Gingivectomy–Gingivoplasty for Oral Physiological Melanosis Depigmentation: A Case Report Involving Human Papillomavirus
Abstract
1. Introduction
2. Patient Information—Case Presentation
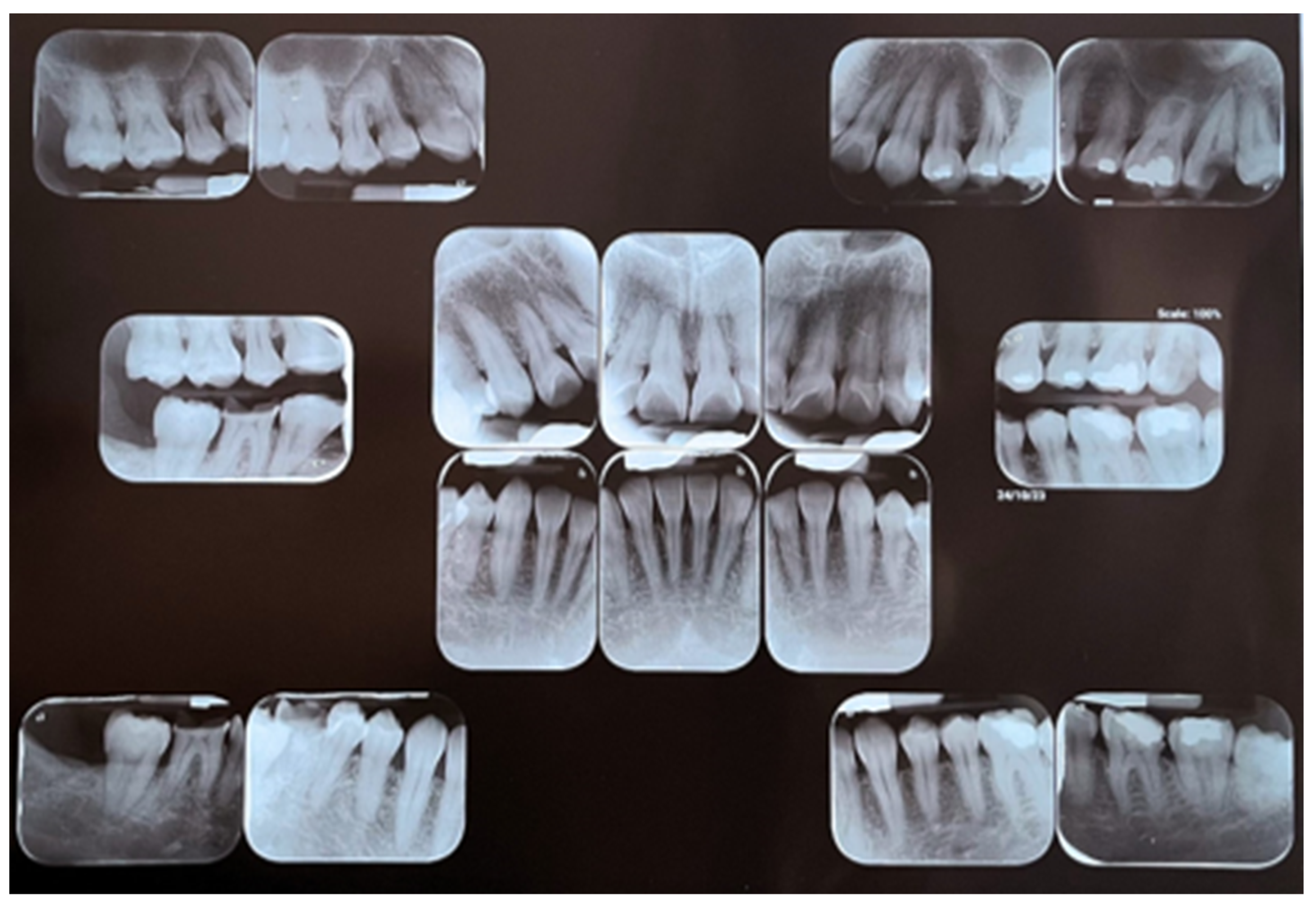
2.1. Clinical Findings
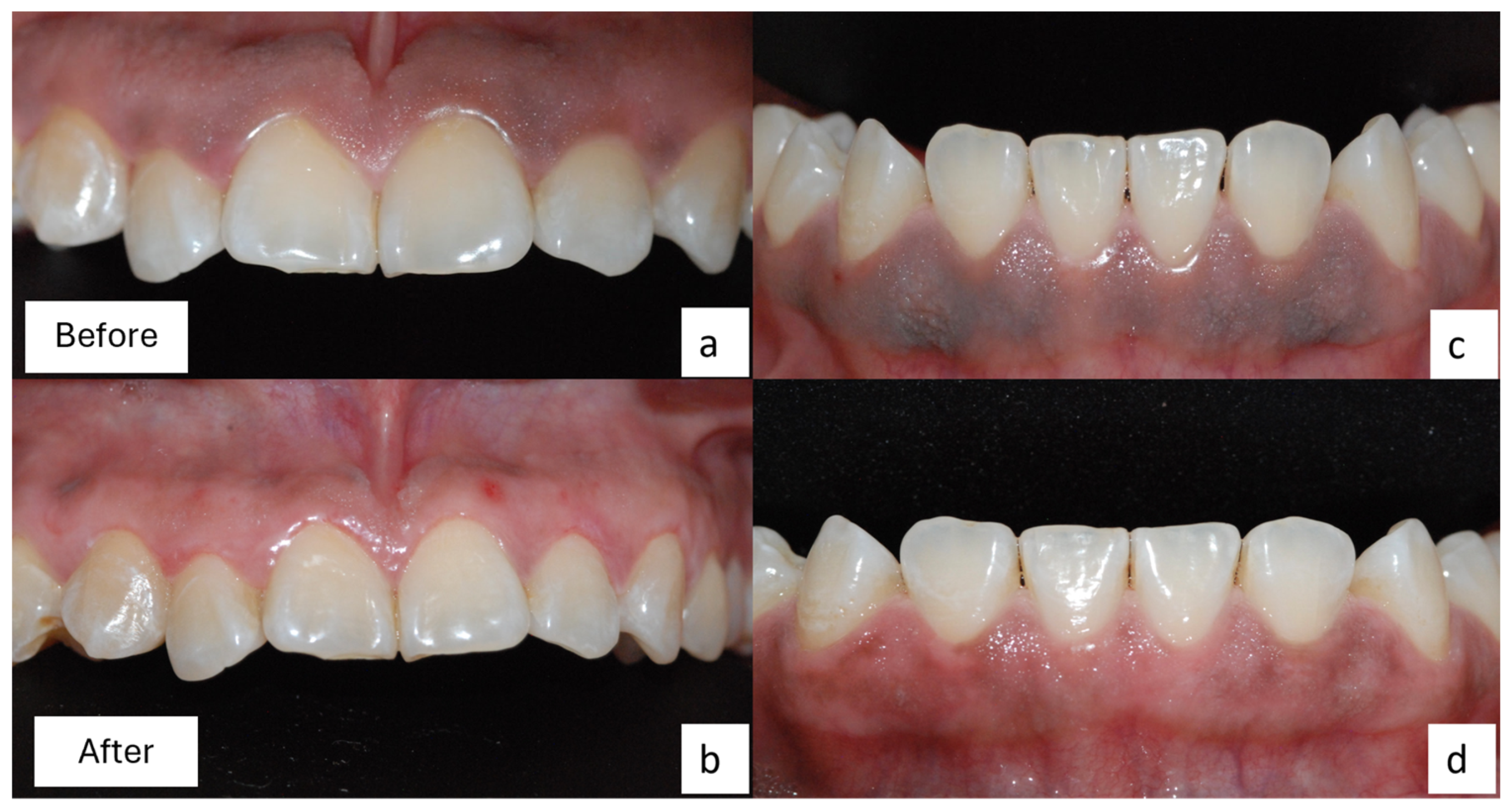
2.2. Surgical Procedure
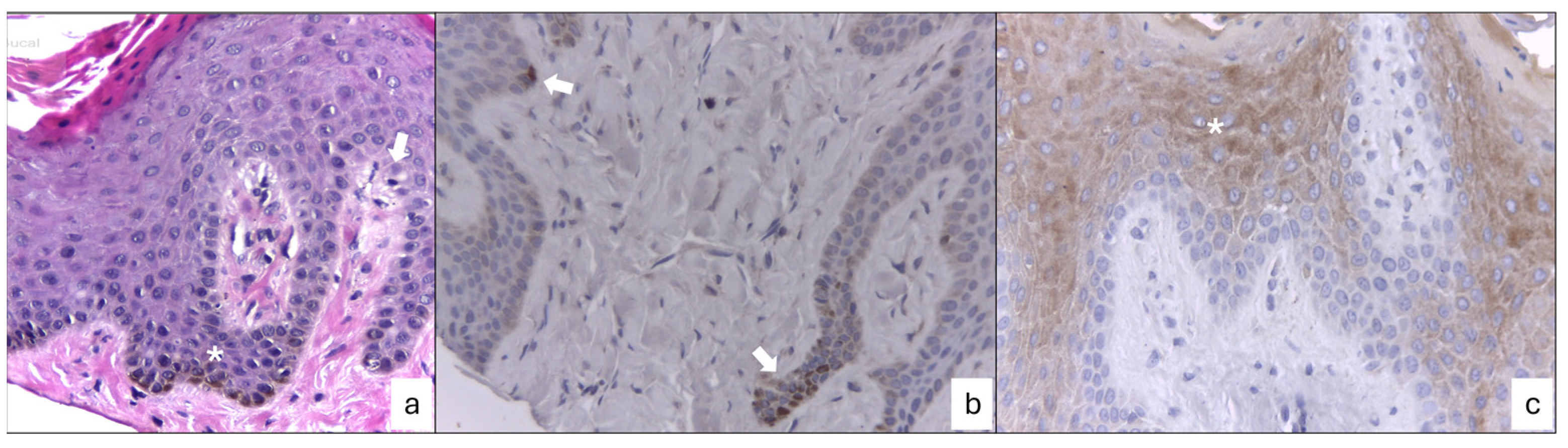
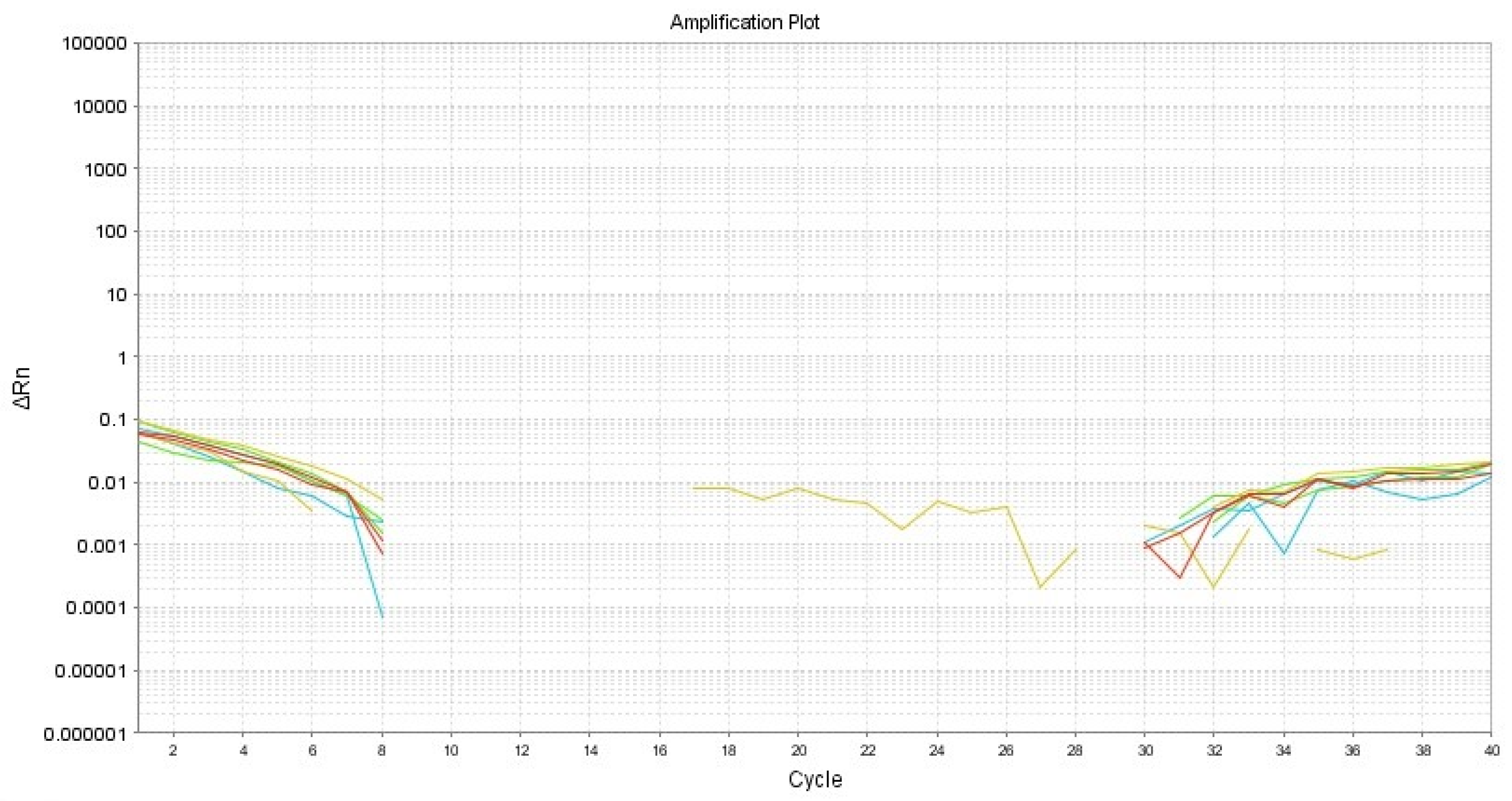
2.3. Clinical and Histopathological Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vargas Casillas, A.P.; Yáñez Ocampo, B.R.; Monteagudo Arrieta, C.A. Periodontología e Implantología, 2nd ed.; Médica Panamericana: México, Mexico, 2021; pp. 5–11. [Google Scholar]
- Gómez de Ferraris, M.E.; Campos Muñóz, A. Histología, Embrióloga e Ingeniera Tisular Bucodental, 4th ed.; Médica Panamericana: México, Mexico, 2019; pp. 247–254. [Google Scholar]
- Carranza, F.A.; Takei, H.H.; Newman, M.G.; Klokkevold, P.R. Periodontología Clínica de Carranza, 11th ed.; Amolca: México, Mexico, 2014; pp. 19–49. [Google Scholar]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Leyva Huerta, E.R.; Gaitán Cepeda, L.A. Patología General e Inmunología, 2nd ed.; Trillas: México, Mexico, 2008; pp. 75–80. [Google Scholar]
