A Novel Device for the Evaluation of In Vitro Bacterial Colonization in Membranes for Guided Tissue and Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Species and Inoculum Preparation
2.2. Experimental Groups
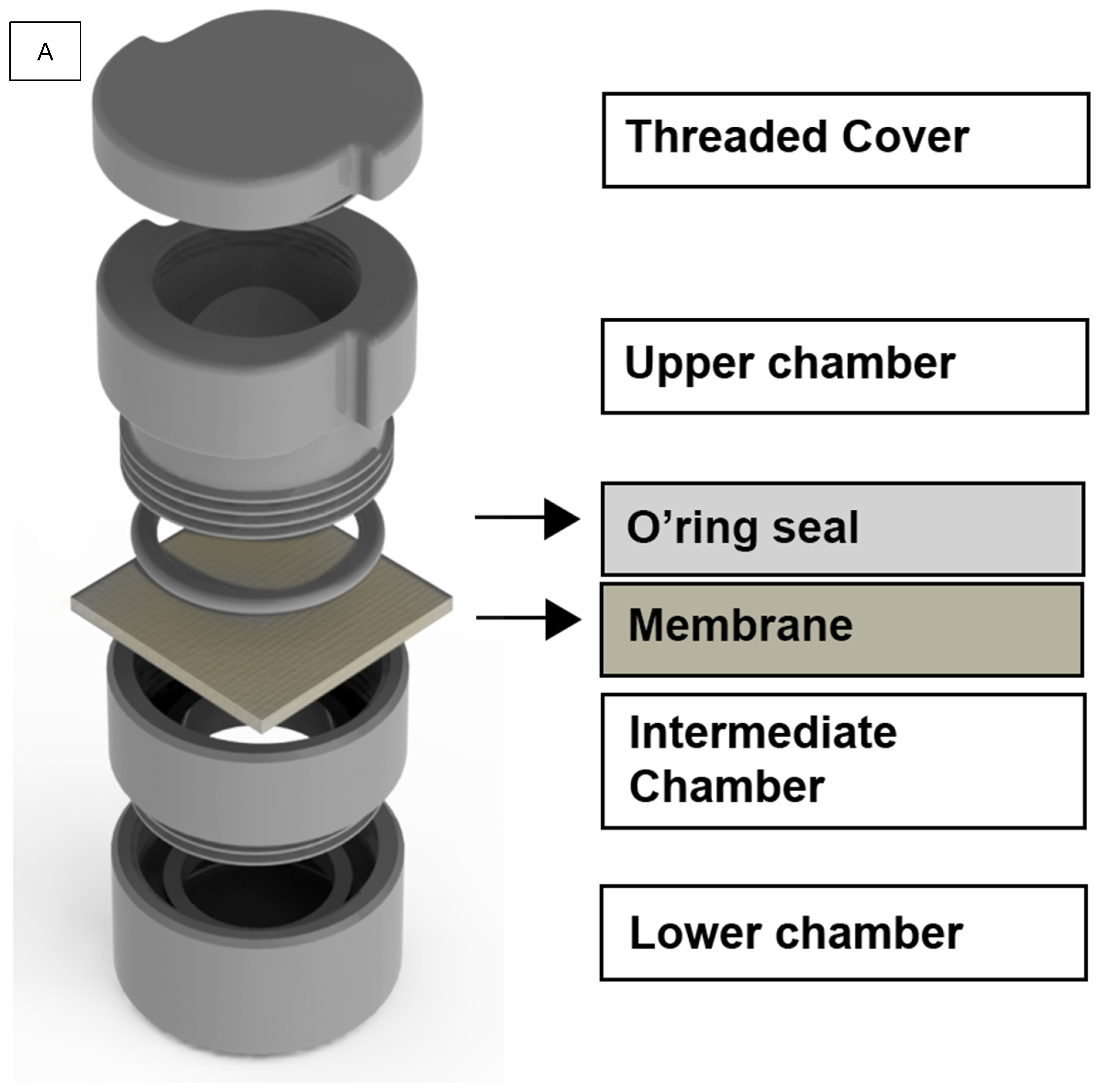
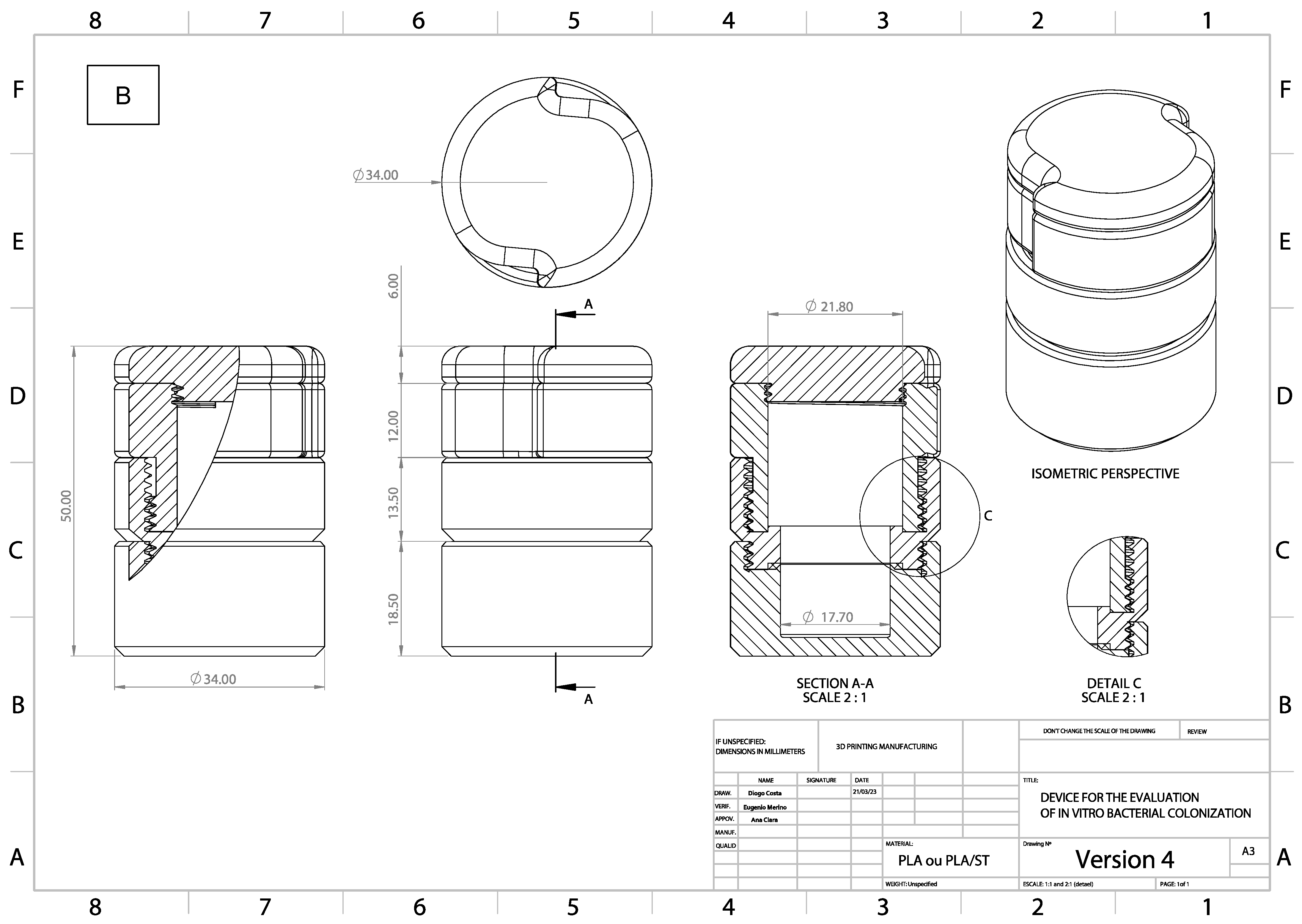
2.3. Methodological Apparatus
2.4. Aseptic Conditions
2.5. Growth of the Bacteria
2.6. Bacterial Adherence Analysis
2.7. Bacterial Viability Analysis—Confocal Laser Confocal Scanning Microscopy
2.8. Bacterial Penetration Analysis—Bacterial Quantification through CFU Count
2.9. Validation of the Apparatus Sealing System
2.10. Statistical Analysis
3. Results
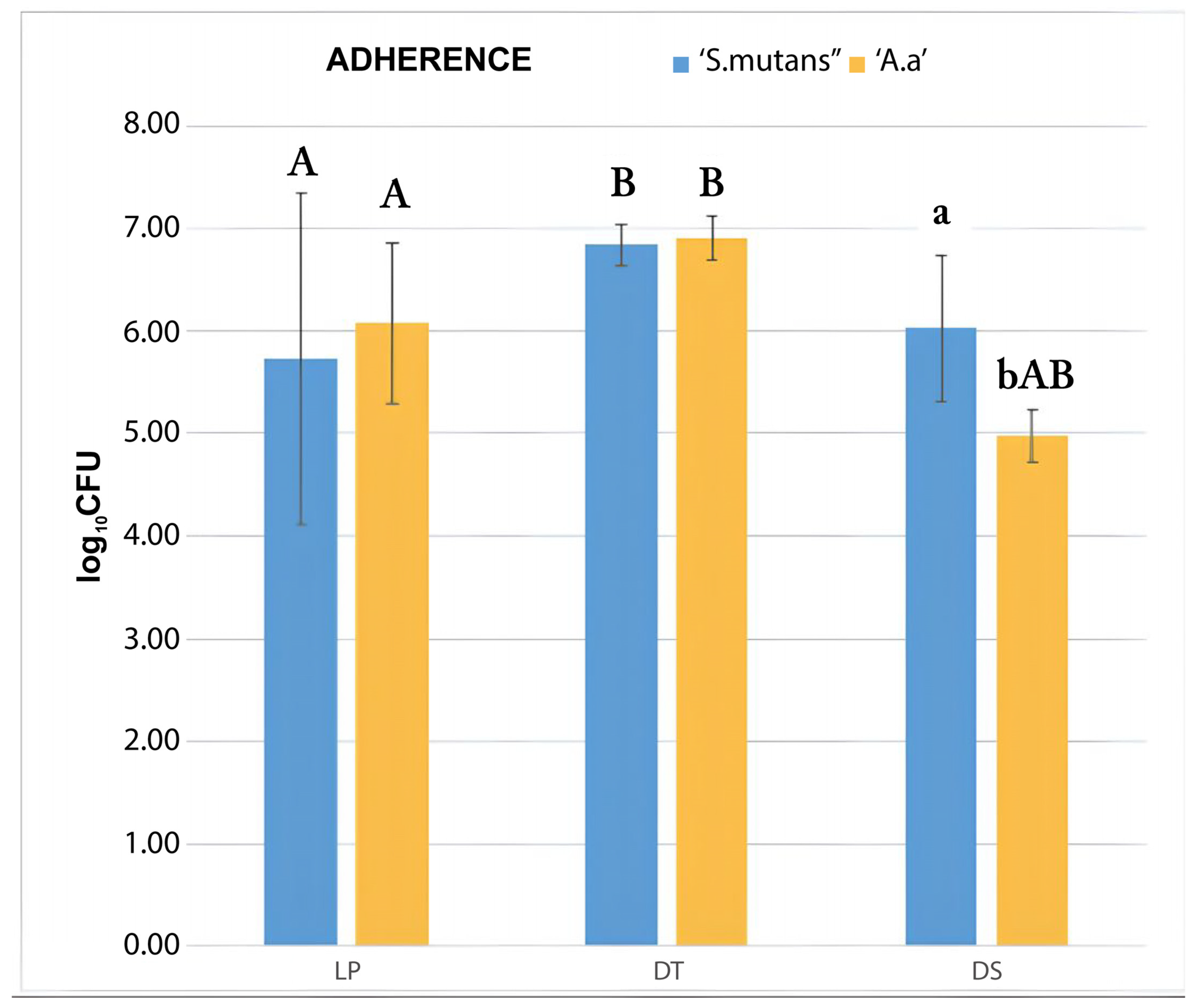
3.1. Bacterial Adherence Test
CFU Count
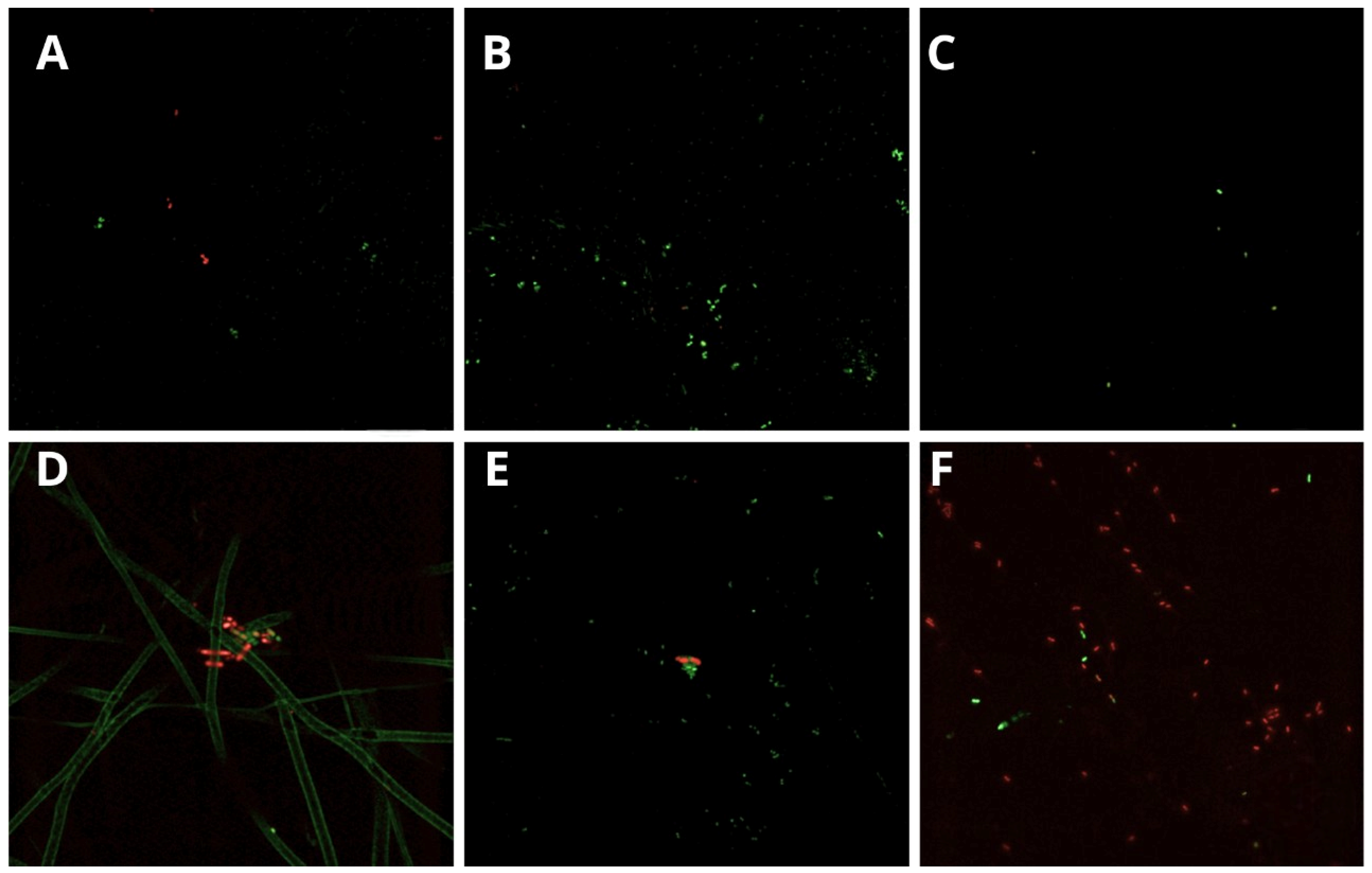
3.2. Bacterial Viability (Confocal Laser Scanning Microscopy)
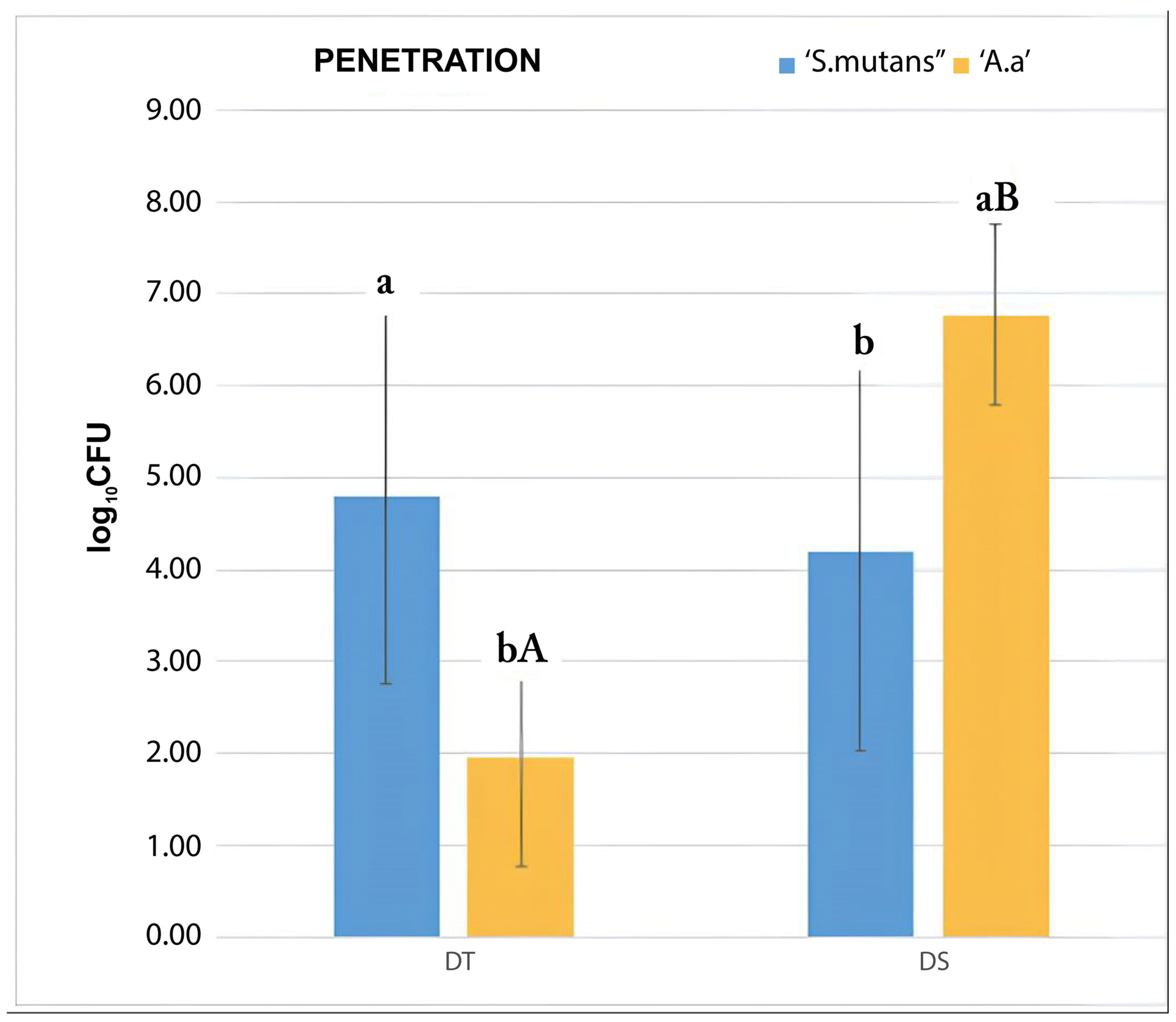
3.3. Bacterial Penetration Test
4. Discussion
5. Conclusions
6. Fomentation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Tumedei, M.; Mourão, C.F.; D’Agostino, S.; Dolci, M.; Di Cosola, M.; Piattelli, A.; Lucchese, A. Histological and Histomorphometric Effectiveness of Barrier Membranes for Jawbone Regeneration: An Overview of More Than 30 Years’ Experience of Research Results of the Italian Implant Retrieval Center (1988–2020). Appl. Sci. 2021, 11, 2438. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, M.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Carroll, M.J. Guided bone regeneration using bone grafts and collagen membranes. Quintessence Int. 2001, 32, 504–515. [Google Scholar] [PubMed]
