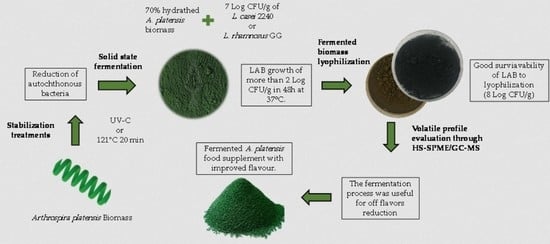
Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction
Abstract
1. Introduction
2. Materials and Methods
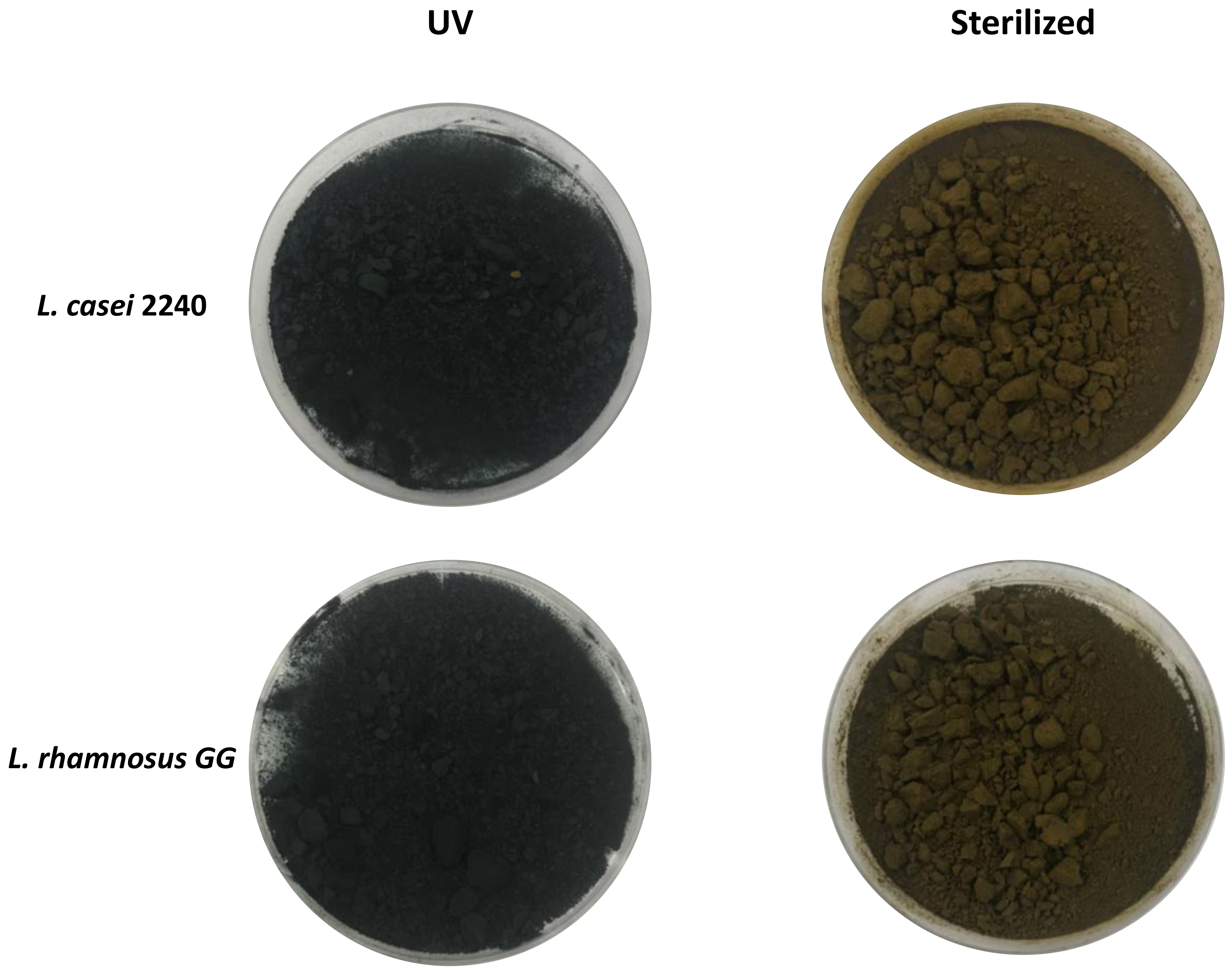
2.1. Arthrospira Platensis Stabilization Treatments
2.2. Lactic Acid Bacteria Strains
2.3. Arthrospira Platensis Biomass Fermentation
2.4. HS-SPME/GC-MS Analysis
2.5. Statistical Analysis
3. Results and Discussion
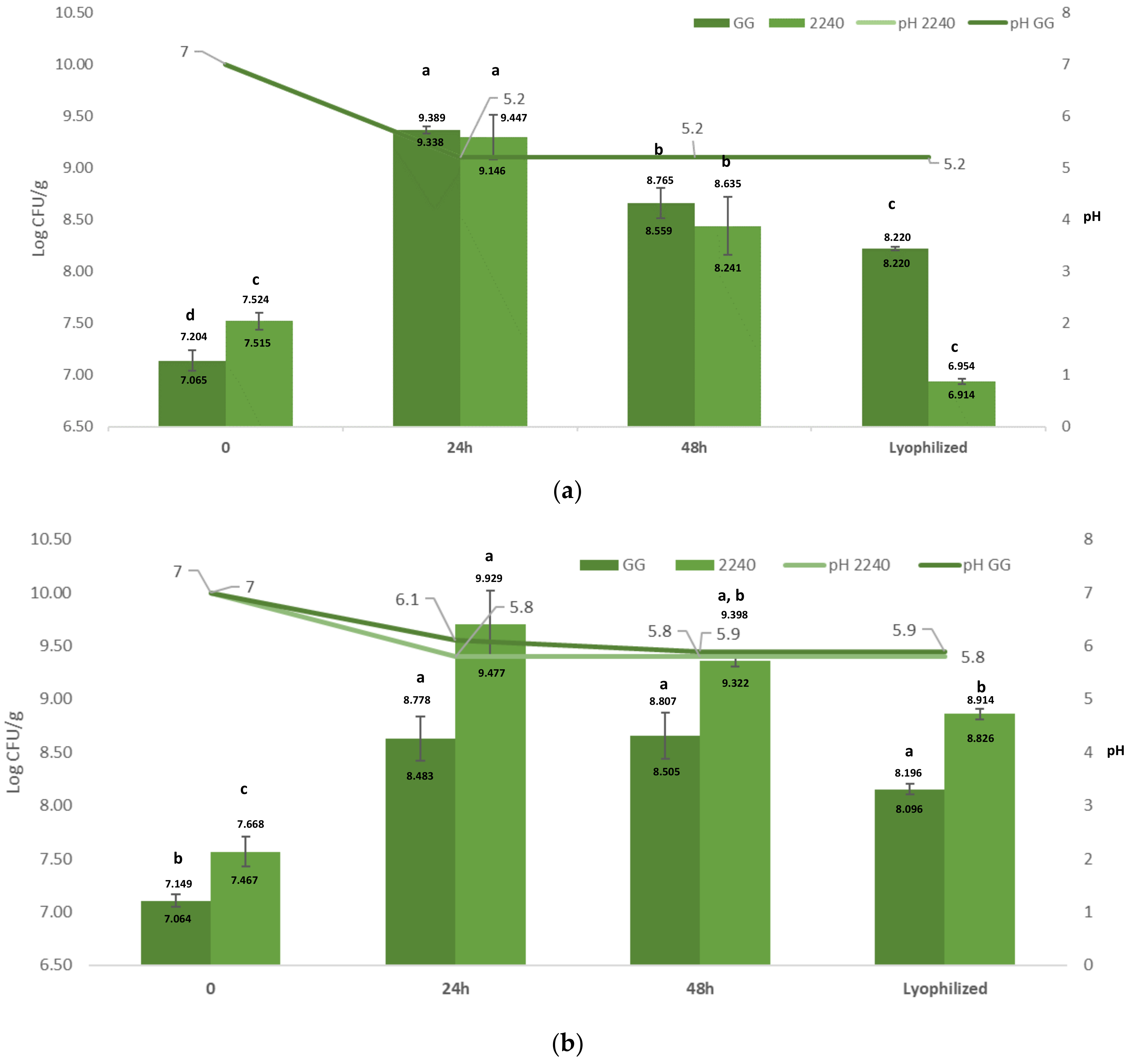
3.1. Arthrospira Platensis Fermentation
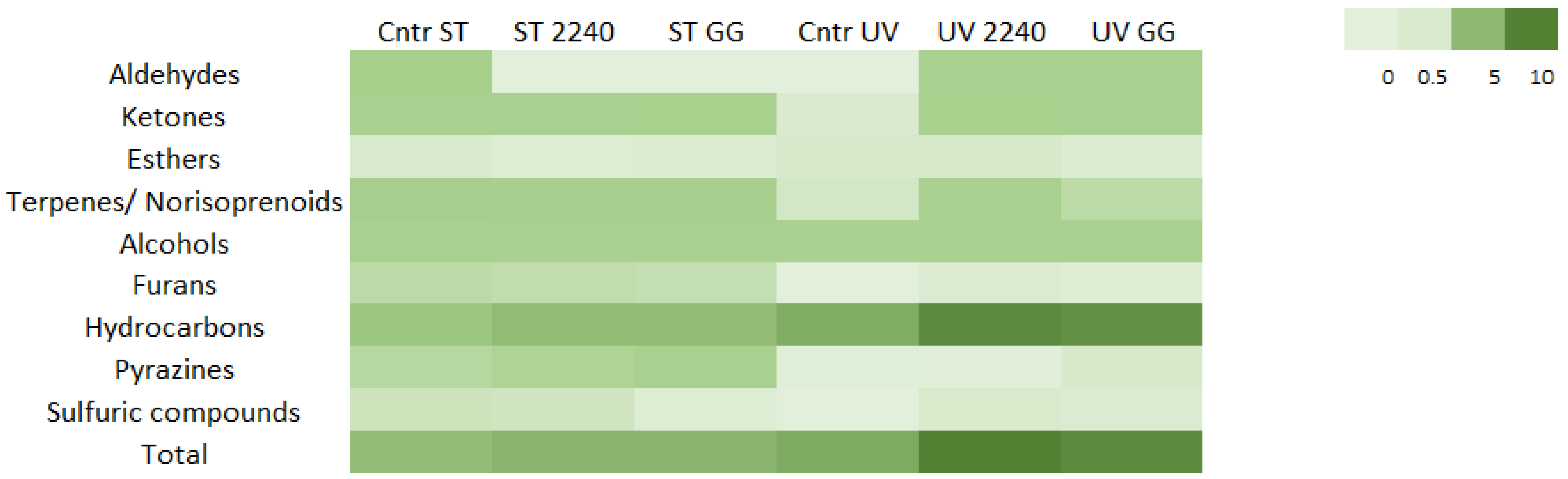
3.2. Volatile Profile Characterization of A. platensis and Changes in Volatile Components after Fermentation
| Chemical Class, Compound Name | Odor Type | Calculated LRI | Reference LRI | Identification Method | Reference | Effect of Stabilization | Effect of Fermentation |
|---|---|---|---|---|---|---|---|
| Aldehydes | |||||||
| Isobutyraldehyde | aldehydic | 805 | 814 | MS + LRI | [52] | n | n |
| 2-Methylbutanal | chocolate | 904 | 903 | MS + LRI | [53] | nd | nd |
| Isovaleraldehyde | aldehydic | 907 | 888 | MS + LRI | [54] | p | p |
| Hexanal | green | 1075 | 1086 | MS + LRI | [55] | n | n |
| Methional | vegetable | 1452 | 1468 | MS + LRI | [56] | p | p |
| Benzaldehyde | fruity | 1523 | 1537 | MS + LRI | [55] | n | n |
| 2,5-Dimethyl benzaldehyde | 1733 | 1705 | MS + LRI | [53] | p | p | |
| Ketones | |||||||
| Acetone | solvent | 810 | 901 | MS + LRI | [52] | p | p |
| 2-Butanone | ethereal | 894 | 901 | MS + LRI | [52] | p | p |
| diacetyl | buttery | 971 | 973 | MS + LRI | [54] | n | p |
| 6-Methyl-2-heptanone | camphoreous | 1229 | 1236 | MS + LRI | [57] | p | p |
| 3-Octanone | herbal | 1245 | 1261 | MS + LRI | [58] | n | p |
| 2-Octanone | earthy | 1277 | 1287 | MS + LRI | [53] | p | n |
| Acetoin | buttery | 1282 | 1300 | MS + LRI | [54] | n | p |
| 2,2,6-Trimethylcyclohexanone | thujonic | 1306 | 1308 | MS + LRI | [59] | p | n |
| Sulcatone | citrus | 1329 | 1335 | MS + LRI | [60] | p | n |
| Esters | |||||||
| Ethyl acetate | ethereal | 872 | 869 | MS + LRI | [61] | n | p |
| Ethyl caprylate | waxy | 1430 | 1438 | MS + LRI | [61] | nd | nd |
| Ethyl decanoate | waxy | 1628 | 1645 | MS + LRI | [61] | p | n |
| Phenethyl acetate | floral | 1804 | 1803 | MS + LRI | [62] | nd | nd |
| Terpenes, norisoprenoids and similar | |||||||
| p-Xylene | 1133 | 1149 | MS + LRI | [63] | p | n | |
| Myrcene | spicy | 1155 | 1143 | MS + LRI | [54] | n | n |
| α-Cyclocitral | citrus | 1427 | 1420 | MS + LRI | [53] | p | p |
