Artificial Intelligence in Periodontology: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question and Study Eligibility
- Which methods were used in these studies to establish datasets; develop, train, and test the model; and report on its performance?
- In cases where these models were tested against human performance:
- a.
- What metrics were used to compare performance?
- b.
- What were the outcomes?
- Original articles published in English.
- Implant- and periodontal-based literature using ML or AI models for diagnostic purposes, detection of abnormalities/pathologies, patient group analysis, or planning of surgical procedures.
- Study designs whereby the use of ML or AI was used as the independent variable.
- Studies not in English.
- Studies using classic software rather than CNN derivative protocols for machine-based learning.
- Studies using AI for purposes other than periodontology and peri-implant health.
2.2. Study Search Strategy and Process
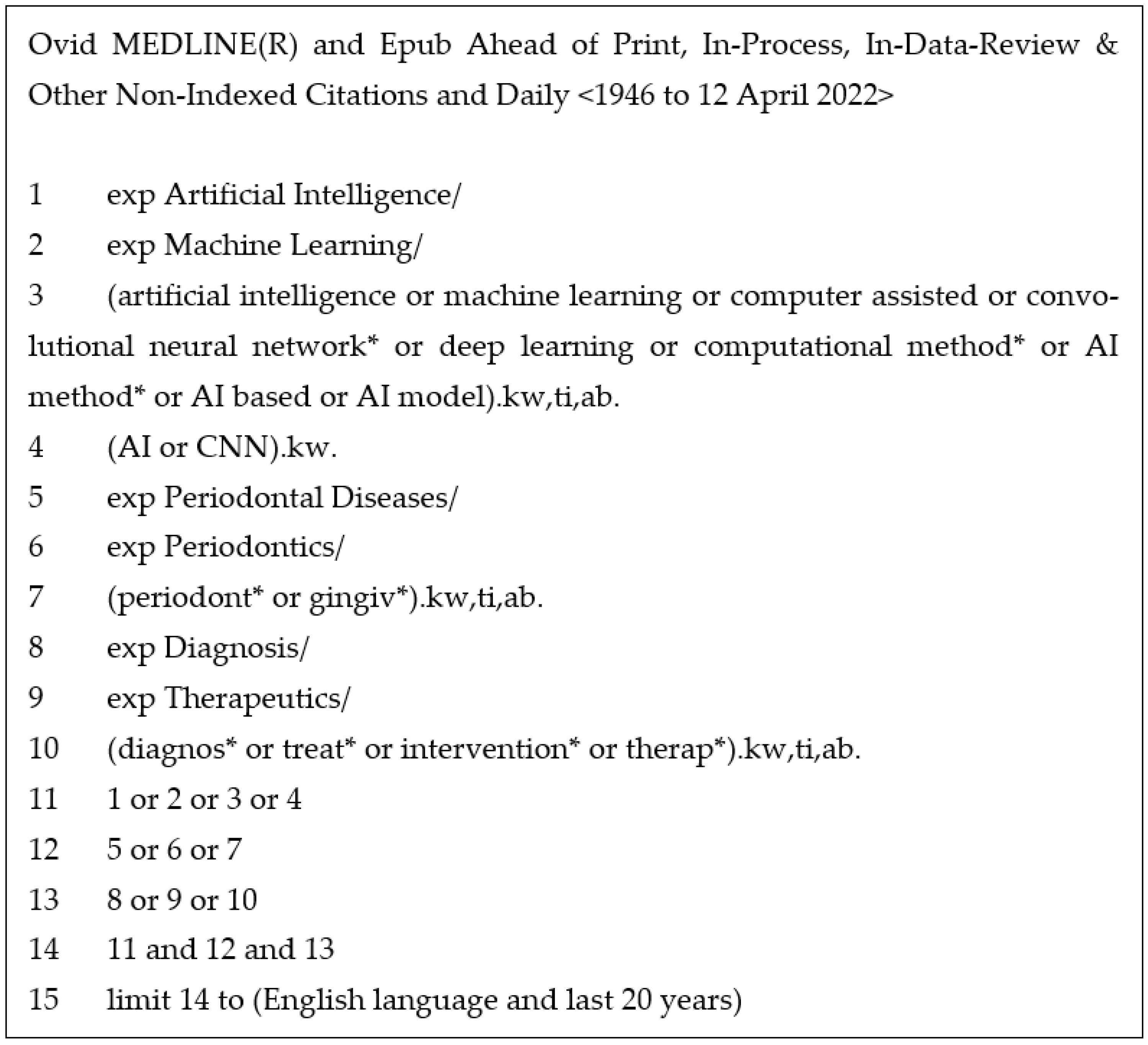
- Medline—the most widely used medical database for publishing journal articles. The search strategy for this is outlined in Figure 1.
- Scopus—the largest database of scientific journals.
- CINAHL—an index that focuses on allied health literature.
- IEEE Xplore—a digital library that includes journal articles, technical standards, conference proceedings, and related materials on computer science.
- arXiv—arXiv is an open-access repository of electronic preprints and postprints approved for posting after moderation but not peer review.
- Google Scholar—Google Scholar is a freely accessible web search engine that indexes the full text or metadata of scholarly literature across an array of publishing formats.