- Gao, Y.; Wang, S.; Shi, B.; Wang, Y.; Chen, Y.; Wang, X.; Lee, E.-S.; Jiang, H.-B. Advances in Modification Methods Based on Biodegradable Membranes in Guided Bone/Tissue Regeneration: A Review. Polymers 2022, 14, 871. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Ruiz, C.; Toledano, M.; Osorio, R. Testing active membranes for bone regeneration: A review. J. Dent. 2021, 105, 103580. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.; Kapila, Y.; Lin, G. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lin GMonje, A.; Chan, H.; Wang, H. Wound healing complications following guided bone regeneration for ridge augmentation: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 41–50. [Google Scholar] [CrossRef]
- Trobos, M.; Juhlin, A.; Shah, F.A.; Hoffman, M.; Sahlin, H.; Dahlin, C. In vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluorethylene (PTFE) membranes for guided bone regeneration. Clin. Implant. Dent. Relat. Res. 2018, 20, 738–748. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, K.; Chen, Y.; Hung, S. Bacterial adhesion to antibiotic-loaded guided tissue regeneration membranes: A scanning electron microscopy study. J. Formos. Med. Assoc. 2015, 114, 35–45. [Google Scholar] [CrossRef]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, A. High-density polytetrafluorethylene membranes in guided bone and tissue regeneration procedures: A literature review. Int. J. Oral Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Leiva-Sabadini, C.; Schuh, C.M.A.P.; Aguayo, S. Bacterial adhesion to collagens: Implications for biofilm formation and disease progression in the oral cavity. Crit. Rev. Microbiol. 2022, 48, 83–95. [Google Scholar] [CrossRef]
