Biocompatible Inorganic PVD MeSiON Thin Films (Me = Cr or Zr) Used to Enhance the Bond Strength Between NiCr-Based Metallic Frameworks and Ceramic in Dental Restorations
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. NiCr Dental Alloy Characterization
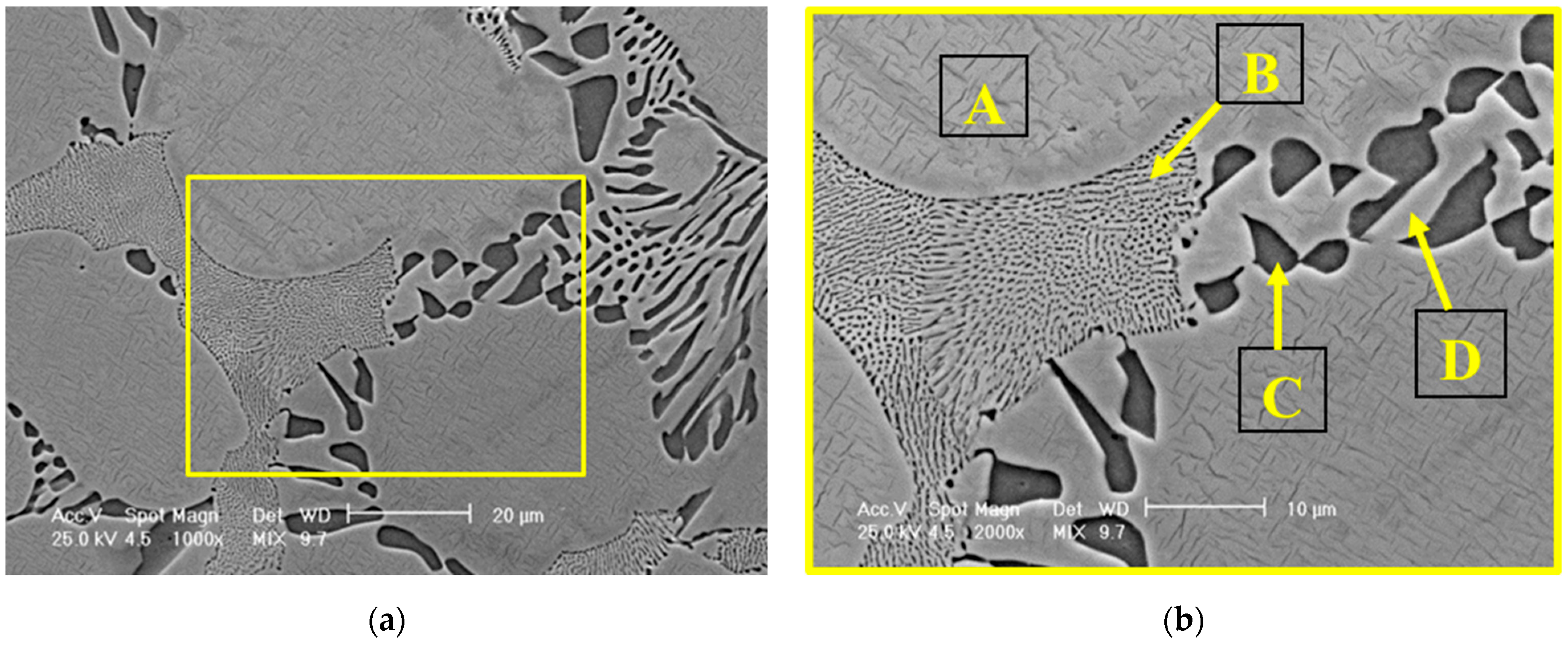
3.1.1. Surface Morphology and Elemental Composition
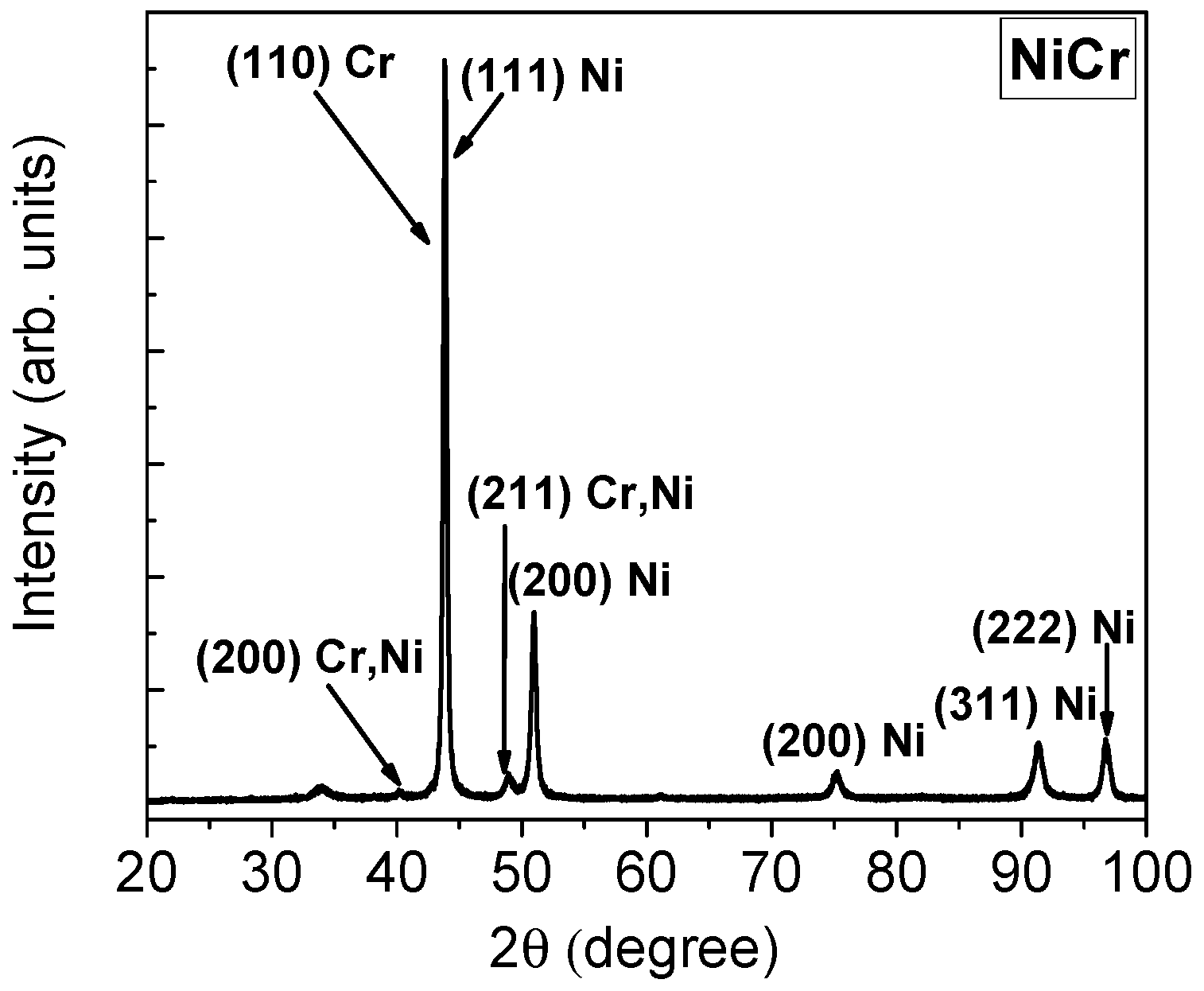
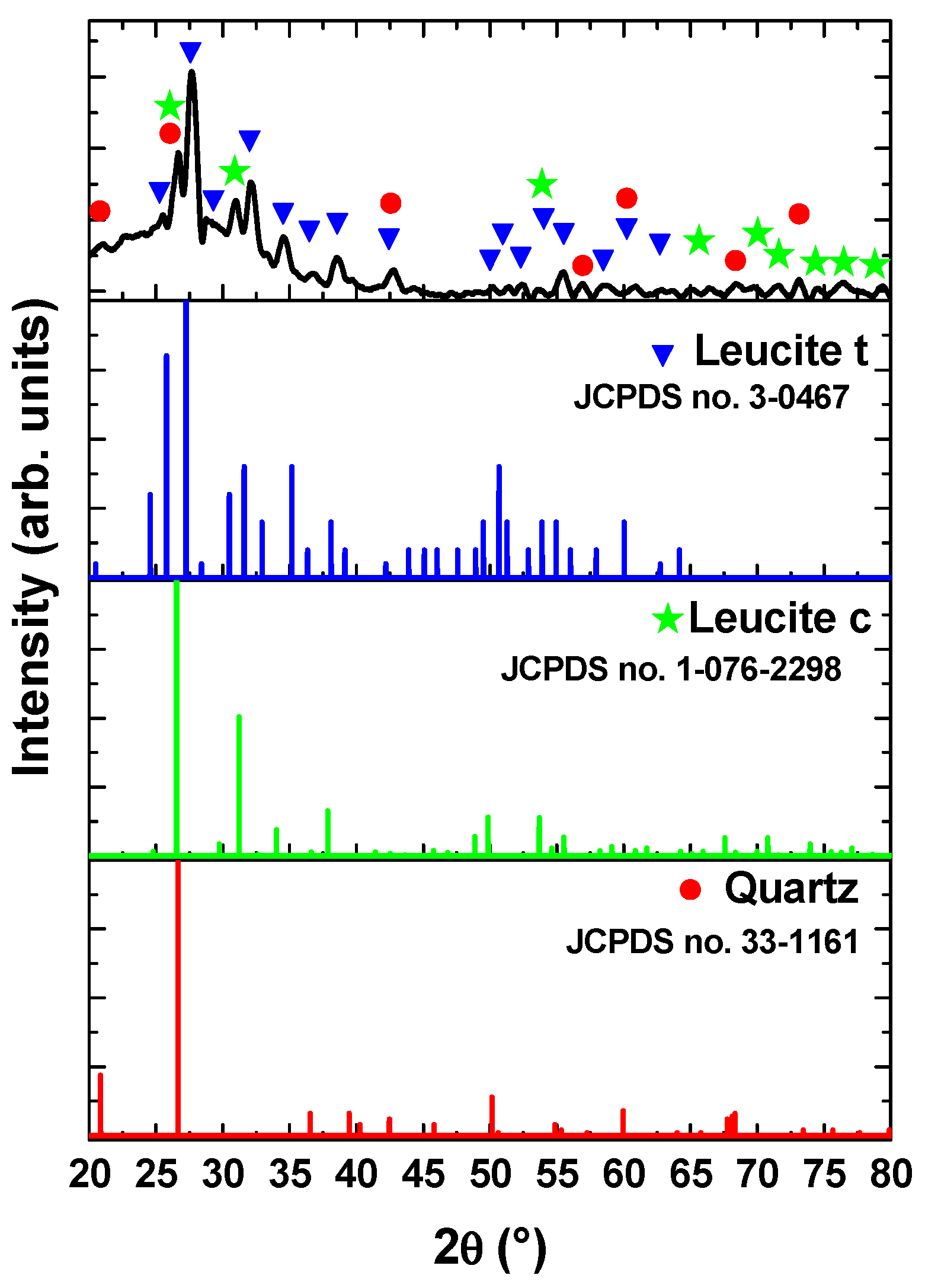
3.1.2. Phase Composition
3.2. Thin Films Characterization
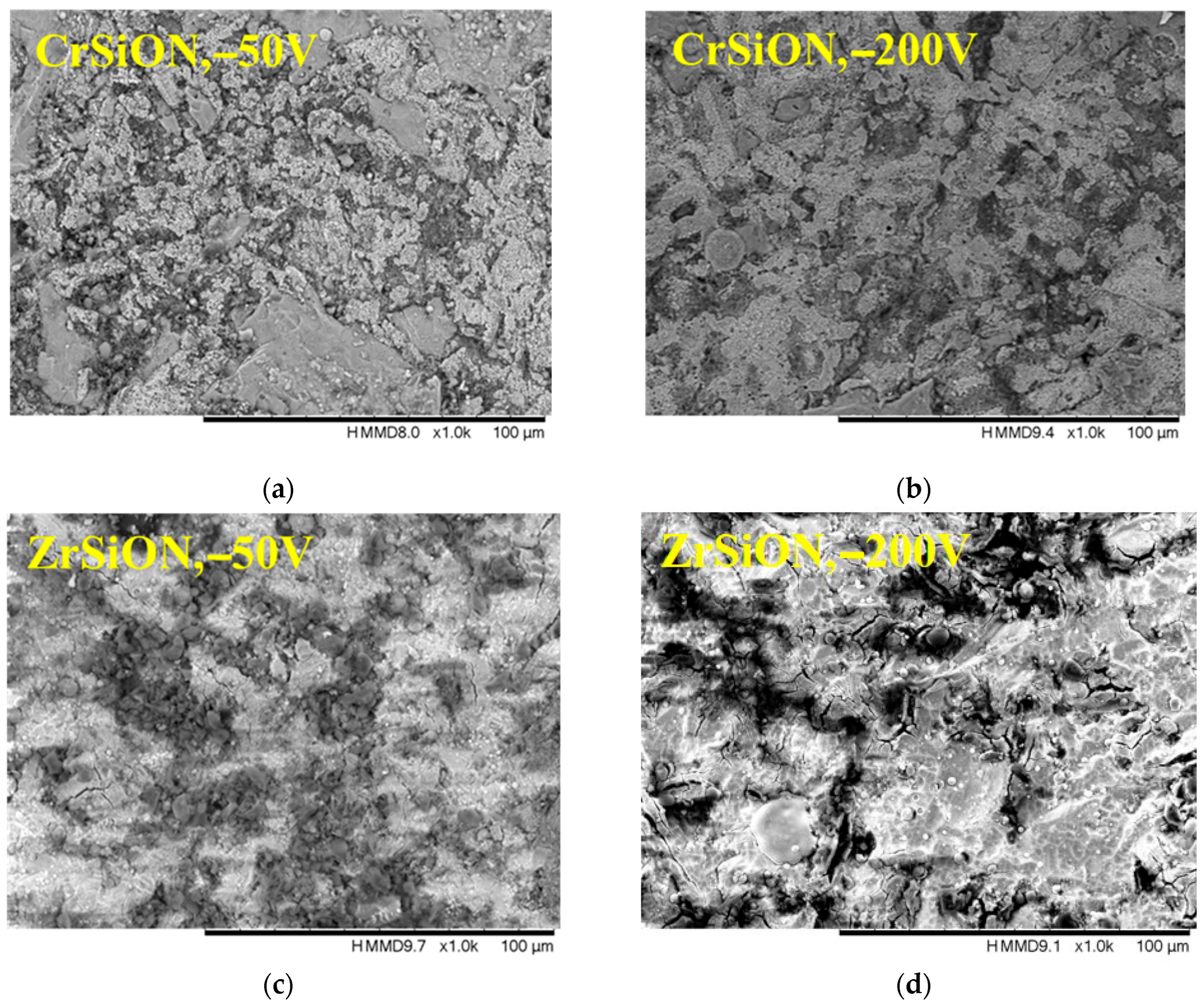
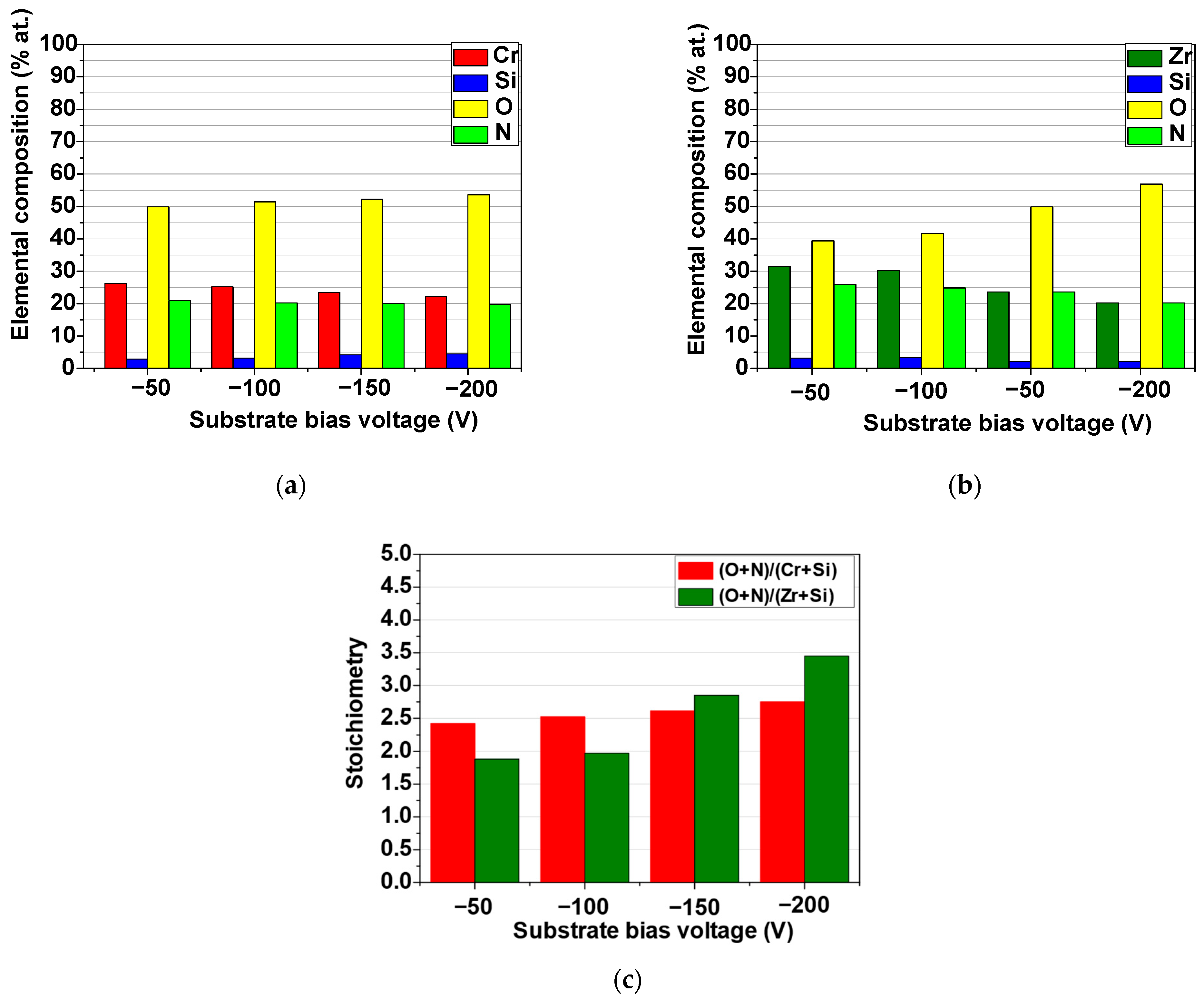
3.2.1. Surface Morphology and Elemental Composition
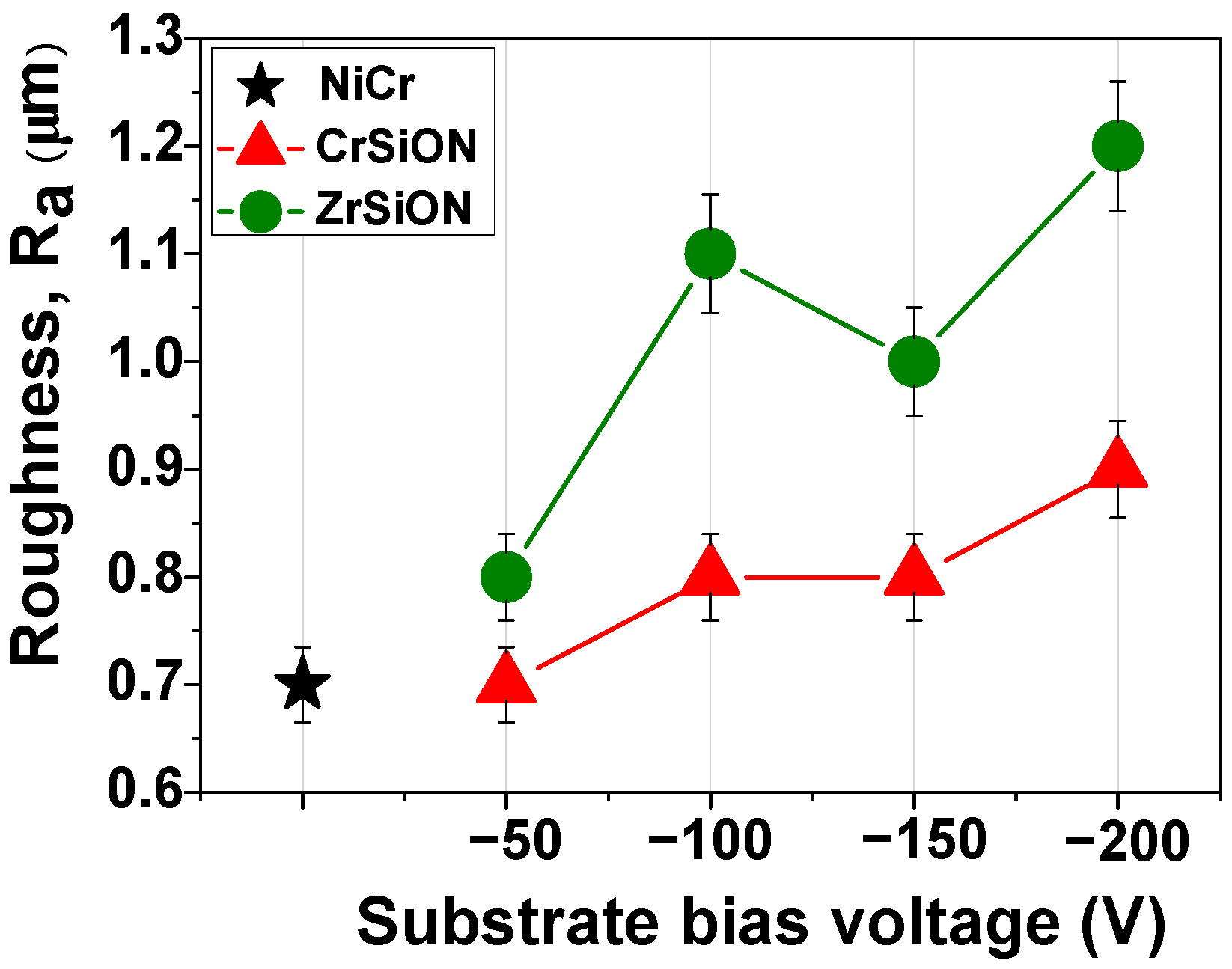
3.2.2. Roughness
3.2.3. Wettability
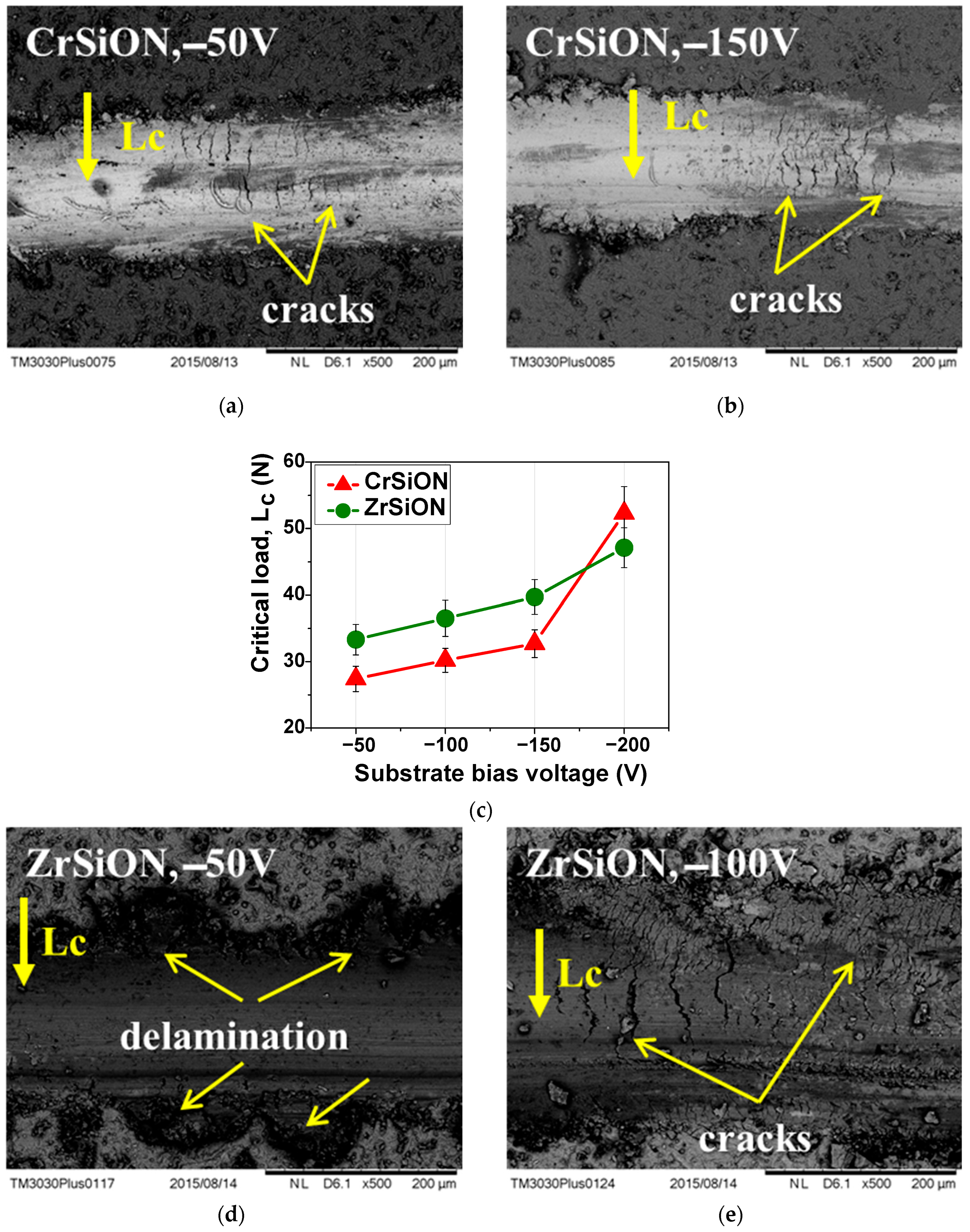
3.2.4. Adhesion to the NiCr Substrate
3.3. Dental Ceramic Characterization
3.3.1. Surface Morphology and Elemental Composition
3.3.2. Phase Composition
3.4. Metal-MeSiON-Ceramic Characterization
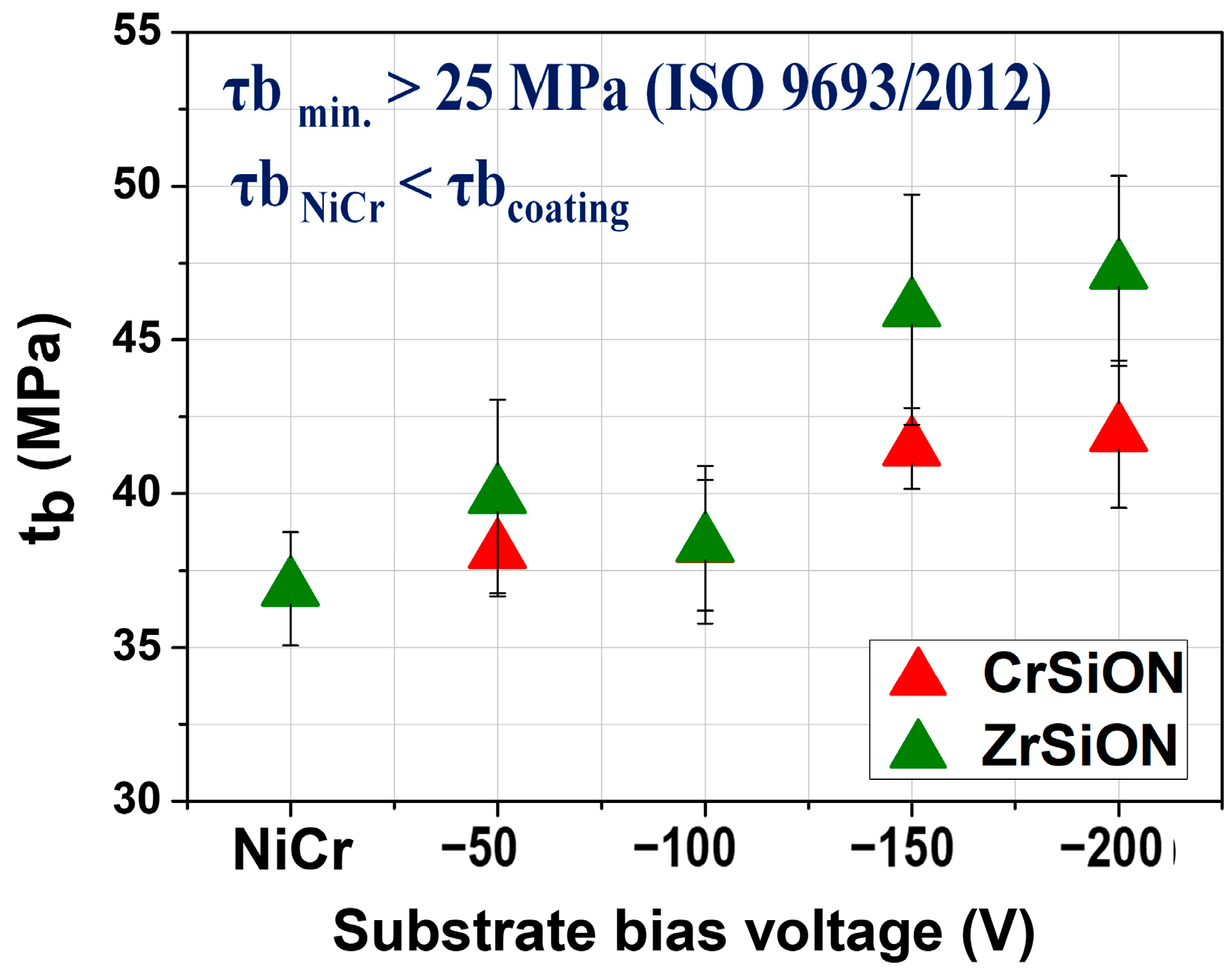
3.4.1. 3-Point Bending Test
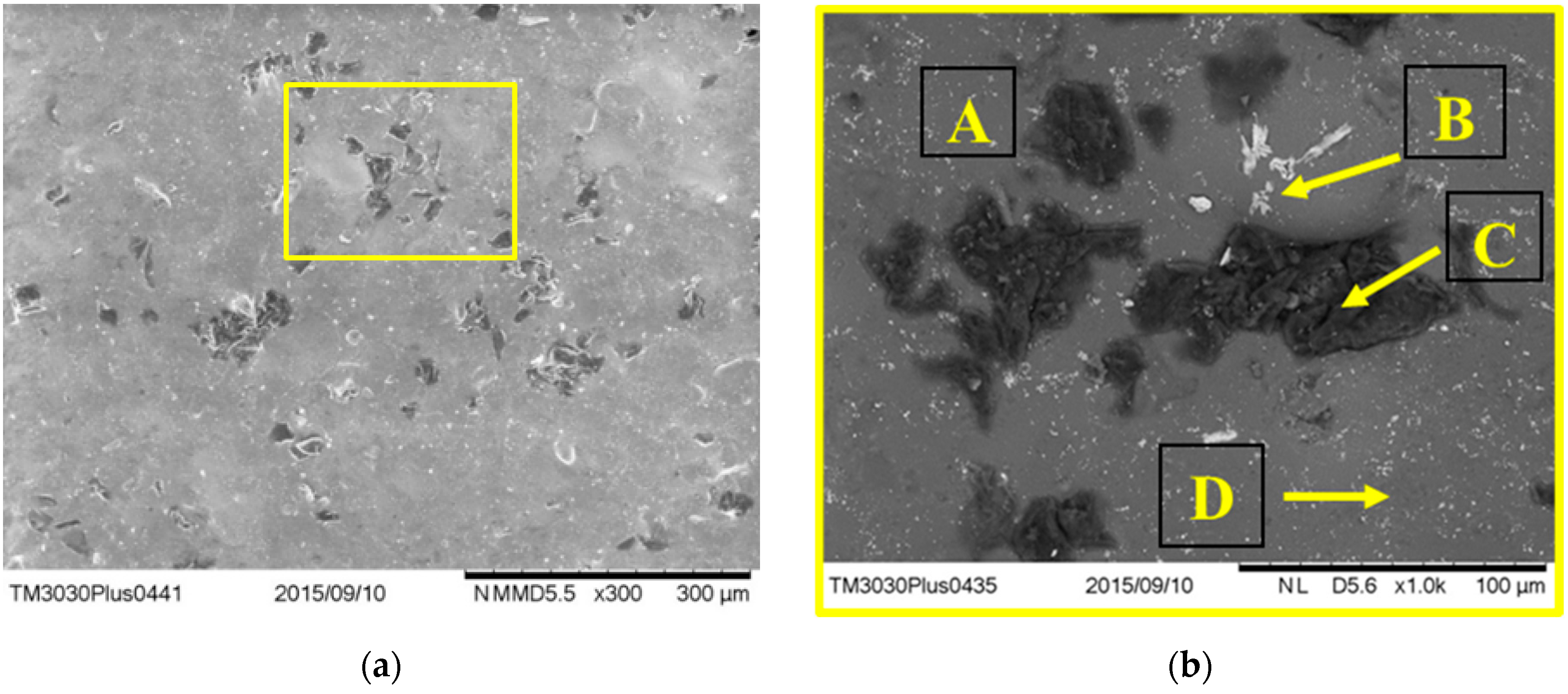
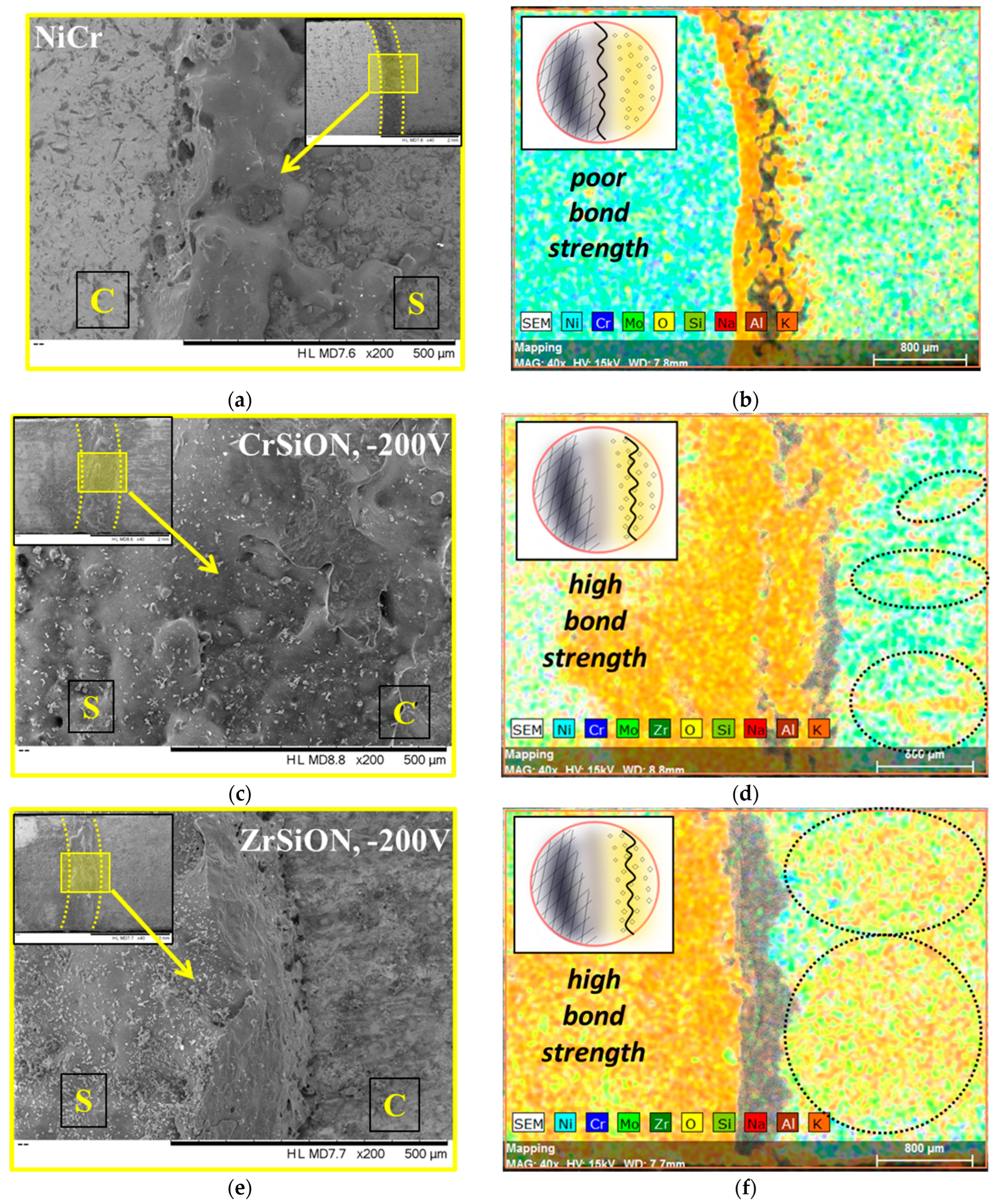
3.4.2. Surface Morphology of Debonded Plates
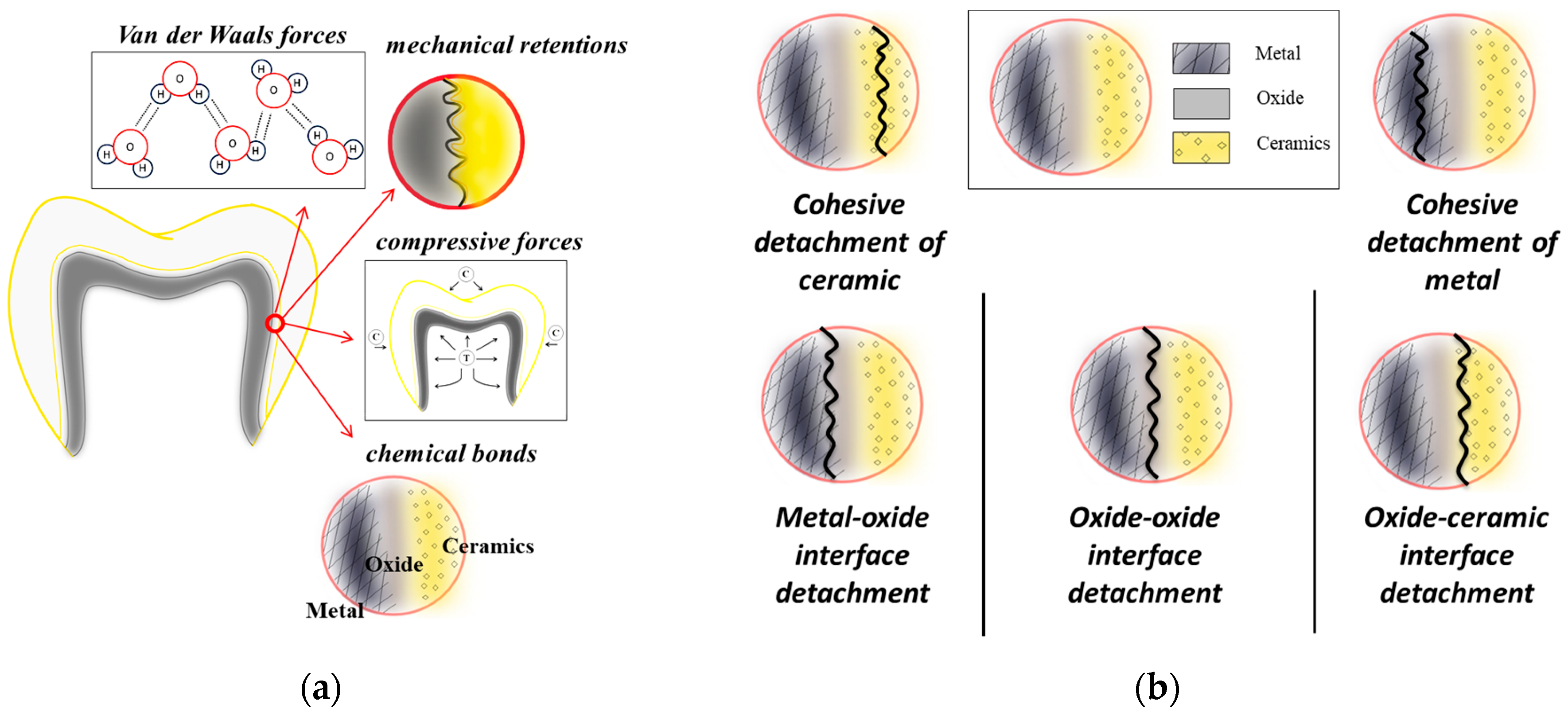