| β-Cyclocitral | tropical | 1609 | 1612 | MS + LRI | [59] | p | n |
| Safranal | herbal | 1635 | 1637 | MS + LRI | [59] | p | p |
| α-Ionene | fruity | 1675 | MS | n | n | ||
| α-Ionone | floral | 1841 | 1848 | MS + LRI | [64] | p | p |
| β-Ionone | floral | 1918 | 1935 | MS + LRI | [64] | n | n |
| β-Ionone-5,6-epoxide | fruity | 1950 | 1989 | MS + LRI | [53] | p | n |
| Alcohols | |||||||
| Ethanol | alcoholic | 923 | 903 | MS + LRI | [54] | p | p |
| Isobutyl alcohol | ethereal | 1080 | 1100 | MS + LRI | [60] | n | n |
| Isoamyl alcohol | fermented | 1195 | 1210 | MS + LRI | [60] | p | p |
| 1-Pentanol | fermented | 1239 | 1260 | MS + LRI | [55] | p | n |
| 1-Hexanol | herbal | 1341 | 1357 | MS + LRI | [61] | p | n |
| 1-Octen-3-ol | earthy | 1437 | 1455 | MS + LRI | [55] | p | n |
| Benzyl alcohol | floral | 1882 | 1896 | MS + LRI | [61] | n | n |
| Furans | |||||||
| 2-Methylfuran | chocolate | 853 | 876 | MS + LRI | [52] | p | p |
| 3-Methylfuran | 881 | 877 | MS + LRI | [53] | n | n | |
| 2-Butylfuran | spicy | 1123 | 1140 | MS + LRI | [65] | p | p |
| 2-Pentylfuran | fruity | 1220 | 1232 | MS + LRI | [64] | p | n |
| Hydrocarbons | |||||||
| 1,2,4,4-Tetramethylcyclopentene | 920 | MS | n | n | |||
| 2,2,4,6,6-Pentamethylheptane | 944 | MS | n | n | |||
| Ethyl benzene | 1119 | 1127 | MS + LRI | [66] | n | n | |
| Tridecane | alkane | 1300 | 1300 | MS + LRI | [56] | p | p |
| Tetradecane | waxy/alkane | 1396 | 1400 | MS + LRI | [56] | p | p |
| 2,6,10-Trimethyltridecane | 1434 | 1442 | MS + LRI | [53] | n | n | |
| Pentadecane | waxy | 1492 | 1500 | MS + LRI | [56] | p | p |
| Hexadecane | alkane | 1590 | 1600 | MS + LRI | [56] | p | p |
| N-acetyl-4(H)-Pyridine | 1644 | MS | p | p | |||
| Heptadecane | alkane | 1687 | 1700 | MS + LRI | [56] | p | p |
| 6,9-Heptadecadiene | 1743 | p | p | ||||
| Pyrazines | |||||||
| 2-Methylpyrazine | nutty | 1267 | 1267 | MS + LRI | [52] | n | n |
| 2,5-Dimethylpyrazine | chocolate | 1318 | 1321 | MS + LRI | [52] | p | n |
| 2-Methyl-5-ethylpyrazine | coffee | 1368 | 1406 | MS + LRI | [52] | n | n |
| 2-Ethyl-6-methylpyrazine | potato | 1383 | 1402 | MS + LRI | [52] | p | p |
| Trimethyl pyrazine | nutty | 1398 | 1401 | MS + LRI | [52] | p | p |
| 2,3-Dimethyl-5-ethylpyrazine | burnt | 1452 | 1460 | MS + LRI | [53] | p | n |
| Tetramethyl pyrazine | nutty | 1468 | 1474 | MS + LRI | [52] | p | n |
| Sulfur compounds | |||||||
| Dimethyl disulfide | sulfurous | 1063 | 1073 | MS + LRI | [54] | p | n |