- Rosebush, M.S.; Briody, A.N.; Cordell, K.G. Black and Brown: Non-neoplastic Pigmentation of the Oral Mucosa. Head Neck Pathol. 2019, 13, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Agurto Mariluz, V.; Castro-Rodríguez, Y. Despigmentación gingival mediante mucoabrasion en el sector anterior. Reporte de caso. Duazary 2021, 18, 107–113. [Google Scholar] [CrossRef]
- Molano, P.E.; Quisoboni, J.F.; Yepes, B.I. Despigmentación gingival y cirugía de alargamiento coronal en erupción pasiva alterada tipo IA e IB en el mismo tiempo quirúrgico. Univ. Odontol. 2015, 34, 19–28. [Google Scholar]
- Castro Rodríguez, Y.; Grados-Pomarino, S. Tratamiento de la melanosis gingival y evaluación de la repigmentación melánica. Reevaluación clínica al cabo de 2 años. Rev. Clín. Periodoncia Implantol. Rehabil. Oral 2015, 8, 139–143. [Google Scholar] [CrossRef]
- Radzki, D.; Kusiak, A.; Ordyniec-Kwaśnica, I.; Bondarczuk, A. Human papillomavirus and leukoplakia of the oral cavity: A systematic review. Postep. Dermatol. Alergol. 2022, 39, 594–600. [Google Scholar] [CrossRef]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef]
