Fatty Acids and Nutraceutical Properties of Lipids in Fallow Deer (Dama dama) Meat Produced in Organic and Conventional Farming Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Meat Samples, Animals, and Treatments
2.2. Chemical Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas-Agustí, A.; Díaz, I.; Sárraga, C.; García-Regueiro, J.A.; Castellari, M. Nutritional properties of organic and conventional beef meat at retail. J. Sci. Food Agric. 2019, 99, 4218–4225. [Google Scholar] [CrossRef]
- Rizzo, G.; Borrello, M.; Dara Guccione, G.; Schifani, G.; Cembalo, L. Organic Food Consumption: The Relevance of the health attribute. Sustainability 2020, 12, 595. [Google Scholar] [CrossRef] [Green Version]
- Sahota, A.; Willer, H.; Lernoud, J. (Eds.) The world of Organic Agriculture. Statistics and Emerging Trends 2018; Research Institute of Organic Agriculture (FiBL) and IFOAM–Organics International: Bonn, Germany, 2018; pp. 145–150. [Google Scholar]
- Tandon, A.; Jabeen, F.; Talwar, S.; Sakashita, M.; Dhir, A. Facilitators and inhibitors of organic food buying behavior. Food Qual. Prefer. 2021, 88, 104077. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 704–714. [Google Scholar] [CrossRef]
- Anisimova, T.; Mavondo, F.; Weiss, J. Controlled and uncontrolled communication stimuli and organic food purchases: The mediating role of perceived communication clarity, perceived health benefits, and trust. J. Mark. Commun. 2017, 25, 180–203. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. II. Fatty acid composition of meat. Livest. Product. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Barański, M.; Rempelos, L.; Iversen, P.O.; Leifert, C. Effects of organic food consumption on human health; the jury is still out! Food Nutr. Res. 2017, 61, 1287333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditlevsen, K.; Sandøe, P.; Lassen, J. Healthy food is nutritious, but organic food is healthy because it is pure: The negotiation of healthy food choices by Danish consumers of organic food. Food Qual. Prefer. 2019, 71, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Kilar, M.; Kilar, J.; Ruda, M. Rolnictwo ekologiczne jako źródło żywności funkcjonalnej. In Innowacyjne Rozwiązania w Technologii Żywności i Żywieniu Człowieka; Tarko, T., Ed.; Wyd. Naukowe Polskiego Towarzystwa Technologów Żywności: Kraków, Poland, 2016; pp. 195–204. ISBN 978-83-937001-8-9. [Google Scholar]
- Kesse-Guyot, E.; Péneau, S.; Méjean, C.; De Edelenyi, F.S.; Galan, P.; Hercberg, S.; Lairon, D. Profiles of organic food consumers in a large sample of French adults: Results from the Nutrinet-Santé cohort study. PLoS ONE 2013, 8, e76998. [Google Scholar] [CrossRef]
- Rembiałkowska, E.; Wiśniewska, K. Jakość mięsa z produkcji ekologicznej. Med. Wet. 2010, 66, 188–191. [Google Scholar]
- Gadomska, J.; Sadowski, S.; Buczkowska, M. Ekologiczna żywność jako czynnik sprzyjający zdrowiu. Probl. Hig. I Epidemiol. 2014, 95, 556–560. [Google Scholar]
- Vargas-Ramella, M.; Munekata, P.E.S.; Gagaoua, M.; Franco, G.; Campagnol, P.C.B.; Pateiro, M.; Da Silva Barretto, A.C.; Domínguez, R.; Lorenzo, J. Inclusion of healthy oils for improving the nutritional characteristics of dry-fermented deer sausage. Foods 2020, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Żochowska-Kujawska, J. Mięso Zwierząt Łownych Jako Potencjalne Źródło Surowca do Produkcji Surowych Wędzonek Dojrzewających; Wyd. Zachodniopomorski Uniwersytet Technologiczny: Szczecin, Poland, 2017; pp. 1–110. ISBN 978-83-7663-233-9. [Google Scholar]
- Kudrnáčová, E.; Bartoň, L.; Bureš, D.; Hoffman, L.C. Carcass and meat characteristics from farm-raised and wild fallow deer (Dama dama) and red deer (Cervus elaphus): A review. Meat Sci. 2018, 141, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Mexia, I.A.; Quaresma, M.A.G.; Coimbra, M.C.P.; Dos Santos, F.A.; Alves, S.P.A.; Bessab, R.J.B.; Antunes, I.C. The influence of habitat and sex on feral fallow deer meat lipid fraction. J. Sci. Food Agric. 2020, 100, 3220–3227. [Google Scholar] [CrossRef]
- Cawthorn, D.M.; Fitzhenry, L.B.; Kotrba, R.; Bureš, D.; Hoffman, L.C. Chemical composition of wild fallow deer (Dama dama) meat from South Africa: A preliminary evaluation. Foods 2020, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Kilar, J. Dziczyzna w jadłospisie człowieka–walory technologiczne, kulinarne i prozdrowotne. In Nowoczesna Produkcja Świń i Stojące Przed nią Wyzwania; Zachodniopomorski Uniwersytet Technologiczny: Szczecin, Poland, 2018; pp. 24–28. ISBN 978-83-7663-247-6. [Google Scholar]