- Ling, L.; Hung, S.; Lee, C.; Chen, Y.; Wu, K. The influence of membrane exposure on the outcomes of guided tissue regeneration: Clinical and microbiological aspects. J. Periodontol. 2003, 38, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Simion, M.; Tristi, P.; Maglione, M.; Piatelli, A. Bacterial penetration in vitro through GTAM membrane with and without topical chlorhexidine application: A light and scanning electron microscopic study. J. Clin. Periodontol. 1995, 22, 321–331. [Google Scholar] [CrossRef]
- De Sanctis, M.; Zucchelli, G.; Clauser, C. Bacterial colonization of barrier material and periodontal regeneration. J. Clin. Periodontol. 1996, 23, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Lee, Y.; Chi, L.; Chen, Y.; Hung, S.; Ling, L. Bacterial penetration through antibiotic-loaded guided tissue regeneration membranes. J. Periodontol. 2009, 80, 1471–1478. [Google Scholar] [CrossRef]
- Rani, S.; Chandra, R.V.; Reddy, A.A.; Reddy, B.H.; Nagarajan, S.; Naveen, A. Evaluation of the Antibacterial Effect of Silver Nanoparticles on Guided Tissue Regeneration Membrane Colonization—An in vitro study. J. Int. Acad. Periodontol. 2015, 17, 66–76. [Google Scholar] [PubMed]
- Yoshinari, N.; Tohya, T.; Mori, A.; Koide, M.; Kawase, H.; Takada, T.; Inagaki, K.; Noguchi, T. Inflammatory cell population and bacterial contamination of membranes used for guided tissue regenerative procedures. J. Periodontol. 1998, 69, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Slutzkey, S.; Kozlovsky, A.; Artzi, Z.; Matalon, S. Collagen barrier membranes may accelerate bacterial growth in vitro: A potential clinical risk to regenerative procedures. Quintessence Int. 2015, 46, 43–50. [Google Scholar] [CrossRef]
- Vallecillo-Rivas, M.; Toledano-Osorio, M.; Vallecillo CToledano, M.; Osorio, R. The Collagen Origin Influences the Degradation Kinetics of Guided Bone Regeneration Membranes. Polymers 2021, 13, 3007. [Google Scholar] [CrossRef]