4. Discussion
Limitations
- In vitro conditions: All experiments were conducted under controlled in vitro laboratory conditions, which do not fully replicate the complex physiological environment of the oral cavity. Critical factors such as the presence of saliva, fluctuating pH levels, thermal cycling, and mechanical fatigue were not simulated, although they significantly influence the long-term clinical performance of the proposed coatings.
- Absence of long-term performance evaluation: While initial bond strength was assessed using standardized mechanical tests according to ISO 9693:2000 [18], the study did not include evaluations of long-term durability, fatigue resistance, or aging behavior. Parameters such as thermocycling, corrosive degradation, and repetitive loading were not addressed, thus limiting the predictive capacity regarding the coatings’ performance over time in the clinical environment.
- Lack of biological testing: Although CrSiON and ZrSiON coatings are considered biocompatible based on their composition and supporting literature, no experimental assessments of cytotoxicity, cell adhesion, or other biological tests were performed in the present study. Therefore, the biological safety and compatibility of these coatings with oral tissue remain to be experimentally assessed. Future studies should include comprehensive biological evaluations to validate the clinical applicability of these coatings.
5. Conclusions
- Thin films of (Cr,Zr)SiON deposited by cathodic arc evaporation significantly improved the bond strength at the metal–ceramic interface in dental restorations.
- Increasing the substrate bias voltage, particularly to −200 V, resulted in denser and more uniform coatings, which exhibited enhanced surface roughness, adhesion, and hydrophilicity.
- ZrSiON coatings demonstrated a superior performance, as evidenced by a higher ceramic coverage area after debonding and an approximate 28% increase in bond strength at −200 V, compared to a ~14% improvement for CrSiON under the same conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawod, N.; Miculescu, M.; Antoniac, I.V.; Miculescu, F.; Agop-Forna, D. Metal–Ceramic Compatibility in Dental Restorations According to the Metallic Component Manufacturing Procedure. Materials 2023, 16, 5556. [Google Scholar] [CrossRef]
