Antimicrobial, Shelf-Life Stability, and Effect of Maltodextrin and Gum Arabic on the Encapsulation Efficiency of Sugarcane Bagasse Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sugarcane Bagasse Characterization
2.3. SCB Extracts
2.4. SCB Lyophilized Powder
2.5. Total Phenolic Content
2.6. DPPH Scavenging Activity
2.7. Ferric Reducing Antioxidant Power (FRAP)
2.8. Superoxide Radical Scavenging (SOD) Method
2.9. Liquid Chromatography-Mass Spectrometry
2.10. Antimicrobial Activity from Sugarcane Bagasse Extracts
2.11. Microencapsulation of Bioactive Compounds
2.12. Microencapsulation Efficiency
2.13. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of Sugarcane Bagasse
3.2. Total Phenolic Content
3.3. Antioxidant Activity of SCB Extracts
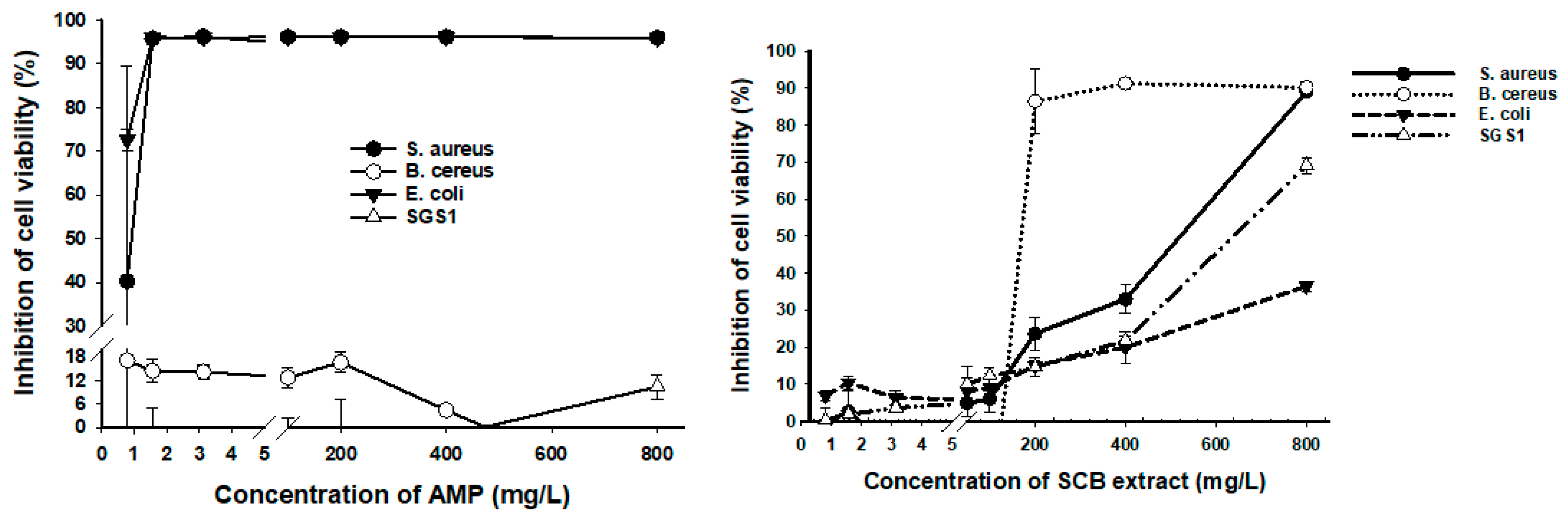
3.4. Antimicrobial Activity of SCB Extracts
3.5. Identification of Bioactive Compounds in Sugarcane Bagasse with HPLC-MS.
3.6. Microencapsulation of Bioactive Compounds
3.7. Shelf-Life Stability of SCB Extracts
3.8. Shelf-Life Stability of Free and Microencapsulated Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado, E.; Reyes-Jaquez, D. Extruded Aquaculture Feed: A Review. In Extrusion of Metals, Polymers and Food Products; IntechOpen: London, UK, 2017; pp. 145–163. [Google Scholar] [CrossRef]
- Araiza-Rosales, E.E.; Delgado-Licón, E.; Carrete-Carreón, F.O.; Medrano-Roldán, H.; Solís-Soto, A.; Rosales-Serna, R.; Haubi-Segura, C.U. Fermentative and nutritional quality of maíz silage complemented with apple and molasses. Ecosistemas Y Recur. Agropecu. 2015, 2, 255–267. [Google Scholar]
- Delgado, E.; Vences-Montaño, M.I.; Rodriguez, J.; Rocha-Guzmán, N.E.; Rodríguez-Vidal, A.; Herrera-González, S.; Medrano-Roldán, H.; Solís-Soto, A.; Ibarra-Pérez, F.J. Inhibition of the growth of rats by extruded snacks from bean (Phaseolus vulgaris) and corn (Zea mays). Emir. J. Food Agric. 2012, 24, 255–263. [Google Scholar]
- Navarro-Cortez, R.O.; Gómez-Aldapa, C.A.; Aguilar-Palazuelos, E.; Delgado-Licon, E.; Rosas, J.C.; Hernández-Ávila, J.; Solís-Soto, A.; Ochoa-Martínez, L.A.; Medrano-Roldán, H. Blue corn (Zea mays L.) with added orange (Citrus sinensis) fruit bagasse: Novel ingredients for extruded snacks. Cyta J. Food 2016, 14, 349–358. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.R. Climate change and sugarcane production: Potential impact and mitigation strategies. Int. J. Agron. 2015. [Google Scholar] [CrossRef]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y.; et al. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.G.; Souza, A.G.; Chiavelli, L.U.R.; Ruiz, A.L.T.G.; Carvalho, J.E.; Pomini, A.M.; Silva, C.C. Phenolic compounds and anticancer activity of commercial sugarcane cultivated in Brazil. An. Acad. Bras. Ciências 2016, 88, 1201–1209. [Google Scholar] [CrossRef]