2.3. Study Selection
2.4. Data Extraction and Outcome of Interest
3. Results
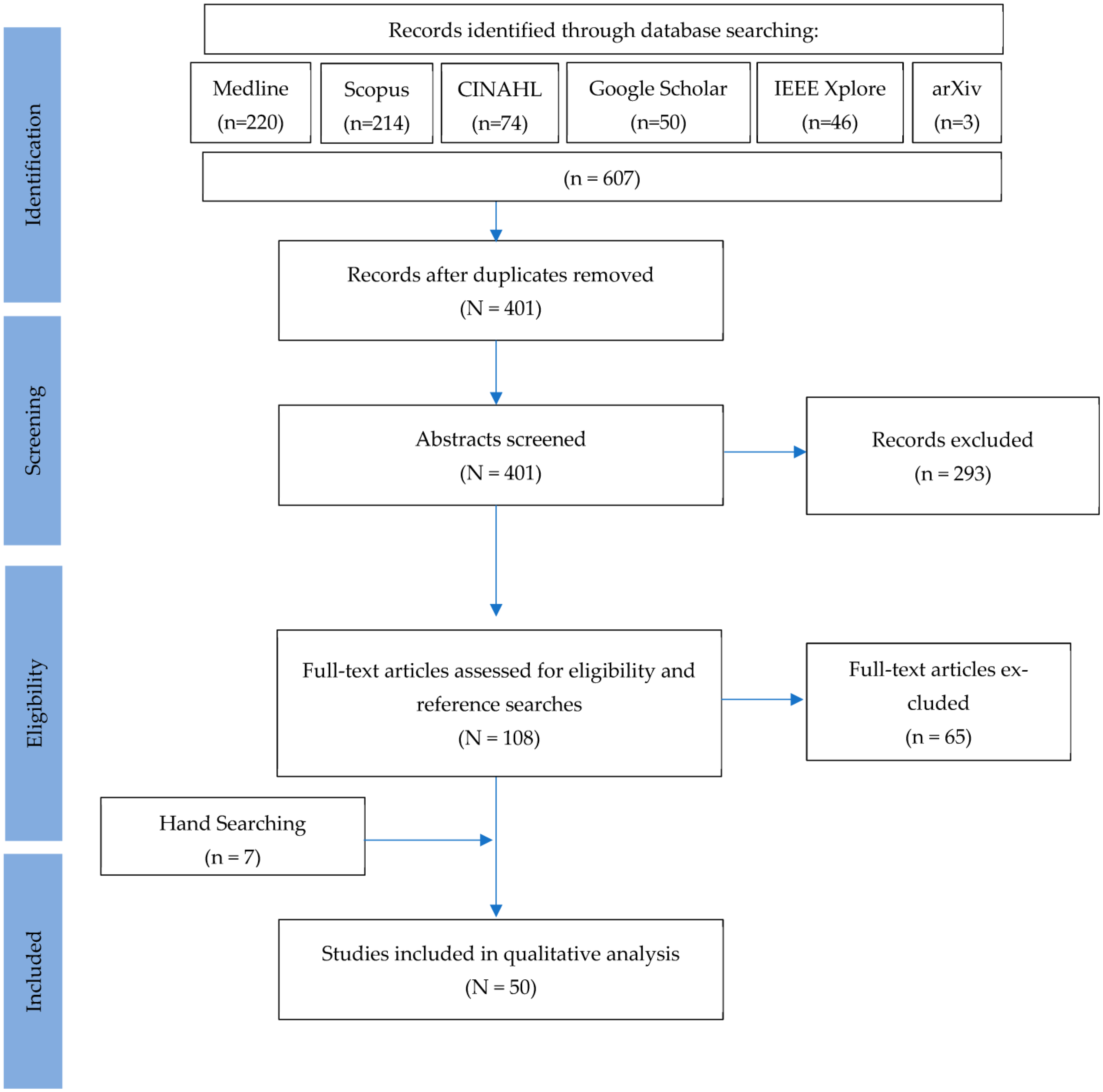
3.1. Study Selection and Data Compilation
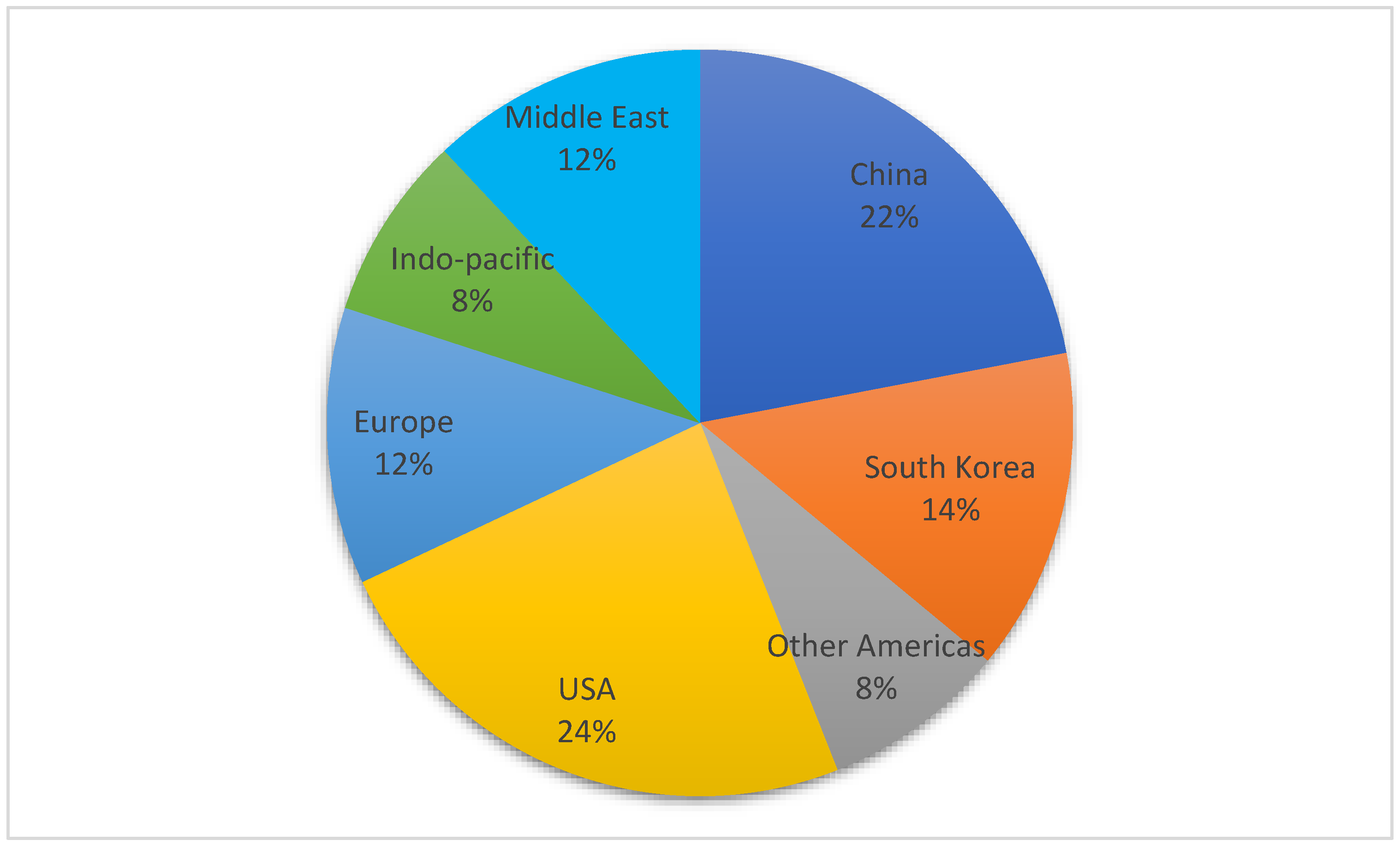
3.2. Location of Research
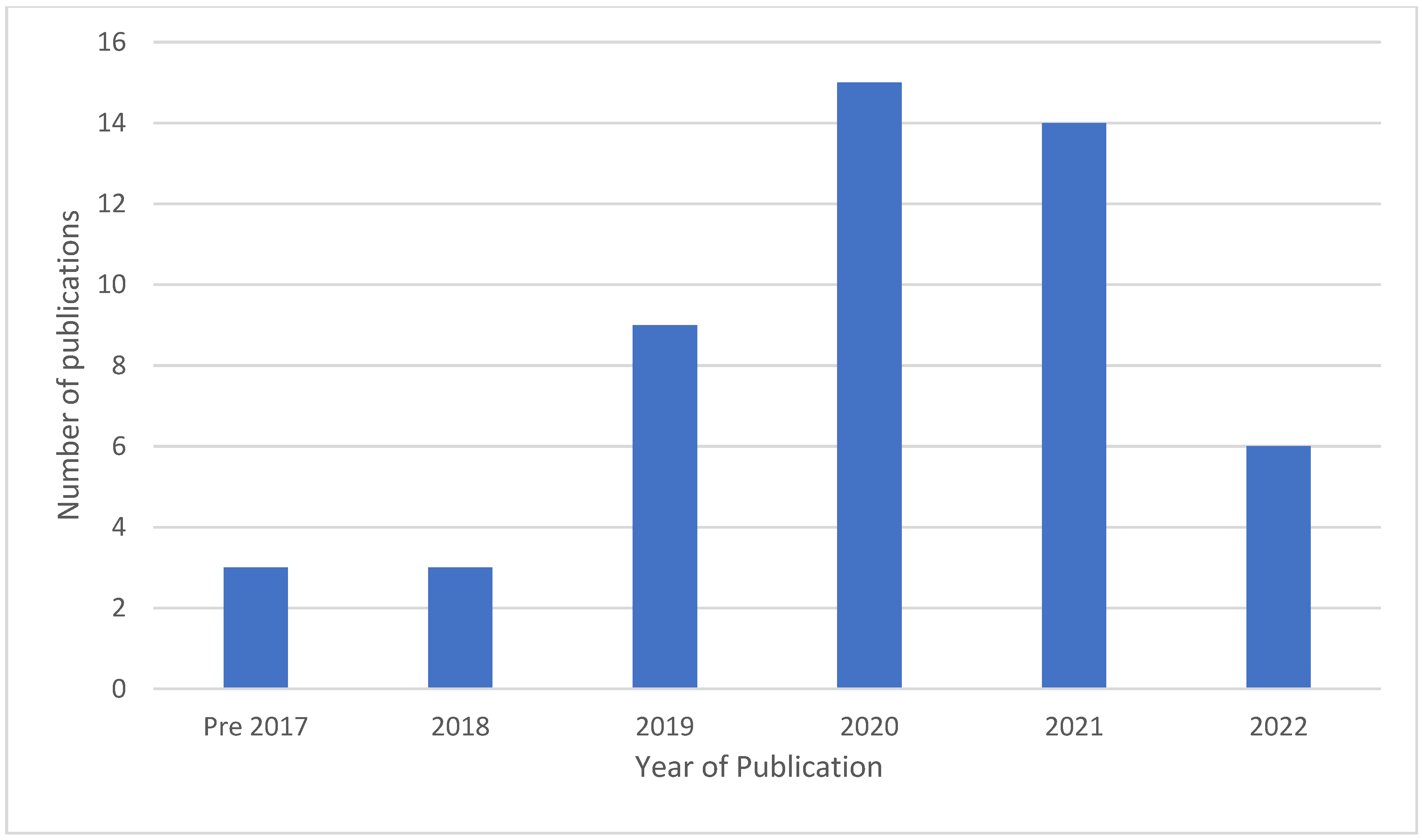
3.3. Year of Publication
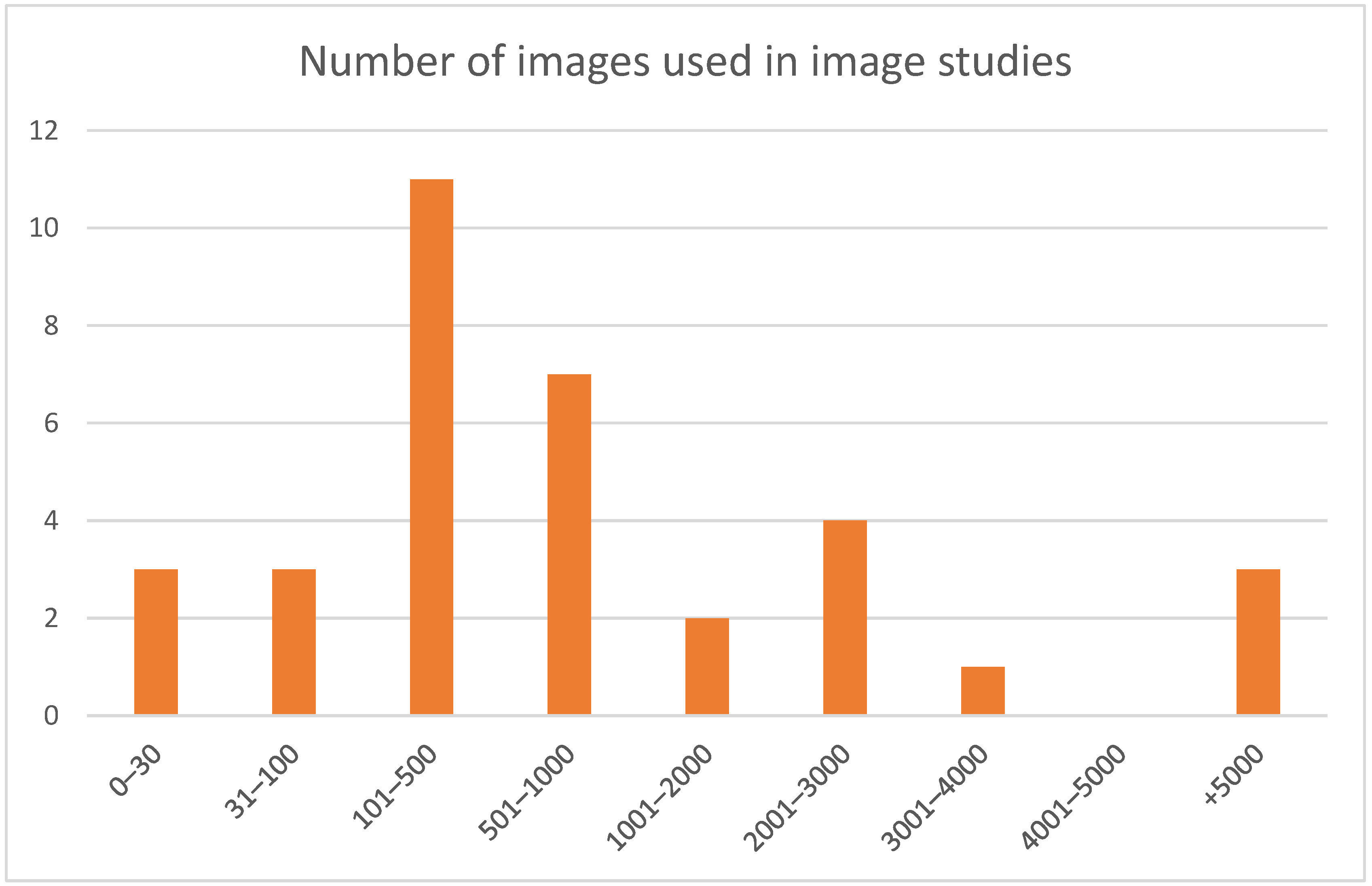
3.4. Input Data
3.5. Datasets
3.6. ML Architectures
3.7. Training and Annotation
3.8. Outcome Metrics and Comparative Texts
| Study | Country | Year | Data Type | Subject Total | ML Architecture | Annotators | Performance Comparison | CNN Performance Comment | Brief Description | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Papantonopoulos [45] | Greece | 2014 | Patient data | 29 | MLP ANN | n.a. | Not comparative | ANN’s gave 90–98% accuracy in classifying patients into AgP or CP. | ANNs used to classify periodontitis by immune response profile to aggressive periodontitis (AgP) or chronic periodontitis (CP) class. |
| 2 | Bezruk [47] | Ukraine | 2017 | Saliva | 141 | CNN (no description) | n.a. | Not comparative | Precision of CNN 0.8 in predicting gingivitis based upon crevicular fluid markers. | CNN was used for the learning task to build an information model of salivary lipid peroxidation and periodontal status and to evaluate the correlation between antioxidant levels in unstimulated saliva and inflammation in periodontal tissues. |
| 3 | Rana [48] | USA | 2017 | Photographs | 405 | CNN Autoencoder | Dentist | Not comparative | AU ROC curve of 0.746 for classifier to distinguish between inflamed and healthy gingiva. | Machine learning classifier used to provide pixel-wise inflammation segmentations from photographs of colour-augmented intraoral images. |
| 4 | Feres [41] | Brazil | 2018 | Plaque | 435 | SVM | n.a. | Not comparative | AUC > 0.95 for SVM to distinguish between disease and health. AUC for ability to distinguish between CP and AgP was 0.83. | SVM was used to assess whether 40 bacterial species could be used to classify patients into CP, AgP, or periodontal health. |
| 5 | Lee [49] | South Korea | 2018 | Periapical | 1740 | CNN encoder + 3 dense layers | Periodontist | Periodontist | CNN showed AU ROC curve of 73.4–82.6 (95% CI 60.9–91.1) in predicting hopeless teeth. | The accuracy of predicting extraction was evaluated and compared between the CNN and blinded board-certified periodontists using 64 premolars and 64 molars diagnosed as severe n the test dataset. For premolars, the deep CNN had an accuracy of 82.8% |
| 6 | Yoon [50] | USA | 2018 | Patient data | 4623 | Deep neural network-BigML | n.a. | Not comparative | DNN used as multi-regressional tool found correlation between ageing and mobility. | 78 variables assessed by DNN were used to find a correlation that can predict tooth mobility. |
| 7 | Aberin [51] | Philippines | 2019 | Plaque | 1000 | AlexNet | Pathologists | Not comparative | Accuracy in predicting health or periodontitis from plaque slides reported at 75%. | CNN was used to classify which microscopic dental plaque images were associated with gingival health. |
| 8 | Askarian [52] | USA | 2019 | Photographs | 30 | SVM | n.a. | Not comparative | 94.3% accuracy of SVM in detection of periodontal infection. | Smartphone-based standardised photograph detection using CNN to classify gingival disease presence. |
| 9 | Duong [31] | Canada | 2019 | Ultrasound | 35 | U-Net | n.a. | Orthodontist | CNN yielded 75% average dicemetric for ultrasound segmentation. | The proposed method was evaluated over 15 ultrasound images of teeth acquired from porcine specimens. |
