Application of Ohmic–Vacuum Combination Heating for the Processing of Senior-Friendly Food (Multiphase Food): Experimental Studies and Numerical Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Model Food
2.2. Ohmic–Vacuum Combination Heating System
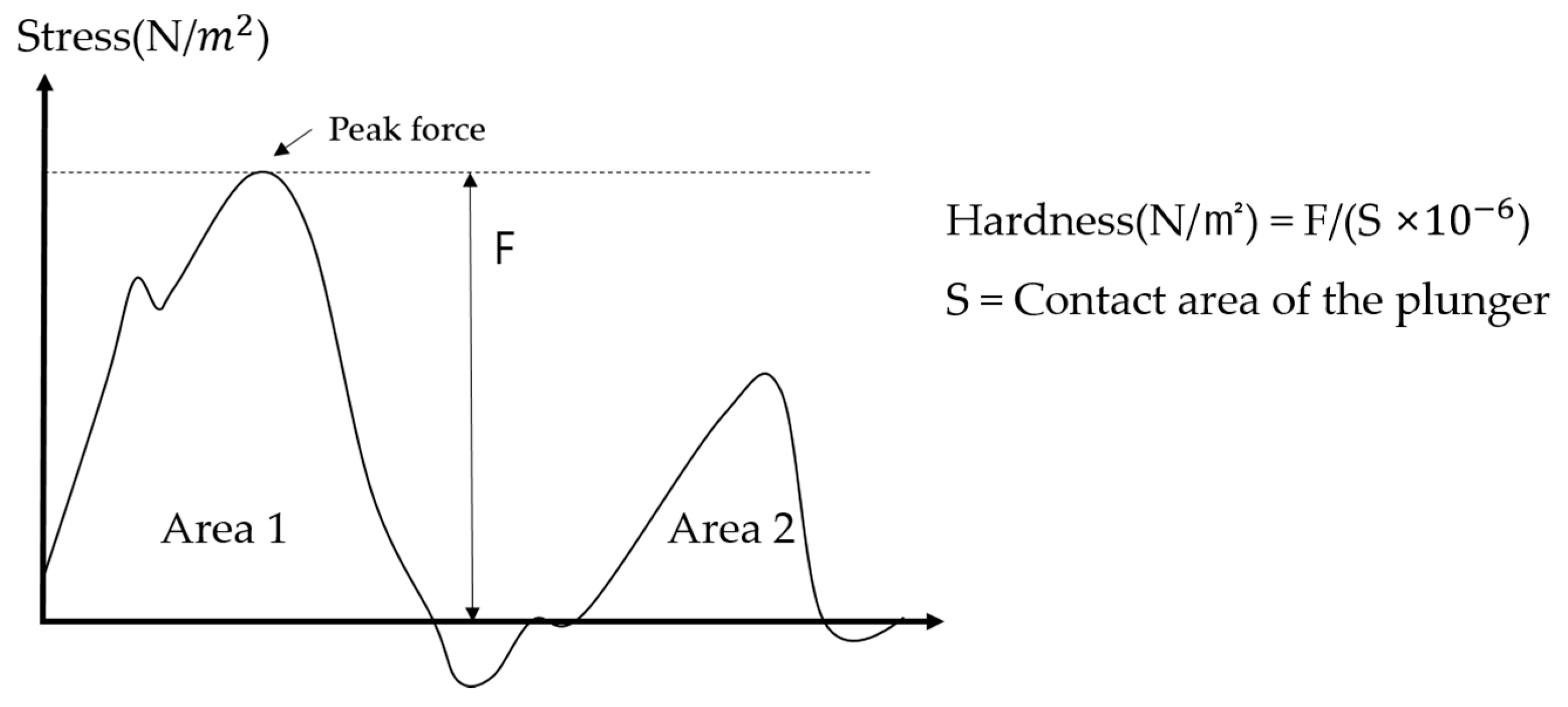
2.3. Measurement of Hardness of Solid Particles
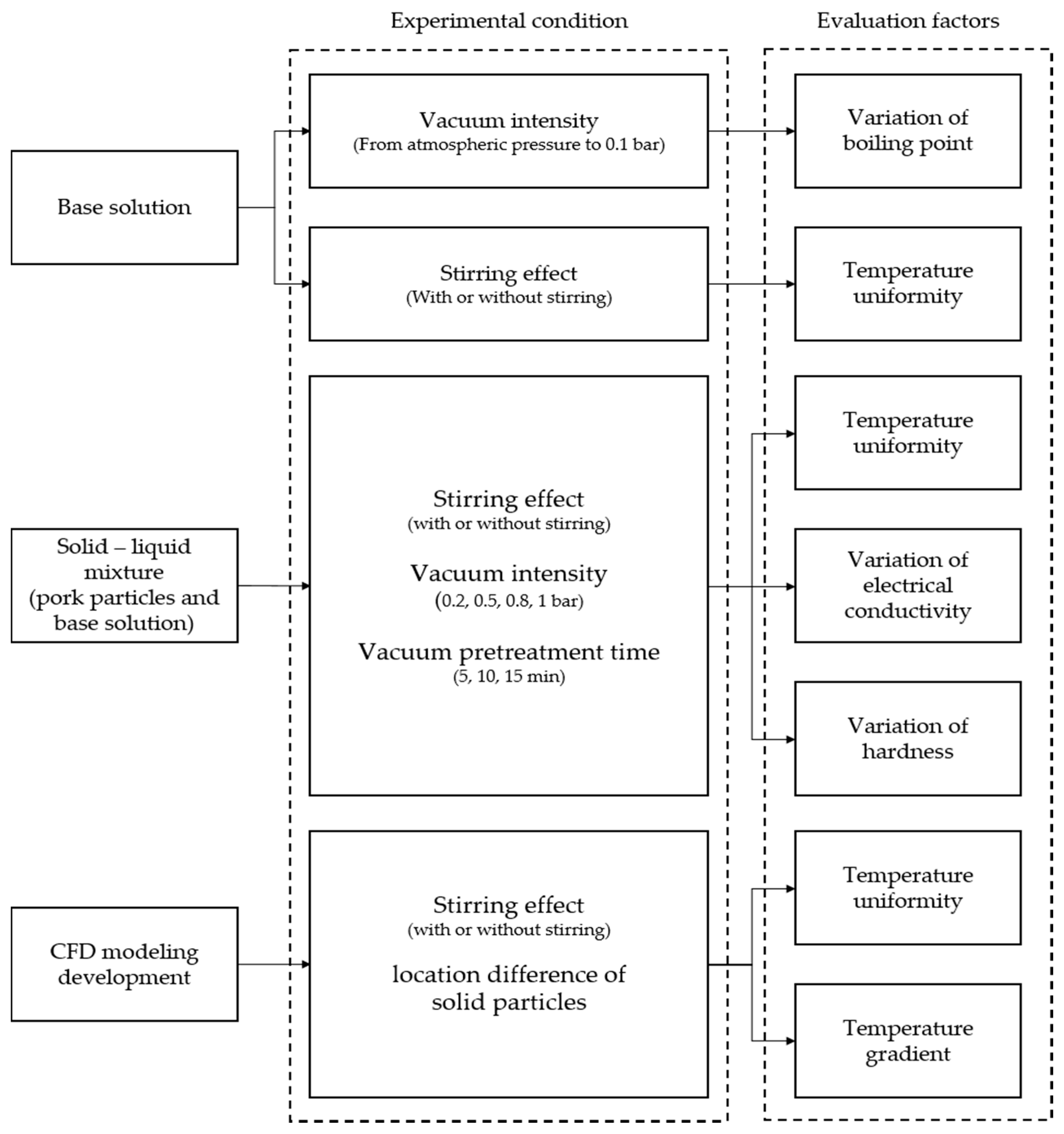
2.4. Experimental Design
2.5. Mathematical Modeling
2.5.1. Governing Equation for Electromagnetic Heat Generation
