Differences in Processing Quality Traits, Protein Content and Composition between Spelt and Bread Wheat Genotypes Grown under Conventional and Organic Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Experiment
2.2. Quality Parameters of the Grain and Flour
2.3. Protein Analysis
2.4. Statistical Analyses
3. Results
3.1. Effect of Wheat Species, Growing Site and Field Management on Processing Quality
3.2. Effect of Wheat Species, Growing Site and Field Management on the Kernel Size, Protein Content and Composition
3.3. Differences in the Genotype and Environmental Effects on Spelt and Bread Wheat Properties
3.4. Principal Component Analysis of Spelt and Bread Wheat Grown under Different Field Managements and in Different Countries
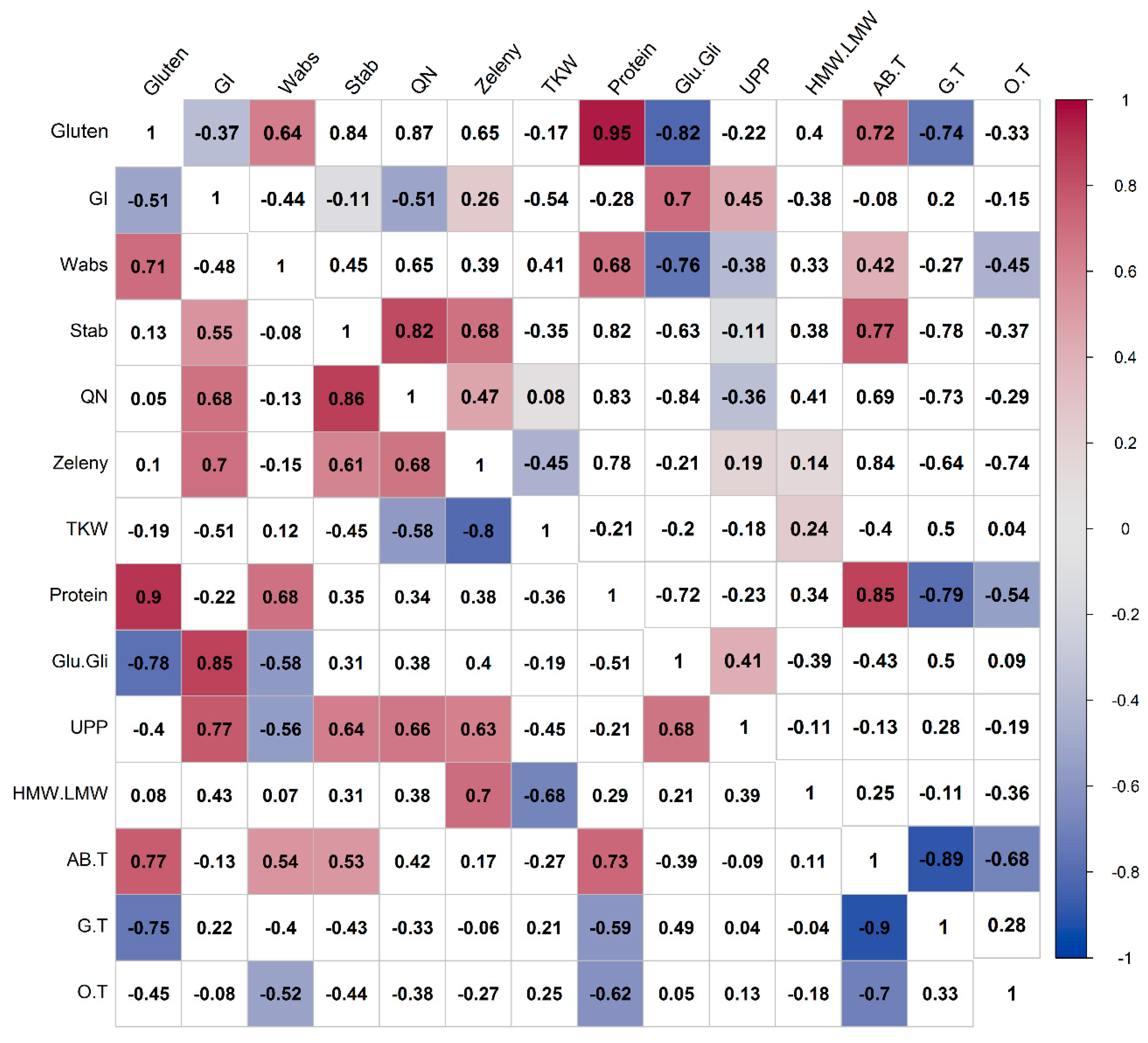
3.5. Correlations of Compositional and Processing Quality Traits in Spelt and Bread Wheat
4. Discussion
4.1. Differences between Spelt and Bread Wheat on Processing Quality, Protein Content and Composition
4.2. The Effect of the Genotype and the Outstanding Characteristics of the Varieties
4.3. The Effect of Environment on Processing Quality, Protein Content and Composition of Spelt and Bread Wheat Varieties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arzani, A.; Ashraf, M. Cultivated Ancient Wheats (Triticum spp.): A Potential Source of Health-Beneficial Food Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R. Re-Discovering Ancient Wheat Varieties as Functional Foods. J. Tradit. Complem. Med. 2015, 5, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Békés, F.; Schoenlechner, R.; Tömösközi, S. Ancient wheats and pseudocereals for possible use in cereal-grain dietary intolerances. In Cereal Grains—Assessing and Managing Quality, 2nd ed.; Wrigley, C., Batey, I., Miskelly, D., Eds.; Woodhead Publishing: Sawston/Cambridge, UK, 2017; pp. 353–389. [Google Scholar] [CrossRef]
- Sugár, E.; Fodor, N.; Sándor, R.; Bónis, P.; Vida, G.; Árendás, T. Spelt Wheat: An Alternative for Sustainable Plant Production at Low N-Levels. Sustainability 2019, 11, 6726. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Galli, V.; Francisci, R.; Mair, V.; Skrabanja, V.; Kreft, I. Characteristics of Spelt Wheat Products and Nutritional Value of Spelt Wheat-Based Bread. Food Chem. 2000, 68, 437–441. [Google Scholar] [CrossRef]
- Kyptova, M.; Konvalina, P.; Khoa, T.D. Technological and Sensory Quality of Grain and Baking Products from Spelt Wheat. Res. Rural Dev. 2017, 2, 46–53. [Google Scholar] [CrossRef]
- Biel, W.; Stankowski, S.; Sobolewska, M.; Sadkiewicz, J.; Jaroszewska, A.; Pużyński, S. Effect of Selected Agronomic Factors on the Baking Quality of Winter Spelt Strains and Cultivars (Triticum aestivum ssp. spelta L.) in Comparison with Common Wheat (Triticum aestivum ssp vulgare L.). Rom. Agric. Res. 2016, 33, 251–258. [Google Scholar]
- He, Z.H.; Liu, L.; Xia, X.C.; Liu, J.J.; Peña, R.J. Composition of HMW and LMW Glutenin Subunits and Their Effects on Dough Properties, Pan Bread, and Noodle Quality of Chinese Bread Wheats. Cereal Chem. J. 2005, 82, 345–350. [Google Scholar] [CrossRef]
- Wieser, H. Comparative Investigations of Gluten Proteins from Different Wheat Species. I. Qualitative and Quantitative Composition of Gluten Protein Types. Eur. Food Res. Technol. 2000, 211, 262–268. [Google Scholar] [CrossRef]
- Shewry, P.R. Plant storage proteins. Biol. Rev. 1995, 70, 375–426. [Google Scholar] [CrossRef]
- Ma, W.; Yu, Z.; She, M.; Zhao, Y.; Islam, S. Wheat Gluten Protein and Its Impacts on Wheat Processing Quality. Front. Agric. Sci. Eng. 2019, 6, 279. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Lafiandra, D. Genetics of wheat gluten proteins. In Advances in Genetics, 1st ed.; Hall, J.C., Dunlap, J.C., Friedmann, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 49, pp. 111–184. [Google Scholar] [CrossRef]
- Schober, T.J.; Bean, S.R.; Kuhn, M. Gluten Proteins from Spelt (Triticum aestivum ssp. spelta) Cultivars: A Rheological and Size-Exclusion High-Performance Liquid Chromatography Study. J. Cereal Sci. 2006, 44, 161–173. [Google Scholar] [CrossRef]
- Rakszegi, M.; Mikó, P.; Löschenberger, F.; Hiltbrunner, J.; Aebi, R.; Knapp, S.; Tremmel-Bede, K.; Megyeri, M.; Kovács, G.; Molnár-Láng, M.; et al. Comparison of Quality Parameters of Wheat Varieties with Different Breeding Origin under Organic and Low-Input Conventional Conditions. J. Cereal Sci. 2016, 69, 297–305. [Google Scholar] [CrossRef][Green Version]
- Mäder, P.; Hahn, D.; Dubois, D.; Gunst, L.; Alföldi, T.; Bergmann, H.; Oehme, M.; Amadò, R.; Schneider, H.; Graf, U.; et al. Wheat Quality in Organic and Conventional Farming: Results of a 21 Year Field Experiment. J. Sci. Food Agric. 2007, 87, 1826–1835. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental Impact of Different Agricultural Management Practices: Conventional vs. Organic Agriculture. Crit. Rev. Plant. Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembiałkowska, E.; Quaglio, G.; Grandjean, P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health 2017, 16, 111. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/GV (accessed on 4 August 2020).