- Lacerda-Bezerra, T.; Ragauskas, A.J. A review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuel Bioprod. Bior. 2016, 10, 634–647. [Google Scholar] [CrossRef]
- Feng, S.; Luo, Z.; Zhang, Y.; Zhong, Z.; Lu, B. Phytochemical contents and antioxidant capacities of different parts of two sugarcane (Saccharum officinarum L.) cultivars. Food Chem. 2014, 151, 452–458. [Google Scholar] [CrossRef]
- Singh, A.; Lal, U.R.; Mukhtar, H.M.; Singh, P.S.; Shah, G.; Dhawan, R.K. Phytochemical profile of sugarcane and its potential health aspects. Pharm. Rev. 2015, 9. [Google Scholar] [CrossRef]
- Zheng, R.; Su, S.; Zhoua, H.; Yan, H.; Ye, J.; Zhao, Z.; You, L.; Fua, X. Antioxidant/antihyperglycemic activity of phenolics from sugarcane (Saccharum officinarum L.) bagasse and identification by UHPLC-HR-TOFMS. Ind. Crop. Prod. 2017, 101, 104–114. [Google Scholar] [CrossRef]
- Beekmann, K.; de Haan, L.H.; Actis-Goretta, L.; van Bladeren, P.J.; Rietjens, I.M. Effect of glucuronidation on the potential of kaempferol to inhibit serine/threonine protein kinases. J. Agric. Food Chem. 2016, 64, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Mehrbod, P.; Abdalla, M.A.; Fotouhi, F.; Heidarzadeh, M.; Aro, A.O.; Eloff, J.N.; McGaw, L.J.; Fasina, F.O. Immunomodulatory properties of quercetin-3-O-α-L-rhamnopyranoside from Rapanea melanophloeos against influenza a virus. BMC Complement. Altern. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Afanasjeva, N. Obtaining phenolic compounds by sugar cane bagasse pyrolysis and its antioxidant capacity measured through chemical and electrochemical methods. J. Phys. Conf. Ser. 2018, 1119. [Google Scholar] [CrossRef]
- Quiroz, J.Q.; Velazquez, V.; Corrales-Garcia, L.L.; Torres, J.D.; Delgado, E.; Ciro, G.; Rojas, J. Use of plant proteins as microencapsulating agents of bioactive compounds extracted from annatto seeds (Bixa orellana L.). Antioxidants 2020, 9, 310. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-drying technique for microencapsulation of Elsholtzia ciliata ethanolic extract using different coating materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Januário, J.G.B.; dos Santos, S.S.; Bergamasco, R.; Madrona, G.S. Microcapsules of ‘jabuticaba’ byproduct: Storage stability and application in gelatin. Rev. Bras. Eng. Agríc. Ambient. 2018, 22, 424–429. [Google Scholar] [CrossRef]