- Daou, E. Bonding Mechanism of Porcelain to Frameworks: Similarities and Dissimilarities between Metal and Zirconia. Br. J. Med. Med. Res. 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Czepułkowska, W.; Wołowiec-Korecka, E.; Klimek, L. The Role of Mechanical, Chemical and Physical Bonds in Metal-Ceramic Bond Strength. Arch. Mater. Sci. Eng. 2018, 1, 5–14. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Mu, Y.; Shao, S.; Wick, C.D.; Ramachandran, B.R.; Meng, W.J. Mechanical Failure of Metal/Ceramic Interfacial Regions under Shear Loading. Acta Mater. 2017, 138, 224–236. [Google Scholar] [CrossRef]
- Özcan, M. Fracture Reasons in Ceramic-fused-to-metal Restorations. J. Oral Rehabil. 2003, 30, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Galiatsatos, P.; Galiatsatos, A.; Asproudi, G. Research on the Role of Surface Treatment of the Metal Surface on the Strength of the Metal–Ceramic Bond. J. Contemp. Dent. Pract. 2023, 24, 188–194. [Google Scholar] [CrossRef]
- Mani, G.; Porter, D.; Collins, S.; Schatz, T.; Ornberg, A.; Shulfer, R. A Review on Manufacturing Processes of cobalt-chromium Alloy Implants and Its Impact on Corrosion Resistance and Biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2024, 112, e35431. [Google Scholar] [CrossRef]