- Organic Farming in the EU. A Fast-Growing Sector. Available online: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/farming/documents/market-brief-organic-farming-in-the-eu_mar2019_en.pdf (accessed on 4 August 2020).
- Agriculture. Database. Organic Farming. Available online: https://ec.europa.eu/eurostat/web/agriculture/data/database (accessed on 4 August 2020).
- Mason, H.; Navabi, A.; Frick, B.; O’Donovan, J.; Niziol, D.; Spaner, D. Does Growing Canadian Western Hard Red Spring Wheat under Organic Management Alter Its Breadmaking Quality? Renew. Agric. Food Syst. 2007, 22, 157–167. [Google Scholar] [CrossRef]
- Vrček, I.V.; Čepo, D.V.; Rašić, D.; Peraica, M.; Žuntar, I.; Bojić, M.; Mendaš, G.; Medić-Šarić, M.A. Comparison of the Nutritional Value and Food Safety of Organically and Conventionally Produced Wheat Flours. Food Chem. 2014, 143, 522–529. [Google Scholar] [CrossRef]
- Mazzoncini, M.; Antichi, D.; Silvestri, N.; Ciantelli, G.; Sgherri, C. Organically vs. Conventionally Grown Winter Wheat: Effects on Grain Yield. Technological Quality, and on Phenolic Composition and Antioxidant Properties of Bran and Refined Flour. Food Chem. 2015, 175, 445–451. [Google Scholar] [CrossRef]
- Mikó, P.; Löschenberger, F.; Hiltbrunner, J.; Aebi, R.; Megyeri, M.; Kovács., G.; Molnár-Láng., M.; Vida, G.; Rakszegi, M. Comparison of Bread Wheat Varieties with Different Breeding Origin under Organic and Low Input Management. Euphytica 2014, 199, 69–80. [Google Scholar] [CrossRef][Green Version]
- Berecz, K.; Simon-Sarkadi, L.; Ragasits, I.; Hoffmann, S. Comparison of Protein Quality and Mineral Element Concentrations in Grain of Spelt (Triticum spelta L.) and Common Wheat (Triticum aestivum L.). Arch. Agron. Soil Sci. 2001, 47, 389–398. [Google Scholar] [CrossRef]
- Ugrenovic, V.; Bodroza-Solarov, M.; Pezo, L.; Djisalov, J.; Popovic, V.; Maric, B.; Filipovic, V. Analysis of Spelt Variability (Triticum spelta L.) Grown in Different Conditions of Serbia by Organic Conditions. Genetika 2018, 50, 635–646. [Google Scholar] [CrossRef]
- Filipčev, B.; Šimurina, O.; Bodroža-Solarov, M.; Obreht, D. Comparison of the Bread-Making Performance of Spelt Varieties Grown under Organic Conditions in the Environment of Northern Serbia and Their Responses to Dough Strengthening Improvers. Hemijska Industrija 2013, 67, 443–453. [Google Scholar] [CrossRef]
- Žilić, S.; Barać, M.; Pešić, M.; Dodig, D.; Ignjatović-Micić, D. Characterization of Proteins from Grain of Different Bread and Durum Wheat Genotypes. Int. J. Mol. Sci. 2011, 12, 5878–5894. [Google Scholar] [CrossRef]
- Šmolková, H.; Gálová, Z.; Gregová, E. Winter Spelt Wheat (Triticum spelta L.) Grain Proteins Genetic Markers. Chem. Pap. 1998, 52, 587–588. [Google Scholar]
- Gálová, Z.; Michalíki, I.; Knoblochová, H.; Gregová, E. Variation in HMW Glutenin Subunits of Different Species of Wheat. Plant Soil Environ. 2011, 48, 15–19. [Google Scholar]
- Yan, Y.; Hsam, S.L.K.; Yu, J.Z.; Jiang, Y.; Ohtsuka, I.; Zeller, F.J. HMW and LMW Glutenin Alleles among Putative Tetraploid and Hexaploid European Spelt Wheat (Triticum spelta L.) Progenitors. Theor. Appl. Genet. 2003, 107, 1321–1330. [Google Scholar] [CrossRef]
- An, X.; Li, Q.; Yan, Y.; Xiao, Y.; Hsam, S.L.K.; Zeller, F.J. Genetic Diversity of European Spelt Wheat (Triticum aestivum ssp. spelta L. Em. Thell.) Revealed by Glutenin Subunit Variations at the Glu-1 and Glu-3 Loci. Euphytica 2005, 146, 193–201. [Google Scholar] [CrossRef]
- Oslovičová, V.; Gálová, Z.; Chňapek, M.; Balážová, Ž. Identification of Triticum aestivum, L. Triticum spelta, L. and Triticum durum DESF, Genotypes on the HMW-GS Base. Plant Soil Environ. 2010, 56, 82–86. [Google Scholar]
- Ormoli, L.; Costa, C.; Negri, S.; Perenzin, M.; Vaccino, P. Diversity Trends in Bread Wheat in Italy during the 20th Century Assessed by Traditional and Multivariate Approaches. Sci. Rep. 2015, 5, 8574. [Google Scholar] [CrossRef]
- Knežević, D.; Šurlan-Momirovć, G.; Ćirić, D. Allelic Variation at Glu-1 Loci in Some Yugoslav Wheat Cultivars. Euphytica 1993, 69, 89–94. [Google Scholar] [CrossRef]
- Marchetti-Deschmann, M.; Lehner, A.; Peterseil, V.; Sövegjarto, F.; Hochegger, R.; Allmaier, G. Fast Wheat Variety Classification by Capillary Gel Electrophoresis-on-a-Chip after Single-Step One-Grain High Molecular Weight Glutenin Extraction. Anal. Bioanal. Chem. 2011, 400, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Hristov, N.; Mladenov, N.; Jockovic, B.; Djuric, V.; Kondic-Spika, A.; Obreht, D. High Molecular Weight (HMW) Glutenin Subunit Composition of NS Wheat Cultivars Released in 1987–2008. Ratarstvo i Povrtarstvo 2013, 50, 29–36. [Google Scholar] [CrossRef]
- Branlard, G.; Dardevet, M.; Amiour, N.; Igrejas, G. Allelic Diversity of HMW and LMW Glutenin Subunits and Omega- Gliadins in French Bread Wheat (Triticum aestivum L.). Genet. Resour. Crop. Evol. 2003, 50, 669–679. [Google Scholar] [CrossRef]
- ACC. Approved Methods of Analysis, 11th ed.; AACC Method 46-30.01; Crude Protein—Combustion Method; Cereals and Grains Association: Saint Paul, MI, USA, 2020; Available online: http://methods.aaccnet.org/summaries/46-30-01.aspx (accessed on 21 July 2020).
