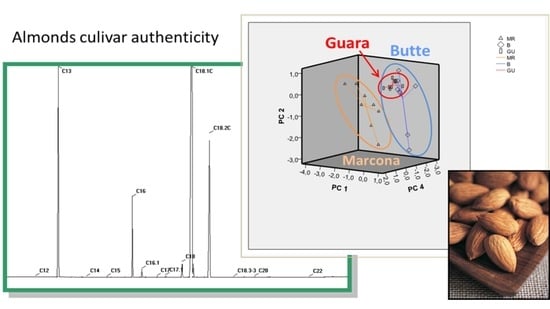
Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins
Abstract
1. Introduction
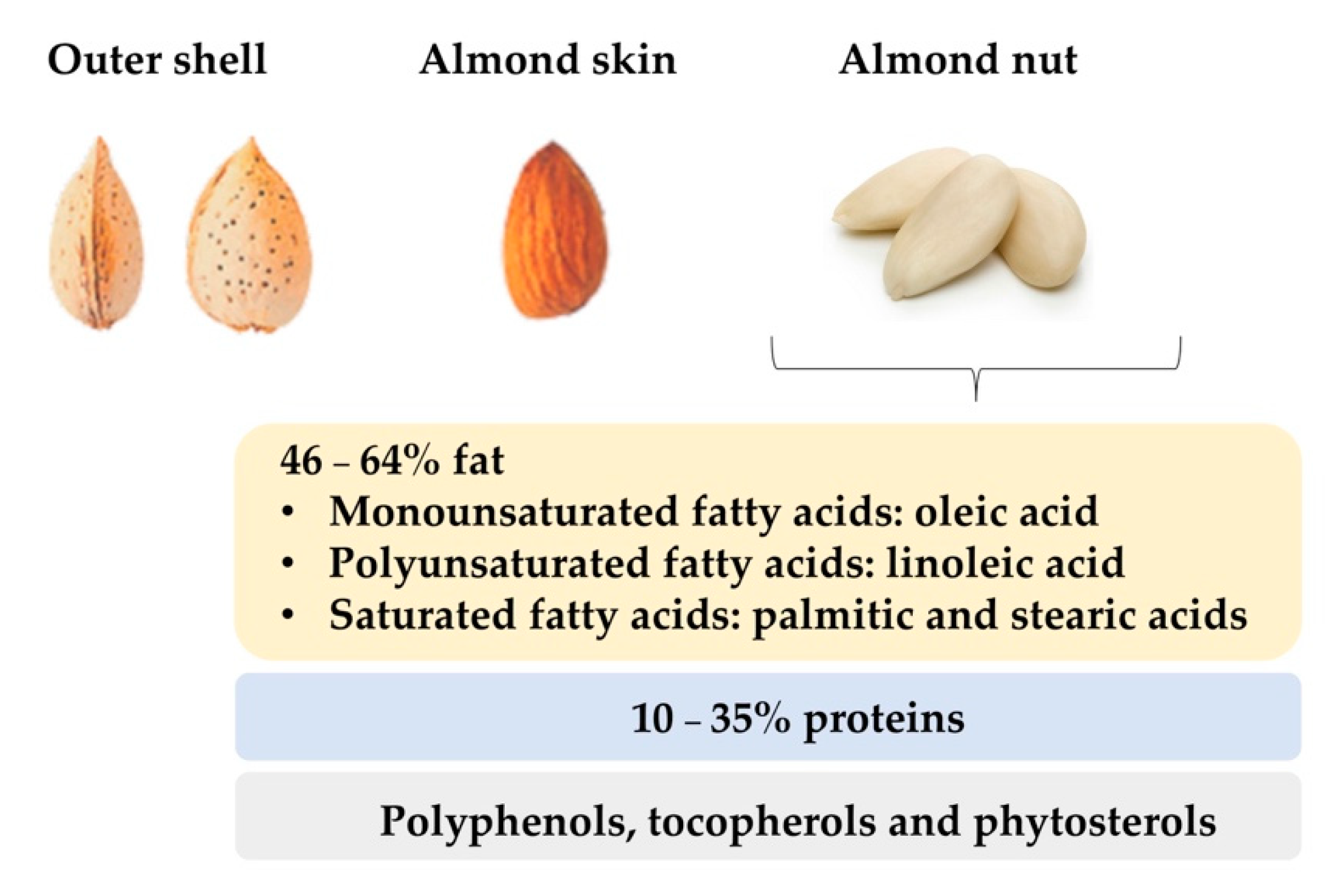
2. Total Fat, Fatty Acids and Triacylglycerides
3. Proteins
4. Amino Acids
5. Carbohydrates and Dietary Fibre
6. Minerals
7. Vitamin E
8. Phytosterols
9. Phenolic Compounds and Antioxidant Activity
10. Volatile Compounds
11. DNA Fingerprinting
| Geographical Origin | Cultivars | Compounds | Analysis | Ref. |
|---|---|---|---|---|
| Different cities in Spain and California | Garrigues, Guara, Marcona and Butte | α, (β + γ), δ- tocopherol | HPLC-PDA ANOVA | [9] |
| Australia, Spain and California | Nonpareil, Johnston, Somerton, Peerless, Price Carmel and Guara | α, (β+γ), δ- tocopherol | HPLC-PDA | [21] |
| Apuglia (Italy) | Barlettana, Cristomorto, Santoro, Catuccia, Filippo Ceo, Piangente,, Pidocchioso, Tuono, Mincone, Catucedda, Fragiulio, Centopezze, Putignano, Ciavea, Santeramo, Galgano, Irene Lanzolla, Cacciola, Catalini, Rana Gentile, Ferrante, Zin Zin, Trianella, Nocella, Cinquanta Vignali, Pizzutella, Pastanella, Pepparuddo, Aloia, Bares, Pappamucco, Rossa, Reale, Senz’arte, A Grappolo, Albanese, Vuoi o non vuoi, Ficarazza, Giunco di Cozze, Alberobello, Cosimo di Bari, Rana, Primecerio, Lorenza Tribuzio, Piscalze, Antonio De Vito, Monaca, Tondina, Pettolecchia, Pulita, Scorza Verde, Gioia, Rachele. | α-tocopherol | HPLC-PDA PCA-LDA | [64] |
| 5 continents | 46 California and Australian, 70 European cultivars from Mediterranean zones, 26 from Iran and wild genotypes. | 17 SSR markets | PCR Cluster analysis | [102] |
| Spain and USA | Marcona, Guara, Planeta, Butte, Colony, Carmel, and Padre | TPC, antioxidant capacity (FRAP), and individual flavonoids content in almond skins | HPLC-ESI-MS/MS LDA | [9] |
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nuts Dried Fruits Statistical Yearbook; INC. International Nut and Dried Fruit Council Foundation: Reus, Spain, 2019. [CrossRef]
- Food and Agriculture Organization of the United States, FAOSTAT Database. Available online: http://www.fao.org/faostat/en/#data (accessed on 2 December 2020).
- Almond Board of California. Almond Almanac 2017, Annual Report. Printed in Modestio, California. Available online: http://www.almonds.com/sites/default/files/2017 (accessed on 20 November 2020).