| 2-Ethyl-4-methylthiazole | nutty | 1336 | 1322 | MS + LRI | [52] | n | n |
| Dimethyl trisulfide | alliaceous | 1369 | 1375 | MS + LRI | [54] | n | n |
3.3. Sensory Properties of the Detected Volatile Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.; Mendes, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2019, 31, 1077–1083. [Google Scholar] [CrossRef]
- Zarbà, C.; Chinnici, G.; D’Amico, M. Novel Food: The Impact of Innovation on the Paths of the Traditional Food Chain. Sustainability 2020, 12, 555. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed: Spirulina supplementation in livestock. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Edible Seaweeds and Spirulina Extracts for Food Application: In Vitro and In Situ Evaluation of Antimicrobial Activity towards Foodborne Pathogenic Bacteria. Foods 2020, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Bancalari, E.; Martelli, F.; Bernini, V.; Neviani, E.; Gatti, M. Bacteriostatic or bactericidal? Impedometric measurements to test the antimicrobial activity of Arthrospira platensis extract. Food Control 2020, 107380. [Google Scholar] [CrossRef]
- Massoud, R.; Khosravi-Darani, K.; Nakhsaz, F.; Varga, L. Evaluation of physicochemical, microbiological and sensory properties of croissants fortified with Arthrospira platensis (Spirulina). Czech J. Food Sci. 2017, 34, 350–355. [Google Scholar] [CrossRef]
- Ak, B.; Avşaroğlu, E.; Işık, O.; Özyurt, G.; Kafkas, E.; Uslu, L. Nutritional and Physicochemical Characteristics of Bread Enriched with Microalgae Spirulina platensis. Int. J. Eng. Res. Appl. 2016, 6, 9. [Google Scholar]
- Zouari, N.; Abid, M.; Fakhfakh, N.; Ayadi, M.A.; Zorgui, L.; Ayadi, M.; Attia, H. Blue-green algae (Arthrospira platensis) as an ingredient in pasta: Free radical scavenging activity, sensory and cooking characteristics evaluation. Int. J. Food Sci. Nutr. 2011, 62, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Golmakani, M.-T.; Soleimanian-Zad, S.; Alavi, N.; Nazari, E.; Eskandari, M.H. Effect of Spirulina (Arthrospira platensis) powder on probiotic bacteriologically acidified feta-type cheese. J. Appl. Phycol. 2019, 31, 1085–1094. [Google Scholar] [CrossRef]
- Mohammadi-Gouraji, E.; Soleimanian-Zad, S.; Ghiaci, M. Phycocyanin-enriched yogurt and its antibacterial and physicochemical properties during 21 days of storage. LWT 2019, 102, 230–236. [Google Scholar] [CrossRef]