- Tömösközi, S.; Nádosi, M.; Balázs, G.; Cavanagh, C.; Morgunov, A.; Salgó, A.; Békés, F. Revival of sedimentation value—Method development, quality prediction and molecular background. In Proceedings of the 10th International Gluten Workshop, INRA, Clermont-Ferrand, France, 7–9 September 2009; pp. 104–108. [Google Scholar]
- Larroque, O.R.; Békés, F. Rapid Size-Exclusion Chromatography Analysis of Molecular Size Distribution for Wheat Endosperm Protein. Cereal Chem. J. 2000, 77, 451–453. [Google Scholar] [CrossRef]
- Marchylo, B.A.; Kruger, J.E.; Hatcher, D.W. Quantitative reversed-phase high-performance liquid chromatographic analysis of wheat storage proteins as a potential quality prediction tool. J. Cereal Sci. 1989, 9, 113–130. [Google Scholar] [CrossRef]
- Virk, D.S.; Pandit, D.B.; Sufian, M.A.; Ahmed, F.; Siddique, M.A.B.; Samad, M.A.; Rahman, M.M.; Islam, M.M.; Ortiz-Ferrara, G.; Joshi, K.D.; et al. REML is an effective analysis for mixed modelling of unbalanced on-farm varietal trials. Exp. Agric. 2009, 45, 77–91. [Google Scholar] [CrossRef]
- Mikos, M.; Podolska, G. Bread-making quality of old common bread (Triticum aestivum ssp. vulgare L.) and spelt (Triticum aestivum ssp. spelta L.) wheat cultivars. J. Food Agric. Environ. 2012, 10, 221–224. [Google Scholar]
- Longin, C.F.H.; Ziegler, J.; Schweiggert, R.; Koehler, P.; Carle, R.; Würschum, T. Comparative study of hulled (einkorn, emmer, and spelt) and naked wheats (durum and bread wheat): Agronomic performance and quality traits. Crop. Sci. 2016, 56, 302–311. [Google Scholar] [CrossRef]
- Ratajczak, K.; Sulewska, H.; Grażyna, S.; Matysik, P. Agronomic traits and grain quality of selected spelt wheat varieties versus common wheat. J. Crop. Improv. 2020, 1–22. [Google Scholar] [CrossRef]
- Hlisnikovský, L.; Hejcman, M.; Kunzová, E.; Menšík, L. The effect of soil-climate conditions on yielding parameters, chemical composition and baking quality of ancient wheat species Triticum monococcum L., Triticum dicoccum Schrank and Triticum spelta L. in comparison with modern Triticum aestivum L. Arch. Agron. Soil Sci. 2019, 65, 152–163. [Google Scholar] [CrossRef]
- Rapp, M.; Beck, H.; Gütler, H.; Heilig, W.; Starck, N.; Römer, P.; Cuendet, C.; Uhlig, F.; Kurz, H.; Würschum, T.; et al. Spelt: Agronomy, quality, and flavor of its breads from 30 varieties tested across multiple environments. Crop. Sci. 2017, 57, 73–747. [Google Scholar] [CrossRef]
- Wiwart, M.; Szafrańska, A.; Wachowska, U.; Suchowilska, E. Quality parameters and rheological dough properties of 15 spelt (Triticum spelta L.) varieties cultivated today. Cereal Chem. 2017, 94, 1037–1044. [Google Scholar]
- Podolska, G.; Aleksandrowicz, E.; Szafrańska, A. Bread making potential of Triticum aestivum and Triticum spelta species. Open Life Sci. 2020, 15, 30–40. [Google Scholar] [CrossRef]
- Petrenko, V.; Spychaj, R.; Prysiazhniuk, O.; Sheiko, T.; Khudolii, L. Evaluation of three wheat species (Triticum aestivum L., T. spelta L., T. dicoccum (Schrank) Schuebl) commonly used in organic cropping systems, considering selected parameters of technological quality. Rom. Agric. Res. 2018, 35, 255–264. [Google Scholar]
- Marconi, E.; Carcea, M.; Schiavone, M.; Cubadda, R. Spelt (Triticum spelta L.) pasta quality: Combined effect of flour properties and drying conditions. Cereal Chem. 2002, 79, 634–639. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D.; Gawlik-Dziki, U.; Różyło, R.; Andruszczak, S.; Kraska, P.; Kowalczyk, D.; Pałys, E.; Baraniak, B. Grinding and nutritional properties of six spelt (Triticum aestivum ssp. Spelta L.) cultivars. Cereal Chem. 2014, 91, 247–254. [Google Scholar] [CrossRef]
- Denčić, S.; Obreht, D.; Kobiljski, B.; Štatkić, S.; Bede, B. Genetic determination of breadmaking quality in wheat. In Proceedings of the 43rd Croatian and 3rd International Symposium on Agriculture, Zagreb, Croatia, 18–21 February 2008; pp. 278–281. [Google Scholar]
- Geneix, N.; Dalgalarrondo, M.; Tassy, C.; Nadaud, I.; Barret, P.; Bakan, B.; Marion, D. Relationships between puroindoline A-prolamin interactions and wheat grain hardness. PLoS ONE 2020, 15, e0225293. [Google Scholar] [CrossRef]
- Szabó, B.P.; Gyimes, E.; Véha, A.; Horváth, Z.H. Flour quality and kernel hardness connection in winter wheat. Acta Universitatis Sapientiae Alimentaria 2016, 9, 33–40. [Google Scholar] [CrossRef]
- Brites, C.M.; Dos Santos, C.A.L.; Bagulho, A.S.; Beirão-da-Costa, M.L. Effect of wheat puroindoline alleles on functional properties of starch. Eur. Food Res. Technol. 2008, 226, 1205–1212. [Google Scholar] [CrossRef]