| 10 | Hegde [39] | USA | 2019 | Patient data | 41,543 | SVM | n.a. | Not comparative | Comparison of ML vs. MLP vs. RF vs. SVM for data analysis. Similar accuracy found between all methods. | The objective was to develop a predictive model using medical-dental data from an integrated electronic health record (iEHR) to identify individuals with undiagnosed diabetes mellitus (DM) in dental settings. |
| 11 | Joo [53] | South Korea | 2019 | Photographs | 451 | CNN encoder + 1 dense layer | n.a. | Not comparative | Reported CNN accuracy of 70–81% for validation data. | Descriptive analysis of preliminary data for concepts of imaging analysis. |
| 12 | Kim [54] | South Korea | 2019 | Panoramic | 12,179 | DeNTNet | Hygienists | Hygienists | Superior F1 score (0.75 vs. 0.69), PPV (0.73 vs. 0.62), and AUC (0.95 vs. 0.85) for balanced setting DeNTNet vs. clinicians for assessing periodontal bone loss. | CNN used to develop an automated diagnostic support system assessing periodontal bone loss in panoramic dental radiographs. |
| 13 | Krois [55] | Germany | 2019 | Panoramic | 85 | CNN encoder + 3 dense layers | Dentist | Dentists | CNN performed less accurately than the original examiner segmentation and independent dentists’ observers. | CNNs used to detect periodontal bone loss (PBL) on panoramic dental radiographs. |
| 14 | Moriyama [56] | Japan | 2019 | Photographs | 820 | AlexNet | Dentist | Not comparative | Changes in ROC curves can have a significant effect on outcomes—looking at predicted pocket depth photographs and distorting images to improve accuracy. | CNN was used to establish if there is a correlation between pocket depth probing and images of the diseased area. |
| 15 | Yauney [57] | USA | 2019 | Patient data | 1215 | EED-net (custom net) | Dentist | Not comparative | AUC of 0.677 for prediction of periodontal disease based on intraoral fluorescent porphyrin biomarker imaging. | CNN was used to establish a link between intraoral fluorescent porphyrin biomarker imaging, clinical examinations, and systemic health conditions with periodontal disease. |
| 16 | Alalharith [58] | Saudi Arabia | 2020 | Photographs | 134 | Faster R-CNN | Dentist | Previously published outcomes | Faster R-CNN had tooth detection accuracy of 100% to determine region of interest and 77.12% accuracy to detect inflammation. | An evaluation of the effectiveness of deep learning based CNNs for the pre-emptive detection and diagnosis of periodontal disease and gingivitis by using intraoral images. |
| 17 | Bayrakdar [59] | Turkey | 2020 | Panoramic | 2276 | GoogLeNet Inception v3 | Radiologist and periodontist | Radiologist and periodontist | CNN showed 0.9 accuracy to detect alveolar bone loss. | CNN used to detect alveolar bone loss from dental panoramic radiographic images. |
| 18 | Chang [60] | South Korea | 2020 | Panoramic | 340 | ResNet | Radiologist | Radiologists | 0.8–0.9 agreement between radiologists and CNN performance. | Automatic method for staging periodontitis on dental panoramic radiographs using the deep learning hybrid method. |
| 19 | Chen [61] | China (and the UK) | 2020 | Photographs | 180 | ANN (no description) | n.a. | Not comparative | ANN accuracy of 71–75.44% for presence of gingivitis from photographs. | Visual recognition of gingivitis testing a novel ANN for binary classification exercise—gingivitis or healthy. |
| 20 | Farhadian [38] | Iran | 2020 | Patient data | 320 | SVM | n.a. | Not comparative | The SVM model gave an 88.4% accuracy to diagnose periodontal disease. | The study aimed to design a support vector machine (SVM)-based decision-making support system to diagnose various periodontal diseases. |
| 21 | Huang [40] | China | 2020 | Gingival crevicular fluid | 25 | SVM | n.a. | n.a. | Classification models achieved greater than or equal to 91% in classifying SP patients, with LDA being the highest at 97.5% accuracy. | This study highlights the potential of antibody arrays to diagnose severe periodontal disease by testing five models (SVM, RF, kNN, LDA, CART). |