- Rafel Socias i Company; Gradziel, T.M. Almonds: Botany, Production and Uses; CABI: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Zhu, Y.; Wilkinson, K.L.; Wirthensohn, M.G. Lipophilic antioxidant content of almonds (Prunus dulcis): A regional and varietal study. J. Food Comp. Anal. 2015, 39, 120–127. [Google Scholar] [CrossRef]
- Yada, S.; Lapsley, K.; Huang, G. A review of composition studies of cultivated almonds: Macronutrients and micronutrients. J. Food Comp. Anal. 2011, 24, 469–480. [Google Scholar] [CrossRef]
- Yildirim, A.N.; Akinci-Yildirim, F.; Şan, B.; Sesli, Y. Total Oil Content and Fatty Acid Profile of some Almond (Amygdalus Communis L.) Cultivars. Pol. J. Food Nutr. Sci. 2016, 66, 173–178. [Google Scholar] [CrossRef]
- Kodad, O.; Socias I Company, R. Variability of oil content and of major fatty acid composition in almond (Prunus amygdalus Batsch) and its relationship with kernel quality. J. Agric. Food Chem. 2008, 56, 4096–4101. [Google Scholar] [CrossRef]
- López-Ortiz, C.M.C.M.; Prats-Moya, S.; Beltrán Sanahuja, A.; Maestre-Pérez, S.E.; Grané-Teruel, N.; Martín-Carratalá, M.L. Comparative study of tocopherol homologue content in four almond oil cultivars during two consecutive years. J. Food Comp. Anal. 2008, 21, 144–151. [Google Scholar] [CrossRef]
- Barreca, D.; Nabavi, S.M.; Sureda, A.; Rasekhian, M.; Raciti, R.; Silva, A.S.; Annunziata, G.; Arnone, A.; Tenore, G.C.; Süntar, I.; et al. Almonds (Prunus dulcis Mill. D. A. webb): A source of nutrients and health-promoting compounds. Nutrients 2020, 12, 672. [Google Scholar] [CrossRef]
- Hojjati, M.; Speziale, M.; Vázquez-Araújo, L.; Mincione, A.; Carbonell-Barrachina, A.A. Instrumental texture properties of Spanish Turrón, Italian Torrone and French Nougat. J. Food Bioproc. Eng. 2015, 1, 15–23. Available online: https://journals.ut.ac.ir/article_58151_0.html (accessed on 7 December 2020).
- Gil Solsona, R.; Boix, C.; Ibáñez, M.; Sancho, J.V. The classification of almonds (Prunus dulcis) by country and variety using UHPLC-HRMS-based untargeted metabolomics. Food Addit. Contam. Part A 2018, 35, 395–403. [Google Scholar] [CrossRef]
- Diella, G.; Di Ciaula, A.; Lorusso, M.P.; Summo, C.; Caggiano, G.; Caponio, F.; Portincasa, P. Distinct effects of two almond cultivars on agreeability and gastrointestinal motility in healthy subjects: More than mere nutraceuticals. J. Gastrointest. Liver Dis. 2018, 27, 31–39. [Google Scholar] [CrossRef]
- USDA. National Nutrient Database for Standard Reference, Release 28. 2015. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 10 December 2020).