- Aydoğan, S.; Şahin, M.; Göçmen Akçacik, A.; Hamzaoğlu, S.; Taner, S. Relationships between farinograph parameters and bread volume, physicochemical traits in bread wheat flours. J. Bahri Dagdas Crop. Res. 2015, 3, 14–18. [Google Scholar]
- Denčić, S.; Mladenov, N.; Kobiljski, B. Effects of Genotype and Environment on Breadmaking Quality in Wheat. Int. J. Plant. Prod. 2011, 5, 71–82. [Google Scholar]
- Sobczyk, A.; Pycia, K.; Stankowski, S.; Jaworska, G.; Kuźniar, P. Evaluation of the Rheological Properties of Dough and Quality of Bread Made with the Flour Obtained from Old Cultivars and Modern Breeding Lines of Spelt (Triticum aestivum ssp. spelta). J. Cereal Sci. 2017, 77, 35–41. [Google Scholar] [CrossRef]
- Żuk-Gołaszewska, K.; Majewska, K.; Tyburski, J.; Goåaszewski, J. Physical and technological properties of kernels and flour made from spelt grown in an organic farming system in north-eastern Poland. J. Cereal Sci. 2018, 79, 501–507. [Google Scholar]
- Zanetti, S.; Winzeler, M.; Feuillet, C.; Keller, B.; Messmer, M. Genetic analysis of bread-making quality in wheat and spelt. Plant Breed. 2001, 120, 13–19. [Google Scholar] [CrossRef]
- Markowski, M.; Żuk-Gołaszewska, K.; Kwiatkowski, D. Influence of variety on selected physical and mechanical properties of wheat. Ind. Crop. Prod. 2013, 47, 113–117. [Google Scholar] [CrossRef]
- Curzon, A.Y.; Chandrasekhar, K.; Nashef, Y.K.; Abbo, S.; Bonfil, D.J.; Reifen, R.; Bar-el, S.; Avneri, A.; Ben-David, R. Distinguishing between bread wheat and spelt grains using molecular markers and spectroscopy. J. Agric. Food Chem. 2019, 67, 3837–3841. [Google Scholar] [CrossRef]
- Southan, M.; MacRitchie, F. Molecular weight distribution of wheat proteins. Cereal Chem. 1999, 76, 827–836. [Google Scholar] [CrossRef]
- Koehler, P.; Wieser, H. Structure and functionality of gluten proteins: An overview. In Proceedings of the 10th International Gluten Workshop, Clermont-Ferrand, France, 7–9 September 2009; pp. 84–88. [Google Scholar]
- Call, L.; Kapeller, M.; Grausgruber, H.; Reiter, E.; Schoenlechner, R.; D’Amico, S. Effects of species and breeding on wheat protein composition. J. Cereal Sci. 2020, 102974. [Google Scholar] [CrossRef]
- Hussain, A.; Larsson, H.; Kuktaite, R.; Prieto-Linde, M.L.; Johansson, E. Influence of organically grown wheat genotypes on protein quality and quantity. Gluten Proteins 2009, 7, 73. [Google Scholar]
- Geisslitz, S.; Wieser, H.; Scherf, K.A.; Koehler, P. Gluten protein composition and aggregation properties as predictors for bread volume of common wheat, spelt, durum wheat, emmer and einkorn. J. Cereal Sci. 2018, 83, 204–212. [Google Scholar] [CrossRef]
- Johansson, E.; Malik, A.H.; Hussain, A.; Rasheed, F.; Newson, W.R.; Plivelic, T.; Hedenqvist, M.S.; Gällstedt, M.; Kuktaite, R. Wheat gluten polymer structures: The impact of genotype, environment, and processing on their functionality in various applications. Cereal Chem. 2013, 90, 367–376. [Google Scholar] [CrossRef]
- Zhang, P.; He, Z.; Zhang, Y.; Xia, X.; Chen, D.; Zhang, Y. Association between% SDS-unextractable polymeric protein (% UPP) and end-use quality in Chinese bread wheat cultivars. Cereal Chem. 2008, 85, 696–700. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Fido, R.J.; Tatham, A.S.; Schofield, J.D. Functional properties of wheat gliadins: 1. Effects on mixing characteristics and bread making quality. J. Cereal Sci. 2002, 35, 299–306. [Google Scholar] [CrossRef]
- Pruska-Kedzior, A.; Kedzior, Z.; Klockiewicz-Kaminska, E. Comparison of viscoelastic properties of gluten from spelt and common wheat. Eur. Food Res. Technol. 2008, 227, 199–207. [Google Scholar] [CrossRef]
- Zhang, P.; He, Z.; Chen, D.; Zhang, Y.; Larroque, O.R.; Xia, X. Contribution of common wheat protein fractions to dough properties and quality of northern-style Chinese steamed bread. J. Cereal Sci. 2007, 46, 1–10. [Google Scholar] [CrossRef]
- Edwards, N.M.; Gianibelli, M.C.; McCaig, T.N.; Clarke, J.M.; Ames, N.P.; Larroque, O.R.; Dexter, J.E. Relationships between dough strength, polymeric protein quantity and composition for diverse durum wheat genotypes. J. Cereal Sci. 2007, 45, 140–149. [Google Scholar] [CrossRef]
