Identification of the Causal Agent of Aqueous Spot Disease of Sweet Cherries (Prunus avium L.) from the Jerte Valley (Cáceres, Spain)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation, Microbiological Counts and Isolation of Microbes
2.3. Identification of Isolated Microbes
2.4. Microscopic Observation of Cherry Tissues
2.5. Inoculation Assays
2.6. Genetic Characterisation of Botrytis Cinerea Morphotypes
2.7. Analysis of Results
3. Results
3.1. Microbial Counts
3.2. Species Identification of Bacteria, Yeast, and Moulds
3.3. Inoculation of Bacteria, Yeast, and Moulds in Wounded Cherries
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture, Fisheries and Food Home Page. Available online: https://www.mapa.gob.es/ (accessed on 20 December 2019).
- Serradilla, M.J.; Martín, A.; Aranda, E.; Hernández, A.; Benito, M.J.; Lopez-Corrales, M.; Córdoba, M.D.G. Authentication of “Cereza del Jerte” sweet cherry varieties by free zone capillary electrophoresis (FZCE). Food Chem. 2008, 111, 457–461. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Hernández, A.; Ruiz-Moyano, S.; Benito, M.J.; Corrales, M.L.; Cordoba, M.D.G. Authentication of ‘Cereza del Jerte’ cherry cultivars using real time PCR. Food Control 2012, 30, 679–685. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Villalobos, M.D.C.; Hernández, A.; Martín, A.; Lozano, M.; Córdoba, M.D.G. Study of microbiological quality of controlled atmosphere packaged ‘Ambrunés’ sweet cherries and subsequent shelf-life. Int. J. Food Microbiol. 2013, 166, 85–92. [Google Scholar] [CrossRef]
- Bai, J.; Plotto, A.; Spotts, R.; Rattanapanone, N. Ethanol vapor and saprophytic yeast treatments reduce decay and maintain quality of intact and fresh-cut sweet cherries. Postharvest Biol. Technol. 2011, 62, 204–212. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Long, L.E. The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem. 2014, 160, 22–30. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Schnabel, G. First report of brown rot caused by Monilinia fructicola in sweet cherry in maryland. Plant Dis. 2013, 97, 145. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bai, H.; Yang, H.; Tang, S.; Wei, M.; Huang, X.; Li, Y. Biological characteristics of Monilia fructigena as pathogen of brown rot in sweet cherry. J. Fruit Sci. 2012, 29, 423–427. [Google Scholar]