- Yamaguchi, S.K.F.; Moreira, J.B.; Costa, J.A.V.; de Souza, C.K.; Bertoli, S.L.; Carvalho, L.F.D. Evaluation of Adding Spirulina to Freeze-Dried Yogurts Before Fermentation and After Freeze-Drying. Ind. Biotechnol. 2019, 15, 89–94. [Google Scholar] [CrossRef]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Guldas, M.; Irkin, R. Influence of Spirulina platensis powder on the microflora of yoghurt and acidophilus milk. Mljekarstvo 2010, 60, 237–243. [Google Scholar]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Bhowmik, D.; Dubey, J.; Mehra, S. Probiotic Efficiency of Spirulina platensis-Stimulating Growth of Lactic Acid Bacteria. World J. Dairy Food Sci. 2009, 4, 160–164. [Google Scholar]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as Natural Fermentation Booster for Milk and Soy Fermented Beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef]
- Parada, J. Lactic acid bacteria growth promoters from Spirulina platensis. Int. J. Food Microbiol. 1998, 45, 225–228. [Google Scholar] [CrossRef]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.E.; Bouchier, P.; O’Sullivan, E.; Kelly, J.; Kevin Collins, J.; Fitzgerald, G.; Paul Ross, R.; Stanton, C. A spray-dried culture for probiotic Cheddar cheese manufacture. Int. Dairy J. 2002, 12, 749–756. [Google Scholar] [CrossRef]
- Wong, S.-S.; Quan Toh, Z.; Dunne, E.M.; Mulholland, E.K.; Tang, M.L.; Robins-Browne, R.M.; Licciardi, P.V.; Satzke, C. Inhibition of Streptococcus pneumoniae adherence to human epithelial cells in vitro by the probiotic Lacticaseibacillus rhamnosus GG. BMC Res. Notes 2013, 6, 135. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galaverna, G.; Neviani, E.; Lazzi, C. Use of Dairy and Plant-Derived Lactobacilli as Starters for Cherry Juice Fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef]
- Smid, E.J.; Kleerebezem, M. Production of Aroma Compounds in Lactic Fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.; Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef]
- Aguero, J.; Lora, J.; Estrada, K.; Concepcion, F.; Nunez, A.; Rodriguez, A.; Pino, J.A. Volatile Components of a Commercial Sample of the Blue-Green Algae Spirulina platensis. J. Essent. Oil Res. 2003, 15, 114–117. [Google Scholar] [CrossRef]
- Cuellar-Bermúdez, S.P.; Barba-Davila, B.; Serna-Saldivar, S.O.; Parra-Saldivar, R.; Rodriguez-Rodriguez, J.; Morales-Davila, S.; Goiris, K.; Muylaert, K.; Chuck-Hernández, C. Deodorization of Arthrospira platensis biomass for further scale-up food applications. J. Sci. Food Agric. 2017, 97, 5123–5130. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.-H.; Ren, D.-F.; Lu, J. Mixed fermentation of Spirulina platensis with Lacticaseibacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]