- Beltran, A.; Prats, M.S.; Maestre, S.E.; Grané, N.; Martín Carratalá, M.L. Classification of four almond cultivars using oil degradation parameters based on FTIR and GC data. J. Am. Oil Chem. Soc. 2009, 86, 51–58. [Google Scholar] [CrossRef]
- Beyhan, Ö.; Aktaş, M.; Yilmaz, N.; Şimşek, N.; Gerçekçioǧlu, R. Determination of fatty acid compositions of some important almond (Prunus amygdalus L.) varieties selected from Tokat province and Eagean region of Turkey. J. Med. Plant. Res. 2011, 5, 4907–4911. [Google Scholar]
- Amorello, D.; Orecchio, S.; Pace, A.; Barreca, S. Discrimination of almonds (Prunus dulcis) geographical origin by minerals and fatty acids profiling. Nat. Prod. Res. 2016, 30, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Čolić, S.D.; Fotirić Akšić, M.M.; Lazarević, K.B.; Zec, G.N.; Gašić, U.M.; Dabić Zagorac, D.; Natić, M.M. Fatty acid and phenolic profiles of almond grown in Serbia. Food Chem. 2017, 234, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, L.; Li, P.; Yu, L.; Mao, J.; Wang, X.; Zhang, Q. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends Food Sci. Technol. 2018, 74, 26–32. [Google Scholar] [CrossRef]
- Zhu, Y.; Wilkinson, K.L.; Wirthensohn, M. Changes in fatty acid and tocopherol content during almond (Prunus dulcis, cv. Nonpareil) kernel development. Sci. Hortic. 2017, 225, 150–155. [Google Scholar] [CrossRef]
- Zhu, Y.; Taylor, C.; Sommer, K.; Wilkinson, K.; Wirthensohn, M. Influence of deficit irrigation strategies on fatty acid and tocopherol concentration of almond (Prunus dulcis). Food Chem. 2015, 173, 821–826. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañan, G.; Juan, T.; Molino, F.; Mamouni, A.; Messaoudi, Z.; Lahlou, M.; Socias I Company, R. Plasticity and stability in the major fatty acid content of almond kernels grown under two Mediterranean climates. J. Hortic. Sci. Biotechnol. 2010, 85, 381–386. [Google Scholar] [CrossRef]
- Rabadán, A.; Álvarez-Ortí, M.; Gómez, R.; Pardo-Giménez, A.; Pardo, J.E. Suitability of Spanish almond cultivars for the industrial production of almond oil and defatted flour. Sci. Hortic. 2017, 225, 539–546. [Google Scholar] [CrossRef]
- Zamany, A.J.; Samadi, G.R.; Kim, D.H.; Keum, Y.-S.; Saini, R.K. Comparative Study of Tocopherol Contents and Fatty Acids Composition in Twenty Almond Cultivars of Afghanistan. J. Am. Oil Chem. Soc. 2017, 94, 805–817. [Google Scholar] [CrossRef]
- Kodad, O.; Alonso, J.M.; Espiau, M.T.; Estopanan, G.; Juan, T.; Socias I Company, R. Chemometric Characterization of Almond Germplasm: Compositional Aspects Involved in Quality and Breeding. J. Am. Oil Chem. Soc. 2011, 136, 273–281. [Google Scholar] [CrossRef]
- Prats-Moya, M.S.; Grané-Teruel, N.; Berenguer-Navarro, V.; Martín-Carratalá, M.L. A Chemometric Study of Genotypic Variation in Triacylglycerol. J. Am. Oil Chem. Soc. 1999, 76, 267–272. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Casal, S.; Ferreira, I.C.F.R.; Peres, A.M.; Pereira, J.A.; Oliveira, M.B.P.P. Supervised chemical pattern recognition in almond (Prunus dulcis) Portuguese PDO cultivars: PCA- and LDA-based triennial study. J. Agric. Food Chem. 2012, 60, 9697–9704. [Google Scholar] [CrossRef]
- Beltrán, A.S.; Ramos, M.S.; Teruel, N.G.; Martín, M.L.C.; Garrigós, M.C.S. Classification of Almond Cultivars Using Oil Volatile Compound Determination by HS-SPME–GC–MS. J. Am. Oil Chem. Soc. 2011, 88, 329–336. [Google Scholar] [CrossRef]
- Kodad, O.; Oukabli, A.; Mamouni, A.; Socias I Company, R.; Estopañán, G.; Juan, T. Study of the genetic diversity of almond seedling populations in Morocco: Application of a chemometric approach. Acta Hortic. 2011, 912, 449–454. [Google Scholar] [CrossRef]
- Soler, L.; Canellas, J.; Saura-Calixto, F. Changes in carbohydrate and protein content and composition of developing almond seeds. J. Agric. Food Chem. 1989, 37, 1400–1404. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Pereira, J.A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Sugars Profiles of Different Chestnut (Castanea sativa Mill.) and Almond (Prunus dulcis) Cultivars by HPLC-RI. Plant. Foods Hum. Nutr. 2010, 65, 38–43. [Google Scholar] [CrossRef]
- Rabadán, A.; Álvarez-Ortí, M.; Pardo, J.E. A comparison of the effect of genotype and weather conditions on the nutritional composition of most important commercial nuts. Sci. Hortic. 2019, 244, 218–224. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Pantelidis, G.; Bacchetta, L.; De Giorgio, D.; Duval, H.; Metzidakis, I.; Spera, D. Protein and mineral nutrient contents in kernels from 72 sweet almond cultivars and accessions grown in France, Greece and Italy. Int. J. Food Sci. Nutr. 2013, 64, 202–209. [Google Scholar] [CrossRef]
- Calixto, F.S.; Cañellas, J.; de Toda, F.M. A chemical study of the protein fraction of mediterranean sweet almond varieties (Prunus amygdalus). Z. Lebensm. Unters. Forsch. 1982, 175, 34–37. [Google Scholar] [CrossRef]
- Yada, S.; Huang, G.; Lapsley, K. Natural variability in the nutrient composition of California-grown almonds. J. Food Comp. Anal. 2013, 30, 80–85. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Fagroud, M.; Juan, T.; Socias I Company, R. Physical and chemical traits of almond kernels of the local almond populations in Morocco: Commercial and industrial end-uses. Acta Hortic. 2014, 1028, 233–238. [Google Scholar] [CrossRef]
- Simsek, M.; Gulsoy, E.; Yavic, A.; Arikan, B.; Yildirim, Y.; Olmez, N.; Erdogmus, B.; Boguc, F. Fatty acid, mineral and proximate compositions of various genotypes and commercial cultivars of sweet almond from the same ecological conditions. Appl. Ecol. Environ. Res. 2018, 16, 2957–2971. [Google Scholar] [CrossRef]
- Forcada, C.F.; Kodad, O.; Juan, T.; Estopañan, G.; Socias I Company, R. Genetic variability and pollen effect on the transmission of the chemical components of the almond kernel. Span. J. Agric. Res. 2011, 3, 781–789. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=3740250 (accessed on 23 November 2020).