- Soriano, A.; Sánchez-García, C. Nutritional Composition of Game Meat from Wild Species Harvested in Europe. Intech Open 2021. Available online: https://www.intechopen.com/online-first/nutritional-composition-of-game-meat-from-wild-species-harvested-in-europe (accessed on 17 May 2021). [CrossRef]
- Kilar, J.; Ruda, M.; Kilar, M.; Grych, K. Dziczyzna. Szanse i bariery zwiększenia spożycia. In Surowce Pochodzenia Zwierzęcego Jako Źródło Składników Bioaktywnych; Słupski, J., Tarko, T., Drożdż, I., Eds.; Wyd. Oddział Małopolski Polskiego Towarzystwa Technologów Żywności: Kraków, Poland, 2018; pp. 53–63. ISBN 978-83-946796-2-0. [Google Scholar]
- Stanisz, M.; Skorupski, M.; Ślósarz, P.; Bykowska-Maciejewska, M.; Składanowska-Baryza, J.; Stańczak, Ł.; Krokowska-Paluszak, M.; Ludwiczak, A. The seasonal variation in the quality of venison from wild fallow deer (Dama dama)—A pilot study. Meat Sci. 2019, 150, 56–64. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Mesinger, D. Fatty acid profile of meat (Longissimus lumborum) from female roe deer (Capreolus capreolus L.) and red deer (Cervus elaphus L.). Int. J. Food Prop. 2018, 21, 2276–2282. [Google Scholar] [CrossRef] [Green Version]
- Ivanović, S.; Pisinov, B.; Pavlović, M.; Pavlović, I. Quality of meat from female fallow deer (Dama dama) and roe deer (Capreolus capreolus) hunted in Serbia. Ann. Anim. Sci. 2020, 20, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Bykowska, M. Influence of selected factors on meat quality from farm-raised and wild fallow deer (Dama dama): A review. Can. J. Anim. Sci. 2018, 98, 405–415. [Google Scholar] [CrossRef]
- Budimir, K.; Mozzon, M.; Toderi, M.; D’Ottavio, P.; Trombetta, M.F. Effect of breed on fatty acid composition of meat and subcutaneous adipose tissue of light lambs. Animals 2020, 10, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamihiro, S.; Stergiadis, S.; Leifert, C.; Eyre, M.D.; Butler, G. Meat quality and health implications of organic and conventional beef production. Meat Sci. 2015, 100, 306–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprzyk, A.; Tyra, M.; Babicz, B. Fatty acid profile of pork from a local and a commercial breed. Arch. Anim. Breed. 2015, 58, 379–385. [Google Scholar] [CrossRef]
- Nogales, S.; Bressan, M.C.; Delgado, J.V.; Telo daGama, L.; Barba, C.; Camacho, M.E. Fatty acid profile of feral cattle meat. Ital. J. Anim. Sci. 2017, 16, 172–184. [Google Scholar] [CrossRef] [Green Version]
- Polak, T.; Rajar, A.; Gaperlin, L.; Lender, B. Cholesterol concentration and fatty acid profile of red deer (Cervus elaphus) meat. Meat Sci. 2008, 80, 864–869. [Google Scholar] [CrossRef]
- Rozbicka-Wieczorek, A.J.; Więsyk, E.; Brzóska, F.; Śliwiński, B.; Kowalczyk, J.; Czauderna, M. Fatty acid profile and oxidative stress of thigh muscles in chickens fed the ration enriched in lycopene, selenium compounds or fish oil. Ann. Anim. Sci. 2014, 14, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Kilar, J.; Ruda, M.; Kilar, M. Dziczyzna. Co o Niej Wiedzą i Czy ją Jedzą Mieszkańcy Podkarpacia; Wyd. PWSZ im. S. Pigonia: Krosno, Poland, 2016; pp. 1–101. ISBN 978-83-64457-21-0. [Google Scholar]
- Kwiecińska, K.; Kosicka-Gębska, M.; Gębski, J. Ocena preferencji konsumentów związanych z wyborem dziczyzny. Handel Wewnętrzny 2016, 1, 53–64. [Google Scholar]
- Regulation (EU) 2018/848—Rules on Organic Production and Labelling of Organic Products. EU Rules on Producing and Labelling Organic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/LSU/?uri=CELEX%3A32018R0848 (accessed on 4 January 2021).
- Dz.U. 2009 nr 116 poz. 975. In Ustawa z Dnia 25 Czerwca 2009 r. o Rolnictwie Ekologicznym; Organic Agriculture Law; Kancelaria Sejmu: Warsaw, Poland. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20091160975/U/D20090975Lj.pdf (accessed on 4 January 2021). (In Polish)
- Braun-Blanguet, J. Pflanzensoziologie, Grundzuge der Vegetationskundle; 3. Aufl; Springer: Vienna, Austria, 1964. [Google Scholar]
- DEFRA Code of Recommendations for the Welfare of Farmed Deer. 2006. Available online: http://www.defra.gov.uk/animalh/welfare/farmed/othersps/deer/pb0055/deercode.htm (accessed on 4 January 2021).
- FEDFA Federation of European Deer Farmers Associations. Available online: https://www.fedfa.com/ (accessed on 4 January 2021).