- Wang, B.N.; Liu, H.F.; Zheng, J.B.; Fan, M.T.; Cao, W. Distribution of phenolic acids in different tissues of jujube and their antioxidant activity. J. Agric. Food Chem. 2011, 59, 1288–1292. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Milac, T.I.; Randolph, T.W.; Wang, P. Analyzing LC-MS/MS data by spectral count and ion abundance: Two case studies. Stat. Interface 2012, 5, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J.W. The role of the signal-to-noise ratio in precision and accuracy. Lc Gc Eur. 2006, 19, 12–16. [Google Scholar]
- Agarwal, D.; Mui, L.; Aldridge, E.; Mottram, R.; McKinney, J.; Fisk, I.D. The impact of nitrogen gas flushing on the stability of seasonings: Volatile compounds and sensory perception of cheese & onion seasoned potato crisps. Food Funct. 2018. [Google Scholar] [CrossRef]
- Van Saun, R.J. Determining Forage Quality: Understanding Feed Analysis. Available online: https://extension.psu.edu/determining-forage-quality-understanding-feed-analysis (accessed on 12 December 2019).
- Delgado, E.; Valverde-Quiroz, L.; Lopez, D.; Cooke, P.H.; Valles-Rosales, D.J.; Flores, N. Characterization of soluble glandless cottonseed meal proteins based on electrophoresis, functional properties, and microscopic structure. J. Food Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.; Martins, G.F.; Antunes, P.A.; Conrrado, R.; Pasquini, D.; Job, A.E.; Curvelo, A.A.S.; Ferreira, M.; Riul, A.; Constantino, C.J.L. Lignin from sugar cane bagasse: Extraction, fabrication of nanostructured films, and application. Langmuir 2007, 23, 6652–6659. [Google Scholar] [CrossRef]
- Reyes-Jáquez, D.; Casillas, F.; Flores, N.; Cooke, P.; Andrade-González, I.; Solís-Soto, A.; Medrano-Roldán, H.; Carrete, F.; Delgado, E. Effect of glandless cottonseed meal content on the microstructure of extruded corn-based snacks. Adv. Food Sci. 2014, 36, 125–130. [Google Scholar]
- Molina-Cortés, A.; Sánchez-Motta, T.; Tobar-Tosse, F.; Quimbaya, M. Spectrophotometric estimation of total phenolic content and antioxidant capacity of molasses and vinasses generated from the sugarcane industry. Waste Biomass Valorization 2019. [Google Scholar] [CrossRef]
- Iqbal, M.; Qamar, M.A.; Bokhari, T.H.; Abbas, M.; Hussain, F.; Masood, N.; Keshavarzi, A.; Qureshi, N.; Nazir, A. Total phenolic, chromium contents and antioxidant activity of raw and processed sugars. Inf. Process. Agric. 2017, 4. [Google Scholar] [CrossRef]
- Damián-Reyna, A.A.; González-Hernández, J.C.; Maya-Yescas, R.; de Jesús Cortés-Penagos, C.; del Carmen Chávez-Parga, M.D. Polyphenolic content and bactericidal effect of Mexican citrus limetta and citrus reticulata. J. Food Sci. Technol. 2017, 54, 531–537. [Google Scholar] [CrossRef]