- Abe, G.L.; Tsuboi, R.; Kitagawa, H.; Sasaki, J.; Li, A.; Kohno, T.; Imazato, S. Poly(lactic acid/caprolactone) bilayer membrane blocks bacterial penetration. J. Periodontal Res. 2022, 57, 510–518. [Google Scholar] [CrossRef]
- Nocca, G.; Filetici, P.; Bugli, F.; Mordente, A.; D’Addona, A.; Dassatti, L. Permeability of P. gingivalis or its metabolic products through collagen and dPTFE membranes and their effects on the viability of osteoblast-like cells: An in vitro study. Odontology 2022, 110, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Nowzari, H.; Slots, J. Microorganisms in polytetrafluorethylene barrier membranes for guided tissue regeneration. J. Clin. Periodontol. 1994, 21, 203–210. [Google Scholar] [CrossRef]
- Simion MTrist, P.; Maglione, M.; Piatelli, A. A preliminary report on a method for studying the permeability of expanded polytetrafluorethylene membrane to bacteria in vitro: A scanning electron microscopic and histological study. J. Periodontol. 1994, 65, 755–761. [Google Scholar] [CrossRef]
- Nowzari, H.; MacDonald, E.S.; Flynn, J.; London, R.M.; Morrison, J.L.; Slots, J. The dynamics of microbial colonization of barrier membranes for guided tissue regeneration. J. Periodontol. 1996, 67, 694–702. [Google Scholar] [CrossRef]
- Hung, S.; Lin, Y.; Wang, Y.; Chen, Y.; Su, C.; Ling, J. Permeability of Streptococcus mutans and Actinobacillus actinomycetemcomitans through guided tissue regeneration membranes and their effects on attachment of periodontal ligament cells. J. Periodontol. 2002, 73, 843–8551. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.C.K.; Schuldt, D.P.V.; Coelho, B.S.; Figueiredo, D.R.; Filho, G.S.; De Almeida, J. Bacterial penetration in barrier membranes used for guided tissue and bone regeneration: A literature review. ImplantNews 2022, 7, 242–250. [Google Scholar]