| 22 | Kim [42] | South Korea | 2020 | Saliva | 692 | SVM | n.a. | n.a. | Accuracy ranged from 0.78 to 0.93 comparing neural network, random forest, and support vector machines with linear kernel, and regularised logistic regression in the R caret package. | CNN was used to assess whether biomarkers can differentiate between healthy controls and those with differing severities of periodontitis. |
| 23 | Kong [46] | China | 2020 | Panoramic | 2602 | EED-net (custom net) | Expert? | Expert? | The custom CNN performed better than U-Net or FCN-8, all with accuracies above 98% for anatomical segmentation. | CNN was used to complete maxillofacial segmentation of images, including periodontal bone loss recognition. |
| 24 | Lee [62] | South Korea | 2020 | Periapicals and panoramic | 10,770 | GoogLeNet Inception v3 | Periodontist | Periodontist | The CNN (0.95) performed better than human (0.90) for OPGs, but the same for PAs (0.97). | CNN used for identification of implants systems and their associated health. |
| 25 | Li [63] | Saudi Arabia | 2020 | Panoramic | 302 | R-CNN | Dentist | Other CNNs and dentist | Proposed architecture gave accuracies of 93% for detecting no periodontitis, 89% for mild, 95% for moderate, and 99% for severe. | This study compared different CNN models for bone loss recognition. |
| 26 | Moran [64] | Brazil | 2020 | Periapicals | 467 | ResNet, Inception | Radiologist and dentist | Compares two CNN approaches for accuracy | AUC ROC curve for ResNet and Inception was 0.86 for identification of regions of periodontal bone destruction. | Assessment of whether a CNN can recognise of periodontal bone loss improve post-image enhancement? |
| 27 | Romm [65] | USA | 2020 | Metabolites | N/A | CNN (No description), PCA | n.a. | n.a. | Oral cancer identified rather than a periodontal disease with 81.28% accuracy. | CNN to analyse metabolite sets for different oral diseases to distinguish between different forms of oral disease. |
| 28 | Shimpi [43] | USA | 2020 | Patient data | N/A | SVM, ANN | n.a. | n.a. | ANN presented more reliable outcomes than NB, LR, and SVM. | The study reviewed classic and CNN regression to assess accuracy in prediction for periodontal risk assessment based on EHIR. |
| 29 | Thanathornwong [66] | Thailand | 2020 | Panoramic | 100 | Faster R-CNN | Periodontist | Periodontist | 0.8 precision for identifying periodontally compromised teeth using radiographs. | CNN used to assess periodontally compromised teeth on OPG. |
| 30 | You [67] | China | 2020 | Photographs | 886 | DeepLabv3+ | Orthodontist | Orthodontist | No statistically significant difference in the ability to discern plaque on photographs compared to clinician. | CNN used to assess plaque presence in paediatric teeth. |
| 31 | Cetiner [68] | Turkey | 2021 | Patient data | 216 | MLP ANN | n.a. | n.a. | The DT was most accurate, with accuracy of 0.871 compared to LR (0.832) and LP (0.852). | Assessment of three models of data mining to provide a predictive decision model for peri-implant health. |
| 32 | Chen [69] | China | 2021 | Periapicals | 2900 | r-CNN | Dentist | n.a. | CNN used to locate periodontitis, caries, and PA pathology on PAs. | |
| 33 | Danks [70] | UK | 2021 | Periapicals | 340 | ResNet | Dentist | Dentist | Predicting periodontitis stage accuracy of 68.3%. | CNN used to find bone loss landmarks using different tools to provide staging of disease. |
| 34 | Kabir [30] | USA | 2021 | Periapicals | 700 | Custom CNN combining Res-Net and U-Net | Periodontitis | Periodontitis | Agreement between professors and HYNETS of 0.69. | CNN calibrated with bone loss on PAs applied to OPGs for staging and grading of whole-mouth periodontal status. |
