Antibiotic Resistance Patterns from Co-Grazing and Non-Co-Grazing Livestock and Wildlife Species from Two Farms in the Western Cape, South Africa. Antibiotics 2021, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Van den Honert, M.S.; Gouws, P.A.; Hoffman, L.C. A Preliminary Study: Antibiotic Resistance of Escherichia coli and Staphylococcus aureus from the Meat and Feces of Various South African Wildlife Species. Food Sci. Anim. Resour. 2021, 41, 135. [Google Scholar] [CrossRef]
- Neethling, J.; Hoffman, L.; Muller, M. Factors influencing the flavour of game meat: A review. Meat Sci. 2016, 113, 139–153. [Google Scholar] [CrossRef]
- Van Schalkwyk, D.L.; Hoffman, L.C. Guidelines for the Harvesting & Processing of Wild Game in Namibia 2016; Ministry of Environment & Tourism: Windhoek, Namibia, 2016.
- Zhang, L.; Ben Said, L.; Diarra, M.S.; Fliss, I. Inhibitory activity of natural synergetic antimicrobial consortia against Salmonella enterica on broiler chicken carcasses. Front. Microbiol. 2021, 12, 972. [Google Scholar]
- Viator, C.L.; Cates, S.C.; Karns, S.A.; Muth, M.K. Food safety practices in the US meat slaughter and processing industry: Changes from 2005 to 2015. J. Food Prot. 2017, 80, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Chen, Y.; Chen, C.; Yang, H.; Chen, Y. Food safety management systems based on ISO 22000: 2018 methodology of hazard analysis compared to ISO 22000: 2005. Accredit. Qual. Assur. 2020, 25, 23–37. [Google Scholar] [CrossRef]
- Da Silva, S.; Farag, K. The impact of lamb cleanliness and line speed on the effectiveness of steam vacuum and carcass wash as decontamination methods after slaughter. Meat Sci. 2021, 171, 108276. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.S.; Mutz, Y.d.S.; Rosario, D.K.A.; Cunha-Neto, A.; Figueiredo, E.E.d.S.; Conte-Junior, C.A. Inactivation of Multi-Drug Resistant Non-Typhoidal Salmonella and Wild-Type Escherichia coli STEC Using Organic Acids: A Potential Alternative to the Food Industry. Pathogens 2020, 9, 849. [Google Scholar] [CrossRef]
- Keeton, J.; Ricke, S.; Anderson, R.; Miller, D.; Azefor, N. Application of Novel Hurdle Technologies to Meat Carcasses and Trimmings for Reduction of Pathogens; University of Arkansas: Fayetteville, AR, USA, 2008; FSIS-C-14-2004. [Google Scholar]
- Dias-Morse, P.; Pohlman, F.; Pinidiya, S.; Coffman, C. Microbial characteristics of ground beef processed from beef trimmings decontaminated by peroxyacetic acid alone or followed by organic acids interventions. Anim. Sci. Ark. Anim. Sci. 2013, 12, 105–109. [Google Scholar]
- Shebs, E.; Lukov, M.; Giotto, F.; Torres, E.; de Mello, A. Efficacy of bacteriophage and organic acids in decreasing STEC O157: H7 populations in beef kept under vacuum and aerobic conditions: A simulated High Event Period scenario. Meat Sci. 2020, 162, 108023. [Google Scholar] [CrossRef] [PubMed]
- Projahn, M.; Sachsenroeder, J.; Correia-Carreira, G.; Becker, E.; Martin, A.; Thomas, C.; Hobe, C.; Reich, F.; Robé, C.; Roesler, U. Impact of On-Farm Interventions against CTX-Resistant Escherichia coli on the Contamination of Carcasses before and during an Experimental Slaughter. Antibiotics 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review. Foods 2021, 10, 2293. https://doi.org/10.3390/foods10102293
Nkosi DV, Bekker JL, Hoffman LC. The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review. Foods. 2021; 10(10):2293. https://doi.org/10.3390/foods10102293
Chicago/Turabian StyleNkosi, Davies Veli, Johan Leon Bekker, and Louwrens Christian Hoffman. 2021. "The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review" Foods 10, no. 10: 2293. https://doi.org/10.3390/foods10102293
APA StyleNkosi, D. V., Bekker, J. L., & Hoffman, L. C. (2021). The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review. Foods, 10(10), 2293. https://doi.org/10.3390/foods10102293