- Sánchez-Bel, P.; Egea, I.; Martínez-Madrid, M.C.; Flores, B.; Romojaro, F. Influence of Irrigation and Organic/Inorganic Fertilization on Chemical Quality of Almond (Prunus amygdalus cv. Guara). J. Agric. Food Chem. 2008, 56, 10056–10062. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Bauza, M.; de Toda, F.; Argamenteria, A. Amino acids, sugars, and inorganic elements in the sweet almond (Prunus amygdalus). J. Agric. Food Chem. 1981, 29, 509–511. [Google Scholar] [CrossRef]
- Ahrens, S.; Venkatachalam, M.; Mistry, A.M.; Lapsley, K.; Sathe, S.K. Almond (Prunus dulcis L.) protein quality. Plant. Foods Hum. Nutr. 2005, 60, 123–128. [Google Scholar] [CrossRef]
- Grané-Teruel, N.; Luna-Amador, M.C.; Prats-Moya, M.S.; Berenguer-Navarro, V.; Martín-Carratalá, M.L. Statistical comparative study of free amino acid HPLC data from a selected almond set. Food Chem. 1999, 65, 23–28. [Google Scholar] [CrossRef]
- Martín Carratalá, M.L.; Prats Moya, M.S.; Grané Teruel, N.; Berenguer Navarro, V. Discriminating Significance of the Free Amino Acid Profile in Almond Seeds. J. Agric. Food Chem. 2002, 50, 6841–6846. [Google Scholar] [CrossRef]
- Seron, L.H.; Poveda, E.G.; Prats Moya, M.S.; Martín Carratalá, M.L.; Berenguer-Navarro, V.; Grané-Teruel, N. Characterisation of 19 almond cultivars on the basis of their free amino acids composition. Food Chem. 1998, 61, 455–459. [Google Scholar] [CrossRef]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Review about Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel. Foods 2020, 9, 1646. [Google Scholar] [CrossRef]
- Socias, R.; Kodad, O.; Alonso, J.M.; Gradziel, T.M. Almond Quality: A Breeding Perspective. Hortic. Rev. 2008, 34, 197–238. [Google Scholar] [CrossRef]
- Romero, A.; Vargas, F.J.; Tous, J.; Ninot, A.; Miarnau, X. New almond varieties from IRTA’s breeding programme: (1) chemical composition. Acta Hortic. 2011, 912, 477–484. [Google Scholar] [CrossRef]
- Gouta, H.; Ksia, E.; Laaribi, I.; Molino, F.; Estopañan, G.; Juan, T.; Kodad, O.; Martínez-Gómez, P.; Martínez-García, P.J. Evaluation of the chemical and nutritional properties of tunisian almond cultivars. Ital. J. Food Sci. 2020, 32, 562–582. [Google Scholar] [CrossRef]
- Kannamkumarath, S.S.; Wróbel, K.K.; Wróbel, K.K.; Caruso, J.A. Speciation of Arsenic in Different Types of Nuts by Ion Chromatography−Inductively Coupled Plasma Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 1458–1463. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Herbello-Hermelo, P.; Domínguez-González, R.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Bioavailability assessment of essential and toxic metals in edible nuts and seeds. Food Chem. 2016, 205, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Musa Özcan, M. Determination of the mineral compositions of some selected oil-bearing seeds and kernels using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES). Grasas Aceites 2006, 57, 211–218. [Google Scholar] [CrossRef]
- Rodushkin, I.; Engström, E.; Sörlin, D.; Baxter, D. Levels of inorganic constituents in raw nuts and seeds on the Swedish market. Sci. Total Environ. 2008, 392, 290–304. [Google Scholar] [CrossRef]
- Piscopo, A.; Romeo, F.V.; Petrovicova, B.; Poiana, M. Effect of the harvest time on kernel quality of several almond varieties (Prunus dulcis (Mill.) D.A. Webb). Sci. Hortic. 2010, 125, 41–46. [Google Scholar] [CrossRef]
- Schirra, M.; Mulas, M.; Nieddu, G.; Virdis, F. Mineral content in “Texas” almonds during fruit growth and ripening. Acta Hortic. 1994. Available online: http://agris.fao.org/agris-search/search.do?recordID=US201301502526 (accessed on 16 November 2020). [CrossRef]
- Prats-Moya, S.; Grané-Teruel, N.; Berenguer-Navarro, V.; Martín-Carratalá, M.L. Inductively coupled plasma application for the classification of 19 almond cultivars using inorganic element composition. J. Agric. Food Chem. 1997, 45, 2093–2097. Available online: http://pubs.acs.org/doi/abs/10.1021/jf960740k (accessed on 16 November 2020). [CrossRef]
- Ayadi, M.; Ghrab, M.; Gargouri, K.; Elloumi, O.; Zribi, F.; Ben Mimoun, M.; Boulares, C.; Guedri, W. Kernel characteristics of almond cultivars under rainfed conditions. Acta Hortic. 2006, 726, 377–381. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ünver, A.; Erkan, E.; Arslan, D. Characteristics of some almond kernel and oils. Sci. Hortic. 2011, 127, 330–333. [Google Scholar] [CrossRef]