| 35 | Khaleel [71] | Iraq | 2021 | Photographs | 120 | BAT algorithm, PCA, SOM | Dentist | n.a. | BAT method provided 95% accuracy against ground truth | Assessment of different algorithms’ efficacy in recognising gingival disease. |
| 36 | Kouznetsova [72] | USA | 2021 | Salivary metabolites | N/A | DNN | n.a. | n.a. | Model performance assessment only of different CNNs. | CNN predicts which molecules should be assessed for metabolic diagnosis of periodontitis or oral cancers. |
| 37 | Lee [35] | South Korea | 2021 | Panoramic | 530 | U-Net, Dense U-Net, ResNet, SegNet | Radiologists | Radiologists | The accuracy of the resulting model was 79.54%. | Assessment of a variety of CNN architectures for detecting and quantifying the missing teeth, bone loss, and staging on panoramic radiographs. |
| 38 | Li [73] | China | 2021 | Photographs | 3932 | Fnet, Lnet, cnet | Dentist | Dentists | Low agreement between three dentists and CNN in heatmap analysis. | CNN used for gingivitis detection photographs. |
| 39 | Li [29] | China | 2021 | Photographs | 110 | DeepLabv3+ | Dentist | n.a. | MobileNetV2 performed in a similar manner to Xception65; however, Mob, was 20× quicker. | Different CNNs trialled for RGB assessment of gingival tissues to assess inflamed gum detection on photographs. |
| 40 | Ma [74] | Taiwan | 2021 | Panoramic | 432 | ConvNet, U-Net | Unknown | n.a. | ConvNet analysis post U-Net segmentation—the model showed moderate levels of agreement (F2 score of between 0.523 and 0.903) and the ability to predict periodontitis and ASCVD. | CNNs used to assess for atherosclerotic cardiovascular disease and periodontitis on OPGs. |
| 41 | Moran [75] | Brazil | 2021 | Periapicals | 5 | Inception and for super-resolution SRCNN | Dentist | n.a. | Minimal enhancement of CNN performance was noted from super resolution, which may introduce additional artefacts. | The study compared the effects of super resolution methods on the ability of CNNs to perform segmentation and bone loss identification. |
| 42 | Ning [76] | Germany | 2021 | Saliva | N/A | DisGeNet, HisgAtlas | n.a. | n.a. | DL-based model able to predict immunosuppression genes in periodontitis with an accuracy of 92.78%. | CNN to identify immune subtypes of periodontitis and pivotal immunosuppression genes that discriminated periodontitis from the healthy. |
| 43 | Shang [32] | China | 2021 | Photographs | 7220 | U-Net | Dentist | Dentist | U-Net to have a 10% increased recognition of calculus, wear facets, gingivitis, and decay | Comparison of U-Net vs. comparison between U-Net and DeepLabV3/PSPNet architecture for image recognition on oral pictures for wear, decay, calculus, and gingivitis. |
| 44 | Wang [77] | USA | 2021 | Metabolites | N/A | FARDEEP | n.a. | n.a. | ML successfully used in logistic regression of plaque samples. | CNN is used as a processing tool for clinical, immune, and microbial profiling of peri-implantitis patients against health. |
| 45 | Jiang [37] | China | 2022 | Panoramic | 640 | U-Net, YOLO-v4 | Periodontist | Periodontist | Compared to the ground truth, accuracy of 0.77 was achieved by the proposed architecture. | CNN used to provide % bone loss and resorption/furcation lesion and staging of periodontal disease from OPGs. |
| 46 | Lee [36] | USA | 2022 | Periapicals | 693 | U-Net, ResNet | Dentist | Dentist | The accuracy of the diagnosis based upon staging and grading was 0.85 | Full mouth PA films were used to review bone loss—staging and grading were then performed. |
| 47 | Li [73] | China | 2022 | Photographs | 2884 | OCNet, Anet | Dentist | Dentist | CNN provided AUC prediction of 87.11% for gingivitis and 80.11% for calculus. | Research trialling different methods of segmentation to assess plaque on photographs of tooth surfaces (inc ‘dye labelling’). |