- Dhaka, V.; Khatkar, B.S. Effects of gliadin/glutenin and HMW-GS/LMW-GS ratio on dough rheological properties and bread-making potential of wheat varieties. J. Food Qual. 2015, 38, 71–82. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Yang, Y.; Liu, X.; Qin, H.; Dong, Z.; Zheng, S.; Zhang, K.; Wang, D. New insight into the function of wheat glutenin proteins as investigated with two series of genetic mutants. Sci. Rep. 2017, 7, 1–14. [Google Scholar]
- Flagella, Z.; Giuliani, M.M.; Giuzio, L.; Volpi, C.; Masci, S. Influence of water deficit on durum wheat storage protein composition and technological quality. Eur. J. Agron. 2010, 33, 197–207. [Google Scholar] [CrossRef]
- Kasarda, D.D.; D’Ovidio, R. Deduced amino acid sequence of an α-gliadin gene from spelt wheat (spelta) includes sequences active in celiac disease. Cereal Chem. 1999, 76, 548–551. [Google Scholar]
- Dubois, B.; Bertin, P.; Mingeot, D. Molecular diversity of α-gliadin expressed genes in genetically contrasted spelt (Triticum aestivum ssp. spelta) accessions and comparison with bread wheat (T. aestivum ssp. aestivum) and related diploid Triticum and Aegilops species. Mol. Breed. 2016, 36, 152. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Salama, D.A.; Hucl, P.; Sosulski, F.W.; Cao, W. Electrophoretic characterization of spring spelt wheat gliadins. J. Agric. Food Chem. 1996, 44, 2117–2123. [Google Scholar] [CrossRef]
- Harsch, S.; Günter, T.; Kling, C.I.; Rozynek, B. Characterization of spelt (Triticum spelta L.) forms by gel-electrophoretic analyses of seed storage proteins. I. The gliadins. Theor. Appl. Genet. 1997, 94, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.D.; Litts, J.C.; Gautier, M.F.; Greene, F.C. Nucleic acid sequence and chromosome assignment of a wheat storage protein gene. Nucleic Acids Res. 1984, 12, 8129–8144. [Google Scholar] [CrossRef]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between the HMW glutenin subunit composition and the breadmaking quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar]
- Sozinov, A.A.; Poperelya, F.A. Polymorphism of prolamins and variability of grain quality. Plant Foods Hum. Nutr. 1982, 31, 243–249. [Google Scholar] [CrossRef]
- Van Lonkhuijsen, H.J.; Hamer, R.J.; Schreuder, C. Influence of specific gliadins on the breadmaking quality of wheat. Cereal Chem. 1992, 69, 174–177. [Google Scholar]
- Uthayakumaran, S.; Tömösközi, S.; Tatham, A.S.; Savage, A.W.J.; Gianibelli, M.C.; Stoddard, F.L.; Bekes, F. Effects of gliadin fractions on functional properties of wheat dough depending on molecular size and hydrophobicity. Cereal Chem. 2001, 78, 138–141. [Google Scholar] [CrossRef]
- Fido, R.J.; Bekes, F.; Gras, P.W.; Tatham, A.S. Effects of α-, β-, γ-and ω-gliadins on the dough mixing properties of wheat flour. J. Cereal Sci. 1997, 26, 271–277. [Google Scholar]
- Hajas, L.; Scherf, K.A.; Török, K.; Bugyi, Z.; Schall, E.; Poms, R.E.; Koehler, P.; Tömösközi, S. Variation in protein composition among wheat (Triticum aestivum L.) cultivars to identify cultivars suitable as reference material for wheat gluten analysis. Food Chem. 2018, 267, 387–394. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative study on gluten protein composition of ancient (einkorn, emmer and spelt) and modern wheat species (durum and common wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Lacko-Bartošová, M.; Konvalina, P.; Lacko-Bartošová, L. Baking quality prediction of spelt wheat based on rheological and mixolab parameters. Acta Aliment. 2019, 48, 213–220. [Google Scholar] [CrossRef]
- Johansson, E.; Nilsson, H.; Mazhar, H.; Skerritt, J.; MacRitchie, F.; Svensson, G. Seasonal effects on storage proteins and gluten strength in four Swedish wheat cultivars. J. Sci. Food Agric. 2002, 82, 1305–1311. [Google Scholar] [CrossRef]
- Noor Hasniza, M.Z.; Wilkes, M.A.; Uthayakumaran, S.; Copeland, L. Growth environment influences grain protein composition and dough functional properties in three Australian wheat cultivars. Cereal Chem. 2014, 91, 169–175. [Google Scholar] [CrossRef]
- Gagliardi, A.; Carucci, F.; Masci, S.; Flagella, Z.; Gatta, G.; Giuliani, M.M. Effects of Genotype, Growing Season and Nitrogen Level on Gluten Protein Assembly of Durum Wheat Grown under Mediterranean Conditions. Agronomy 2020, 10, 755. [Google Scholar] [CrossRef]