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- PN-ISO 1444:2000. Meat and Meat Products. Determination of Fat Content; Polish Committee for Standardization: Warsaw, Poland, 2000. (In Polish)
- SOP. Cholesterol in meat by GC method; M.023a wersja 1 z 20.10.2011; SOP: Kraków, Poland, 2011. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Domaradzki, P.; Żółkiewski, P.; Litwińczuk, A.; Florek, M.; Dmoch, M. Profil i wartość odżywcza kwasów tłuszczowych w wybranych mięśniach szkieletowych buhajków rasy polskiej holsztyńsko-fryzyjskiej. Med. Weter. 2019, 75, 310–315. [Google Scholar]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Zilversmit, D.B. Cholesterol index of foods. J. Am. Diet. Assoc. 1979, 74, 562–565. [Google Scholar] [PubMed]
- Connor, S.L.; Gustafson, J.R.; Artaud-Wild, S.M.; Favell, D.P.; Classick-Kohn, C.J.; Hatcher, L.F.; Connor, W.E. The cholesterol/saturated-fat index an indication of the hypercholesterolemic and atherogenic potential of food. Lancet 1986, 327, 1229–1232. [Google Scholar] [CrossRef]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation. FAO Food Nutr. Pap. 2010, 91, 1–180. [Google Scholar]
- European Food Safety Authority. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef] [Green Version]
- WHO/FAO. Diet, nutrition and the prevention of chronic diseases. In Report of a Joint WHO/FAO Expert Consultation; World Health Organization Technical Report Series; 916. Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; pp. 54–60. [Google Scholar]
- Domaradzki, P.; Florek, M.; Skałecki, P.; Litwińczuk, A.; Kędzierska-Matysek, M.; Wolanciuk, A.; Tajchman, K. Fatty acid composition, cholesterol content and lipid oxidation indices of intramuscular fat from skeletal muscles of beaver (Castor fiber L.). Meat Sci. 2019, 150, 131–140. [Google Scholar] [CrossRef]
- Janiszewski, P.; Bogdaszewska, Z.; Bogdaszewski, M.; Bogdaszewski, P.; Ciululko-Dołęga, J.; Nasiadka, P.; Steiner, Ż. Rearing and Breeding of Cervids on Farms; Wydawnictwo UWM: Olsztyn, Poland, 2014; p. 133. [Google Scholar]
- Volpelli, L.A.; Valusso, R.; Piasentier, E. Carcass quality in male fallow deer (Dama dama): Effects of age and supplementary feeding. Meat Sci. 2002, 60, 427–432. [Google Scholar] [CrossRef]
- Razmaitė, V.; Pileckas, V.; Šiukščius, A.; Juškiene, V. Fatty acid composition of meat and edible offal from free-living red deer (Cervus elaphus). Foods 2020, 9, 923. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Hnatyk, N.; Dąbrowski, D.; Janiszewski, P.; Gugołek, A.; Kubiak, D.; Śmiecińska, K.; Winarski, R.; Koba-Kowalczyk, M. A comparison of the quality of the Longissimus lumborum muscle from wild and farm-raised fallow deer (Dama dama L.). Small Rumin. Res. 2015, 129, 77–83. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Joo, S.H.; Lee, K.W.; Hwang, Y.H.; Joo, S.T. Histochemical characteristics in relation to meat quality traits of eight major muscles from Hanwoo steers. Korean J. Food Sci. Anim. Resour. 2017, 37, 716–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.Y.; Lunt, D.K.; Choi, C.B.; Chae, S.H.; Rhoades, R.D.; Adams, T.H. Lipid characteristics of subcutaneous adipose tissue and M. Longissimus thoracis of Angus and Wagyu steers fed to US and Japanese endpoints. Meat Sci. 2006, 73, 432–441. [Google Scholar] [CrossRef]