2.5.2. Governing Equation for Turbulent Flow
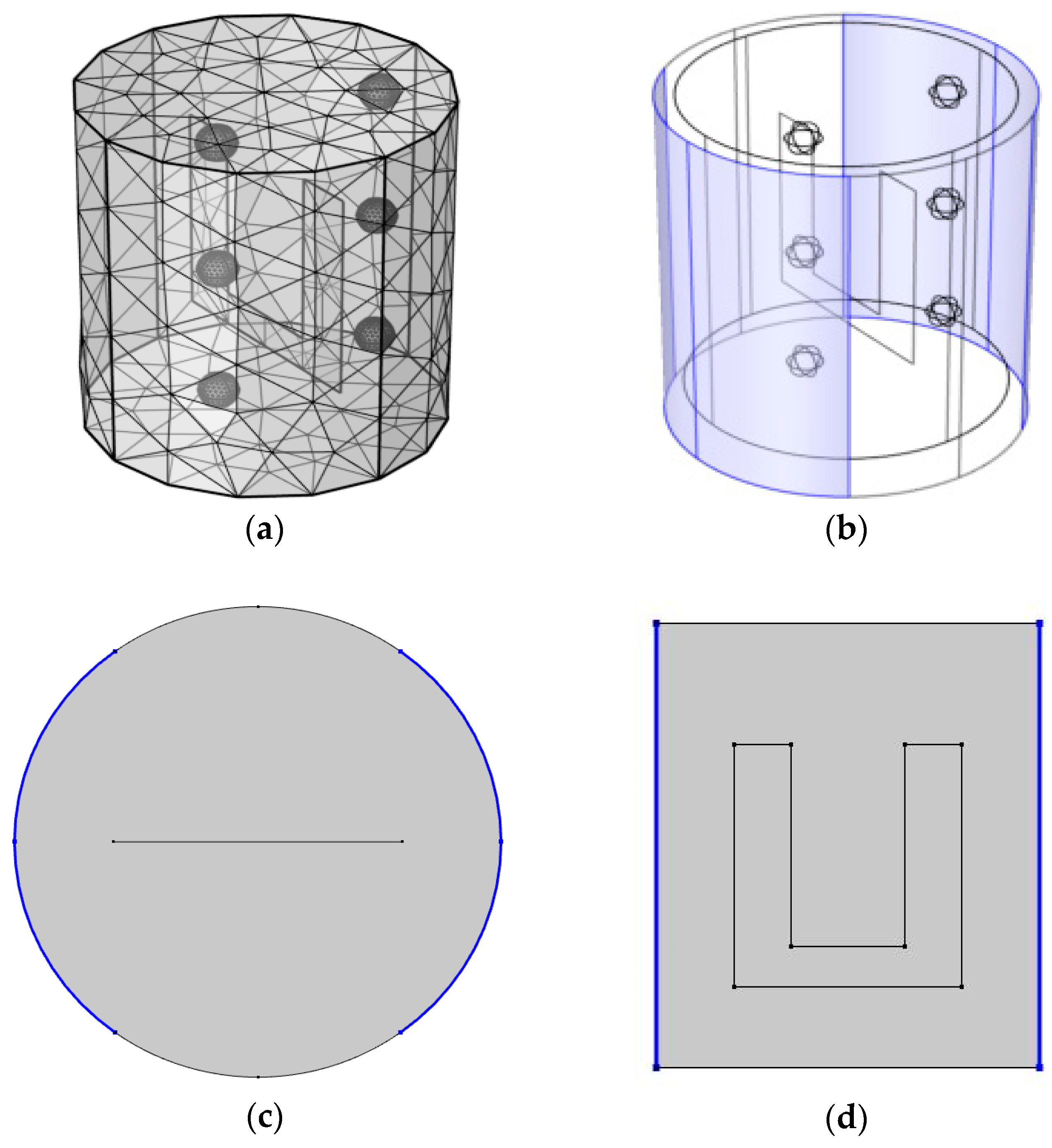
2.5.3. Mathematical Modeling Setup
3. Results and Discussion
3.1. The Effect of Vacuum Pretreatment on Change in Electrical Conductivities of Pork Particle and Base Solution
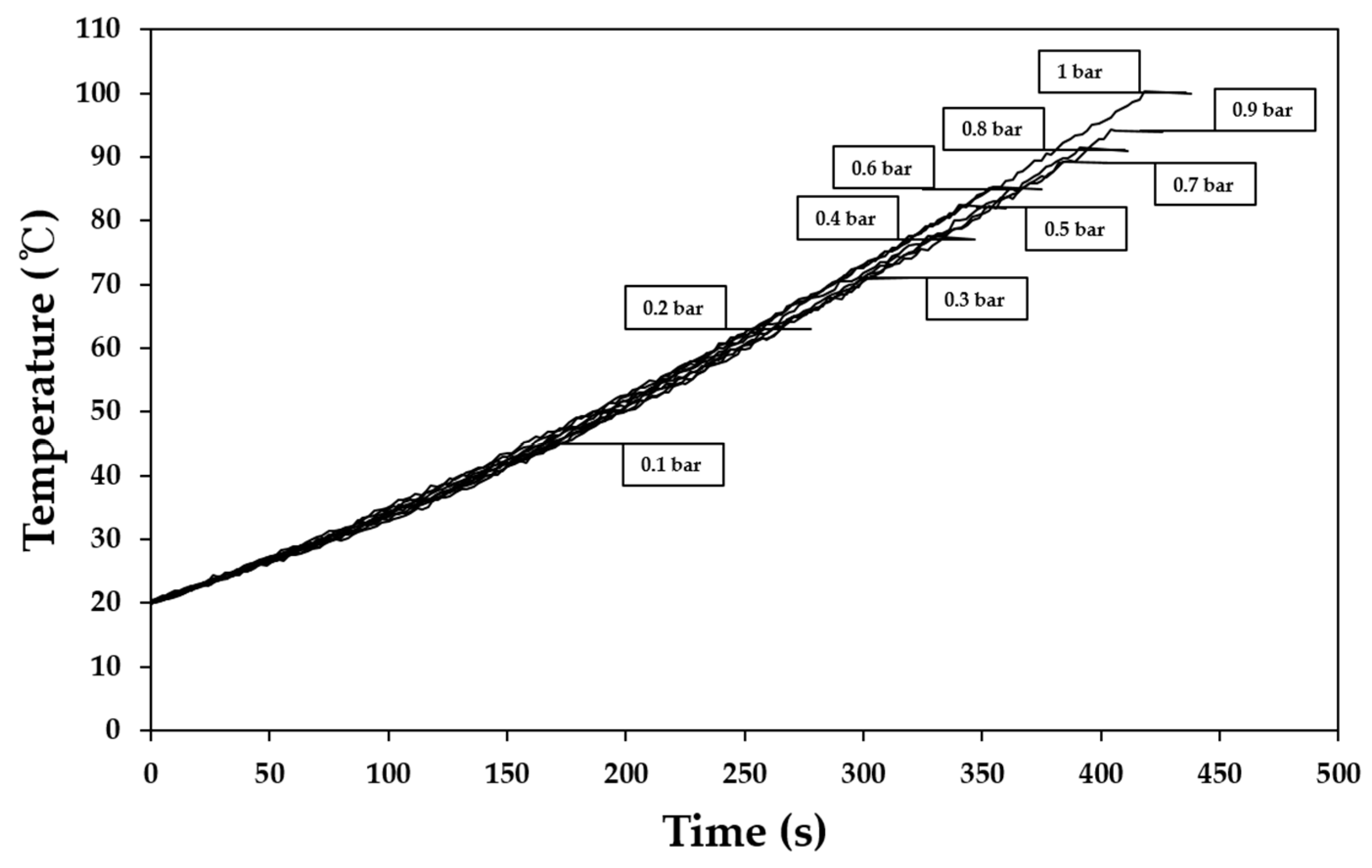
3.2. Change of Boiling Point of Base Solution Depending on Vacuum Intensity
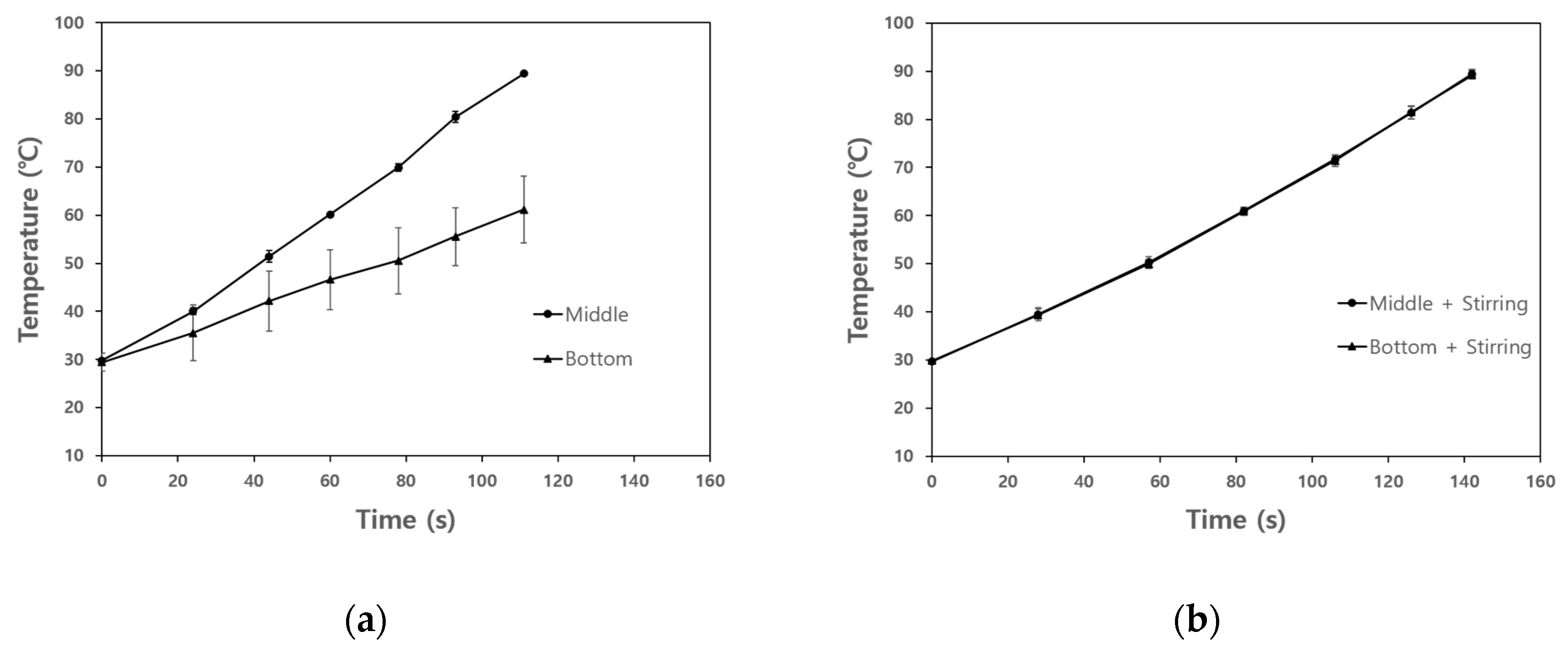