- Kodad, O.; Socias I Company, R.; Alonso, J.M. Genotypic and environmental effects on tocopherol content in almond. Antioxidants 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- De Souza, N.E.; Rodrigues, A.C.; Souza, A.H.P.; Matsushita, M.; Pedrão, M.R.; Dias, L.F. Quantification of minerals and tocopherols isomers in chestnuts approach chemometrics. Semin. Cienc. Agrar. 2014, 35, 2427–2436. [Google Scholar] [CrossRef][Green Version]
- Fernandes, G.D.; Gómez-Coca, R.B.; Pérez-Camino, M.D.C.; Moreda, W.; Barrera-Arellano, D. Chemical Characterization of Major and Minor Compounds of Nut Oils: Almond, Hazelnut, and Pecan Nut. J. Chem. 2017, 2017, 2609549. [Google Scholar] [CrossRef]
- Carballo, S.; Prats, M.S.; Maestre, S.E.; Todolí, J.L. Determination of fat-soluble vitamins in vegetable oils through microwave-assisted high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 1073–1081. [Google Scholar] [CrossRef][Green Version]
- Maestri, D.; Martínez, M.; Bodoira, R.; Rossi, Y.; Oviedo, A.; Pierantozzi, P.; Torres, M. Variability in almond oil chemical traits from traditional cultivars and native genetic resources from Argentina. Food Chem. 2015, 170, 55–61. [Google Scholar] [CrossRef]
- Chen, H.; Angiuli, M.; Ferrari, C.; Tombari, E.; Salvetti, G.; Bramanti, E. Tocopherol speciation as first screening for the assessment of extra virgin olive oil quality by reversed-phase high-performance liquid chromatography/fluorescence detector. Food Chem. 2011, 125, 1423–1429. [Google Scholar] [CrossRef]
- Giorgio, D.D.; Leo, L.; Zacheo, G.; Lamascese, N. Evaluation of 52 almond (Prunus amygdalus Batsch) cultivars from the Apulia region in southern Italy. J. Hortic. Sci. Biotechnol. 2007, 82, 541–546. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Juan, T.; Alonso, J.M.M.; Espiau, M.T.T.; Socias I Company, R. Oil content, fatty acid composition and tocopherol concentration in the Spanish almond genebank collection. Sci. Hortic. 2014, 177, 99–107. [Google Scholar] [CrossRef]
- Schlörmann, W.; Birringer, M.; Böhm, V.; Löber, K.; Jahreis, G.; Lorkowski, S.; Müller, A.K.; Schöne, F.; Glei, M. Influence of roasting conditions on health-related compounds in different nuts. Food Chem. 2015, 180, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, W.; Schlörmann, W.; Glei, M. B-vitamins, carotenoids and α-/γ-tocopherol in raw and roasted nuts. Food Chem. 2017, 221, 222–227. [Google Scholar] [CrossRef] [PubMed]
- FSA Panel on Dietetic Products, N. and A. (NDA). Scientific Opinion on the substantiation of health claims related to plant sterols and plant stanols and maintenance of normal blood cholesterol concentrations and maintenance of normal prostate size and normal urination. EFSA J. 2010, 8, 1813. [Google Scholar] [CrossRef]
- Fernández-Cuesta, Á.; Kodad, O.; Socias I Company, R.; Velasco, L. Phytosterol variability in almond germplasm. J. Am. Soc. Hortic. Sci. 2012, 137, 343–348. [Google Scholar] [CrossRef]
- Rabadán, A.; Álvarez-Ortí, M.; Gómez, R.; de Miguel, C.; Pardo, J.E. Influence of genotype and crop year in the chemometrics of almond and pistachio oils. J. Sci. Food Agric. 2018, 98, 2402–2410. [Google Scholar] [CrossRef]
- Kodad, O.; Fernández-Cuesta, Á.; Karima, B.; Velasco, L.; Ercislil, S.; Socias I Company, R. Natural variability in phytosterols in almond (Prunus amygdalus) trees growing under a southern Mediterranean climate. J. Hortic. Sci. Biotechnol. 2015, 90, 543–549. [Google Scholar] [CrossRef]
- López Ortíz, C.M.; Prats Moya, M.S.; Berenguer Navarro, V. A rapid chromatographic method for simultaneous determination of β-sitosterol and tocopherol homologues in vegetable oils. J. Food Comp. Anal. 2006, 19, 141–149. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolomics and antioxidant activity of the leaves of Prunus dulcis Mill. (Italian vs. Toritto and Avola). J. Pharm. Biomed. Anal. 2018, 158, 54–65. [Google Scholar] [CrossRef]