- Kiprovski, B.; Borković, B.; Malenčić, D.; Veberic, R.; Štampar, F.; Mikulic-Petkovsek, M. Postharvest changes in primary and secondary metabolites of sweet cherry cultivars induced by Monilinia laxa. Postharvest Biol. Technol. 2018, 144, 46–54. [Google Scholar] [CrossRef]
- De Paiva, E.; Serradilla, M.J.; Ruiz-Moyano, S.; Cordoba, M.D.G.; Del Villalobos, M.; Casquete, R.; Hernández, A. Combined effect of antagonistic yeast and modified atmosphere to control Penicillium expansum infection in sweet cherries cv. Ambrunés. Int. J. Food Microbiol. 2017, 241, 276–282. [Google Scholar] [CrossRef]
- López, S.N.; Sangorrín, M.P.; Pildain, M.B. Fruit rot of sweet cherries and raspberries caused by Penicillium crustosum and Mucor piriformis in South Patagonia, Argentina. Can. J. Plant Pathol. 2016, 38, 511–516. [Google Scholar] [CrossRef]
- Wang, C.; Niu, Y.; Meng, Q.; Zhang, L. Ethyl pyruvate (EP) suppressed post-harvest blue mold of sweet cherry fruit by inhibiting the growth of Penicillium oxalicum. J. Sci. Food Agric. 2019, 99, 3517–3524. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Afroz, T.; Kim, B.-S.; Lee, Y.-G. Occurrence of postharvest gray mold rot of sweet cherry due to Botrytis cinerea in Korea. J. Plant Dis. Prot. 2016, 124, 93–96. [Google Scholar] [CrossRef]
- Tarbath, M.; Measham, P.F.; Glen, M.; Barry, K.M. Host factors related to fruit rot of sweet cherry (Prunus avium L.) caused by Botrytis cinerea. Australas. Plant Pathol. 2014, 43, 513–522. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Liu, Z.H. First report of black spot disease caused by Alternaria alternata on cherry fruits in China. Plant Dis. 2012, 96, 1580. [Google Scholar] [CrossRef]
- Romanazzi, G.; Nigro, F.; Ippolito, A.; Salerno, M. Effect of short hypobaric treatments on postharvest rots of sweet cherries, strawberries and table grapes. Postharvest Biol. Technol. 2001, 22, 1–6. [Google Scholar] [CrossRef]
- Ayyanath, M.-M.; Zurowski, C.L.; Scott, I.M.; Lowery, D.T.; Watson, M.C.; O’Gorman, D.T.; MacKenzie, K.E.; Úrbez-Torres, J.R. Relationship between Drosophila suzukiiand postharvest disorders of sweet cherry (Prunus avium). Phytobiomes J. 2018, 2, 42–50. [Google Scholar] [CrossRef] [Green Version]
- O’Gorman, D.T.; Walker, M.; Fraser, J.; Boulé, J.; Úrbez-Torres, J.R.; Toivonen, P.M. Unravelling cherry slip-skin maceration disorder. Can. J. Plant Pathol. 2016, 38, 533–540. [Google Scholar]