3.3. The Effect of Agitation on Temperature Uniformity of Base Solution and Model Food
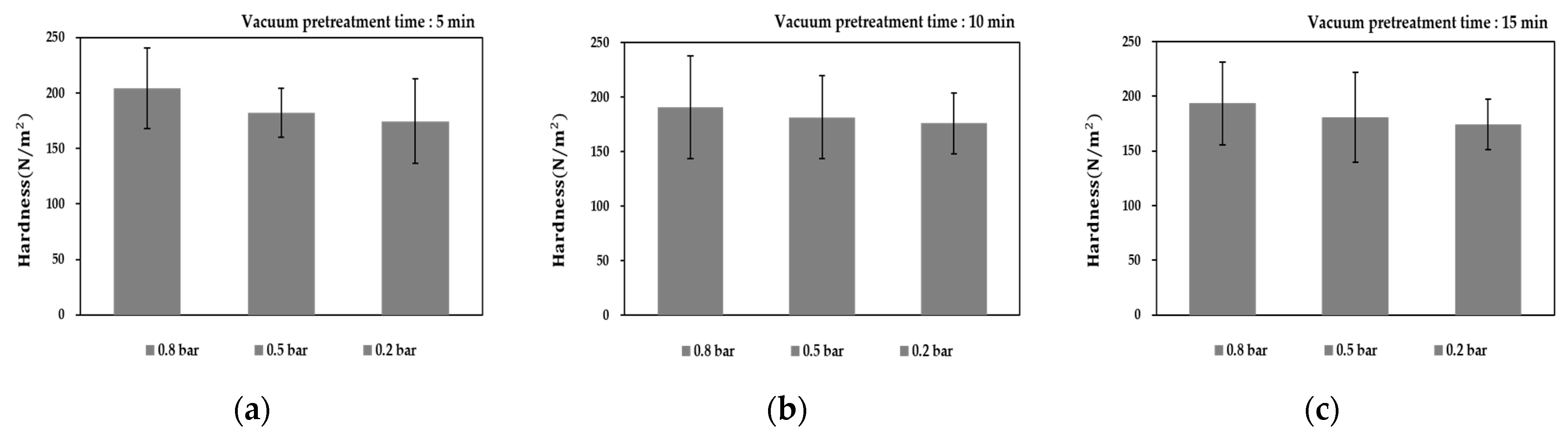
3.4. Variation of Particle Hardness
3.5. Simulation Verification
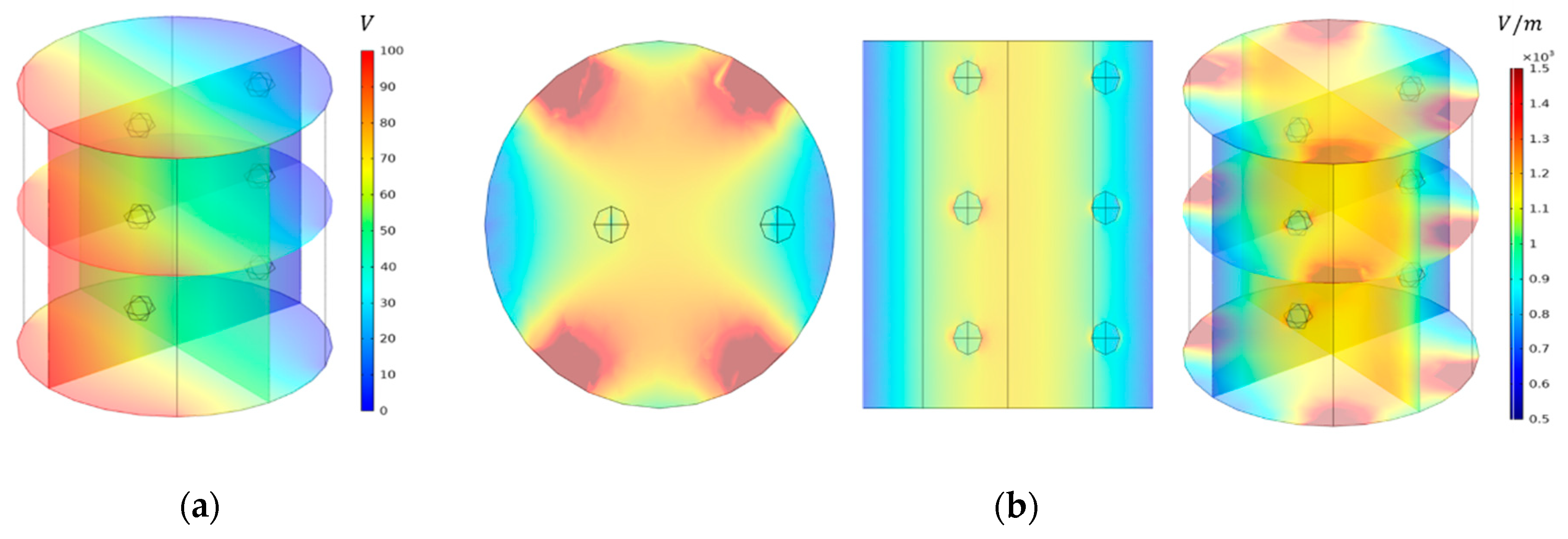
3.5.1. The Simulated Electric Field Strength Distribution in Ohmic Chamber
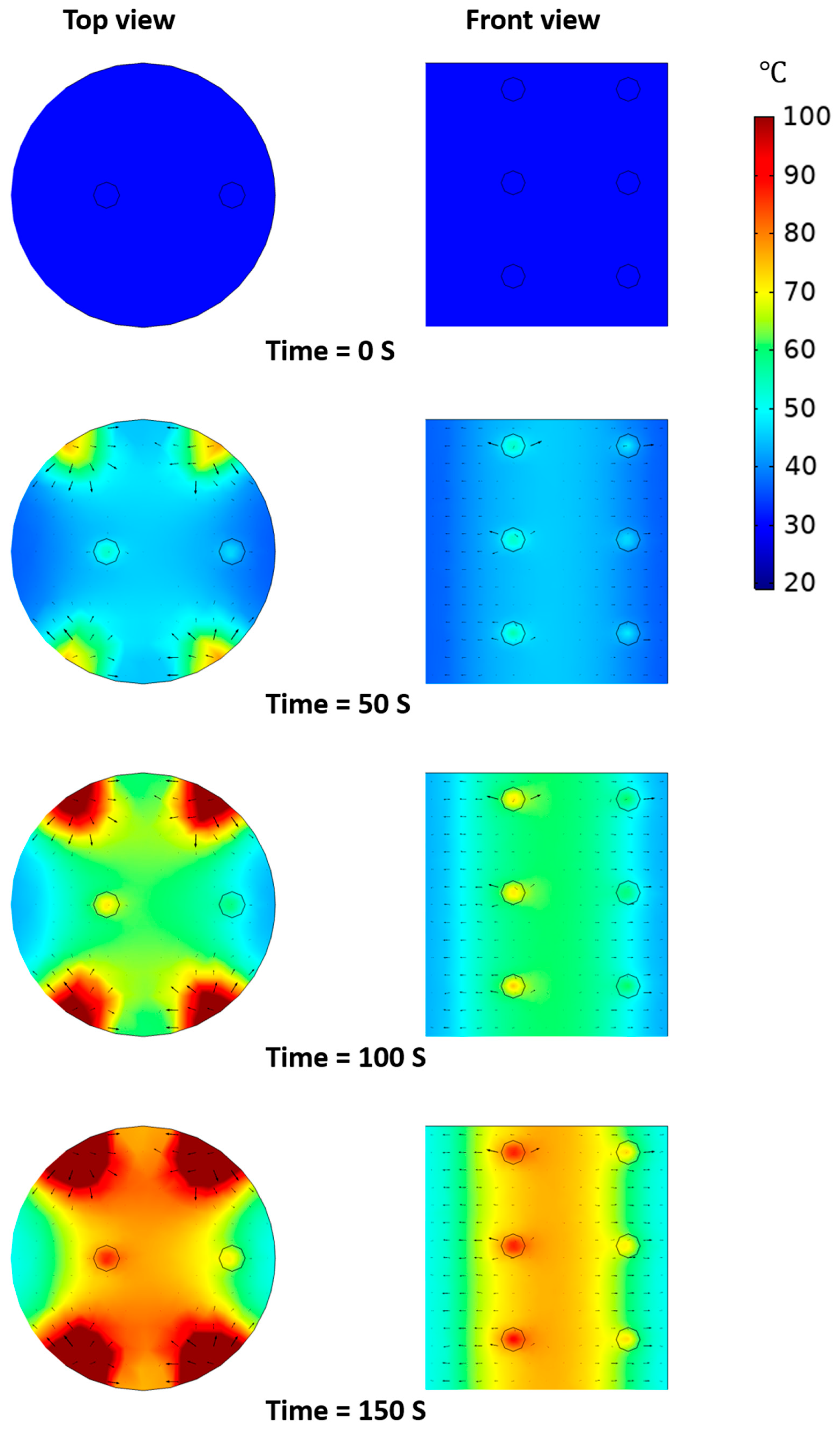
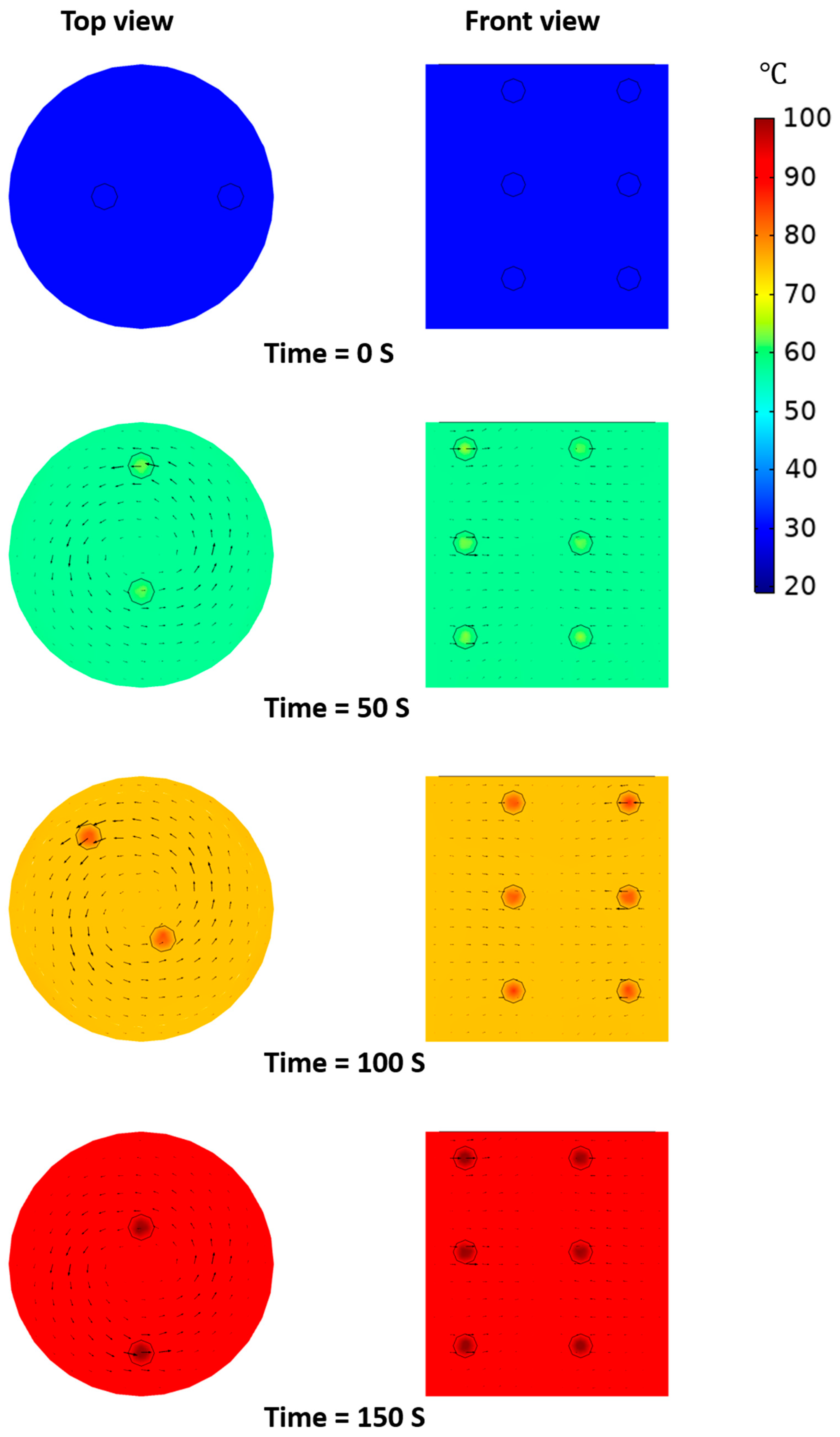
3.5.2. Temperature Distribution of Model Food under OH Depending on Presence of Agitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Canning, D.; Lubet, A. Global Population Aging: Fact, Challenges, Solutions & Perspectives. Chall. Solut. Perspect. Daedalus 2015, 144, 80–92. [Google Scholar]
- Suzuki, T. Low Fertility and Population Aging in Germany and Japan: Prospects and Policies. In Fertility and Public Policy; Springer: Tokyo, Japan, 2013; pp. 1–87. ISBN 9784431547808. [Google Scholar]
- Maestas, N.; Mullen, K.J.; Powell, D. The Effect of Population Aging on Economic Growth, the Labor Force and Productivity (No. w22452); National Bureau of Economic Research: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Baek, J. Trends in elderly-friendly home-style alternative food. Inst. Electron. Inf. Eng. 2017, 44, 44–51. [Google Scholar]
- Lee, S.B. Trend of Japan’s Care Food Industry; Seoul, Korea. 2017. Available online: https://www.kati.net/board/pubilshedMaterialsView.do?menu_dept2=48&board_seq=85502 (accessed on 15 November 2020).