- Aksoy, Y.; Çiçek, Ü.; Sen, U.; Sirin, E.; Ugurlu, M.; Önenç, A.; Kuran, M.; Ulutas, Z. Meat production characteristics of Turkish native breeds: II. meat quality, fatty acid, and cholesterol profile of lambs. Arch. Anim. Breed. 2019, 62, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C.; Deckelbaum, R.J. Fat as a physiological regulator: The news gets better. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 127–131. [Google Scholar] [CrossRef]
- Simopoulos, A. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revilla, I.; Plaza, J.; Palacios, C. The effect of grazing level and ageing time on the physicochemical and sensory characteristics of beef meat in organic and conventional production. Animals 2021, 11, 635. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.M. Fatty acid and cholesterol profiles, hypocholesterolemic, atherogenic, and thrombogenic incides of broiler meat in the retail market. Lipids Health Dis. 2017, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Dayrit, F.D. The properties of lauric acid and their significance in coconut oil. Review. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Bureš, D.; Bartoň, L.; Kotrba, R.; Hakl, J. Quality attributes and composition of meat from red deer (Cervus elaphus), fallow deer (Dama dama) and Aberdeen Angus and Holstein cattle (Bos taurus). J. Sci. Food Agric. 2015, 95, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, M.; Kaczor, U. Stearoyl-CoA desaturase—The lipid metabolism regulator. Postepy Hig. Med. Dosw. 2014, 68, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Guerendiain, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martinez-González, M.A.; Aros, F.; Lamuela-Raventós, R.M.; et al. Plasma fatty acid composition, estimated desaturase activities, and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin. Nutr. 2014, 33, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Von Roemeling, C.A.; Marlow, L.A.; Wei, J.J.; Cooper, S.J.; Caulfield, T.R.; Wu, K.; Tan, W.W.; Copland, J.A. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcino ma. Clin. Cancer Res. 2013, 19, 2368–2380. [Google Scholar] [CrossRef] [Green Version]
- Kolanowski, W. Long chain polyunsaturated omega-3 fatty acids and their role in reducing the risk of life-style related diseases. Bromat. Chem. Toksykol. 2007, 11, 229–237, (In Polish, In English abstract). [Google Scholar]
- Turner, T.D.; Jensen, J.; Jessica, L.; Pilfold, J.; Prema, D.; Donkor, K.K.; Cinel, B.; Thompson, D.J.; Dugan, M.E.R.; Church, J.S. Comparison of fatty acids in beef tissues from conventional, organic and natural feeding systems in western Canada. Can. J. Anim. Sci. 2015, 95, 4958. [Google Scholar] [CrossRef]