- Kim, Y.K. First report of a new postharvest rot in sweet cherries caused by Aureobasidium pullulans. Plant Dis. 2014, 98, 424. [Google Scholar] [CrossRef]
- Michailides, T.J.; Morgan, D.P.; Day, K.R. First report of sour rot of California peaches and nectarines caused by yeasts. Plant Dis. 2004, 88, 222. [Google Scholar] [CrossRef]
- Alam, M.W.; Rehman, A.; Malik, A.U.; Iqbal, Z.; Amin, M.; Ali, S.; Hameed, A.; Sarfraz, S. First report of Geotrichum candidum causing postharvest sour rot of peach in Punjab, Pakistan. Plant Dis. 2017, 101, 1543. [Google Scholar] [CrossRef]
- Ménard, M.; Sutra, L.; Luisetti, J.; Prunier, J.P.; Gardan, L. Pseudomonas syringae pv. avii (pv. nov.), the causal agent of bacterial canker of wild cherries (Prunus avium) in France. Eur. J. Plant Pathol. 2003, 109, 565–576. [Google Scholar] [CrossRef]
- Vicente, J.G.; Roberts, S.J. Discrimination of Pseudomonas syringae isolates from sweet and wild cherry using rep-PCR. Eur. J. Plant Pathol. 2007, 117, 383–392. [Google Scholar] [CrossRef]
- Spadoni, A.; Ippolito, A.; Sanzani, S.M. First report of Stemphylium eturmiunum causing postharvest rot of sweet cherry in Italy. Crop. Prot. 2020, 132, 105112. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; Corrales, M.L.; González-Gómez, D. Physicochemical and bioactive properties evolution during ripening of ‘Ambrunés’ sweet cherry cultivar. LWT 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Totten, S.M.; Garrido, D.A.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl. Environ. Microbiol. 2013, 79, 6040–6049. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, G.; Ruiz-Moyano, S.; Hernández, A.; Benito, M.; Córdoba, M.; Pérez-Nevado, F.; Martín, A. Application of ISSR-PCR for rapid strain typing of Debaryomyces hansenii isolated from dry-cured Iberian ham. Food Microbiol. 2014, 42, 205–211. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequence of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematic; Reynolds, R., Taylor, J.W., Eds.; CBA International: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Hong, S.-B.; Go, S.-J.; Shin, H.-D.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Aspergillus fumigatusand related species. Mycologia 2005, 97, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K.; Battistuzzi, F.U. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Ali, H.M.; El-Aziz, A.R.M.; Al-Othman, M.R.; Al-Wadai, A.S. Molecular characterization of aflatoxigenic and non-aflatoxigenic Aspergillus flavus isolates collected from corn grains. Genet. Mol. Res. 2014, 13, 9352–9370. [Google Scholar] [CrossRef]