- Hsu, M.; Yamada, T. Population Aging, Health Care, and Fiscal Policy Reform: The Challenges for Japan. Scand. J. Econ. 2019, 121, 547–577. [Google Scholar] [CrossRef]
- García, J.; Méndez, D.; Álvarez, M.; Sanmartin, B.; Vazquez Sobrado, R.; Regueiro, L.; Atanassova, M. Design of novel functional food products enriched with bioactive extracts from holothurians for meeting the nutritional needs of the elderly. LWT Food Sci. Technol. 2019, 109, 55–62. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Akobundu, U.; Bailey, R.L.; Shlisky, J.; Beaudreault, A.R.; Bergeron, G.; Blancato, R.B.; Blumberg, J.B.; Bourassa, M.W.; Gomes, F.; et al. Hidden hunger: Solutions for America’s aging populations. Nutrients 2018, 10, 1210. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Harris Ledikwe, J.; Smiciklas-Wright, H.; Mitchell, D.C.; Jensen, G.L. Persistent oral health problems associated with comorbidity and impaired diet quality in older adults. J. Am. Diet. Assoc. 2004, 104, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Haboubi, N. Assessment and management of nutrition in older people and its importance to health. Clin. Interv. Aging 2010, 5, 207–216. [Google Scholar]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Graham, B.G. Taste and smell perception affect appetite and immunity in the elderly. Eur. J. Clin. Nutr. 2000, 54, S54–S63. [Google Scholar] [CrossRef]
- Scharfstein, M. Gaurf Food Industry for The Aging Society. Food Sci. Ind. 2006, 39, 47–51. [Google Scholar]
- Torkian Boldaji, M.; Borghei, A.M.; Beheshti, B.; Hosseini, S.E. The process of producing tomato paste by ohmic heating method. J. Food Sci. Technol. 2015, 52, 3598–3606. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Gholamhosseinpour, A.; Niakousari, M. Application of microwave and ohmic heating for pasteurization of cantaloupe juice: Microbial inactivation and chemical properties. J. Sci. Food Agric. 2019, 99, 4276–4286. [Google Scholar] [CrossRef] [PubMed]
- Fadavi, A.; Yousefi, S.; Darvishi, H.; Mirsaeedghazi, H. Comparative study of ohmic vacuum, ohmic, and conventional-vacuum heating methods on the quality of tomato concentrate. Innov. Food Sci. Emerg. Technol. 2018, 47, 225–230. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Choi, W.; Lee, S.H.; Jun, S. Exploring the heating patterns of multiphase foods in a continuous flow, simultaneous microwave and ohmic combination heater. J. Food Eng. 2013, 116, 65–71. [Google Scholar] [CrossRef]
- Jun, S.; Sastry, S. Modeling and optimization of ohmic heating of foods inside a flexible package. J. Food Process Eng. 2005, 28, 417–436. [Google Scholar] [CrossRef]
- Sastry, S.K.; Palaniappan, S. Mathematical Modeling and Experimental Studies on Ohmic Heating of Liquid-Particle Mixtures in a Static Heater. J. Food Process Eng. 1992, 15, 241–261. [Google Scholar] [CrossRef]
- Benabderrahmane, Y.; Pain, J.P. Thermal behaviour of a solid/liquid mixture in an ohmic heating sterilizer-slip phase model. Chem. Eng. Sci. 2000, 55, 1371–1384. [Google Scholar] [CrossRef]
- Fadavi, A.; Salari, S. Ohmic Heating of Lemon and Grapefruit Juices Under Vacuum Pressure—Comparison of Electrical Conductivity and Heating Rate. J. Food Sci. 2019, 84, 2868–2875. [Google Scholar] [CrossRef]
- Wang, L.; Sun, D.-W. Modelling vacuum cooling process of cooked meat—part 1: Analysis of vacuum cooling system Mode ` me de pour la viande cuite—Partie 1: Analyse du syste refroidissement sous vide. Int. J. Refrig. 2002, 25, 854–861. [Google Scholar] [CrossRef]
- Yaghmaee, P.; Durance, T.D. Destruction and injury of Escherichia coli during microwave heating under vacuum. J. Appl. Microbiol. 2005, 98, 498–506. [Google Scholar] [CrossRef]
- Paranjpe, S.S.; Ferruzzi, M.; Morgan, M.T. Effect of a flash vacuum expansion process on grape juice yield and quality. LWT Food Sci. Technol. 2012, 48, 147–155. [Google Scholar] [CrossRef]
- Mariscal, M.; Bouchon, P. Comparison between atmospheric and vacuum frying of apple slices. Food Chem. 2008, 107, 1561–1569. [Google Scholar] [CrossRef]
- Darvishi, H.; Mohammadi, P.; Fadavi, A.; Koushesh Saba, M.; Behroozi-Khazaei, N. Quality preservation of orange concentrate by using hybrid ohmic—Vacuum heating. Food Chem. 2019, 289, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kim, S.S.; Park, S.H.; Ahn, J.B.; Kang, D.H. Numerical analysis of rectangular type batch ohmic heater to identify the cold point. Food Sci. Nutr. 2020, 8, 648–658. [Google Scholar] [CrossRef]
- Ye, X.; Ruan, R.; Chen, P.; Doona, C. Simulation and verification of ohmic heating in static heater using MRI temperature mapping. LWT Food Sci. Technol. 2004, 37, 49–58. [Google Scholar] [CrossRef]