- Trošt, K.; Klančnik, A.; Vodopivec, B.M.; Lemut, M.S.; Novšak, K.J.; Raspor, P.; Možina, S.S. Polyphenol, antioxidant and antimicrobial potential of six different white and red wine grape processing leftovers. J. Sci. Food Agric. 2016, 4809–4820. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Novoa, A.V.; Linares, A.F.; Lajolo, F.M.; Genovese, M.I. Antioxidant activity of phenolics compounds from sugar cane (Saccharum officinarum L.) juice. Plant Foods Hum. Nutr. 2006, 61, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wu, C.; Wang, M. The jujube (Ziziphus Jujuba Mill.) Fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.T.; Tu, H.C.; Chang, W.N.; Chen, B.H.; Shi, Y.Y.; Chang, T.C.; Chen, B.H.; Shi, Y.Y.; Chang, T.C.; Fu, T.F. Grape seed extract inhibits the growth and pathogenicity of Staphylococcus aureus by interfering with dihydrofolate reductase activity and folate-mediated one-carbon metabolism. Int. J. Food Microbiol. 2010, 141, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.D.; Han, W.J.; Rui, Y.; Dai, Y.; Xia, Y.F. In vivo disposition and metabolism of madecassoside, a major bioactive constituent in Centella asiatica. J. Ethnopharmacol. 2013, 150, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Bhardwaj, R.; Sharma, P.; Yadav, A.; Singh, B. Antimicrobial activity of sennosides from Cassia pumila Lamk. J. Med. Plants Res. 2012, 6, 3591–3595. [Google Scholar] [CrossRef]
- Arul, A.B.; Paulraj, M.G.; Ignacimuthu, S.; Al-Numair, K.S. Cancer chemopreventive potential of luteolin-7-O-glucoside isolated from Ophiorrhiza mungos. Nutr. Cancer 2011, 63, 130–138. [Google Scholar] [CrossRef]
- Boubaker, J.; Bhouri, W.; Sghaier, B.; Ghedira, K.; Dijoux, F.M.G.; Chekir-Ghedira, L. Ethyl acetate extract and its major constituent, isorhamnetin 3-O-rutinoside, from Nitraria retusa leaves, promote apoptosis of human myelogenous erythroleukaemia cells. Cell Prolif. Basic Clin. Sci. 2011, 44. [Google Scholar] [CrossRef]
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A novel potential natural anticancer agent. Avicenna J. Phytomed. 2017, 7, 285–294. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. J. Food Sci. Technol. 2005, 41, 1–21. [Google Scholar] [CrossRef]
- Franceschinis, L.; Salvatori, D.M.; Sosa, N.; Schebor, C. Physical and functional properties of blackberry freeze- and spray-dried powders. Dry Technol. 2014, 32. [Google Scholar] [CrossRef]
- Farías-Cervantes, V.S.; Chávez-Rodríguez, A.; Delgado-Licon, E.; Aguilar, J.; Medrano-Roldan, H.; Andrade-González, I. Effect of spray drying of agave fructans, nopal mucilage and aloe vera juice. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Gutiérrez-Lomelí, M.; Lugo-Cervantes, E.; Zurita, F.; Robles-García, M.A.; Ruiz-Cruz, S.; Aguilar, J.A.; Morales-Del Rio, J.A.; Guerrero-Medina, P.J. Storage effect on phenols and on the antioxidant activity of extracts from anemopsis californica and inhibition of elastase enzyme. J. Chem. 2015. [Google Scholar] [CrossRef]