- Muñoz, G.; Hinrichsen, P.; Brygoo, Y.; Giraud, T. Genetic characterisation of Botrytis cinerea populations in Chile. Mycol. Res. 2002, 106, 594–601. [Google Scholar] [CrossRef]
- Sipiczki, M.; Pfliegler, W.P.; Holb, I.J. Metschnikowia species share a pool of diverse rRNA genes differing in regions that determine hairpin-loop structures and evolve by reticulation. PLoS ONE 2013, 8, e67384. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Liu, Y.; Shujian, H.; Moosa, A. First record of Alternaria alternata causing postharvest fruit rot of sweet cherry (Prunus avium) in China. Plant Dis. 2020, 104, 2030. [Google Scholar] [CrossRef] [Green Version]
- Úrbez-Torres, J.R.; Boulé, J.; Haag, P.; Hampson, C.; O’Gorman, D.T. First report of root and crown rot caused by Fusarium oxysporum on sweet cherry (Prunus avium) in British Columbia. Plant Dis. 2016, 100, 855. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Förster, H.; Thompson, D.F. Identification and etiology of visible quiescent infections of Monilinia fructicola and Botrytis cinerea in sweet cherry fruit. Plant Dis. 2000, 84, 328–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.K.; Wilcox, W.F. Evaluation of cherry genotypes for resistance to fruit infection by Monilinia fructicola (Wint.) honey. HortScience 1989, 24, 1013–1015. [Google Scholar]
- Param, N.; Zoffoli, J.P. Genotypic differences in sweet cherries are associated with the susceptibility to mechanical damage. Sci. Hortic. 2016, 211, 410–419. [Google Scholar] [CrossRef]
- Mirzaei, S.; Goltapeh, E.M.; Shams-Bakhsh, M.; Safaie, N.; Chaichi, M. Genetic and phenotypic diversity among Botrytis cinerea Isolates in Iran. J. Phytopathol. 2009, 157, 474–482. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. Genetic structure of Botrytis cinerea populations from different host plants in California. Plant Dis. 2005, 89, 1083–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoffoli, J.P.; Toivonen, P.; Wang, Y. Postharvest biology and handling for fresh markets. In Cherries: Botany, Production and Uses; Quero-García, J., Lezzoni, A., Puławska, J., Lang, G., Eds.; CABI: Wallingford, UK, 2017; pp. 460–484. [Google Scholar]