- Ladani, L.; Palmieri, M. Review of the Use of Metals in Biomedical Applications: Biocompatibility, Additive Manufacturing Technologies, and Standards and Regulations. Metals 2024, 14, 1039. [Google Scholar] [CrossRef]
- Ramírez-Ledesma, A.L.; Roncagliolo, P.; Álvarez-Pérez, M.A.; Lopez, H.F.; Juárez-Islas, J.A. Corrosion Assessment of an Implantable Dental Co-Cr Alloy in Artificial Saliva and Biocompatibility Behavior. J. Mater. Eng. Perform. 2020, 29, 1657–1670. [Google Scholar] [CrossRef]
- Tomova, Z.; Vlahova, A.; Zlatev, S.; Stoeva, I.; Tomov, D.; Davcheva, D.; Hadzhigaev, V. Clinical Evaluation of Corrosion Resistance, Ion Release, and Biocompatibility of CoCr Alloy for Metal-Ceramic Restorations Produced by CAD/CAM Technologies. Dent. J. 2023, 11, 166. [Google Scholar] [CrossRef]
- Achitei, D.C.; Baltatu, M.S.; Vizureanu, P.; Sandu, A.V.; Benchea, M.; Istrate, B. Ni-Cr Alloys Assessment for Dental Implants Suitability. Appl. Sci. 2022, 12, 12814. [Google Scholar] [CrossRef]