- Shigeishi, H. Association between human papillomavirus and oral cancer: A literature review. Int. J. Clin. Oncol. 2023, 28, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.C.; Bain, C.J.; Smith, D.D.; Whiteman, D.C.; Antonsson, A. Oral human papillomavirus infection incidence and clearance: A systematic review of the literature. J. Gen. Virol. 2017, 98, 519–526. [Google Scholar] [CrossRef]
- Correa-Arzate, L.; Portilla-Robertson, J.; Ramírez-Jarquín, J.O.; Jacinto-Alemán, L.F.; Mejía-Velázquez, C.P.; Villanueva-Sánchez, F.G.; Rodríguez-Vázquez, M. LRP5, SLC6A3, and SOX10 Expression in Conventional Ameloblastoma. Genes 2023, 14, 1524. [Google Scholar] [CrossRef]
- Sedeh, S.A.; Badihi, S.; Esfahaniyan, V. Comparison of recurrent rate of gingival pigmentation after treatment by liquid nitrogen and cryoprob in 18 months follows-up. Dent. Res. J. 2014, 11, 592–598. [Google Scholar]
- Osorio Ayala, L.D.; Cantos Tello, P.M.; Carvajal Endara, A.S. Gingival Melanosis: Diagnosis and Therapy of Its Aesthetic Involvement. Literature Review. Odovtos—Int. J. Dent. Sci. 2021, 23, 39–51. [Google Scholar] [CrossRef]
- González-Rodríguez, A.J.; Lorente-Gual, R. Current indications and new applications of intense pulsed light. Actas Dermosifiliogr. 2015, 106, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Atsawasuwan, P.; Greethong, K.; Nimmanon, V. Treatment of gingival hyperpigmentation for esthetic purposes by Nd:YAG laser: Report of 4 cases. J. Periodontol. 2000, 71, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Mahayni, M.; Kujan, O.; Hamadah, O. Aesthetic Gingival Melanin Pigmentation Treatment in Smokers and Non-Smokers: A Comparison Study Using Nd:YAG Laser and Ceramic Bur. J. Pers. Med. 2023, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. Surgical plume in dermatology: An insidious and often overlooked hazard. Clin. Exp. Dermatol. 2020, 45, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Tai, H.; Tanaka, A.; Ikezawa-Suzuki, I.; Takagi, K.; Yoshida, Y.; Yoshie, H. Effects of ascorbic acid on gingival melanin pigmentation in vitro and in vivo. J. Periodontol. 