- Webb, E.C.; O’Neill, H.A. The animal fat paradox and meat quality. Meat Sci. 2008, 80, 28–36. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet. The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Kim, W.; Khan, N.A.; McMurray, D.N.; Prior, I.A.; Wang, N.; Chapkin, R.S. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid Res. 2010, 49, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Cichosz, G.; Czeczot, H. Trans fatty acids in the human diet. Bromat. Chem. Toksykol. 2012, 2, 181–190, (In Polish, In English abstract). [Google Scholar]
- Bałasińska, B.; Jank, M.; Kulasek, G. Properties and the role of polyunsaturated fatty acids in health protection of human and animal. Życie Wet. 2010, 85, 749–756, (In Polish, In English abstract). [Google Scholar]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic potential of n-3 polyunsaturated fatty acids in human autoimmune diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kritchevsky, D.; Tepper, S.A.; Wright, S.; Czarnecki, S.K.; Wilson, T.A.; Nicolosi, R.J. Conjugated linoleic acid isomer effects in atherosclerosis: Growth and regression of lesions. Lipids 2004, 39, 611. [Google Scholar] [CrossRef]
- Benjamin, S.; Prakasan, P.; Sreedharan, S.; Wright, A.D.; Spener, F. Pros and cons of CLA consumption: An insight from clinical evidences. Nutr. Metab. 2015, 21, 4. [Google Scholar] [CrossRef] [Green Version]
- Pogorzelska-Nowicka, E.; Atanasov, A.G.; Horbańczuk, J.; Wierzbicka, A. Bioactive compounds in functional meat products. Molecules 2018, 23, 307. [Google Scholar] [CrossRef] [Green Version]
- Tocci, R.; Sargentini, C. Meat quality of Maremmana young bulls. Acta Sci. Anim. Sci. 2020, 42, e46515. [Google Scholar] [CrossRef] [Green Version]
- Hmso, U. Nutritional Aspects of Cardiovascular Disease (Report on Health and Social Subjects No. 46); HMSO: London, UK, 1994. [Google Scholar]
- Harris, W.S. The omega-6:omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 34–40. [Google Scholar] [CrossRef]
- Popova, T.; Ignatova, M.; Petkov, E.; Stanišić, N. Difference in fatty acid composition and related nutritional indices of meat between two lines of slow-growing chickens slaughtered at different ages. Arch. Anim. Breed. 2016, 59, 319–327. [Google Scholar] [CrossRef]
- Švrčula, V.; Košinová, K.; Okrouhlá, M.; Chodová, D.; Hart, V. The effect of sex on meat quality of fallow deer (Dama dama) from the farm located in the Middle Bohemia. Ital. J. Anim. Sci. 2019, 18, 498–504. [Google Scholar] [CrossRef] [Green Version]
- Valfre, F.; Caprino, F.; Turchini, G.M. The health benefit of sea food. Vet. Res. Commun. 2003, 27, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kilar, J. Zasoby zwierząt łownych w Polsce w aspekcie bazy surowcowej przetwórstwa mięsnego. In Ochrona Środowiska Produkcji Rolniczej; Uniwersytet Rzeszowski: Rzeszów, Poland, 2020; pp. 60–74. ISBN 978-83-7996-818-3. [Google Scholar]
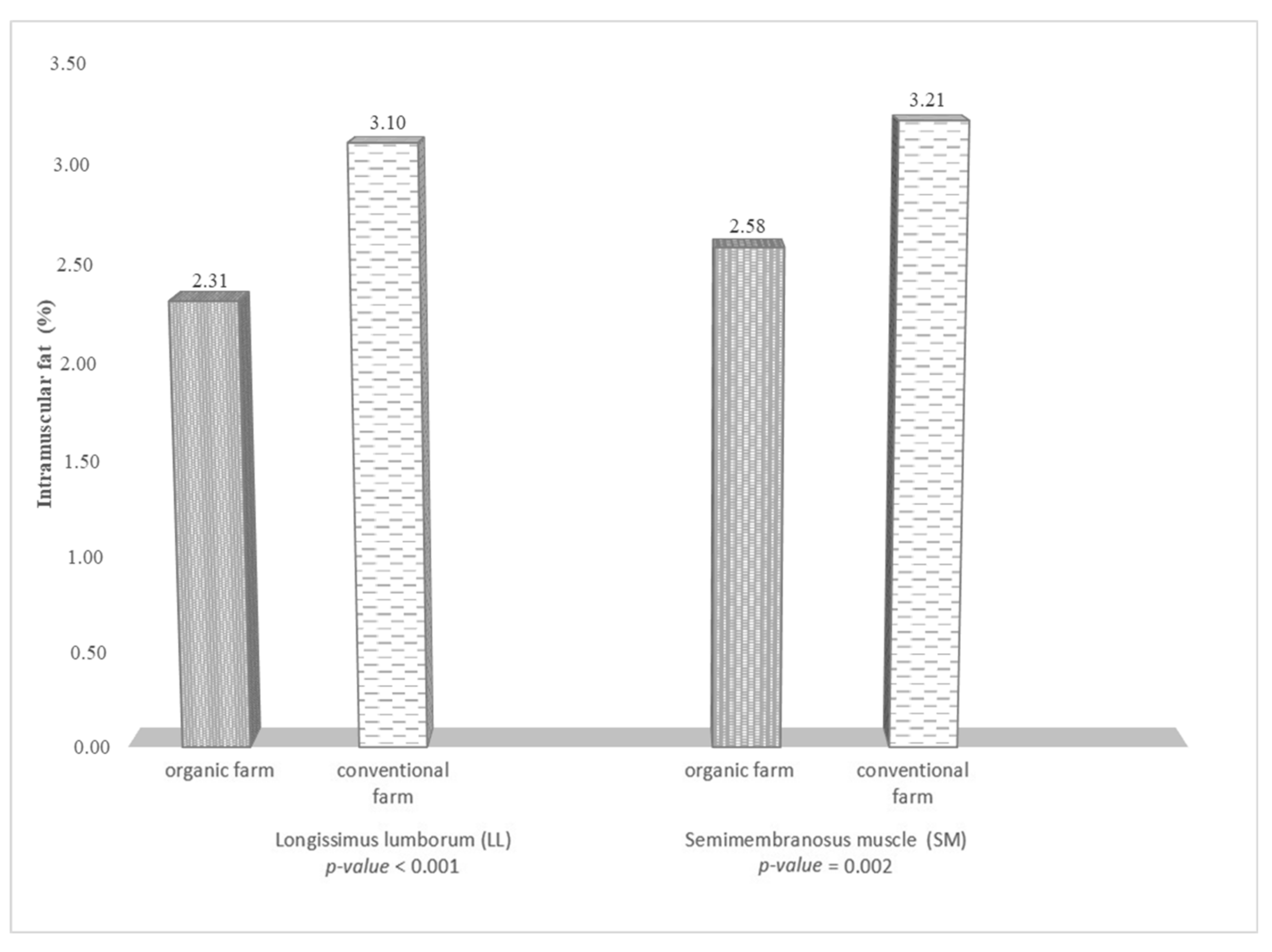
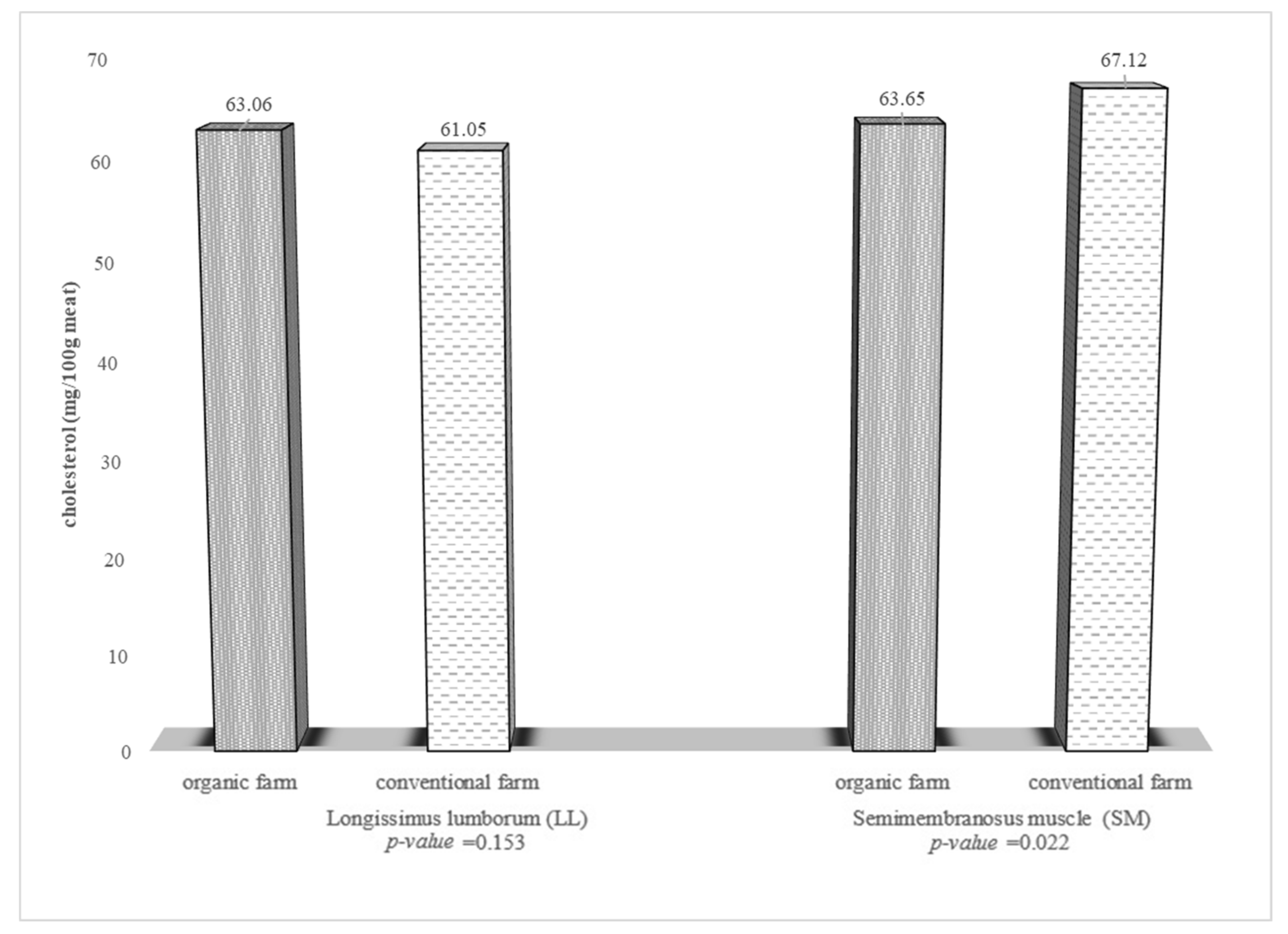