- Gil, A.C.K.; Prado, M.M.; Da Rocha, L.R.; Benfatti, C.; Filho, G.S.; De Almeida, J. In vitro evaluation of membranes for regenerative procedures against oral bacteria. Braz. Dent. J. 2023, 34, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Yuan, K.; Burgett, F.; Shyr, Y.; Syed, S. Adherence of oral microorganisms to guided tissue membranes: An in vitro study. J. Periodontol. 1994, 65, 211–218. [Google Scholar] [CrossRef]
- Begic, G.; Didovic, M.P.; Blagojevic, S.L.; Badovinac, I.J.; Zigon, J.; Percic, M.; Peloza, O.C.; Gobin, I. Adhesion of oral bacteria to commercial d-PTFE membranes: Polymer microstructure makes a difference. Int. J. Mol. Sci. 2022, 23, 2983. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier membranes for guided bone regeneration (GBR): A focus on recent advances in collagen membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Quantitatively predicting bacterial adhesion using surface free energy determined with spectrophotometric method. Environ. Sci. Technol. 2015, 49, 6164–6171. [Google Scholar] [CrossRef] [PubMed]
- Zelikman, H.; Slutzkey, G.; Rosner, O.; Levartovsky, S.; Matalon, S.; Beitlitu, I. Bacterial growth on three non-resorbable polytetrafluorethylene (PTFE) membranes—An in vitro study. Materials 2022, 15, 5705. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gibbons, R.J. Binding of streptococci of the “mutans” group to type I collagen associated with apatitic surfaces. Oral Microbiol. Immunol. 1990, 5, 131–136. [Google Scholar] [CrossRef]