- Mann, G.; Diffey, S.; Cullis, B.; Azanza, F.; Martin, D.; Kelly, A.; McIntyre, L.; Schmidt, A.; Ma, W.; Nath, Z.; et al. Genetic control of wheat quality: Interactions between chromosomal regions determining protein content and composition, dough rheology, and sponge and dough baking properties. Theor. Appl. Genet. 2009, 118, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Becerra, H.F.; Abugalieva, A.; Morgounov, A.; Abdullayev, K.; Bekenova, L.; Yessimbekova, M.; Sereda, G.; Shpigun, S.; Tsygankov, V.; Zelenskiy, Y.; et al. Phenotypic correlations, G x E interactions and broad sense heritability analysis of grain and flour quality characteristics in high latitude spring bread wheats from Kazakhstan and Siberia. Euphytica 2010, 171, 23. [Google Scholar] [CrossRef]
- Löschenberger, F.; Fleck, A.; Grausgruber, H.; Hetzendorfer, H.; Hof, G.; Lafferty, J.; Marn, M.; Neumayer, A.; Pfaffinger, G.; Birschitzky, J. Breeding for organic agriculture: The example of winter wheat in Austria. Euphytica 2008, 163, 469. [Google Scholar] [CrossRef]
- Hildermann, I.; Thommen, A.; Dubois, D.; Boller, T.; Wimken, A.; Mader, P. Yield and baking quality of winter wheat cultivars in different farming systems of the DOK longterm trial. J. Sci. Food Agric. 2009, 89, 2477–2491. [Google Scholar] [CrossRef]
- Warman, P.R. Results of the long-term vegetable crop production trials: Conventional vs. compost-amended soils. Acta Hort. 1998, 469, 333–341. [Google Scholar] [CrossRef]
- Wilson, J.D.; Bechtel, D.B.; Wilson, G.W.T.; Seib, P.A. Bread quality of spelt wheat and its starch. Cereal Chem. 2008, 85, 629–638. [Google Scholar] [CrossRef]
- Cubadda, R.; Marconi, E. Spelt wheat. In Pseudocereals and Less Common Cereals; Belton, P.S., Taylor, J.R.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 153–175. [Google Scholar]
- Mangova, M.; Rachovska, G. Technological characteristics of newly developed mutant common winter wheat lines. Plant Soil Environ. 2004, 50, 84–87. [Google Scholar] [CrossRef]
- Rakita, S.; Dokić, L.; Dapčević Hadnađev, T.; Hadnađev, M.; Torbica, A. Predicting rheological behavior and baking quality of wheat flour using a GlutoPeak test. J. Texture Stud. 2018, 49, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Hristov, N.; Mladenov, N.; Djuric, V.; Kondic-Spika, A.; Marjanovic-Jeromela, A.; Simic, D. Genotype by environment interactions in wheat quality breeding programs in southeast Europe. Euphytica 2010, 174, 315–324. [Google Scholar] [CrossRef]
- Schober, T.J.; Kuhn, M. Capillary zone electrophoresis for gliadin separation: Applications in a spelt breeding program. Eur. Food Res. Technol. 2003, 217, 350–359. [Google Scholar] [CrossRef]
- Gupta, R.B.; MacRitchie, F. Allelic variation at glutenin subunit and gliadin loci, Glu- 1, Glu-3 and Gli-1 of common wheats. II. Biochemical basis of the allelic effects on dough properties. J. Cereal Sci. 1994, 19, 19–29. [Google Scholar] [CrossRef]
- Caballero, L.; Martin, L.M.; Alvarez, J.B. Variation and genetic diversity for gliadins in Spanish spelt wheat accessions. Genet. Resour. Crop. Evol. 2004, 51, 667–686. [Google Scholar] [CrossRef]
- Seilmeier, W.; Valdez, I.; Mendez, E.; Wieser, H. Comparative investigations of gluten proteins from different wheat species II. characterization of ω-gliadins. Eur. Food Res. Technol. 2001, 212, 355–363. [Google Scholar] [CrossRef]
- Wieser, H. Comparative investigations of gluten proteins from different wheat species. III. N-terminal amino acid sequences of α-gliadins potentially toxic for coeliac patients. Eur. Food Res. Technol. 2001, 213, 183–186. [Google Scholar]
- Rodríguez-Quijano, M.; Vargas-Kostiuk, M.E.; Ribeiro, M.; Callejo, M.J. Triticum aestivum ssp. vulgare and ssp. spelta cultivars. 1. Functional evaluation. Eur. Res. Technol. 2019, 245, 1561–1570. [Google Scholar]
- Metakovsky, E.V. Gliadin allele identification in common wheat. 2. Catalogue of gliadin alleles in common wheat. J. Genet. Breed. 1991, 45, 325–344. [Google Scholar]
- Ingver, A.; Tamm, I.; Tamm, Ü. Effect of organic and conventional production on yield and the quality of spring cereals. Latv. J. Agron. 2008, 11, 61–67. [Google Scholar]
- Sobolewska, M.; Stankowski, S. The influence of farming systems on the technological quality of grain and flour cultivars of winter wheat. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2017, 332, 73–82. [Google Scholar] [CrossRef]
- Fares, C.; Menga, V.; Codianni, P.; Russo, M.; Perrone, D.; Suriano, S.; Savino, M.; Rascio, A. Phenolic Acids Variability and Grain Quality of Organically and Conventionally Fertilised Old Wheats under a Warm Climate. J. Sci. Food Agric. 2019, 99, 4615–4623. [Google Scholar] [CrossRef]
- Abedi, T.; Alemzadeh, A.; Kazemeini, S.A. Wheat yield and grain protein response to nitrogen amount and timing. Aust. J. Crop. Sci. 2011, 5, 330–336. [Google Scholar]