| Fatty Acids | Muscle | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| longissimus lumborum (LL) | semimembranosus (SM) | |||||||||||||
| Organic Farm | Conventional Farm | organic Farm | Conventional Farm | |||||||||||
| SE | mg·100 g−1 | SE | mg·100g−1 | p-Value | SE | mg·100g−1 | SE | mg·100g−1 | p-Value | |||||
| C10:0 | 0.09 | 0.022 | 1.45 | 0.07 | 0.013 | 1.66 | 0.123 | 0.09 | 0.026 | 1.64 | 0.08 | 0.013 | 1.71 | 0.180 |
| C12:0 | 0.16 | 0.020 | 2.65 | 0.23 | 0.082 | 5.10 | 0.006 | 0.16 | 0.033 | 2.94 | 0.23 | 0.083 | 5.23 | 0.019 |
| C13:0 | 0.19 | 0.223 | 3.09 | 0.18 | 0.261 | 3.87 | 0.967 | 0.17 | 0.207 | 2.96 | 0.24 | 0.344 | 5.31 | 0.571 |
| C14:0 | 3.44 | 1.316 | 58.38 | 3.84 | 1.531 | 83.02 | 0.503 | 3.39 | 1.534 | 63.57 | 3.72 | 1.045 | 87.25 | 0.551 |
| C15:0 | 0.87 | 0.393 | 13.90 | 1.49 | 0.492 | 33.11 | 0.002 | 0.38 | 0.256 | 7.33 | 1.74 | 0.253 | 40.14 | <0.0001 |
| C16:0 | 23.96 | 2.424 | 398.55 | 22.49 | 2.376 | 500.72 | 0.146 | 21.66 | 2.732 | 399.67 | 20.46 | 3.288 | 467.10 | 0.341 |
| C17:0 | 1.10 | 0.506 | 17.33 | 0.86 | 0.281 | 18.70 | 0.154 | 0.51 | 0.238 | 9.59 | 1.23 | 0.418 | 28.99 | <0.0001 |
| C18:0 | 15.52 | 4.780 | 254.27 | 19.07 | 3.491 | 472.42 | 0.050 | 17.80 | 2.896 | 328.77 | 20.53 | 3.405 | 477.84 | 0.046 |
| C20:0 | 0.30 | 0.220 | 5.12 | 0.50 | 0.298 | 11.51 | 0.069 | 0.21 | 0.067 | 3.83 | 0.23 | 0.103 | 5.33 | 0.578 |
| C21:0 | 0.23 | 0.119 | 4.02 | 0.75 | 0.110 | 6.44 | <0.0001 | 0.33 | 0.106 | 5.97 | 0.22 | 0.073 | 4.96 | 0.006 |
| C22:0 | 0.07 | 0.029 | 1.18 | 0.08 | 0.013 | 1.75 | 0.593 | 0.13 | 0.041 | 2.34 | 0.22 | 0.144 | 5.00 | 0.055 |
| C24:0 | 0.18 | 0.033 | 2.91 | 0.09 | 0.023 | 1.90 | <0.0001 | 0.17 | 0.034 | 3.07 | 0.10 | 0.027 | 2.32 | <0.0001 |
| C14:1 | 2.57 | 0.232 | 42.65 | 2.87 | 0.839 | 64.09 | 0.248 | 1.63 | 0.409 | 29.60 | 3.29 | 1.186 | 76.82 | <0.0001 |
| C15:1 | 1.23 | 0.386 | 20.93 | 2.50 | 0.816 | 54.28 | <0.0001 | 1.08 | 0.896 | 19.87 | 3.59 | 1.277 | 84.18 | <0.0001 |
| C16:1 | 3.56 | 0.927 | 57.83 | 1.90 | 1.155 | 41.53 | 0.001 | 3.89 | 0.942 | 70.99 | 2.11 | 1.138 | 49.58 | <0.0001 |
| C17:1 | 1.31 | 0.360 | 21.19 | 0.67 | 0.336 | 14.61 | <0.0001 | 1.04 | 0.302 | 18.94 | 0.86 | 0.342 | 20.22 | 0.186 |
| C18:1n9c | 19.95 | 4.833 | 336.86 | 22.86 | 3.039 | 513.16 | 0.092 | 23.26 | 3.239 | 432.12 | 22.77 | 3.227 | 522.55 | 0.716 |
| C18:1n9t | 0.48 | 0.250 | 8.28 | 0.70 | 0.282 | 15.88 | 0.057 | 0.75 | 0.994 | 13.04 | 0.62 | 0.390 | 14.89 | 0.683 |
| C20:1 | 0.23 | 0.119 | 4.01 | 0.75 | 0.110 | 16.82 | <0.0001 | 0.16 | 0.073 | 2.98 | 0.64 | 0.088 | 14.80 | <0.0001 |
| C24:1n9 | 0.19 | 0.034 | 3.13 | 0.23 | 0.036 | 5.17 | 0.004 | 0.19 | 0.050 | 3.41 | 0.16 | 0.041 | 3.69 | 0.183 |
| C18:2n6c (LA) | 7.64 | 0.966 | 128.31 | 6.99 | 1.419 | 156.99 | 0.208 | 7.24 | 1.022 | 133.61 | 5.97 | 1.659 | 142.21 | 0.035 |
| C18:2n6t | 0.63 | 0.052 | 10.54 | 0.36 | 0.061 | 7.95 | <0.0001 | 0.60 | 0.036 | 10.99 | 0.33 | 0.068 | 7.88 | <0.0001 |