- Halpern, M.; Fridman, S.; Atamna-Ismaeel, N.; Izhaki, I. Rosenbergiella nectarea gen. nov., sp. nov., in the family Enterobacteriaceae, isolated from floral nectar. Int. J. Syst. Evol. Microbiol. 2013, 63, 4259–4265. [Google Scholar] [CrossRef]
- Palacio-Bielsa, A.; Roselló, M.; Llop, P.; López, M.M. Erwinia spp. from pome fruit trees: Similarities and differences among pathogenic and non-pathogenic species. Trees 2011, 26, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Chandler, J.A.; James, P.M.; Jospin, G.; Lang, J.M. The bacterial communities of Drosophila suzukii collected from undamaged cherries. PeerJ 2014, 2, e474. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jia, Y.; Tian, Y.; Zhou, L.; Sun, W.; Deng, J.; Liu, F. First report of necrotic disease caused by Pantoea agglomerans on plum (Prunus salicina) in China. Plant Dis. 2020, 104, 1248. [Google Scholar] [CrossRef]
- Tóth, T.; Lakatos, T.; Koltay, A. Lonsdalea quercina subsp. populi subsp. nov., isolated from bark canker of poplar trees. Int. J. Syst. Evol. Microbiol. 2013, 63, 2309–2313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamby, K.A.; Hernández, A.; Boundy-Mills, K.; Zalom, F.G. Associations of yeasts with spotted-wing drosophila (Drosophila suzukii; Diptera: Drosophilidae) in cherries and raspberries. Appl. Environ. Microbiol. 2012, 78, 4869–4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Moyano, S.; Martín, A.; Villalobos, M.; Calle, A.; Serradilla, M.; Cordoba, M.D.G.; Hernández, A. Yeasts isolated from figs (Ficus carica L.) as biocontrol agents of postharvest fruit diseases. Food Microbiol. 2016, 57, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and application of antifungal VOCs-producing yeasts as biocontrol agents of grey mould in fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Saligkarias, I.; Gravanis, F.; Epton, H.A. Biological control of Botrytis cinerea on tomato plants by the use of epiphytic yeasts Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I-182: II. A study on mode of action. Biol. Control. 2002, 25, 151–161. [Google Scholar] [CrossRef]
- Mari, M.; Martini, C.; Spadoni, A.; Rouissi, W.; Bertolini, P. Biocontrol of apple postharvest decay by Aureobasidium pullulans. Postharvest Biol. Technol. 2012, 73, 56–62. [Google Scholar] [CrossRef]
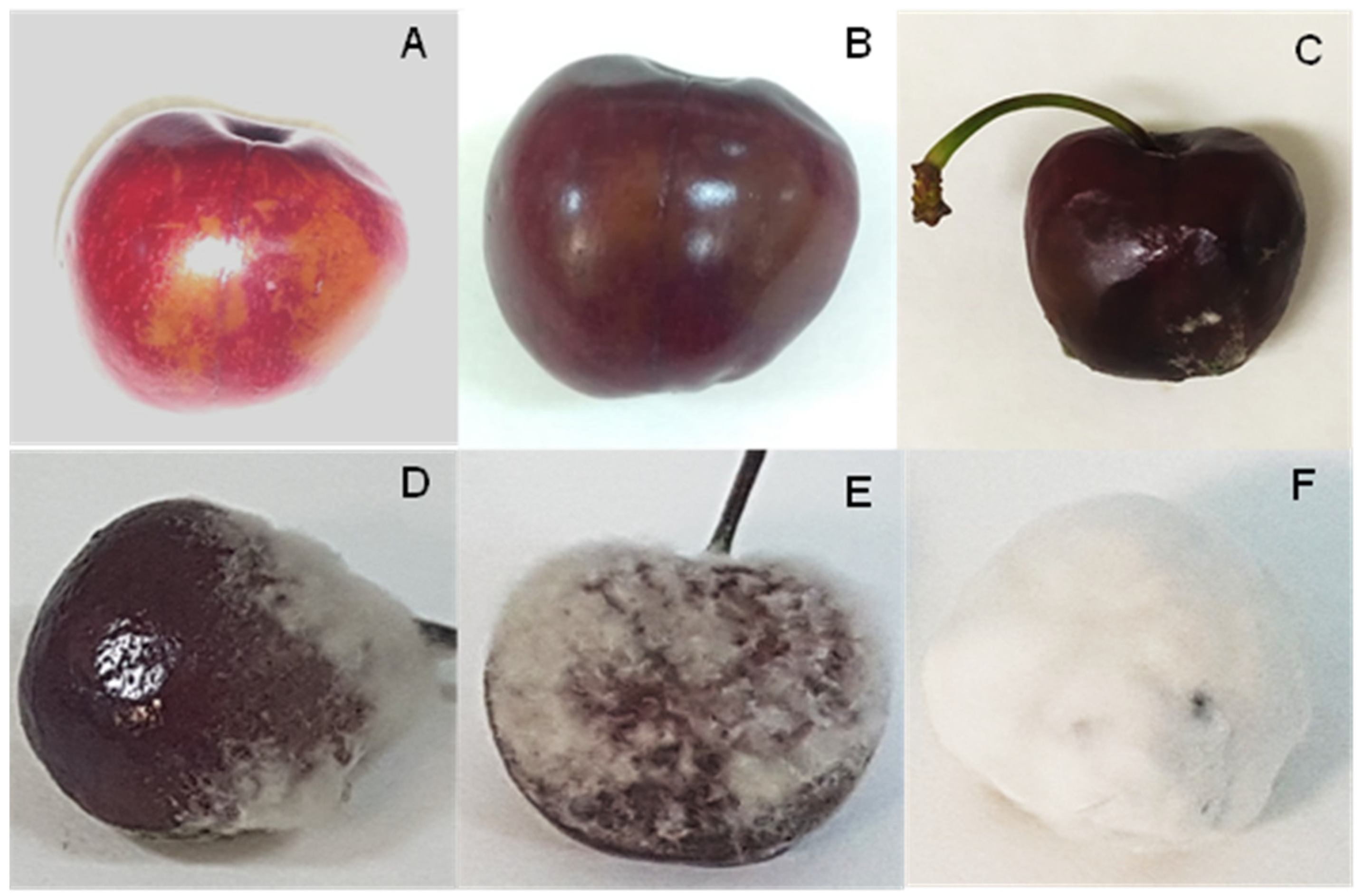




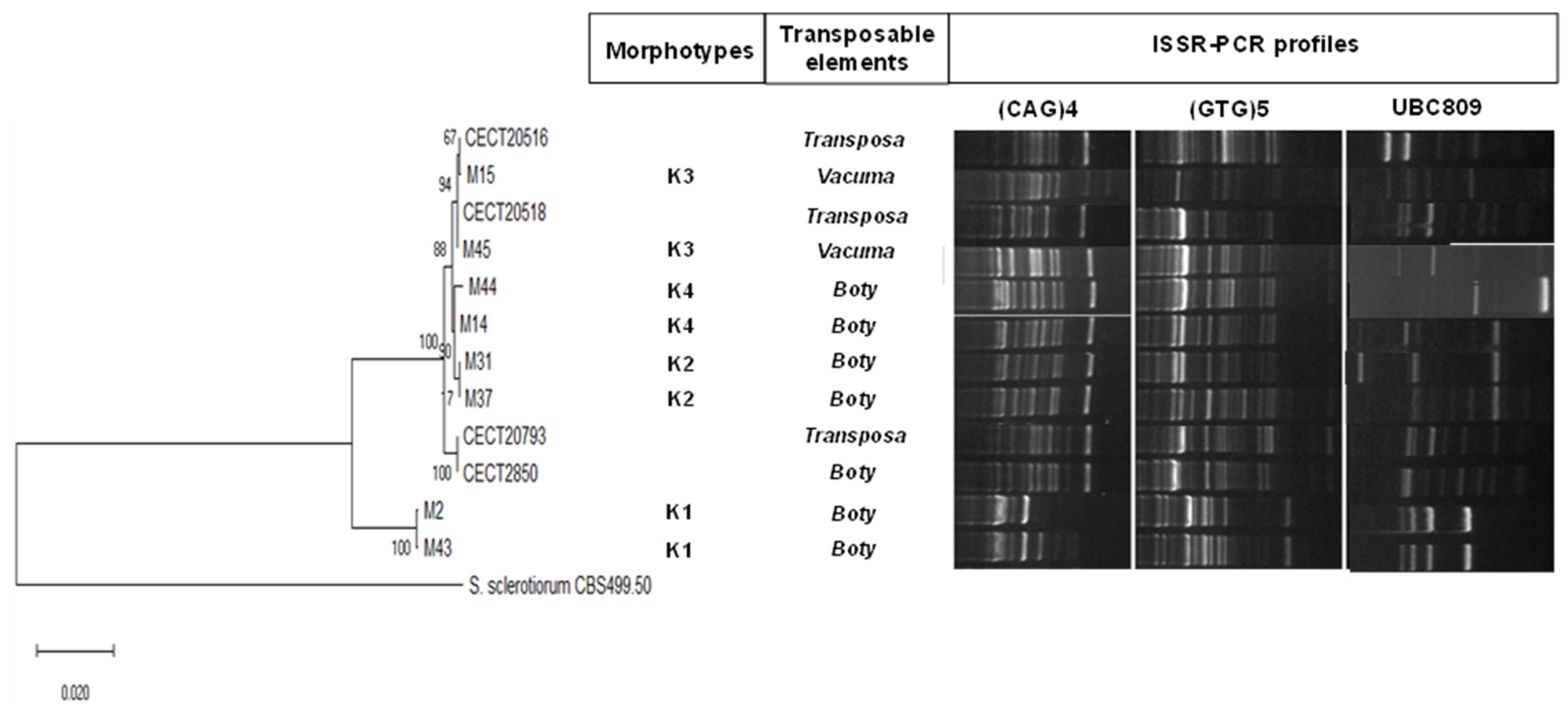
| Season | Microbial Group | Species Identification | % of Identification (Reference Accession Number) | Disease Incidence (%) |