- Neto, H.G.; Cândido, M.S.M.; Júnior, A.L.R.; Garcia, P.P.N.S. Analysis of Depth of the Microporosity in a Nickel–Chromium System Alloy—Effects of Electrolytic, Chemical and Sandblasting Etching. J. Oral Rehabil. 2003, 30, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, A.; Shetty, G. An In Vitro Study to Compare the Effect of Two Etching Techniques on the Tensile Bond Strength of Resin Cement Bonded to Base Metal Alloy and Enamel. J. Indian Prosthodont. Soc. 2013, 13, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Moslehifard, E.; Seyyedashrafi, M.M.; Khosronejad, N. Evaluation of Surface Roughness of a Ni-Cr Alloy Treated With the Nd/YAG Laser and the Sandblast Technique. J. Lasers Med. Sci. 2021, 12, e69. [Google Scholar] [CrossRef] [PubMed]
- Vladescu, A.; Dinu, M.; Braic, M.; Vitelaru, C.; Balaceanu, M.; Tarcolea, M.; Braic, V.; Baciu, F.; Cotrut, C.M. The Effect of TiSiN Interlayers on the Bond Strength of Ceramic to NiCr and CoCr Alloys. Ceram. Int. 2015, 41, 8051–8058. [Google Scholar] [CrossRef]
- ISO 6892-1:2019; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- ISO 9693-1:2012; Dentistry—Compatibility Testing—Part 1: Metal-Ceramic Systems. International Organization for Standardization (ISO): Geneva, Switzerland, 2012.