| C18:2c9t11 (CLA) | 2.29 | 0.123 | 37.97 | 1.60 | 0.078 | 35.86 | <0.0001 | 2.14 | 0.343 | 39.22 | 1.66 | 0.073 | 38.50 | <0.0001 |
| C20:2n6 | 0.16 | 0.031 | 2.63 | nd | nd | nd | - | 0.15 | 0.046 | 2.74 | nd | nd | nd | - |
| C22:2n6 | 0.32 | 0.236 | 5.02 | nd | nd | nd | - | 0.16 | 0.065 | 2.89 | nd | nd | nd | - |
| C18:3n6 (GLA) | 1.31 | 0.157 | 21.58 | 0.36 | 0.196 | 8.21 | <0.0001 | 1.43 | 0.461 | 25.99 | 1.38 | 0.235 | 32.39 | 0.745 |
| C18:3n3 (ALA) | 4.32 | 0.691 | 71.93 | 2.15 | 0.694 | 48.92 | <0.0001 | 3.87 | 0.742 | 70.81 | 1.96 | 0.737 | 46.63 | <0.0001 |
| C20:3n6 | 0.91 | 0.237 | 15.49 | 1.65 | 0.263 | 39.94 | <0.0001 | 0.85 | 0.170 | 15.64 | 0.45 | 0.221 | 10.43 | <0.0001 |
| C20:3n3 | 0.64 | 0.142 | 10.60 | 0.73 | 0.114 | 16.38 | 0.079 | 0.91 | 0.547 | 16.35 | 0.81 | 0.102 | 18.69 | 0.544 |
| C20:4n6 (AA) | 1.68 | 0.146 | 28.04 | 1.71 | 0.065 | 38.21 | 0.545 | 1.75 | 0.077 | 32.31 | 1.74 | 0.068 | 40.36 | 0.781 |
| C20:5n3 (EPA) | 1.56 | 0.464 | 26.17 | 1.14 | 0.037 | 25.34 | 0.005 | 1.23 | 0.129 | 22.88 | 1.19 | 0.028 | 25.50 | 0.240 |
| C22:6n3 (DHA) | 2.83 | 0.093 | 47.22 | 1.63 | 0.072 | 36.42 | <0.0001 | 2.60 | 0.083 | 47.01 | 1.50 | 0.029 | 34.82 | <0.0001 |
| Specification | Muscle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Longissimus lumborum (LL) | Semimembranosus (SM) | |||||||||
| Organic Farm | Conventional Farm | p-Value | Organic Farm | Conventional Farm | p-Value | |||||
| SE | SE | SE | SE | |||||||
| Sums of FA groups | ||||||||||
| SFA | 46.12 | 5.742 | 49.19 | 4.790 | 0.169 | 44.98 | 3.673 | 48.96 | 3.332 | 0.011 |
| UFA | 53.88 | 5.742 | 50.81 | 4.791 | 0.169 | 55.02 | 3.673 | 51.04 | 3.332 | 0.011 |
| MUFA | 29.62 | 5.096 | 32.49 | 3.390 | 0.119 | 32.10 | 3.610 | 34.04 | 3.294 | 0.183 |
| PUFA | 24.26 | 1.822 | 18.33 | 2.256 | <0.0001 | 22.92 | 2.350 | 17.00 | 2.502 | <0.0001 |
| PUFA n-6 | 12.59 | 1.457 | 11.05 | 1.624 | 0.023 | 12.18 | 1264 | 9.88 | 1.991 | 0.003 |
| PUFA n-3 | 9.34 | 0.565 | 5.65 | 0.744 | <0.0001 | 8.60 | 1.180 | 5.46 | 0.740 | <0.0001 |
| OFA | 27.56 | 1.631 | 26.56 | 2.988 | 0.318 | 25.21 | 3.090 | 24.40 | 3.421 | 0.548 |
| FA Ratios | ||||||||||
| SFA/UFA | 0.88 | 0.199 | 0.99 | 0.218 | 0.204 | 0.83 | 0.123 | 0.97 | 0.139 | 0.014 |
| MUFA/SFA | 0.66 | 0.208 | 0.67 | 0.115 | 0.938 | 0.72 | 0.129 | 0.70 | 0.099 | 0.647 |
| PUFA/SFA | 0.54 | 0.094 | 0.38 | 0.073 | <0.0001 | 0.51 | 0.081 | 0.35 | 0.064 | <0.0001 |
| PUFA n-6/PUFA n-3 | 1.35 | 0.123 | 1.96 | 0.176 | <0.0001 | 1.42 | 0.124 | 1.81 | 0.269 | <0.0001 |
| Nutraceutical indices | ||||||||||
| DFA | 69.40 | 1.921 | 69.88 | 3.261 | 0.666 | 72.81 | 3.265 | 71.57 | 3.284 | 0.361 |
| AI | 0.54 | 0.090 | 0.54 | 0.132 | 0.979 | 0.47 | 0.116 | 0.50 | 0.090 | 0.561 |
| TI | 0.73 | 0.153 | 0.92 | 0.209 | 0.022 | 0.72 | 0.112 | 0.90 | 0.118 | <0.0001 |
| h/H | 2.53 | 0.227 | 2.68 | 0.426 | 0.310 | 2.94 | 0.470 | 3.00 | 0.530 | 0.771 |
| ∆9-desaturase C16 | 13.01 | 3.692 | 7.59 | 3.978 | 0.002 | 15.24 | 3.527 | 9.24 | 4.082 | <0.0001 |