| 48 | Liu [78] | China | 2022 | Periapicals | 1670 | Faster R-CNN | Dentist | Dentist | The results confirm the advantage of utilising multiple CNN architectures for joint optimisation to increase UTC ROC boosts of up to 8%. | CNN used to assess implant marginal bone loss with dichotomous outcomes. |
| 49 | Pan [33] | USA | 2022 | Ultrasound | 627 | U-Net | Dentist | Dentist | Showed a significant difference between CNN outcome and dental experts’ labelling. | CNN was used to provide an estimation of gingival height in porcine models. |
| 50 | Zadrozny [34] | Poland | 2022 | Panoramic | 30 | U-Net | Radiologists | Dentists | Tested CNN showed unacceptable reliability for assessment of caries (ICC = 0.681) and periapical lesions (ICC = 0.619), but acceptable for fillings (ICC = 0.920), endodontically treated teeth (ICC = 0.948), and periodontal bone loss (ICC = 0.764). | Testing of commercially available product Diagnocat in the evaluation of panoramic radiographs. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, P.C. The Post-Physician Era: Medicine in the 21st Century. JAMA 1977, 237, 2336. [Google Scholar] [CrossRef]
- Park, W.J.; Park, J.-B. History and application of artificial neural networks in dentistry. Eur. J. Dent. 2018, 12, 594–601. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S149–S161. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Susin, C. Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontology 2000 2017, 75, 45–51. [Google Scholar] [CrossRef]
- Pirih, F.Q.; Monajemzadeh, S.; Singh, N.; Sinacola, R.S.; Shin, J.M.; Chen, T.; Fenno, J.C.; Kamarajan, P.; Rickard, A.H.; Travan, S.; et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontology 2000 2021, 87, 50–75. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.; Hughes, F.; Preshaw, P.M. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef]
- Kebschull, M.; Demmer, R.T.; Papapanou, P.N. “Gum bug, leave my heart alone!”--epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 2010, 89, 879–902. [Google Scholar] [PubMed]
- Leroy, R.; Eaton, K.A.; Savage, A. Methodological issues in epidemiological studies of periodontitis--how can it be improved? BMC Oral. Health. 2010, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Wilson, N.H.F. Manifesto for a paradigm shift: Periodontal health for a better life. Br. Dent. J. 2014, 216, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, R.P.; Coelho, L.; Silva, A.; Pereira, J.; Pinto, M.; Baptista, I. Validation of a dental image-analyzer tool to measure the radiographic defect angle of the intrabony defect in periodontitis patients. J. Periodontal Res. 2012, 47, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- LeCun, Y.; Boser, B.; Denker, J.; Henderson, D.; Howard, R.; Hubbard, W.; Jackel, L. Handwritten digit recognition with a back-propagation network. Adv. Neural Inf. Process. Systems 1989, 2, 303–318. [Google Scholar]
- Du, G.; Cao, X.; Liang, J.; Chen, X.; Zhan, Y. Medical Image Segmentation based on U-Net: A Review. J. Imaging Sci. Technol. 2020, 64, 20508-1. [Google Scholar] [CrossRef]
- Astley, J.R.; Wild, J.M.; Tahir, B.A. Deep learning in structural and functional lung image analysis. Br. J. Radiol. 2022, 95, 20201107. [Google Scholar]
- Haenssle, H.A.; Fink, C.; Schneiderbauer, R.; Toberer, F.; Buhl, T.; Blum, A.; Kalloo, A.; Hassen, A.B.H.; Thomas, L.; Enk, A.; et al. Man against machine: Diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1836–1842. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunović, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar]
- Marcus, G. Deep learning: A critical appraisal. arXiv 2018, arXiv:180100631. [Google Scholar]