|---|---|---|---|---|
| 2019 | Bacteria | Bacillus cereus | 100 (KF835392.1) | 0.0 |
| 2019 | Agrococcus lahaulensis | 99.87 (MT214266.1) | 0.0 | |
| 2019, 2020 | Rahnella aquatilis | 100 (MN826597.1) | 13.3 | |
| 2019, 2020 | Tatumella terrea | 99.73 (LC505503.1) | 43.3 | |
| 2019, 2020 | Pantoea agglomerans | 99.73 (FJ611832.1) | 20.0 | |
| 2019, 2020 | Lonsdalea quercitina | 100 (JF311446.1) | 83.3 | |
| 2019, 2020 | Erwinia bilingiae | 100 (CP031695.1) | 33.3 | |
| 2019, 2020 | Erwinia persicina | 100 (MN540710.1) | 13.3 | |
| 2019, 2020 | Rosenbergiella nectarea | 100 (HQ284897.1) | 36.6 | |
| 2019, 2020 | Hafnia paralvei | 99.77 (MF111316.1) | 13.3 | |
| 2020 | Pseudomonas spp. | 100 (LC548103.1) | 26.7 | |
| 2020 | Leuconostoc mesenteroides | 100 (MT544938.1) | 36.7 | |
| 2019 | Yeasts | Yarrowia lipolytica | 100 (MH459414.1) | 0.0 |
| 2019 | Rhodotorula nothofagi | 100 (KX811212.1) | 0.0 | |
| 2019, 2020 | Candida oleophila | 99.83 (MF940128.1) | 0.0 | |
| 2019, 2020 | Aureobasidium pullulans | 100 (MT035961.1) | 0.0 | |
| 2019, 2020 | Filobasidium wieringae | 100 (KY103450.1) | 0.0 | |
| 2019, 2020 | Filobasidium magnum | 100 (MH197140.1) | 0.0 | |
| 2019, 2020 | Candida railenensis | 99.52 (KY102357.1) | 0.0 | |
| 2019, 2020 | Pichia kluyveri | 99.54 (KY104557.1) | 0.0 | |
| 2019, 2020 | Hanseniaspora uvarum | 99.86 (KY103554.1) | 13.3 | |
| 2020 | Metschnikowia pulcherrima | 97.02 (CP034457.1) | 20.0 | |
| 99.40 (CP034456.1) * | ||||
| 2020 | Metschnikowia viticola | 92.66 (KY104213.1) | 33.3 | |
| 100 (JN544019.1) * | ||||
| 2020 | Geotrichum candidum | 99.80 (MN861070.1) | 100 | |
| 2019 | Moulds | Schizophyllum commune | 100 (MN218205.1) | 100 |
| 2019 | Trichoderma atroviride | 100 (KU942400.1) | 0.0 | |
| 2019, 2020 | Penicillium crustosum | 100 (MK102704.1) | 100 | |
| 2019, 2020 | Mucor plumbeus | 99.67 (MK268150.1) | 0.0 | |
| 2019, 2020 | Alternaria alternata | 100 (MT446174.1) | 100 | |
| 2019, 2020 | Cladosporium cladosporioides | 100 (MF475952.1) | 36.7 | |
| 2019, 2020 | Botrytis cinerea | 100 (MH860108.1) | 100 | |
| 2020 | Fusarium oxysporum | 100 (MT529329.1) | 100 | |
| 2020 | Mucor racemosus | 99.84 (HM641690.1) | 56.7 | |
| 2020 | Monilia laxa | 100 (MN049483.1) | 100 | |
| 2020 | Alternaria infectoria | 100 (MH205934.1) | 56.7 |
| Inoculation Combinations | Symptoms a | Disease Incidence (%) | ||
|---|---|---|---|---|