- Vojnov, B.; Manojlović, M.; Latković, D.; Milošev, D.; Dolijanović, Ž.; Simić, M.; Babec, B.; Šeremešić, S. Grain yield, yield components and protein content of organic spelt wheat (Triticum spelta L.) grown in different agro-ecological conditions of northern Serbia. Ratarstvo i Povrtarstvo 2020, 57, 1–7. [Google Scholar] [CrossRef]
- Dupont, F.M.; Hurkman, W.J.; Vensel, W.H.; Tanaka, C.; Kothari, K.M.; Chung, O.K.; Altenbach, S.B. Protein accumulation and composition in wheat grains: Effects of mineral nutrients and high temperature. Eur. J. Agron. 2006, 25, 96–107. [Google Scholar] [CrossRef]
- Roozeboom, K.L.; Schapaugh, W.T.; Tuinstra, M.R.; Vanderlip, R.L.; Milliken, G.A. Testing wheat in variable environments: Genotype, environment, interaction effects, and grouping test locations. Crop. Sci. 2008, 48, 317–330. [Google Scholar] [CrossRef]
- Gil, D.H.; Bonfil, D.J.; Svoray, T. Multi scale analysis of the factors influencing wheat quality as determined by Gluten Index. Field Crop. Res. 2011, 123, 1–9. [Google Scholar] [CrossRef]
- Malik, A.H.; Prieto-Linde, M.L.; Kuktaite, R.; Andersson, A.; Johansson, E. Individual and interactive effects of cultivar maturation time, nitrogen regime and temperature level on accumulation of wheat proteins. J. Sci. Food Agric. 2011, 91, 2192–2200. [Google Scholar] [CrossRef]
- Vida, G.; Szunics, L.; Veisz, O.; Bedő, Z.; Láng, L.; Árendás, T.; Bónis, P.; Rakszegi, M. Effect of genotypic, meteorological and agronomic factors on the gluten index of winter durum wheat. Euphytica 2014, 197, 61–71. [Google Scholar] [CrossRef]
- Wang, J.; Chatzidimitriou, E.; Wood, L.; Hasanalieva, G.; Markellou, E.; Iversen, P.O.; Seal, C.; Baranski, M.; Vigar, V.; Ernst, L.; et al. Effect of Wheat Species (Triticum aestivum vs. T. spelta), Farming System (Organic vs. Conventional) and Flour Type (Wholegrain vs. White) on Composition of Wheat Flour—Results of a Retail Survey in the UK and Germany—2. Antioxidant Activity, and Phenolic and Mineral Content. Food Chem. 2020, 6, 100091. [Google Scholar] [CrossRef]
- Fares, C.; Codianni, P.; Menga, V. Effects of organic fertilization on quality and antioxidant properties of hulled wheats. Ital. J. Food Sci. 2012, 24, 188–193. [Google Scholar]
- Triboi, E.; Abad, A.; Michelena, A.; Lloveras, J.; Ollier, J.L.; Daniel, C. Environmental effects on the quality of two wheat genotypes: 1. Quantitative and qualitative variation of storage proteins. Eur. J. Agron. 2000, 13, 47–64. [Google Scholar] [CrossRef]

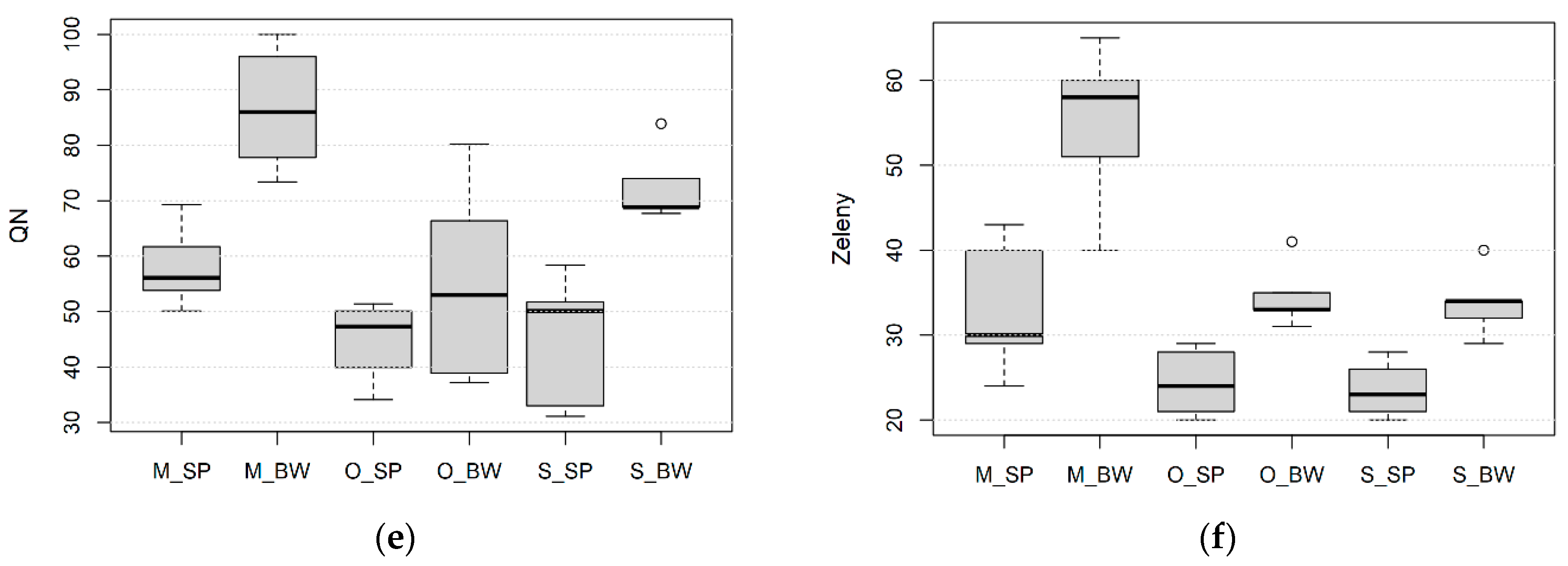

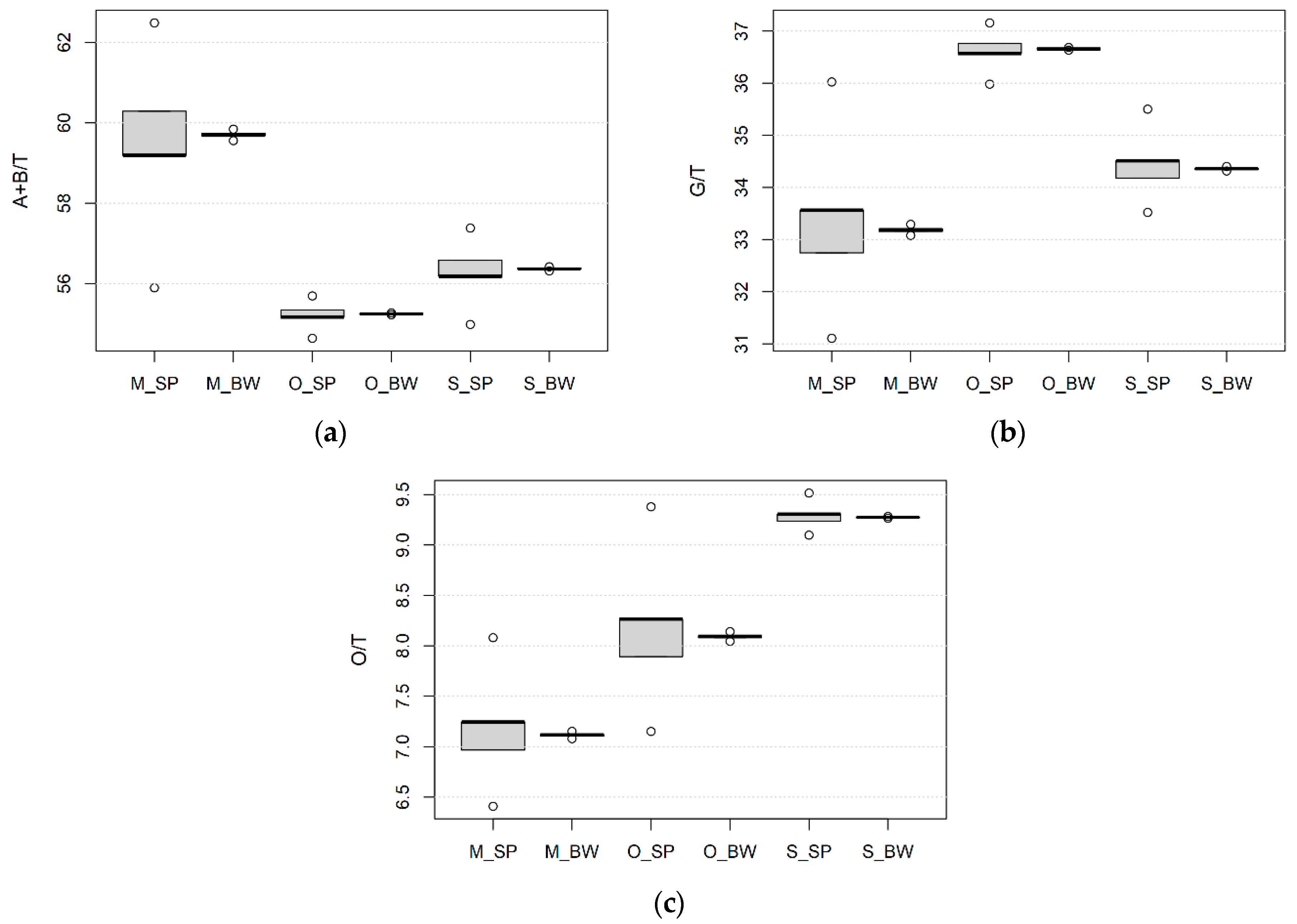
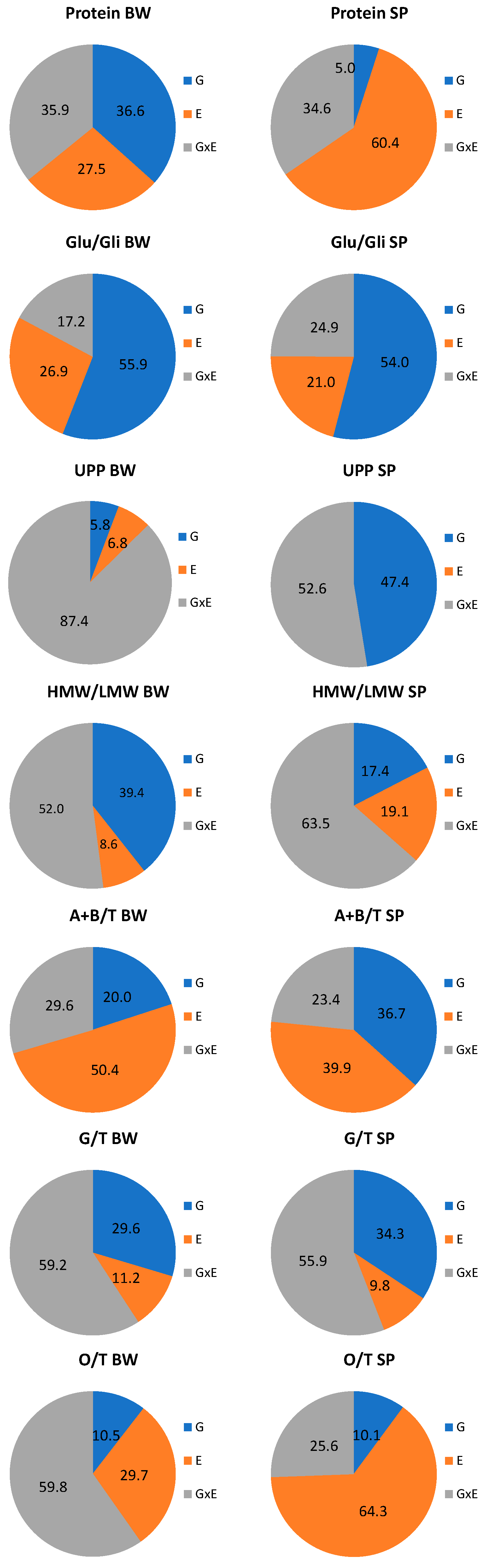
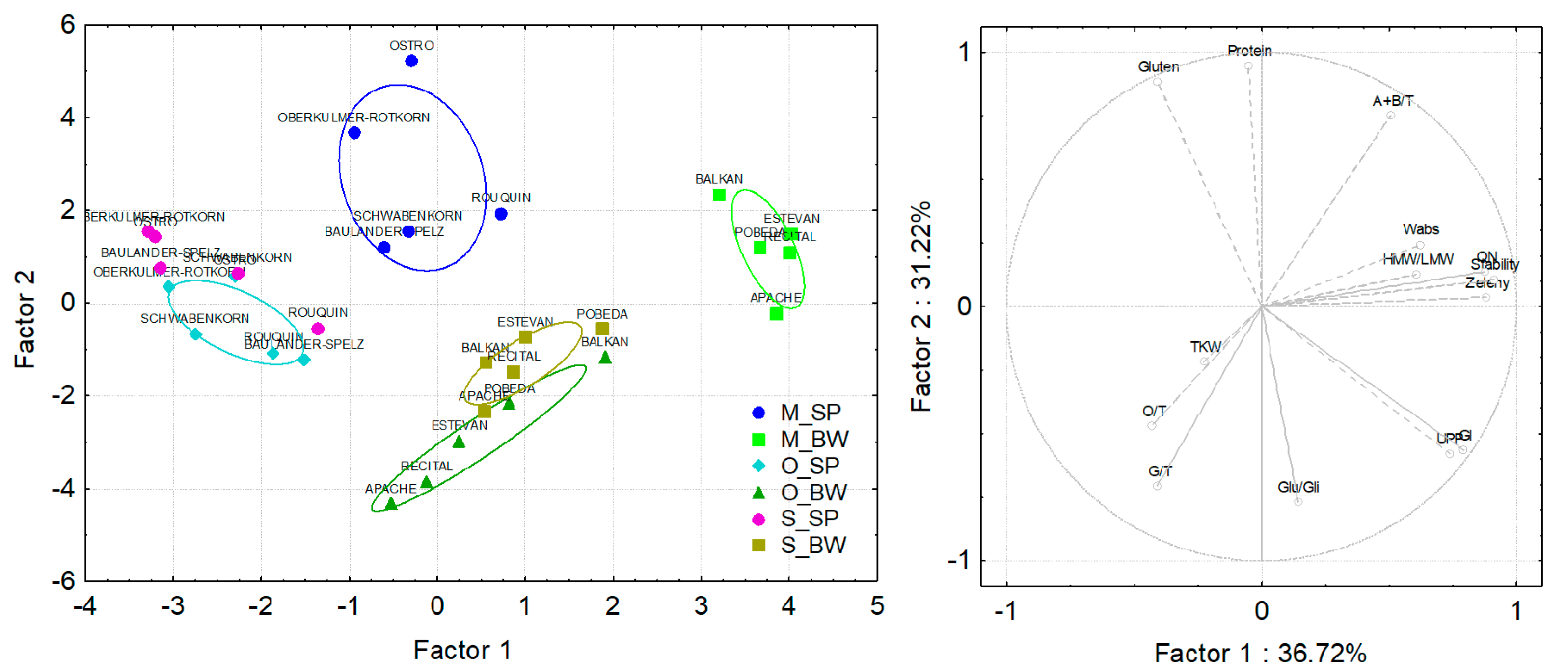
| Species/Variety | Pedigree | Origin | Year | HMW-GS | Glu-Score | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Glu-A1 | Glu-B1 | Glu-D1 | ||||||
| T. aestivum L. subsp. spelta | ||||||||
| Baulander Spelz | Geiberger Spelz | DEU | 1926 | 1 | 13 + 16 | 2 + 12 | 8 | [29,30,31,32] |
| Ostro † | Oberkulmer-Rotkorn/Steins-Roter-Tiroler | CHE | 1978 | 1 | 6 + 8 | 2 + 12 | 6 | [29,30] |
| 2 * | 6 + 8 | 2 + 12 | 6 | [33] | ||||
| Rouquin † | Lignée-24/Ardenne//Altgol | BEL | 1979 | 1 | 6 + 8 | 2 + 12 | 6 | [33] |
| 1 | 6 + 8 | 5 + 10 | 8 | [29] | ||||
| Schwabenkorn | (S)LV | DEU | 1988 | 1 | 6 + 8 | 2 + 12 | 6 | [29,30,33] |
| Oberkulmer-Rotkorn | (S)LV-CHE | CHE | 1948 | 1 | 6 + 8 | 2 + 12 | 6 | [29] |
| T. aestivum L. subsp. aestivum | ||||||||
| Apache | Axial/NRPB-84-4233 | FRA | 1998 | N | 7 + 9 | 2 + 12 | 5 | [34] |
| Balkan | Bačka/Bez1//Miron808/3/NS433/4/Skor35 | SRB | 1979 | 2 * | 7 + 9 | 5 + 10 | 9 | [35] |
| Estevan | Capo/SE-24090 | DEU | 2009 | 1 | 7 + 9 | 5 + 10 | 9 | [36] |
| Pobeda | Sremica/Balkan | SRB | 1990 | 2 * | 7 + 9 | 5 + 10 | 9 | [37] |
| Recital | Mexique-267(R-267)/9369 | FRA | 1986 | 2 * | 6 + 8 | 5 + 10 | 8 | [38] |
| 2 * | 7 + 9 | 5 + 10 | 9 | [30] | ||||
| Growing Conditions | Hungary | Serbia | |
|---|---|---|---|
| Location | geographic coordinates | 47°18′ N, 18°47′ E | 45°20′ N, 19°51′ E |
| Altitude | 115 m | 84 m | |
| Growing parameters | previous crop: conventional organic | oil radish phacelia | soybean |
| sowing density | 550 seeds/m2 | 550 seeds/m2 | |
| Soil parameters | soil type | chernozem | chernozem |
| pH (KCl) | 7.25 | 7.41 | |
| humus (m/m%) | 2.8 | 2.6 | |
| P2O5 (mg/kg) | 210 | 208 | |
| K2O (mg/kg) | 210 | 176 | |
| yearly average nitrogen input through nitrogen, phosphorus and potassium (NPK) combined fertilizer (conventional) (active ingredient, kg/ha) | 120 | 100 | |