- Bottone, A.; Mantoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite profiling and antioxidant activity of the polar fraction of Italian almonds (Toritto and Avola): Analysis of seeds, skins, and blanching water. J. Pharm. Biomed. Anal. 2020, 190, 113518. [Google Scholar] [CrossRef]
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V.M.; Caponio, F.; Pasqualone, A. Evaluation of the chemical and nutritional characteristics of almonds (Prunus dulcis (Mill). D.A. Webb) as influenced by harvest time and cultivar. J. Sci. Food. Agric. 2018, 98, 5647–5655. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem. Toxicol. 2008, 46, 2230–2235. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.-Y.O. Polyphenol content and antioxidant activity of California almonds depend on cultivar and harvest year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.Y.O. Quantification of almond skin polyphenols by liquid chromatography-mass spectrometry. J. Food Sci. 2009, 74, C326–C332. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.N.; Yildirim, F.; Şan, B.; Polat, M.; Sesli, Y. Variability of phenolic composition and tocopherol content of some commercial Almond cultivars. J. Appl. Bot. Food Qual. 2016, 170, 163–170. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B.; Lumberg, J.E.B.B. Determination of Flavonoids and Phenolics and Their Distribution in Almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Erten, E.S.; Cadwallader, K.R. Identification of predominant aroma components of raw, dry roasted and oil roasted almonds. Food Chem. 2017, 217, 244–253. [Google Scholar] [CrossRef]
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S.E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A.E. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis). Food Chem. 2014, 151, 31–39. [Google Scholar] [CrossRef]
- Beck, J.J.; Willett, D.S.; Gee, W.S.; Mahoney, N.E.; Higbee, B.S. Differentiation of Volatile Profiles from Stockpiled Almonds at Varying Relative Humidity Levels Using Benchtop and Portable GC-MS. J. Agric. Food Chem. 2016, 64, 9286–9292. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Beltrán, A.; Karabagias, I.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Monitoring the oxidative stability and volatiles in blanched, roasted and fried almonds under normal and accelerated storage conditions by DSC, thermogravimetric analysis and ATR-FTIR. Eur. J. Lipid Sci. Technol. 2015, 117, 1199–1213. [Google Scholar] [CrossRef]
- Lee, J.; Xiao, L.; Zhang, G.; Ebeler, S.E.; Mitchell, A.E. Influence of storage on volatile profiles in roasted almonds (prunus dulcis). J. Agric. Food Chem. 2014, 62, 11236–11245. [Google Scholar] [CrossRef] [PubMed]
- Franklin, L.M.; King, E.S.; Chapman, D.; Byrnes, N.; Huang, G.; Mitchell, A.E. Flavor and Acceptance of Roasted California Almonds during Accelerated Storage. J. Agric. Food Chem. 2018, 66, 1222–1232. [Google Scholar] [CrossRef]
- Yang, J.; Pan, Z.; Takeoka, G.; MacKey, B.; Bingol, G.; Brandl, M.T.; Garcin, K.; McHugh, T.H.; Wang, H. Shelf-life of infrared dry-roasted almonds. Food Chem. 2013, 138, 671–678. [Google Scholar] [CrossRef]
- Oliveira, I.; Malheiro, R.; Meyer, A.S.; Pereira, J.A.; Gonçalves, B. Application of chemometric tools for the comparison of volatile profile from raw and roasted regional and foreign almond cultivar (Prunus dulcis). J. Food Sci. Technol. 2019, 56, 3764–3776. [Google Scholar] [CrossRef]
- Beltrán, A.; Ramos, M.; Grané, N.; Martín, M.L.; Garrigós, M.C. Monitoring the oxidation of almond oils by HS-SPME–GC–MS and ATR-FTIR: Application of volatile compounds determination to cultivar authenticity. Food Chem. 2011, 126, 603–609. [Google Scholar] [CrossRef]
- Vichi, S.; Mayer, M.N.; León-Cárdenas, M.G.; Quintanilla-Casas, B.; Tres, A.; Guardiola, F.; Batlle, I.; Romero, A. Chemical Markers to Distinguish the Homo- and Heterozygous Bitter Genotype in Sweet Almond Kernels. Foods 2020, 9, 747. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Palombo, V.; Loor, J.J. What are omics sciences? In Periparturient Diseases of Dairy Cows: A Systems Biology Approach; Springer: Cham, Switzerland, 2017; pp. 1–7. ISBN 9783319430331. [Google Scholar]
- Sánchez-Pérez, R.; Pavan, S.; Mazzeo, R.; Moldovan, C.; Aiese Cigliano, R.; Del Cueto, J.; Ricciardi, F.; Lotti, C.; Ricciardi, L.; Dicenta, F.; et al. Mutation of a bHLH transcription factor allowed almond domestication. Science 2019, 364, 1095–1098. [Google Scholar] [CrossRef]