| Mould | Bacteria | Yeasts | ||
| Botrytis cinerea M2 | Erwinia bilingiae B1 | - b | + (gas production) | 100 |
| Lonsdalea quercina B22 | - | ++ | 100 | |
| Leuconostoc mesenteroides B24 | - | ++ | 100 | |
| Tatumella terrea B27 | - | + | 100 | |
| Rosenbergiella nectarea B45 | - | + | 100 | |
| - | Yarrowia lipolytica L2 | +/− | 100 | |
| - | Aureobasidium pullulans L12 | +/− | 70.0 | |
| - | Candida oleophila L69 | +/− | 50.0 | |
| - | Candida railenensis L193 | +/− | 100 | |
| - | Hanseniaspora uvarum L92 | +/− | 100 | |
| - | Metschnikowia pulcherrima L25 | +/− | 26.7 | |
| - | Metschnikowia viticola L211 | +/− | 100 | |
| - | Pichia kluyveri L223 | +/− | 46.7 | |
| Lonsdalea quercitina B22 | Yarrowia lipolytica L2 | +/− | 100 | |
| Aureobasidium pullulans L12 | +/− | 100 | ||
| Metschnikowia viticola L211 | +/− | 100 | ||
| Hanseniaspora uvarum L92 | +/− | 100 | ||
| Tatumella terrea B27 | Yarrowia lipolytica L2 | +/− | 100 | |
| Aureobasidium pullulans L12 | +/− | 100 | ||
| Metschnikowia viticola L211 | +/− | 100 | ||
| Hanseniaspora uvarum L92 | +/− | 100 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serradilla, M.J.; Moraga, C.; Ruiz-Moyano, S.; Tejero, P.; Córdoba, M.d.G.; Martín, A.; Hernández, A. Identification of the Causal Agent of Aqueous Spot Disease of Sweet Cherries (Prunus avium L.) from the Jerte Valley (Cáceres, Spain). Foods 2021, 10, 2281. https://doi.org/10.3390/foods10102281
Serradilla MJ, Moraga C, Ruiz-Moyano S, Tejero P, Córdoba MdG, Martín A, Hernández A. Identification of the Causal Agent of Aqueous Spot Disease of Sweet Cherries (Prunus avium L.) from the Jerte Valley (Cáceres, Spain). Foods. 2021; 10(10):2281. https://doi.org/10.3390/foods10102281
Chicago/Turabian StyleSerradilla, Manuel Joaquín, Carlos Moraga, Santiago Ruiz-Moyano, Paula Tejero, María de Guía Córdoba, Alberto Martín, and Alejandro Hernández. 2021. "Identification of the Causal Agent of Aqueous Spot Disease of Sweet Cherries (Prunus avium L.) from the Jerte Valley (Cáceres, Spain)" Foods 10, no. 10: 2281. https://doi.org/10.3390/foods10102281
APA StyleSerradilla, M. J., Moraga, C., Ruiz-Moyano, S., Tejero, P., Córdoba, M. d. G., Martín, A., & Hernández, A. (2021). Identification of the Causal Agent of Aqueous Spot Disease of Sweet Cherries (Prunus avium L.) from the Jerte Valley (Cáceres, Spain). Foods, 10(10), 2281. https://doi.org/10.3390/foods10102281