| ∆9-desaturase C18 | 56.27 | 13.044 | 54.61 | 6.913 | 0.701 | 56.68 | 4.607 | 52.62 | 7.262 | 0.116 |
| Elongase | 56.31 | 3.369 | 63.25 | 3.638 | <0.0001 | 61.52 | 5.237 | 65.88 | 3.909 | 0.030 |
| NV | 1.04 | 0.224 | 0.91 | 0.221 | 0.186 | 0.84 | 0.180 | 0.86 | 0.142 | 0.835 |
| DHA + EPA | 4.38 | 0.477 | 2.77 | 0.076 | <0.0001 | 3.83 | 0.137 | 2.69 | 0.027 | <0.0001 |
| HPI | 1.37 | 0.190 | 1.34 | 0.304 | 0.747 | 1.55 | 0.354 | 1.42 | 0.226 | 0.277 |
| CI | 4.35 | 0.221 | 4.56 | 0.218 | 0.420 | 4.44 | 0.224 | 4.96 | 0.270 | <0.0001 |
| CSI | 3.92 | 0.194 | 4.15 | 0.221 | 0.067 | 4.02 | 0.207 | 4.49 | 0.276 | <0.0001 |
| Specification | Percent (%) of Energy Requirements Recommended by FAO a | g/day (in a 2000-Kcal Diet) b | Mean Content (g/100g of Fresh Meat)c | Percent (%) of Contribution to a 2000-Kcal Diet | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| longissimus lumborum (LL) | semimembranosus (SM) | longissimus lumborum (LL) | semimembranosus (SM) | |||||||
| organic Farm | conventional Farm | Organic Farm | Conventional Farm | Organic Farm | Conventional Farm | Organic Farm | Conventional Farm | |||
| Total fat | 20.0–35.0 | 44.0–78.0 | 2.31 | 3.10 | 2.58 | 3.21 | 2.96–5.25 | 3.97–7.04 | 3.30–5.86 | 4.11–7.29 |
| ∑ SFA | <10.0 | <22.0 | 0.768 | 1.100 | 0.837 | 1.133 | ≥3.49 | ≥5.02 | ≥3.81 | ≥5.14 |
| ∑ MUFA | 15.0–20.0 | 33.0–44.0 | 0.493 | 0.726 | 0.597 | 0.788 | 1.12–1.49 | 1.65–2.20 | 1.36–1.81 | 1.79–2.39 |
| ∑ PUFA | 6.0–11.0 | 13.0–24.0 | 0.404 | 0.410 | 0.426 | 0.393 | 1.68–3.11 | 1.71–3.15 | 1.77–3.27 | 1.64–3.02 |
| ∑ PUFA n-6 | 2.5–9.0 | 5.6–20.0 | 0.210 | 0.247 | 0.226 | 0.228 | 1.05–3.75 | 1.23–4.42 | 1.13–4.03 | 1.14–4.07 |
| ∑ PUFA n-3 | 0.5–2.0 | 1.1–4.4 | 0.155 | 0.126 | 0.161 | 0.126 | 3.52–14.09 | 2.86–11.45 | 3.66–14.64 | 2.86–11.54 |
| EPA + DHA d | 250 mg | 0.250 | 0.073 | 0.062 | 0.071 | 0.062 | 29.23 | 24.81 | 28.41 | 24.81 |
| Cholesterol e | <300 mg | <0.300 | 0.063 | 0.061 | 0.063 | 0.067 | ≥21.02 | ≥20.35 | ≥21.22 | ≥22.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilar, J.; Kasprzyk, A. Fatty Acids and Nutraceutical Properties of Lipids in Fallow Deer (Dama dama) Meat Produced in Organic and Conventional Farming Systems. Foods 2021, 10, 2290. https://doi.org/10.3390/foods10102290
Kilar J, Kasprzyk A. Fatty Acids and Nutraceutical Properties of Lipids in Fallow Deer (Dama dama) Meat Produced in Organic and Conventional Farming Systems. Foods. 2021; 10(10):2290. https://doi.org/10.3390/foods10102290
Chicago/Turabian StyleKilar, Janusz, and Anna Kasprzyk. 2021. "Fatty Acids and Nutraceutical Properties of Lipids in Fallow Deer (Dama dama) Meat Produced in Organic and Conventional Farming Systems" Foods 10, no. 10: 2290. https://doi.org/10.3390/foods10102290
APA StyleKilar, J., & Kasprzyk, A. (2021). Fatty Acids and Nutraceutical Properties of Lipids in Fallow Deer (Dama dama) Meat Produced in Organic and Conventional Farming Systems. Foods, 10(10), 2290. https://doi.org/10.3390/foods10102290