- ISO 9693:2000; Metal-Ceramic Dental Restorative Systems. International Organization for Standardization (ISO): Geneva, Switzerland, 2000.
- BS EN 1071-3:2005; Advanced Technical Ceramics. Methods of Test for Ceramic Coatings—Determination of Adhesion and Other Mechanical Failure Modes by a Scratch Test. British Standards Institution: London, UK, 2006.
- Ghasemi, A.; Pouranvari, M. Thermal Processing Strategies Enabling Boride Dissolution and Gamma Prime Precipitation in Dissimilar Nickel-Based Superalloys Transient Liquid Phase Bond. Mater. Des. 2019, 182, 108008. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, M.; Yang, G.; Liu, H.; Ye, H.; Deng, C.; Zhang, L. The Microstructure, Solidification Path, and Microhardness of As-Cast Ni-Al-Cr-Os Alloys in a Ni-Rich Region. Materials 2023, 16, 6777. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Grudzień-Rakoczy, M.; Cygan, R.; Kargul, T.; Maj, Ł.; Zielińska-Lipiec, A. Analysis of the As-Cast Microstructure and Properties of the Ni-Based Superalloy MAR-M247® Produced Via Directional Solidification. Metall. Mater. Trans. A 2023, 54, 3630–3652. [Google Scholar] [CrossRef]
- Mathieu, S.; Aranda, L.; Portebois, L.; Mathieu, S.; Vilasi, M. On the Pre-Oxidation Treatments of Four Commercial Ni-Based Superalloys in Air and in Ar–H2O at 950 °C. Oxid. Met. 2018, 90, 43–63. [Google Scholar] [CrossRef]
- Lu, H.; Yang, M.; Zhou, L.; Ma, Z.; Cui, B.; Yin, F.; Li, D. Effects of Heat Treatment on the Microstructure and Properties of a Cast Nickel-Based High-Cr Superalloy. Metals 2022, 12, 2176. [Google Scholar] [CrossRef]
- Yu, J.-M.; Kang, S.-Y.; Lee, J.-S.; Jeong, H.-S.; Lee, S.-Y. Mechanical Properties of Dental Alloys According to Manufacturing Process. Materials 2021, 14, 3367. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bowers, B.; Wolan, J.; Cai, Z.; Bumgardner, J. Metallurgical, Surface, and Corrosion Analysis of Ni–Cr Dental Casting Alloys before and after Porcelain Firing. Dent. Mater. 2008, 24, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yu, W.; Zhang, F.; Smales, R.J.; Zhang, Y.; Lu, C. Corrosion Behaviour and Surface Analysis of a Co–Cr and Two Ni–Cr Dental Alloys before and after Simulated Porcelain Firing. Eur. J. Oral Sci. 2011, 119, 93–101. [Google Scholar] [CrossRef]
- Dinu, M.; Hauffman, T.; Cordioli, C.; Vladescu, A.; Braic, M.; Hubin, A.; Cotrut, C.M. Protective Performance of Zr and Cr Based Silico-Oxynitrides Used for Dental Applications by Means of Potentiodynamic Polarization and Odd Random Phase Multisine Electrochemical Impedance Spectroscopy. Corros. Sci. 2017, 115, 118–128. [Google Scholar] [CrossRef]
- Muhammed, M.; Javidani, M.; Ebrahimi Sadrabadi, T.; Heidari, M.; Levasseur, T.; Jahazi, M. A Comprehensive Review of Cathodic Arc Evaporation Physical Vapour Deposition (CAE-PVD) Coatings for Enhanced Tribological Performance. Coatings 2024, 14, 246. [Google Scholar] [CrossRef]
- CHUN, S.-Y. Bias Voltage Effect on the Properties of TiN Films by Reactive Magnetron Sputtering. J. Korean Phys. Soc. 2010, 56, 1134–1139. [Google Scholar] [CrossRef]
- Niu, E.W.; Li, L.; Lv, G.H.; Chen, H.; Feng, W.R.; Fan, S.H.; Yang, S.Z.; Yang, X.Z. Influence of Substrate Bias on the Structure and Properties of ZrN Films Deposited by Cathodic Vacuum Arc. Mater. Sci. Eng. A 2007, 460–461, 135–139. [Google Scholar] [CrossRef]
- Ul-Hamid, A. The Effect of Deposition Conditions on the Properties of Zr-Carbide, Zr-Nitride and Zr-Carbonitride Coatings—A Review. Mater. Adv. 2020, 1, 988–1011. [Google Scholar] [CrossRef]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, K.S.; Taylor, M.; Castillo, A.; Cisneros-Zevallos, L.; Oh, J.K.; et al. Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef]
- Lingyu, S.; Guo, J.; Chen, H.; Zhang, D.; Shang, L.; Zhang, B.; Zhao, Y. Tailoring Materials with Specific Wettability in Biomedical Engineering. Adv. Sci. 2021, 8, 2100126. [Google Scholar] [CrossRef]
- Elias, C.; Oshida, Y.; Lima, J.; Muller, C. Relationship between Surface Properties (Roughness, Wettability and Morphology) of Titanium and Dental Implant Removal Torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Aslan, N.; Aksakal, B.; Cihangir, S.; Cetin, F.; Yilmazer, Y. ZrN and Ta-C Coatings on Titanium for Biomedical Applications: Improved Adhesion, Corrosion, Antibacterial Activity, and Cytotoxicity Properties. J. Mater. Res. 2023, 38, 3923–3936. [Google Scholar] [CrossRef]
- Hassan, M.A.; Bushroa, A.R.; Mahmoodian, R. Identification of Critical Load for Scratch Adhesion Strength of Nitride-Based Thin Films Using Wavelet Analysis and a Proposed Analytical Model. Surf. Coat. Technol. 2015, 277, 216–221. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Shtansky, D.V. Comparative Study of Electrochemical and Impact Wear Behavior of TiCN, TiSiCN, TiCrSiCN, and TiAlSiCN Coatings. Surf. Coat. Technol. 2013, 216, 273–281. [Google Scholar] [CrossRef]
- Fu, L.; Engqvist, H.; Xia, W. Glass–Ceramics in Dentistry: A Review. Materials 2020, 13, 1049. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Ghulam, O.; Krsoum, M.; Binmahmoud, S.; Taher, H.; Elmalky, W.; Zafar, M.S. Revolution of Current Dental Zirconia: A Comprehensive Review. Molecules 2022, 27, 1699. [Google Scholar] [CrossRef]
- Cesar, P.F.; Soki, F.N.; Yoshimura, H.N.; Gonzaga, C.C.; Styopkin, V. Influence of Leucite Content on Slow Crack Growth of Dental Porcelains. Dent. Mater. 2008, 24, 1114–1122. [Google Scholar] [CrossRef]
- Ho, G.W.; Matinlinna, J.P. Insights on Ceramics as Dental Materials. Part I: Ceramic Material Types in Dentistry. Silicon 2011, 3, 109–115. [Google Scholar] [CrossRef]
- Fredericci, C.; Yoshimura, H.N.; Molisani, A.L.; Pinto, M.M.; Cesar, P.F. Effect of Temperature and Heating Rate on the Sintering of Leucite-Based Dental Porcelains. Ceram. Int. 2011, 37, 1073–1078. [Google Scholar] [CrossRef]