- Alioto, T.; Alexiou, K.; Bardil, A.; Barteri, F.; Castanera, R.; Cruz, F.; Dhingra, A.; Duval, H.; Fernández i Martí, Á.; Frias, L.; et al. Transposons played a major role in the diversification between the closely related almond (Prunus dulcis) and peach (P. persica) genomes: Results from the almond genome sequence. Plant. J. 2020, 101, 455–472. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, J.Y.; Moon, J.C.; Kwon, K.; Jang, C.S. Development of molecular markers for detecting almond, peanut, pine nut, and walnut in commercial food using quantitative real-time PCR. Appl. Biol. Chem. 2018, 61, 345–354. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Sánchez-Pérez, R.; Rubio, M. Clarifying omics concepts, challenges, and opportunities for Prunus breeding in the postgenomic era. OMICS J. Integr. Biol. 2012, 16, 268–283. [Google Scholar] [CrossRef]
- Wünsch, A.; Hormaza, J.I. Cultivar identification and genetic fingerprinting of temperate fruit tree species using DNA markers. Euphytica 2002, 125, 59–67. [Google Scholar] [CrossRef]
- Wooley, F.M.; Collins, G.G.; Sedgley, M. Application of DNA fingerprinting for the classification of selected almond [Prunus dulcis (Miller) D.A. Webb] cultivars. Aust. J. Exp. Agric. 2000, 40, 995–1001. [Google Scholar] [CrossRef]
- Gouta, H.; Ksia, E.; Buhner, T.; Moreno, M.Á.; Zarrouk, M.; Mliki, A.; Gogorcena, Y. Assessment of genetic diversity and relatedness among Tunisian almond germplasm using SSR markers. Hereditas 2010, 147, 283–292. [Google Scholar] [CrossRef]
- Shiran, B.; Amirbakhtiar, N.; Kiani, S.; Mohammadi, S.; Sayed-Tabatabaei, B.E.; Moradi, H. Molecular characterization and genetic relationship among almond cultivars assessed by RAPD and SSR markers. Sci. Hortic. 2007, 111, 280–292. [Google Scholar] [CrossRef]
- Rigoldi, M.P.; Rapposelli, E.; De Giorgio, D.; Resta, P.; Porceddu, A. Genetic diversity in two Italian almond collections. Electron. J. Biotechnol. 2015, 18, 40–45. [Google Scholar] [CrossRef]
- Fathi, A.; Ghareyazi, B.; Haghnazari, A.; Ghaffari, M.R.; Pirseyedi, M.; Kadkhodaei, S.; Naghavi, M.R.; Mardi, M. Assessment of the genetic diversity of almond (Prunus dulcis) using microsatellite markers and morphological traits. Iran. J. Biotechnol. 2008, 6, 98–106. [Google Scholar]
- Fernández i Martí, À.; Alonso, J.M.; Espiau, M.T.; Rubio-Cabetas, M.J.; Socias I Company, R. Genetic diversity in Spanish and Foreign almond germplasm assessed by molecular characterization with simple sequence repeats. J. Am. Soc. Hortic. Sci. 2009, 134, 535–542. [Google Scholar] [CrossRef]
- Fernández i Martí, A.; Font i Forcada, C.; Kamali, K.; Rubio-Cabetas, M.J.; Wirthensohn, M.; Socias I Company, R. Molecular analyses of evolution and population structure in a worldwide almond [Prunus dulcis (Mill.) D.A. Webb syn. P. Amygdalus Batsch] pool assessed by microsatellite markers. Genet. Resour. Crop. Evol. 2015, 62, 205–219. [Google Scholar] [CrossRef]
- Pérez de los Cobos, F.; Martínez-García, P.J.; Romero, A.; Miarnau, X.; Eduardo, I.; Howad, W.; Mnejja, M.; Dicenta, F.; Socias I Company, R.; Rubio-Cabetas, M.J.; et al. Pedigree analysis of 220 almond genotypes reveals two world mainstream breeding lines based on only three different cultivars. Hortic. Res. 2021, 8, 11. [Google Scholar] [CrossRef]

| Country | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|
| United States | 1,545,500 | 1,302,998 | 1,376,337 | 1,476,539 | 1,872,500 | 1,936,840 |
| Spain | 195,704 | 211,084 | 199,167 | 255,503 | 339,033 | 340,420 |
| Australia | 55,978 | 63,331 | 72,902 | 75,373 | 69,880 | 146,410 |
| Morocco | 101,026 | 97,723 | 112,681 | 116,923 | 117,270 | 102,185 |
| Iran | 136,338 | 146,000 | 111,845 | 129,566 | 139,029 | 177,015 |
| Italy | 74,016 | 70,399 | 74,584 | 79,599 | 79,801 | 77,300 |
| Turkey | 73,230 | 80,000 | 85,000 | 90,000 | 100,000 | 150,000 |
| Geographical Origin Confirm the Color of Back Ground | Cultivars-Country of Origin | Compounds | Analysis | Ref. |
|---|---|---|---|---|
| Turkey | Cristomorto, Largueta, Ferraduel, Ferragnes, Ferrastar, Glorieta, Lauranne, Masbovera, Nonpareil, Picantili, Sonora, Supernova, Texas, Tuono, and Yaltinski | Palmitic, Palmitoleic, Heptadecanoic, Stearic, Oleic, Linoleic, Arachidic | GC-FID PCA | [7] |