- Högnadóttir, Á. Flavor Perception and Volatile Compounds in Fish. 2000, p. 25. Available online: http://www.matis.is/media/matis/utgafa/Skyrsla_01-00_Flavor_Perception_and_Volatile_Compounds_in_Fish.pdf (accessed on 30 December 2020).
- Fink, P. Ecological functions of volatile organic compounds in aquatic systems. Mar. Freshw. Behav. Physiol. 2007, 40, 155–168. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Bae, H.-N.; Eom, S.-H.; Lim, K.-S.; Yun, I.-H.; Chung, Y.-H.; Jeon, J.-M.; Kim, H.-W.; Lee, M.-S.; Lee, Y.-B.; et al. Removal of off-flavors from sea tangle (Laminaria japonica) extract by fermentation with Aspergillus oryzae. Bioresour. Technol. 2012, 121, 475–479. [Google Scholar] [CrossRef]
- Pala, Ç.U.; Toklucu, A.K. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar] [CrossRef]
- Koutchma, T. UV Light for Processing Foods. Ozone Sci. Eng. 2008, 30, 93–98. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.; de Morais, A.; de Morais, R. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Martelli, F.; Favari, C.; Mena, P.; Guazzetti, S.; Ricci, A.; Rio, D.D.; Lazzi, C.; Neviani, E.; Bernini, V. Antimicrobial and Fermentation Potential of Himanthalia elongata in Food Applications. Microorganisms 2020, 8, 248. [Google Scholar] [CrossRef]
- Jia, R.; Chen, H.; Chen, H.; Ding, W. Effects of fermentation with Lacticaseibacillus rhamnosus GG on product quality and fatty acids of goat milk yogurt. J. Dairy Sci. 2016, 99, 221–227. [Google Scholar] [CrossRef]
- Rubio, R.; Aymerich, T.; Bover-Cid, S.; Guàrdia, M.D.; Arnau, J.; Garriga, M. Probiotic strains Lacticaseibacillus plantarum 299V and Lacticaseibacillus rhamnosus GG as starter cultures for fermented sausages. LWT Food Sci. Technol. 2013, 54, 51–56. [Google Scholar] [CrossRef]
- Antelo, F.S.; Costa, J.A.V.; Kalil, S.J. Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem. Eng. J. 2008, 41, 43–47. [Google Scholar] [CrossRef]
- Dutta, D.; Dutta, A.; Raychaudhuri, U.; Chakraborty, R. Rheological characteristics and thermal degradation kinetics of beta-carotene in pumpkin puree. J. Food Eng. 2006, 76, 538–546. [Google Scholar] [CrossRef]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of Chlorophyll Degradation and Color Loss in Heated Broccoli Juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef]