- Zhu, S.M.; Zareifard, M.R.; Chen, C.R.; Marcotte, M.; Grabowski, S. Electrical conductivity of particle-fluid mixtures in ohmic heating: Measurement and simulation. Food Res. Int. 2010, 43, 1666–1672. [Google Scholar] [CrossRef]
- Chen, C.; Abdelrahim, K.; Beckerich, I. Sensitivity analysis of continuous ohmic heating process for multiphase foods. J. Food Eng. 2010, 98, 257–265. [Google Scholar] [CrossRef]
- Marra, F.; Zell, M.; Lyng, J.G.; Morgan, D.J.; Cronin, D.A. Analysis of heat transfer during ohmic processing of a solid food. J. Food Eng. 2009, 91, 56–63. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Roohi, R. Ohmic heating of blended citrus juice: Numerical modeling of process and bacterial inactivation kinetics. Innov. Food Sci. Emerg. Technol. 2019, 52, 313–324. [Google Scholar] [CrossRef]
- Salengke, S.; Sastry, S.K. Models for ohmic heating of solid-liquid mixtures under worst-case heating scenarios. J. Food Eng. 2007, 83, 337–355. [Google Scholar] [CrossRef]
- Zell, M.; Cronin, D.A.; Morgan, D.J.; Marra, F.; Lyng, J.G. Solid Food Pasteurization by Ohmic Heating: Influence of Process Parameters. In Proceedings of the COMSOL Conference, Boston, MA, USA, 9–11 October 2008. [Google Scholar]
- Jun, S.; Sastry, S. Reusable pouch development for long term space missions: A 3D ohmic model for verification of sterilization efficacy. J. Food Eng. 2007, 80, 1199–1205. [Google Scholar] [CrossRef]
- Wilcox, D.C. Turbulence Modeling for CFD, 2nd ed.; DCW Industries: Palm Drive La Cañada, CA, USA, 1998; pp. 87–89. [Google Scholar]
- Wang, W.C.; Sastry, S.K. Salt diffusion into vegetable tissue as a pretreatment for ohmic heating: Electrical conductivity profiles and vacuum infusion studies. J. Food Eng. 1993, 20, 299–309. [Google Scholar] [CrossRef]
- Shim, J.; Lee, S.H.; Jun, S. Modeling of ohmic heating patterns of multiphase food products using computational fluid dynamics codes. J. Food Eng. 2010, 99, 136–141. [Google Scholar] [CrossRef]
- Allali, H.; Marchal, L.; Vorobiev, E. Effects of vacuum impregnation and ohmic heating with citric acid on the behaviour of osmotic dehydration and structural changes of apple fruit. Biosyst. Eng. 2010, 106, 6–13. [Google Scholar] [CrossRef]
- Moreno, J.; Bugueño, G.; Velasco, V.; Petzold, G.; Tabilo-Munizaga, G. Osmotic dehydration and vacuum impregnation on physicochemical properties of Chilean papaya (Carica candamarcensis). J. Food Sci. 2004, 69, 102–106. [Google Scholar] [CrossRef]
- Moreno, J.; Simpson, R.; Estrada, D.; Lorenzen, S.; Moraga, D.; Almonacid, S. Effect of pulsed-vacuum and ohmic heating on the osmodehydration kinetics, physical properties and microstructure of apples (cv. Granny Smith). Innov. Food Sci. Emerg. Technol. 2011, 12, 562–568. [Google Scholar] [CrossRef]
- Moreno, J.; Simpson, R.; Baeza, A.; Morales, J.; Muñoz, C.; Sastry, S.; Almonacid, S. Effect of ohmic heating and vacuum impregnation on the osmodehydration kinetics and microstructure of strawberries (cv. Camarosa). LWT Food Sci. Technol. 2012, 45, 148–154. [Google Scholar] [CrossRef]
- Moreno, J.; Zúñiga, P.; Dorvil, F.; Petzold, G.; Mella, K.; Bugueño, G. Osmodehydration assisted by ohmic heating/pulse vacuum in apples (cv. Fuji): Retention of polyphenols during refrigerated storage. Int. J. Food Sci. Technol. 2017, 52, 1203–1210. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; Tárrega, A.; García-Segovia, P.; Martínez-Monzó, J. Comparison of Vacuum Treatments and Traditional Cooking Using Instrumental and Sensory Analysis. Food Anal. Methods 2014, 7, 400–408. [Google Scholar] [CrossRef]

| Temperature (°C) | A | B | C | D | E | F | Base Solution |
|---|---|---|---|---|---|---|---|
| 30 | 0.76 ± 0.07 | 1.11 ± 0.01 | 0.92 ± 0.03 | 0.39 ± 0.01 | 0.27 ± 0.01 | 0.75 ± 0.04 | 0.79 ± 0.03 |
| 40 | 0.91 ± 0.05 | 1.32 ± 0.01 | 1.10 ± 0.05 | 0.45 ± 0.02 | 0.31 ± 0.01 | 0.93 ± 0.06 | 0.90 ± 0.02 |
| 50 | 1.08 ± 0.07 | 1.56 ± 0.02 | 1.23 ± 0.04 | 0.53 ± 0.02 | 0.37 ± 0.01 | 1.12 ± 0.07 | 1.01 ± 0.02 |
| 60 | 1.20 ± 0.06 | 1.82 ± 0.04 | 1.37 ± 0.08 | 0.60 ± 0.02 | 0.43 ± 0.01 | 1.33 ± 0.08 | 1.12 ± 0.03 |
| 70 | 1.35 ± 0.08 | 2.00 ± 0.08 | 1.62 ± 0.11 | 0.69 ± 0.02 | 0.47 ± 0.01 | 1.53 ± 0.09 | 1.22 ± 0.03 |
| 80 | 1.48 ± 0.06 | 2.29 ± 0.07 | 1.76 ± 0.05 | 0.75 ± 0.04 | 0.53 ± 0.02 | 1.67 ± 0.11 | 1.31 ± 0.04 |
| 90 | 1.58 ± 0.04 | 2.39 ± 0.07 | 1.85 ± 0.09 | 0.82 ± 0.04 | 0.60 ± 0.01 | 1.86 ± 0.10 | 1.45 ± 0.06 |
| 30 °C | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | |||
|---|---|---|---|---|---|---|---|---|---|
| Base solution (A) | 0.80 ± 0.03 | 0.90 ± 0.02 | 1.01 ± 0.02 | 1.13 ± 0.03 | 1.22 ± 0.03 | 1.31 ± 0.04 | 1.45 ± 0.06 | ||