- Ali, A.; Chong, C.H.; Mah, S.H.; Abdullah, L.C.; Choong, T.S.Y.; Chua, B.L. Impact of storage conditions on the stability of predominant phenolic constituents and antioxidant activity of dried piper betle extracts. Molecules 2018, 23, 484. [Google Scholar] [CrossRef]

| As Fed | Dry Matter | |
|---|---|---|
| Moisture % | 0.90 ± 0.07 | --- |
| Dry Matter % | 99.10 ± 0.07 | --- |
| Crude Protein % | 2.20 ± 0.35 | 2.20 ± 0.42 |
| Available Protein % | 1.40 ± 0.42 | 1.40 ± 0.42 |
| ADICP % | 0.80 ± 0.00 | 0.80 ± 0.00 |
| Adjusted Crude Protein % | 2.20 ± 0.35 | 2.20 ± 0.42 |
| ADF % | 55.30 ± 4.95 | 55.80 ± 4.95 |
| aNDF % | 81.90 ± 11.24 | 82.60 ± 11.31 |
| Crude Fat % | 0.50 ± 0.14 | 0.50 ± 0.14 |
| TDN % | 52.00 ± 0.71 | 52.00 ± 0.00 |
| NEL, Mcal/Lb | 0.19 ± 0.04 | 0.19 ± 0.04 |
| NEM, Mcal/Lb | 0.39 ± 0.02 | 0.40 ± 0.03 |
| NEG, Mcal/Lb | 0.15 ± 0.04 | 0.15 ± 0.04 |
| Calcium % | 0.07 ± 0.01 | 0.07 ± 0.00 |
| Phosphorus % | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Magnesium % | 0.04 ± 0.03 | 0.04 ± 0.03 |
| Potassium % | 0.20 ± 0.06 | 0.21 ± 0.05 |
| Sodium % | 0.006 ± 0.00 | 0.006 ± 0.00 |
| Iron ppm | 1110.00 ± 63.64 | 1120.00 ± 77.78 |
| Zinc ppm | 9.00 ± 0.71 | 9.00 ± 1.41 |
| Copper ppm | 4.00 ± 0.71 | 4.00 ± 0.71 |
| Manganese ppm | 36.00 ± 1.41 | 36.00 ± 2.12 |
| Molybdenum ppm | 8.20 ± 0.85 | 8.30 ± 0.92 |
| Sulfur % | 0.04 ± 0.00 | 0.04 ± 0.00 |
| Ash % | 4.47 ± 0.71 | 4.51 ± 0.00 |
| Soluble Protein % CP | --- | 42.00 ± 3.54 |
| NFC % | 10.00 ± 0.85 | 10.10 ± 0.85 |
| Assay | Fresh Extract | Extract after 30 Days of Storage | Statistical Difference | T Statistic |
|---|---|---|---|---|
| DPPH (%) | 71.32 ± 1.47 * | 78.71 ± 1.16 | −7.39 ± 2.04 | −10.84 |
| FRAP (µmol/g) | 941.61 ± 7.03 | 1005.50 ± 10.53 | −44.52 ± 30.82 | −4.33 |
| SOD (%) | 27.10 ± 2.32 | 29.69 ± 3.04 | −2.59 ± 3.71 | −2.09 |
| Biocompound | m/z Similarity | Mass (Da) | AA | RSD (%) | Classification |
|---|---|---|---|---|---|
| Isorhamnetin-3-O-rutinoside | 979 | 623.42 | 651 | 0.2 | Flavonoids |
| Madecassoside | 999 | 973.39 | 469 | 5.4 | Terpenes |
| Sennoside B | 999 | 861.28 | 443 | 5.7 | Anthranoids |
| Luteolin-7-O-glucoside (Cynaroside) | 969 | 447.34 | 260 | 9.6 | Glycosyloxyflavones |
| Kaempferol-3-Glucuronide | 968 | 461.33 | 135 | 0.4 | Flavonoids |
| Phosphatidylcholine 18 | 1000 | 842.65 | 134 | 0.4 | Phosphatidylcholines |
| Hesperidin | 983 | 609.41 | 119 | 1.6 | Flavonoids |
| Thalsimidine | 993 | 621.4 | 104 | 1.7 | Alkaloids |