- Wu, S.-C.; Wang, F.-J.; Pan, C.-L. The comparison of antioxidative properties of seaweed oligosaccharides fermented by two lactic acid bacteria. J. Mar. Sci. Technol. 2010, 18, 9. [Google Scholar]
- Pereira, A.L.F.; Maciel, T.C.; Rodrigues, S. Probiotic beverage from cashew apple juice fermented with Lacticaseibacillus casei. Food Res. Int. 2011, 44, 1276–1283. [Google Scholar] [CrossRef]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable By-Product Lacto-Fermentation as a New Source of Antimicrobial Compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Giulio, B.D.; Orlando, P.; Barba, G.; Coppola, R.; Rosa, M.D.; Sada, A.; Prisco, P.P.D.; Nazzaro, F. Use of alginate and cryo-protective sugars to improve the viability of lactic acid bacteria after freezing and freeze-drying. World J. Microbiol. Biotechnol. 2005, 21, 739–746. [Google Scholar] [CrossRef]
- Reddy, K.B.P.K.; Awasthi, S.P.; Madhu, A.N.; Prapulla, S.G. Role of Cryoprotectants on the Viability and Functional Properties of Probiotic Lactic Acid Bacteria during Freeze Drying. Food Biotechnol. 2009, 23, 243–265. [Google Scholar] [CrossRef]
- Varga, L.; Szigeti, J.; Kovács, R.; Földes, T.; Buti, S. Influence of a Spirulina platensis Biomass on the Microflora of Fermented ABT Milks During Storage (R1). J. Dairy Sci. 2002, 85, 1031–1038. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Dave, R.I.; Shah, N.P. Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int. Dairy J. 1997, 7, 31–41. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007, 30, 563–572. [Google Scholar] [CrossRef]
- sherena.johnson@nist.gov Standard Reference Data. Available online: https://www.nist.gov/srd (accessed on 8 October 2020).
- Ricci, A.; Cirlini, M.; Levante, A.; Dall’Asta, C.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Cirlini, M.; Dall’Asta, C.; Silvanini, A.; Beghè, D.; Fabbri, A.; Galaverna, G.; Ganino, T. Volatile fingerprinting of chestnut flours from traditional Emilia Romagna (Italy) cultivars. Food Chem. 2012, 134, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Tanaka, T.; Yamaguchi, T.; Katsumata, R.; Kiuchi, K. Comparison of volatile components in commercial Itohiki-Natto by solid phase microextraction and gas chromatography. Nippon Shokuhin Kagaku Kogaku Kaishi 2003, 50, 278–285. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Yamamoto, M.; Baldermann, S.; Yoshikawa, K.; Fujita, A.; Mase, N.; Watanabe, N. Determination of Volatile Compounds in Four Commercial Samples of Japanese Green Algae Using Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.; Ganino, T.; Lazzi, C.; Chiancone, B. From Byproduct to Resource: Fermented Apple Pomace as Beer Flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Cirlini, M.; Morini, E.; Galaverna, G. Brand-dependent volatile fingerprinting of Italian wines from Valpolicella. J. Chromatogr. A 2011, 1218, 7557–7565. [Google Scholar] [CrossRef]
- Ong, P.K.C.; Acree, T.E. Similarities in the Aroma Chemistry of Gewu1 rztraminer Variety Wines and Lychee (Litchi chinesis Sonn.) Fruit. J. Agric. Food. Chem. 1999, 47, 665–670. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.-G.; Shibamoto, T. Antioxidative Activities of Fractions Obtained from Brewed Coffee. J. Agric. Food Chem. 2004, 52, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Brunton, N.P.; Cronin, D.A.; Monahan, F.J. Volatile components associated with freshly cooked and oxidized off-flavours in turkey breast meat. Flavour Fragr. J. 2002, 17, 327–334. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.; Nieman, K.; Dall’Asta, C.; Del Rio, D. Phenolic and Volatile Composition of a Dry Spearmint (Mentha spicata L.) Extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef]
- Schneider, H.; Gelpi, E.; Bennett, E.O.; Oró, J. Fatty acids of geochemical significance in microscopic algae. Phytochemistry 1970, 9, 613–617. [Google Scholar] [CrossRef]
- Stoyanova, L.G.; Ustyugova, E.A.; Netrusov, A.I. Antibacterial metabolites of lactic acid bacteria: Their diversity and properties. Appl. Biochem. Microbiol. 2012, 48, 229–243. [Google Scholar] [CrossRef]
- Höckelmann, C.; Jüttner, F. Off-flavours in water: Hydroxyketones and β-ionone derivatives as new odour compounds of freshwater cyanobacteria. Flavour Fragr. J. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Do Nascimento, T.C.; Nass, P.P.; Fernandes, A.S.; Vieira, K.R.; Wagner, R.; Jacob-Lopes, E.; Zepka, L.Q. Exploratory data of the microalgae compounds for food purposes. Data Brief 2020, 29, 105182. [Google Scholar] [CrossRef]
- Van Durme, J.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the Volatile Composition and Sensory Properties of Five Species of Microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef]