| Hungary | Serbia | ||
|---|---|---|---|
| full season | Growing period (days) | 279 | 264 |
| Cumulative precipitation (mm) | 365.6 | 435.3 | |
| Mean temperature (°C) | 9.3 | 11.5 | |
| Absolute min temperature (°C) | −14.4 | −16.3 | |
| Absolute max temperature (°C) | 36.0 | 35.0 | |
| last 100 days | Cum. precipitation in the last 100 days before harvest (mm) | 225.0 | 265.4 |
| Mean temperature in the last 100 days (°C) | 17.1 | 17.5 | |
| Absolute min temp in the last 100 days (°C) | −0.7 | −0.9 | |
| Absolute max temp in the last 100 days (°C) | 36.0 | 35.0 | |
| abs. min-max | No of days with Tmin ≤ 0 °C | 90 | 75 |
| No of days with Tmin ≤ −10 °C | 6 | 3 | |
| No of days with Tmax ≥ 25 °C | 42 | 38 | |
| No of days with Tmax ≥ 30 °C | 16 | 17 | |
| No of days with Tmax ≥ 35 °C | 1 | 1 |
| Variety/Growing Site | Protein (%) | Glu/Gli | UPP (%) | HMW/LMW | A+B/T | G/T | O/T |
|---|---|---|---|---|---|---|---|
| T. aestivum L. subsp. spelta | |||||||
| Baulander Spelz | 15.22 c | 0.88 b,c | 34.87 b,c | 0.42 c | 55.17 b | 35.84 b | 8.99 b |
| Ostro | 15.90 d | 0.80 a | 25.87 a | 0.38 b,c | 58.52 d | 33.93 a | 7.55 a |
| Rouquin | 14.77 b | 0.97 d | 38.00 c | 0.38 b,c | 53.96 a | 37.65 c | 8.48 a,b |
| Schwabenkorn | 14.34 a | 0.90 c,d | 35.81 b,c | 0.35 a,b | 57.36 c | 34.97 b | 7.67 a |
| Oberkulmer-Rotkorn | 15.97 d | 0.82 a,b | 30.43 a,b | 0.33 a | 58.70 d | 33.76 a | 7.53 a |
| Average | 15.2 B | 0.87 A | 33.00 A | 0.37 A | 56.74 A | 35.23 B | 8.04 A |
| M | 16.83 c | 0.87 a | 33.62 a | 0.41 b | 59.02 c | 34.40 a | 6.58 a |
| O | 14.07 a | 0.92 b | 32.28 a | 0.36 a | 54.89 a | 36.30 b | 8.81 b |
| S | 14.81 b | 0.84 a | 33.09 a | 0.35 a | 56.32 b | 34.93 a | 8.74 b |
| Genotype (G) | <0.0001 *** | <0.0001 | 0.0002 *** | 0.0001 *** | <0.0001 *** | <0.0001 *** | 0.0004 *** |
| Growing site (E) | <0.0001 *** | 0.0012 ** | 0.7744 n.s. | 0.0002 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| G × E | <0.0001 *** | 0.1282 n.s. | 0.0383 * | 0.0005 *** | <0.0001 *** | <0.0001 *** | 0.0094 ** |
| T. aestivum L. subsp. aestivum | |||||||
| Apache | 11.47 a | 1.29 d | 56.90 a | 0.42 a,b | 53.76 a | 33.91 b,c | 12.33 c |
| Balkan | 13.48 c | 0.75 a | 51.89 a | 0.55 c | 55.40 b | 30.31 a | 14.29 d |
| Estevan | 13.52 c | 1.00 b | 56.45 a | 0.41 a | 54.03 a | 35.70 c | 10.26 b |
| Pobeda | 13.56 c | 0.96 b | 48.94 a | 0.40 a | 58.13 c | 32.96 b | 8.91 a |
| Recital | 12.26 b | 1.12 c | 53.02 a | 0.43 b | 58.32 c | 30.93 a | 10.75 b |
| Average | 12.9 A | 1.02 B | 53.44 B | 0.44 B | 55.93 A | 32.76 A | 11.31 B |
| M | 15.07 c | 0.91 a | 53.50 a,b | 0.47 c | 58.51 c | 31.38 a | 9.71 a |
| O | 11.15 a | 1.18 c | 58.01 b | 0.41 a | 52.63 a | 33.27 b | 10.11 a |
| S | 12.36 b | 0.99 b | 48.81 a | 0.44 b | 56.64 b | 33.64 b | 14.10 b |
| Genotype (G) | <0.0001 *** | <0.0001 *** | 0.2297 n.s. | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| Growing site (E) | <0.0001 *** | <0.0001 *** | 0.0157 * | <0.0001 *** | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| G × E | <0.0001 *** | <0.0001 *** | 0.1947 n.s. | <0.0001 *** | <0.0001 *** | 0.0002 *** | <0.0001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takač, V.; Tóth, V.; Rakszegi, M.; Mikić, S.; Mirosavljević, M.; Kondić-Špika, A. Differences in Processing Quality Traits, Protein Content and Composition between Spelt and Bread Wheat Genotypes Grown under Conventional and Organic Production. Foods 2021, 10, 156. https://doi.org/10.3390/foods10010156
Takač V, Tóth V, Rakszegi M, Mikić S, Mirosavljević M, Kondić-Špika A. Differences in Processing Quality Traits, Protein Content and Composition between Spelt and Bread Wheat Genotypes Grown under Conventional and Organic Production. Foods. 2021; 10(1):156. https://doi.org/10.3390/foods10010156
Chicago/Turabian StyleTakač, Verica, Viola Tóth, Marianna Rakszegi, Sanja Mikić, Milan Mirosavljević, and Ankica Kondić-Špika. 2021. "Differences in Processing Quality Traits, Protein Content and Composition between Spelt and Bread Wheat Genotypes Grown under Conventional and Organic Production" Foods 10, no. 1: 156. https://doi.org/10.3390/foods10010156
APA StyleTakač, V., Tóth, V., Rakszegi, M., Mikić, S., Mirosavljević, M., & Kondić-Špika, A. (2021). Differences in Processing Quality Traits, Protein Content and Composition between Spelt and Bread Wheat Genotypes Grown under Conventional and Organic Production. Foods, 10(1), 156. https://doi.org/10.3390/foods10010156