| Sicily, Spain, California | Not specified | Oleic, Linoleic, Palmitic, Stearic, Myristic, Arachidonic, Arachidic | GC-MS PCA | [17] |
| Serbia | Marcona-Spain, Texas- USA, Troito-Italy and 17 selections from the large spontaneous population of almonds in North Serbia, called Slankamen Hill | Oleic, Linoleic, Palmitic, Stearic, Myristic, Arachidic, Palmitoleic, Heptadecanoic, Cis-10-heptadecenoic, Linolenic, Eicosenoic, Tricosanoic, Behenic, Pentadecanoic, Docosadienoic, Lignoceric | GC-FID PCA | [18] |
| Afghanistan | Khairodini samangani, Pista Badam, Kaghazai Siah Dana, Qaharbai, Sangak Shashum, Shokorbai, Carmel, Kaf Samangani, Khairodini, Kaghazai Kalan, Sattarbai, Belabai, Marawaja Du Maghza, Sattarbai Doum, Shakh-i- Buz Safid, Sangak Dahum, Qambari Kunduzi, Sattarbai Bakhmali, Khairodini-161 Samangan, Sattarbai Saiz Talkhak | Palmitic, Tridecanoic, Palmitoleic, Stearic, Oleic, Linoleic, Arachidic, Linolenic, Henicosanoic, Behenic, Tricosanoic, Lignoceric | GC-FID PCA | [24] |
| Argentina, France, Greece, India, Italy, Portugal, Spain, Syria, Ukraine, USA | Emilito-Argentina; Marcona Argentina-Argentina, Ai-France, Ardechoise-France, Bartre-France, Belle d’Aurons-France, Cristar-France, Ferragnes-France, Ferralise-France, Fourcouronne-France, Fournat de Brezenaud-France, Pointu d’Aureille-France, Princesse-France, Stelliette-France, Tardive de la Verdiere-France, Tournefort-France, Exinograd-Greece, Phyllis-Greece, Pagrati-Greece, Symmetriki-Greece, Truoito-Greece, Tsotoliou-Greece, Kata-India, Spilo-India, Talengy-India, Bonifacio-Italy, Cavaliera-Italy, Cristomorto-Italy, Filippo Ceo-Italy, Fiori-Italy, Fragiulio-Italy, Mollese-Italy, Olla-Italy, Prouvista-Italy, Rachele-Italy, Rana-Italy, Supernova-Italy, Tuono-Italy, Carreirinha-Portugal, Cosa Nova-Portugal, Gama-Portugal, Rameira-Portugal, Raposa-Portugal, Verdeal-Portugal, Atocha-Spain, Del Cid-Spain, Desmayo Largueta-Spain, Desmayo Rojo-Spain, Garbí-Spain, Garrigues-Spain, Mollar Arbeca-Spain, Marcona-Spain, Mollar-Spain, Ramillete-Spain, Verdereta-Spain, Siria-1-Syria, Siria-3-Syria, Nikitskij-Ukraine, Primorskij-Ukraine, Sovietskij-Ukraine, Drake-USA, IXL-USA, LeGrand-USA, Mono-USA, Nec Plus Ultra-USA, Nonpareil-USA, Peerless-USA, Tardy Nonpareil-USA, Texas-USA, Thompsom-USA, Tioga-USA, Tokyo-USA, Yosemite-USA | Palmitic, Palmitoleic, Stearic, Oleic, Linoleic | GC-FID PCA | [25] |
| Portugal | Protected Designation of Origin: Casa Nova, Duro Italiano, Pegarinhos, Refego. Non PDO: Ferraduel, Ferragnes, Ferrastar, Gloriette and Marcona | Palmitic, Palmitoleic, Cis-10-heptadecenoic, Stearic, Oleic, Linoleic, Arachidic | GC-FID PCA | [27] |
| Spain and California | Marcona-Spain, Guara-Spain Garrigues-Spain and Butte-California | Palmitic, Palmitoleic, Stearic, Oleic, Linoleic | GC-FID PCA | [28] |
| Morocco; Spain, France and Tunisia | Marcona-Spain, Desmayo Largueta-Spain, Ferragnès-France, Fournat de Brézenaud-France, Ferraduel-France, Khoukhi’-Tunisia and 46 local genotypes from the Rif mountains (north of Morocco), the Atlas mountains and the valley of Tadla (central-south Morocco) | Palmitic, Palmitoleic, Stearic, Oleic, Linoleic | GC-FID PCA | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán Sanahuja, A.; Maestre Pérez, S.E.; Grané Teruel, N.; Valdés García, A.; Prats Moya, M.S. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods 2021, 10, 153. https://doi.org/10.3390/foods10010153
Beltrán Sanahuja A, Maestre Pérez SE, Grané Teruel N, Valdés García A, Prats Moya MS. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods. 2021; 10(1):153. https://doi.org/10.3390/foods10010153
Chicago/Turabian StyleBeltrán Sanahuja, Ana, Salvador E. Maestre Pérez, Nuria Grané Teruel, Arantzazu Valdés García, and María Soledad Prats Moya. 2021. "Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins" Foods 10, no. 1: 153. https://doi.org/10.3390/foods10010153
APA StyleBeltrán Sanahuja, A., Maestre Pérez, S. E., Grané Teruel, N., Valdés García, A., & Prats Moya, M. S. (2021). Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods, 10(1), 153. https://doi.org/10.3390/foods10010153