2009, 80, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, D.S. An insight into gingival depigmentation techniques: The pros and cons. Int. J. Health Sci. 2018, 12, 84–89. [Google Scholar]
- Lin, Y.H.; Tu, Y.K.; Lu, C.T.; Chung, W.C.; Huang, C.F.; Huang, M.S.; Lu, H.K. Systematic review of treatment modalities for gingival depigmentation: A random-effects poisson regression analysis. J. Esthet. Restor. Dent. 2014, 26, 162–178. [Google Scholar] [CrossRef]
- Castro-Rodríguez, Y.; Bravo-Castagnola, F.; Grados-Pomarino, S. Repigmentación melánica de la melanosis gingival: Revisión sistemática. Rev. Clin. Periodoncia Implantol. Rehabil. Oral. 2016, 9, 238–243. [Google Scholar] [CrossRef]
- Pavlic, V.; Brkic, Z.; Marin, S.; Cicmil, S.; Gojkov-Vukelic, M.; Aoki, A. Gingival melanin depigmentation by Er:YAG laser: A literature review. J. Cosmet. Laser Ther. 2018, 20, 85–90. [Google Scholar] [CrossRef] [PubMed]
- HPV (BSB-66), MMab. Available online: https://www.biosb.com/biosb-products/hpv-antibody-mmab-bsb-66/#1612989340760-d265bd99-c22c37f4-9c5f4bf9-7739a8cb-e736 (accessed on 6 November 2023).