| Pork particle (B) | 0.74 ± 0.03 | 0.83 ± 0.04 | 0.95 ± 0.06 | 1.03 ± 0.05 | 1.12 ± 0.06 | 1.24 ± 0.06 | 1.35 ± 0.07 | ||
| 1 bar | 5 min | (A) | 0.86 ± 0.02 | 0.96 ± 0.03 | 1.06 ± 0.03 | 1.16 ± 0.01 | 1.28 ± 0.03 | 1.39 ± 0.01 | 1.54 ± 0.01 |
| (B) | 0.79 ± 0.79 | 0.90 ± 0.09 | 1.00 ± 0.10 | 1.10 ± 0.10 | 1.19 ± 0.10 | 1.28 ± 0.11 | 1.38 ± 0.11 | ||
| 10 min | (A) | 0.86 ± 0.01 | 0.95 ± 0.01 | 1.05 ± 0.01 | 1.17 ± 0.02 | 1.29 ± 0.02 | 1.39 ± 0.04 | 1.47 ± 0.02 | |
| (B) | 0.72 ± 0.05 | 0.88 ± 0.04 | 0.98 ± 0.04 | 1.07 ± 0.05 | 1.17 ± 0.05 | 1.27 ± 0.07 | 1.38 ± 0.06 | ||
| 15 min | (A) | 0.86 ± 0.04 | 0.95 ± 0.03 | 1.06 ± 0.03 | 1.18 ± 0.03 | 1.30 ± 0.06 | 1.39 ± 0.05 | 1.48 ± 0.04 | |
| (B) | 0.78 ± 0.05 | 0.94 ± 0.08 | 1.06 ± 0.09 | 1.14 ± 0.08 | 1.23 ± 0.09 | 1.32 ± 0.09 | 1.43 ± 0.10 | ||
| 0.8 bar | 5 min | (A) | 0.82 ± 0.04 | 0.92 ± 0.02 | 1.04 ± 0.04 | 1.15 ± 0.02 | 1.26 ± 0.05 | 1.37 ± 0.05 | 1.49 ± 0.06 |
| (B) | 0.79 ± 0.09 | 0.99 ± 0.12 | 1.15 ± 0.14 | 1.24 ± 0.15 | 1.32 ± 0.13 | 1.43 ± 0.12 | 1.51 ± 0.11 | ||
| 10 min | (A) | 0.84 ± 0.06 | 0.93 ± 0.05 | 1.03 ± 0.04 | 1.14 ± 0.03 | 1.26 ± 0.03 | 1.37 ± 0.04 | 1.48 ± 0.05 | |
| (B) | 0.75 ± 0.09 | 0.89 ± 0.10 | 1.02 ± 0.07 | 1.13 ± 0.07 | 1.22 ± 0.09 | 1.33 ± 0.08 | 1.44 ± 0.09 | ||
| 15 min | (A) | 0.84 ± 0.02 | 0.94 ± 0.02 | 1.04 ± 0.02 | 1.15 ± 0.02 | 1.26 ± 0.03 | 1.36 ± 0.03 | 1.46 ± 0.01 | |
| (B) | 0.75 ± 0.06 | 0.88 ± 0.05 | 1.00 ± 0.04 | 1.09 ± 0.05 | 1.20 ± 0.03 | 1.31 ± 0.03 | 1.41 ± 0.03 | ||
| 0.5 bar | 5 min | (A) | 0.84 ± 0.02 | 0.95 ± 0.02 | 1.06 ± 0.02 | 1.17 ± 0.03 | 1.29 ± 0.05 | 1.40 ± 0.08 | 1.48 ± 0.07 |
| (B) | 0.72 ± 0.07 | 0.83 ± 0.05 | 0.97 ± 0.04 | 1.09 ± 0.03 | 1.23 ± 0.07 | 1.35 ± 0.08 | 1.47 ± 0.07 | ||
| 10 min | (A) | 0.81 ± 0.05 | 0.91 ± 0.04 | 1.02 ± 0.04 | 1.13 ± 0.04 | 1.25 ± 0.03 | 1.37 ± 0.02 | 1.47 ± 0.03 | |
| (B) | 0.70 ± 0.06 | 0.81 ± 0.06 | 0.93 ± 0.06 | 1.04 ± 0.04 | 1.16 ± 0.05 | 1.25 ± 0.05 | 1.35 ± 0.06 | ||
| 15 min | (A) | 0.84 ± 0.02 | 0.94 ± 0.02 | 1.05 ± 0.03 | 1.16 ± 0.03 | 1.28 ± 0.03 | 1.38 ± 0.03 | 1.48 ± 0.04 | |
| (B) | 0.74 ± 0.04 | 0.85 ± 0.03 | 0.96 ± 0.04 | 1.05 ± 0.03 | 1.14 ± 0.03 | 1.23 ± 0.03 | 1.33 ± 0.04 | ||
| 0.2 bar | 5 min | (A) | 0.84 ± 0.04 | 0.98 ± 0.08 | 1.10 ± 0.10 | 1.22 ± 0.11 | 1.31 ± 0.12 | 1.43 ± 0.10 | 1.51 ± 0.06 |
| (B) | 0.75 ± 0.04 | 0.86 ± 0.06 | 0.98 ± 0.06 | 1.08 ± 0.05 | 1.18 ± 0.04 | 1.28 ± 0.04 | 1.39 ± 0.04 | ||
| 10 min | (A) | 0.87 ± 0.04 | 0.98 ± 0.05 | 1.09 ± 0.07 | 1.22 ± 0.07 | 1.35 ± 0.08 | 1.48 ± 0.09 | 1.55 ± 0.05 | |
| (B) | 0.72 ± 0.08 | 0.85 ± 0.07 | 0.98 ± 0.07 | 1.10 ± 0.09 | 1.23 ± 0.10 | 1.35 ± 0.11 | 1.44 ± 0.08 | ||
| 15 min | (A) | 0.82 ± 0.06 | 0.93 ± 0.03 | 1.03 ± 0.02 | 1.13 ± 0.02 | 1.25 ± 0.05 | 1.38 ± 0.07 | 1.47 ± 0.04 | |
| (B) | 0.74 ± 0.06 | 0.85 ± 0.06 | 0.97 ± 0.07 | 1.07 ± 0.07 | 1.15 ± 0.07 | 1.24 ± 0.09 | 1.36 ± 0.11 | ||
| Vacuum Pretreatment Time | ||||||
|---|---|---|---|---|---|---|
| 0 min | 5 min | 10 min | 15 min | |||
| Vacuum intensity | 0.8 bar | (A) | 88.99 ± 0.42 | 88.65 ± 0.15 | 89.14 ± 0.72 | 87.77 ± 0.13 |
| (B) | 88.19 ± 2.26 | 86.68 ± 1.15 | 86.75 ± 1.27 | 86.09 ± 0.37 | ||
| 0.5 bar | (A) | 77.89 ± 0.69 | 77.12 ± 0.66 | 76.62 ± 0.34 | 76.95 ± 0.08 | |
| (B) | 78.23 ± 1.58 | 75.97 ± 0.50 | 76.18 ± 0.21 | 74.81 ± 2.75 | ||
| 0.2 bar | (A) | 54.93 ± 0.69 | 54.59 ± 0.19 | 54.4 ± 0.25 | 54.43 ± 0.50 | |
| (B) | 54.18 ± 1.93 | 53.82 ± 0.04 | 53.85 ± 1.51 | 53.81 ± 1.76 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joe, S.Y.; So, J.H.; Hwang, S.H.; Cho, B.-K.; Lee, W.-H.; Kang, T.; Lee, S.H. Application of Ohmic–Vacuum Combination Heating for the Processing of Senior-Friendly Food (Multiphase Food): Experimental Studies and Numerical Simulation. Foods 2021, 10, 138. https://doi.org/10.3390/foods10010138
Joe SY, So JH, Hwang SH, Cho B-K, Lee W-H, Kang T, Lee SH. Application of Ohmic–Vacuum Combination Heating for the Processing of Senior-Friendly Food (Multiphase Food): Experimental Studies and Numerical Simulation. Foods. 2021; 10(1):138. https://doi.org/10.3390/foods10010138
Chicago/Turabian StyleJoe, Sung Yong, Jun Hwi So, Seon Ho Hwang, Byoung-Kwan Cho, Wang-Hee Lee, Taiyoung Kang, and Seung Hyun Lee. 2021. "Application of Ohmic–Vacuum Combination Heating for the Processing of Senior-Friendly Food (Multiphase Food): Experimental Studies and Numerical Simulation" Foods 10, no. 1: 138. https://doi.org/10.3390/foods10010138
APA StyleJoe, S. Y., So, J. H., Hwang, S. H., Cho, B.-K., Lee, W.-H., Kang, T., & Lee, S. H. (2021). Application of Ohmic–Vacuum Combination Heating for the Processing of Senior-Friendly Food (Multiphase Food): Experimental Studies and Numerical Simulation. Foods, 10(1), 138. https://doi.org/10.3390/foods10010138