- Zucchelli, G.; Cesari, C.; Clauser, C.; De Sanctis, M. Early bacterial accumulation on guided tissue regeneration memebrane materials: An in vivo study. J. Periodontol. 1998, 69, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Barber, H.D.; Lignelli, J.; Smith, B.M.; Bartee, B.K. Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J. Oral Maxillofac. Surg. 2007, 65, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Turri, A.; Čirgić, E.; Shah, F.A.; Hoffman, M.; Omar, O.; Dahlin, C.; Trobos, M. Early plaque formation on PTFE membranes with expanded or dense surface structures applied in the oral cavity of human volunteers. Clin. Exp. Dent. Res. 2021, 7, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, N.; Trajkovski, B.; Rahman, B.; Zafiropoulos, G. Clinical applications and outcomes of non-resorbable polytetrafluorethylene (PTFE) membranes in guided bone regeneration: Review. J. Int. Dent. Med. Res. 2019, 12, 1626–1635. [Google Scholar]
- Milella, E.; Ramires, P.A.; Brescia, E.; La Sala, G.; Di Paola, L.; Bruno, V. Physicochemical, mechanical and biological properties of commercial membranes for GTR. J. Biomed. Mater. Res. 2001, 58, 427–435. [Google Scholar] [CrossRef]
- Bartee, B.K.; Carr, J.A. Evaluation of a high-density polytetrfluorethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regenerarion in the rat mandible. J. Oral Implantol. 1995, 21, 88–95. [Google Scholar]
- Yaghobee, S.; Samadi, N.; Khorsand, A.; Ghahroudi, A.A.R.; Kadkhodazadeh, M. Comparison of the passage of Streptococcus mutans and Aggregatibacter through membranes loaded with tetracycline, amoxicillin, and chlorhexidine: An in vitro study. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Shi, R.; Niu, Y.; Gong, M.; Coates, P.; Crawford, A.; Chen, D.; Tian, W.; Zhang, L. Fabrication of drug-loaded anti-infective guided tissue regeneration membrane with adjustable biodegradation property. Colloids Surf. B Biointerfaces 2015, 135, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Saarani, N.N.; Jamuna-Thevi, K.; Shahab, N.; Hermawan, H.; Saidin, S. Antibacterial efficacy of triple-layered poly(lactic-co-glycolic acid)/nanoparticle/lauric acid guided bone regeneration membrane on periodontal bacteria. Dent. Mater. J. 2017, 36, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Sun, B.; Qiao, Z.; Zhao, K.; Zhou, X.; Zhang, Q.; Zou, D.; He, C.; Zhang, X. Bi-layered electrospun nanofibrous membrane with osteogenic and antibacterial properties for guided bone regeneration. Colloids Surf. B Biointerfaces 2019, 176, 219–229. [Google Scholar] [CrossRef] [PubMed]
| 3D Printer | |
|---|---|
| Layer height of the material | 0.25 mm |
| Extruder temperature | 190 °C |
| Heated layer temperature | 34 °C |
| Printings Setup | |
|---|---|
| Nozzle size | 0.4 |
| Bottom thickness | 0.25 |
| Filament diameter | 1.75 |
| Filament flow | 100 |
| Solid top | 1.0 |
| Solid bottom | 1.0 |
| Solid thickness of layer | 0.9 |
| Fill overlap | 15 |
| Fan activation | 1.0 |
| Fan speed | 100 |
| Adherence Test (MANOVA) | ||||
|---|---|---|---|---|
| Experimental Group | S. mutans (β) | A. actinomycetemcomitans b (Δ) | Pvalue between Species | Pvalue between Groups |
| LP | 5.73 (1.62) | 6.08 (0.78) | ** | (LP; DT) = 0.01 *β |
| DT | 6.84 (0.21) | 6.91 (0.21) | ** | (DT; DS) < 0.01 *Δ |
| DS | 6.03 (0.71) | 4.98 (0.25) | 0.005 * | (LP; DS) = 0.011 *Δ |
| Penetration Test (MANOVA) | ||||
|---|---|---|---|---|