- Santa Arango, A.M.; Escobar Garcés, C.M.; Agudelo Valderrama, J.L.; Guzmán Monsalve, A.; Palacio Santos, L.A.; Echavarría Isaza, A. Oligomerization of Propene over ZSM-5 Modified with Cr and W. Rev. Fac. Ing. Univ. Antioq. 2013, 57, 57–65. [Google Scholar] [CrossRef]
- Anders, A. Cathodic Arcs; Springer Series on Atomic, Optical, and Plasma Physics; Springer: New York, NY, USA, 2008; Volume 50, ISBN 978-0-387-79107-4. [Google Scholar]
- Jiang, J.; Hao, J.; Pang, X.; Wang, P.; Liu, W. Structure and Characteristics of Amorphous (Ti,Si)-C:H Films Deposited by Reactive Magnetron Sputtering. Diam. Relat. Mater. 2010, 19, 1172–1177. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Ubuo, E.E.; Udoetok, I.A.; Tyowua, A.T.; Ekwere, I.O.; Al-Shehri, H.S. The Direct Cause of Amplified Wettability: Roughness or Surface Chemistry? J. Compos. Sci. 2021, 5, 213. [Google Scholar] [CrossRef]
- Choi, H.; Liang, H. Wettability and Spontaneous Penetration of a Water Drop into Hydrophobic Pores. J. Colloid Interface Sci. 2016, 477, 176–180. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C.; Koch, K. Micro-, Nano- and Hierarchical Structures for Superhydrophobicity, Self-Cleaning and Low Adhesion. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1631–1672. [Google Scholar] [CrossRef]
- Zahiri, B.; Sow, P.K.; Kung, C.H.; Mérida, W. Understanding the Wettability of Rough Surfaces Using Simultaneous Optical and Electrochemical Analysis of Sessile Droplets. J. Colloid Interface Sci. 2017, 501, 34–44. [Google Scholar] [CrossRef]


| Sample | Dimensions | Type of Investigations |
|---|---|---|
Specimens 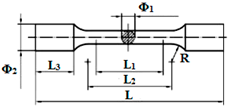 | Φ1 = 3 ± 0.1 mm; Φ2 = 6 mm L = 42 mm; L1 = 15 ± 0.1 mm; L2 = 18 ± 0.1 mm; L3 = 8.5 mm | Mechanical tensile tests for determining Young’s modulus according to ISO 6892 [16] |
Plates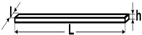 | L = 25 ± 1 mm l = 3 ± 0.1 mm h = 0.5 ± 0.05 mm | 3-point bending tests for determining ceramic-to-metal adhesion according to ISO 9693/2012 [17] |
Disc1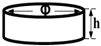 | Φ = 20 mm h = 5 mm | Surface morphology and elemental composition |
Disc2 | Φ = 25 mm h = 2 mm | Phase composition, Adhesion |
Disc3 | Φ = 12 mm h = 2 mm | Roughness, Wettability |
| Materials/Machines | Brand Name | Manufacturer Details | Composition of Materials |
|---|---|---|---|
| NiCr alloy | Argeloy NP | Argent, San Diego, CA USA | 76 wt. % Ni, 14 wt. % Cr, 6 wt. % Mo, 2 wt. % Al, 1.8 wt. % Be |
| CrSi cathode | - | Cathay Advanced Materials Ltd., Huizhou, Guangdong Province, China | 84 at.% Cr, 16 at.% Si |
| ZrSi cathode | - | Cathay Advanced Materials Ltd., Huizhou, Guangdong Province, China | 84 at.% Cr, 16 at.% |
| Ceramic | Vision Classic | Wohlwend AG, Fürstentum, Liechtenstein | - |
| 3D printer | ProJet DP3000 | 3D Systems, Rock Hill, SC, USA | - |
| Induction furnace with centrifugal force | Ducatron Serie 3 | Ugin Dentaire, Seyssinet-Pariset, France | - |
| Computer-controlled dental ceramic furnace | JELRUS VIP Universal, | Air Techniques, Inc., New York, NY, USA | - |
| Energy dispersive X-ray spectrometer | - | Bruker, Billerica, MA, USA | - |
| Scanning electron microscope | TM3030 Plus | Hitachi, Tokyo, Japan | - |
| Glow discharge optical emission spectroscopy | SPECTRUMA GDA-750HP | Spectruma Analytik GmbH, Hof, Germany | - |
| X-ray diffractometer | Miniflex II | Rigaku, Tokyo, Japan | - |
| Profilometer | Dektak 150 | Bruker, Billerica, MA, USA | |
| Optical tensiometer | Attension Theta Lite 101 | Biolin Scientific AB, Göteborg, Sweden | |
| Scratch platform | UMT TriboLab™ | Bruker, Billerica, MA, USA |
| Element | Solid Solution (A) | Fine Eutectic (B) | Compounds Within the Coarse Eutectic Structure (C) | Lamellas Within the Coarse Eutectic Structure (D) |
|---|---|---|---|---|
| Al | 6.43 | 4.30 | - | 5.19 |
| Mo | 3.31 | 7.94 | - | 4.09 |
| Cr | 16.78 | 15.85 | 2.46 | 16.33 |
| Ni | 73.48 | 71.92 | 97.54 | 74.39 |
| Area | O | Si | K | Al | Na | C | Ba | Zr | S | Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| (A) | 47.84 | 20.96 | 4.61 | 6.05 | 4.73 | 14.80 | - | - | - | 1.01 |
| (B) | 47.94 | 12.21 | 2.84 | 3.25 | 3.06 | 24.83 | 3.26 | - | 2.61 | - |
| (C) | 24.21 | 9.59 | 0.99 | 1.23 | 2.08 | 61.90 | - | - | - | - |
| (D) | 44.72 | 8.41 | 2.77 | 2.57 | 2.01 | 34.47 | - | 5.05 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, M.; Cotrut, C.M.; Vladescu, A.; Baciu, F.; Parau, A.C.; Pana, I.; Constantin, L.R.; Vitelaru, C. Biocompatible Inorganic PVD MeSiON Thin Films (Me = Cr or Zr) Used to Enhance the Bond Strength Between NiCr-Based Metallic Frameworks and Ceramic in Dental Restorations. Dent. J. 2025, 13, 318. https://doi.org/10.3390/dj13070318
Dinu M, Cotrut CM, Vladescu A, Baciu F, Parau AC, Pana I, Constantin LR, Vitelaru C. Biocompatible Inorganic PVD MeSiON Thin Films (Me = Cr or Zr) Used to Enhance the Bond Strength Between NiCr-Based Metallic Frameworks and Ceramic in Dental Restorations. Dentistry Journal. 2025; 13(7):318. https://doi.org/10.3390/dj13070318
Chicago/Turabian StyleDinu, Mihaela, Cosmin Mihai Cotrut, Alina Vladescu (Dragomir), Florin Baciu, Anca Constantina Parau, Iulian Pana, Lidia Ruxandra Constantin, and Catalin Vitelaru. 2025. "Biocompatible Inorganic PVD MeSiON Thin Films (Me = Cr or Zr) Used to Enhance the Bond Strength Between NiCr-Based Metallic Frameworks and Ceramic in Dental Restorations" Dentistry Journal 13, no. 7: 318. https://doi.org/10.3390/dj13070318
APA StyleDinu, M., Cotrut, C. M., Vladescu, A., Baciu, F., Parau, A. C., Pana, I., Constantin, L. R., & Vitelaru, C. (2025). Biocompatible Inorganic PVD MeSiON Thin Films (Me = Cr or Zr) Used to Enhance the Bond Strength Between NiCr-Based Metallic Frameworks and Ceramic in Dental Restorations. Dentistry Journal, 13(7), 318. https://doi.org/10.3390/dj13070318






.jpg)