- Isleten Hosoglu, M. Aroma characterization of five microalgae species using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem. 2018, 240, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Achyuthan, K.; Harper, J.; Manginell, R.; Moorman, M. Volatile Metabolites Emission by In Vivo Microalgae—An Overlooked Opportunity? Metabolites 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Amàrita, F.; Fernàndez-Esplà, D.; Requena, T.; Pelaez, C. Conversion of methionine to methional by Lactococcus lactis. FEMS Microbiol. Lett. 2001, 204, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Amárita, F.; Martínez-Cuesta, C.M.; Taborda, G.; Soto-Yárritu, P.L.; Requena, T.; Peláez, C. Formation of methional by Lactococcus lactis IFPL730 under cheese model conditions. Eur. Food Res. Technol. 2002, 214, 58–62. [Google Scholar] [CrossRef][Green Version]
- Helinck, S.; Le Bars, D.; Moreau, D.; Yvon, M. Ability of Thermophilic Lactic Acid Bacteria To Produce Aroma Compounds from Amino Acids. Appl. Environ. Microbiol. 2004, 70, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; da Silva Vale, A.; de Carvalho Neto, D.P.; Muynarsk, E.S.; Soccol, V.T.; Soccol, C.R. Lactic acid bacteria: What coffee industry should know? Curr. Opin. Food Sci. 2020, 31, 1–8. [Google Scholar] [CrossRef]
- El-Gendy, S.M.; Abdel-Galil, H.; Shahin, Y.; Hegazi, F.Z. Acetoin and Diacetyl Production by Homo- and Heterofermentative Lactic Acid Bacteria. J. Food Prot. 1983, 46, 420–425. [Google Scholar] [CrossRef]
- Milovanović, I.; Mišan, A.; Simeunović, J.; Kovač, D.; Jambrec, D.; Mandić, A. Determination of Volatile Organic Compounds in Selected Strains of Cyanobacteria. J. Chem. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, S.; Cui, W.; Lv, J. Identification of Antifungal Compounds Produced by Lacticaseibacillus casei AST18. Curr. Microbiol. 2012, 65, 156–161. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods 2021, 10, 67. https://doi.org/10.3390/foods10010067
Martelli F, Cirlini M, Lazzi C, Neviani E, Bernini V. Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods. 2021; 10(1):67. https://doi.org/10.3390/foods10010067
Chicago/Turabian StyleMartelli, Francesco, Martina Cirlini, Camilla Lazzi, Erasmo Neviani, and Valentina Bernini. 2021. "Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction" Foods 10, no. 1: 67. https://doi.org/10.3390/foods10010067
APA StyleMartelli, F., Cirlini, M., Lazzi, C., Neviani, E., & Bernini, V. (2021). Solid-State Fermentation of Arthrospira platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods, 10(1), 67. https://doi.org/10.3390/foods10010067