| Experimental Group | S. mutans (β) | A. actinomycetemcomitans b (Δ) | Pvalue between Species | Pvalue between Groups |
| LP | 0 | 0 | ** | (LP; DT) = 0.003 *β (LP; DS) = 0.012 *β (LP; DS) < 0.01 *Δ |
| DT | 4.8 (2.04) | 1.94 (1.19) | 0.009 * | (DT;LP) = 0.03 *β (DT; DS) < 0.01 *Δ |
| DS | 4.19 (2.16) | 6.77 (0.98) | 0.016 * | (DS;LP) = 0.012 *β (DS; LP) < 0.01 *Δ (DS; DT) < 0.01 *Δ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, A.C.K.; Merino, E.A.D.; Costa, D.P.; Giracca, C.N.; Mazzon, R.; Magrin, G.L.; de Almeida, J.; Benfatti, C.A.M. A Novel Device for the Evaluation of In Vitro Bacterial Colonization in Membranes for Guided Tissue and Bone Regeneration. Dent. J. 2024, 12, 202. https://doi.org/10.3390/dj12070202
Gil ACK, Merino EAD, Costa DP, Giracca CN, Mazzon R, Magrin GL, de Almeida J, Benfatti CAM. A Novel Device for the Evaluation of In Vitro Bacterial Colonization in Membranes for Guided Tissue and Bone Regeneration. Dentistry Journal. 2024; 12(7):202. https://doi.org/10.3390/dj12070202
Chicago/Turabian StyleGil, Ana Clara Kuerten, Eugenio A. D. Merino, Diogo Pontes Costa, César Nunes Giracca, Ricardo Mazzon, Gabriel Leonardo Magrin, Josiane de Almeida, and Cesar Augusto Magalhães Benfatti. 2024. "A Novel Device for the Evaluation of In Vitro Bacterial Colonization in Membranes for Guided Tissue and Bone Regeneration" Dentistry Journal 12, no. 7: 202. https://doi.org/10.3390/dj12070202
APA StyleGil, A. C. K., Merino, E. A. D., Costa, D. P., Giracca, C. N., Mazzon, R., Magrin, G. L., de Almeida, J., & Benfatti, C. A. M. (2024). A Novel Device for the Evaluation of In Vitro Bacterial Colonization in Membranes for Guided Tissue and Bone Regeneration. Dentistry Journal, 12(7), 202. https://doi.org/10.3390/dj12070202