- Oliveira, L.H.; Santos, L.S.; Silva, C.O.; Augusto, E.F.; Neves, F.P. Papillomavirus infections in the oral and genital mucosa of asymptomatic women. Braz. J. Infect. Dis. 2017, 21, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.C.; Markham, C.M.; Ross, M.W.; Mullen, P.D. Examining the association between oral health and oral HPV infection. Cancer Prev. Res. 2013, 6, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Troconis, J.; Delgado, M.; González, G.; Rivas, A.; Molero, K. Melanosis of the vagina and human papillomavirus infection, an uncommon pathology: Case report. Investig. Clin. 2011, 52, 268–273. [Google Scholar] [PubMed]
- Taylor, C.O.; Lewis, J.S. Histologically documented transformation of benign oral melanosis into malignant melanoma: A case report. J. Oral Maxillofac. Surg. 1990, 48, 732–734. [Google Scholar] [CrossRef]
- Kahn, M.A.; Weathers, D.R.; Hoffman, J.G. Transformation of a benign oral pigmentation to primary oral melanoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 454–459. [Google Scholar] [CrossRef]


| Gene | Primer Sequence | GenBank Accession |
|---|---|---|
| B2M-Forward | AATGCTTGGCTGTGATAC | NG_012920.2 |
| B2M-Reverse | CTATGGCGGAAGATAACTG | |
| E2-Forward | AGGACGGATTAACTGTAA | OP712097.1 |
| E2-Reverse | GTTGCCATTCACTATCATA | |
| E6-Forward | ATTAGAACAGCAATACAACAA | |
| E6-Reverse | GCAACAAGACATACATCG | |
| E7-Forward | ACAGAGCCCATTACAATA | |
| E7-Reverse | CATTAACAGGTCTTCCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Martínez, L.; Mendoza-Espinosa, B.I.; Jacinto-Alemán, L.F.; Molotla-Fragoso, A.; Mejía-Velázquez, C.P.; Alonso-Moctezuma, A.; Ramírez-Martínez, C.M.; Trejo-Remigio, D.A.; Toriz-Pichardo, E.M. Gingivectomy–Gingivoplasty for Oral Physiological Melanosis Depigmentation: A Case Report Involving Human Papillomavirus. Dent. J. 2024, 12, 203. https://doi.org/10.3390/dj12070203
Villa-Martínez L, Mendoza-Espinosa BI, Jacinto-Alemán LF, Molotla-Fragoso A, Mejía-Velázquez CP, Alonso-Moctezuma A, Ramírez-Martínez CM, Trejo-Remigio DA, Toriz-Pichardo EM. Gingivectomy–Gingivoplasty for Oral Physiological Melanosis Depigmentation: A Case Report Involving Human Papillomavirus. Dentistry Journal. 2024; 12(7):203. https://doi.org/10.3390/dj12070203
Chicago/Turabian StyleVilla-Martínez, Leslie, Blanca Itzel Mendoza-Espinosa, Luis Fernando Jacinto-Alemán, Adriana Molotla-Fragoso, Claudia Patricia Mejía-Velázquez, Alejandro Alonso-Moctezuma, Carla Monserrat Ramírez-Martínez, David Alonso Trejo-Remigio, and Elsa Mónica Toriz-Pichardo. 2024. "Gingivectomy–Gingivoplasty for Oral Physiological Melanosis Depigmentation: A Case Report Involving Human Papillomavirus" Dentistry Journal 12, no. 7: 203. https://doi.org/10.3390/dj12070203
APA StyleVilla-Martínez, L., Mendoza-Espinosa, B. I., Jacinto-Alemán, L. F., Molotla-Fragoso, A., Mejía-Velázquez, C. P., Alonso-Moctezuma, A., Ramírez-Martínez, C. M., Trejo-Remigio, D. A., & Toriz-Pichardo, E. M. (2024). Gingivectomy–Gingivoplasty for Oral Physiological Melanosis Depigmentation: A Case Report Involving Human Papillomavirus. Dentistry Journal, 12(7), 203. https://doi.org/10.3390/dj12070203