| Isorhamnetin-3-O-galactoside-6″-rhamnoside | 987 | 623.36 | 93 | 0.5 | Flavonoids |
| (-)-Podophyllotoxin | 983 | 459.31 | 72 | 0.7 | Lignans |
| Speciofiline (Uncarine F) | 999 | 367.21 | 56 | 2.7 | Alkaloids |
| Gardnerine | 992 | 369.31 | 44 | 1.1 | Alkaloids |
| Licoricesaponin G2 | 998 | 837.51 | 30 | 1.9 | Saponiin |
| Pseudojervine | 999 | 632.39 | 28 | 5 | Alkaloids |
| Ginsenoside Rb1 | 994 | 1107.36 | 26 | 2.6 | Saponin |
| Saikosaponin a | 833 | 778.52 | 23 | 2.1 | Saponin |
| EE (%) | FRAP (µmol/gr) | DPPH Inhibition (%) | ||||
|---|---|---|---|---|---|---|
| Coefficient | p-Value * | Coefficient | p-Value * | Coefficient | p-Value * | |
| X1 | 43.73 | <0.0001 | 104.9 | 0.0004 | 30.24 | 0.0003 |
| X2 | 83.11 | <0.0001 | 107.53 | 0.0029 | 35.52 | 0.0002 |
| X1 X2 | 48.22 | <0.0001 | −39.95 | 0.0101 | −6.36 | 0.2736 |
| X1X2(X1-X2) | 49.30 | 0.0057 | −111.02 | 0.0006 | −38.13 | 0.0032 |
| SD | 2.83 | 4.56 | 1.98 | |||
| R2 | 0.9644 | 0.8274 | 0.8331 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velazquez-Martinez, V.; Valles-Rosales, D.; Rodriguez-Uribe, L.; Holguin, O.; Quintero-Quiroz, J.; Reyes-Jaquez, D.; Rodriguez-Borbon, M.I.; Villagrán-Villegas, L.Y.; Delgado, E. Antimicrobial, Shelf-Life Stability, and Effect of Maltodextrin and Gum Arabic on the Encapsulation Efficiency of Sugarcane Bagasse Bioactive Compounds. Foods 2021, 10, 116. https://doi.org/10.3390/foods10010116
Velazquez-Martinez V, Valles-Rosales D, Rodriguez-Uribe L, Holguin O, Quintero-Quiroz J, Reyes-Jaquez D, Rodriguez-Borbon MI, Villagrán-Villegas LY, Delgado E. Antimicrobial, Shelf-Life Stability, and Effect of Maltodextrin and Gum Arabic on the Encapsulation Efficiency of Sugarcane Bagasse Bioactive Compounds. Foods. 2021; 10(1):116. https://doi.org/10.3390/foods10010116
Chicago/Turabian StyleVelazquez-Martinez, Victor, Delia Valles-Rosales, Laura Rodriguez-Uribe, Omar Holguin, Julian Quintero-Quiroz, Damian Reyes-Jaquez, Manuel Ivan Rodriguez-Borbon, Luz Yazmin Villagrán-Villegas, and Efren Delgado. 2021. "Antimicrobial, Shelf-Life Stability, and Effect of Maltodextrin and Gum Arabic on the Encapsulation Efficiency of Sugarcane Bagasse Bioactive Compounds" Foods 10, no. 1: 116. https://doi.org/10.3390/foods10010116
APA StyleVelazquez-Martinez, V., Valles-Rosales, D., Rodriguez-Uribe, L., Holguin, O., Quintero-Quiroz, J., Reyes-Jaquez, D., Rodriguez-Borbon, M. I., Villagrán-Villegas, L. Y., & Delgado, E. (2021). Antimicrobial, Shelf-Life Stability, and Effect of Maltodextrin and Gum Arabic on the Encapsulation Efficiency of Sugarcane Bagasse Bioactive Compounds. Foods, 10(1), 116. https://doi.org/10.3390/foods10010116