- Pendlebury, M.; Horner, K.; Eaton, K. Selection Criteria for Dental Radiography; Faculty of General Dental Practitioners: London, UK; Royal College of Surgeons: London, UK, 2004. [Google Scholar]
- Dietrich, T.; Ower, P.; Tank, M.; West, N.X.; Walter, C.; Needleman, I.; Hughes, F.J.; Wadia, R.; Milward, M.R.; Hodge, P.J.; et al. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions–implementation in clinical practice. Br. Dent. J. 2019, 226, 16–22. [Google Scholar] [CrossRef]
- Endodontology ESo. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef]
- Langlais, R.P.; Skoczylas, L.J.; Prihoda, T.J.; Langland, O.E.; Schiff, T. Interpretation of bitewing radiographs: Application of the kappa statistic to determine rater agreements. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 751–756. [Google Scholar] [CrossRef]
- Gröndahl, K.; Sundén, S.; Gröndahl, H.-G. Inter- and intraobserver variability in radiographic bone level assessment at Brånemark fixtures. Clin. Oral Implant. Res. 1998, 9, 243–250. [Google Scholar] [CrossRef]
- Schwendicke, F.; Samek, W.; Krois, J. Artificial Intelligence in Dentistry: Chances and Challenges. J. Dent. Res. 2020, 99, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Hupe, M. EndNote X9. Journal of Electronic Resources in Medical Libraries. 2019, 16, 117–119. [Google Scholar] [CrossRef]
- Li, G.-H.; Hsung, T.-C.; Ling, W.-K.; Lam, W.Y.-H.; Pelekos, G.; McGrath, C. Automatic Site-Specific Multiple Level Gum Disease Detection Based on Deep Neural Network. 15th ISMICT 2021, 15, 201–205. [Google Scholar] [CrossRef]
- Kabir, T.; Lee, C.-T.; Nelson, J.; Sheng, S.; Meng, H.-W.; Chen, L.; Walji, M.F.; Jiang, X.; Shams, S. An End-to-end Entangled Segmentation and Classification Convolutional Neural Network for Periodontitis Stage Grading from Periapical Radiographic Images. arXiv 2021, arXiv:2109.13120. [Google Scholar]
- Duong, D.Q.; Nguyen, K.-C.T.; Kaipatur, N.R.; Lou, E.H.M.; Noga, M.; Major, P.W.; Punithakumar, K.; Le, L.H. Fully automated segmentation of alveolar bone using deep convolutional neural networks from intraoral ultrasound images. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference, Berlin, Germany, 23–27 July 2019; pp. 6632–6635. [Google Scholar]
- Shang, W.; Li, Z.; Li, Y. (Eds.) Identification of common oral disease lesions based on U-Net. In Proceedings of the 2021 IEEE 3rd International Conference on Frontiers Technology of Information and Computer (ICFTIC), Greenville, SC, USA, 12–14 November 2021. [Google Scholar]
- Pan, Y.-C.; Chan, H.-L.; Kong, X.; Hadjiiski, L.M.; Kripfgans, O.D. Multi-class deep learning segmentation and automated measurements in periodontal sonograms of a porcine model. Dentomaxillofacial Radiol. 2022, 51, 214–218. [Google Scholar] [CrossRef]
- Zadrożny, L.; Regulski, P.; Brus-Sawczuk, K.; Czajkowska, M.; Parkanyi, L.; Ganz, S.; Mijiritsky, E. Artificial Intelligence Application in Assessment of Panoramic Radiographs. Diagnostics 2022, 12, 224. [Google Scholar] [CrossRef]
- Lee, S. A deep learning-based computer-aided diagnosis method for radiographic bone loss and periodontitis stage: A multi-device study. In Proceedings of the 2021 52nd Korean Electric Society Summer Conference, July 2021. [Google Scholar]
- Lee, C.; Kabir, T.; Nelson, J.; Sheng, S.; Meng, H.; Van Dyke, T.E.; Walji, M.F.; Jiang, X.; Shams, S. Use of the deep learning approach to measure alveolar bone level. J. Clin. Periodontol. 2021, 49, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, D.; Cao, Z.; Wu, F.; Zhu, H.; Zhu, F. A two-stage deep learning architecture for radiographic staging of periodontal bone loss. BMC Oral Heal. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Farhadian, M.; Shokouhi, P.; Torkzaban, P. A decision support system based on support vector machine for diagnosis of periodontal disease. BMC Res. Notes 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Hegde, H.; Shimpi, N.; Panny, A.; Glurich, I.; Christie, P.; Acharya, A. Development of non-invasive diabetes risk prediction models as decision support tools designed for application in the dental clinical environment. Inform. Med. Unlocked 2019, 17, 100254. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wu, J.; Mao, Y.; Zhu, S.; Huang, G.F.; Petritis, B.; Huang, R. Developing a periodontal disease antibody array for the prediction of severe periodontal disease using machine learning classifiers. J. Periodontol. 2019, 91, 232–243. [Google Scholar] [CrossRef]
- Feres, M.; Louzoun, Y.; Haber, S.; Faveri, M.; Figueiredo, L.C.; Levin, L. Support vector machine-based differentiation between aggressive and chronic periodontitis using microbial profiles. Int. Dent. J. 2018, 68, 39–46. [Google Scholar] [CrossRef]
- Kim, E.-H.; Kim, S.; Kim, H.-J.; Jeong, H.-O.; Lee, J.; Jang, J.; Joo, J.-Y.; Shin, Y.; Kang, J.; Park, A.K.; et al. Prediction of Chronic Periodontitis Severity Using Machine Learning Models Based On Salivary Bacterial Copy Number. Front. Cell. Infect. Microbiol. 2020, 10, 571515. [Google Scholar] [CrossRef]
- Shimpi, N.; McRoy, S.; Zhao, H.; Wu, M.; Acharya, A. Development of a periodontitis risk assessment model for primary care providers in an interdisciplinary setting. Technol. Heal. Care 2020, 28, 143–154. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhou, Z.; Zhou, Z.; Wu, X.; Li, Y.; Wang, S.; Liao, W.; Ying, S.; Zhao, Z. Artificial intelligence for caries and periapical periodontitis detection. J. Dent. 2022, 122, 104107. [Google Scholar] [CrossRef]
- Papantonopoulos, G.; Takahashi, K.; Bountis, T.; Loos, B.G. Artificial Neural Networks for the Diagnosis of Aggressive Periodontitis Trained by Immunologic Parameters. PLoS ONE 2014, 9, e89757. [Google Scholar] [CrossRef]
- Kong, Z.; Xiong, F.; Zhang, C.; Fu, Z.; Zhang, M.; Weng, J.; Fan, M. Automated Maxillofacial Segmentation in Panoramic Dental X-Ray Images Using an Efficient Encoder-Decoder Network. IEEE Access 2020, 8, 207822–207833. [Google Scholar] [CrossRef]
- Bezruk, V.; Krivenko, S.; Kryvenko, L. (Eds.) Salivary lipid peroxidation and periodontal status detection in ukrainian atopic children with convolutional neural networks. In Proceedings of the 2017 4th International Scientific-Practical Conference Problems of Infocommunications Science and Technology (PIC S&T), Kharkov, Ukraine, 10–13 October 2017. [Google Scholar]
- Rana, A.; Yauney, G.; Wong, L.C.; Gupta, O.; Muftu, A.; Shah, P. (Eds.) Automated segmentation of gingival diseases from oral images. In Proceedings of the 2017 IEEE Healthcare Innovations and Point of Care Technologies, HI-POCT 2017, Bethesda, MD, USA, 6–8 November 2017. [Google Scholar]
- Lee, J.-H.; Kim, D.-H.; Jeong, S.-N.; Choi, S.-H. Diagnosis and prediction of periodontally compromised teeth using a deep learning-based convolutional neural network algorithm. J. Periodontal Implant. Sci. 2018, 48, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Odlum, M.; Lee, Y.; Choi, T.; Kronish, I.M.; Davidson, K.W.; Finkelstein, J. Applying Deep Learning to Understand Predictors of Tooth Mobility Among Urban Latinos. Stud. Health Technol. Inform. 2018, 251, 241–244. [Google Scholar] [PubMed]
- Aberin, S.T.A.; De Goma, J.C. Detecting periodontal disease using convolutional neural networks. In Proceedings of the 2018 IEEE 10th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM), Baguio City, Philippines, 29 November–2 December 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Askarian, B.; Tabei, F.; Tipton, G.A.; Chong, J.W. (Eds.) Smartphone-based method for detecting periodontal disease. In Proceedings of the 2019 IEEE Healthcare Innovations and Point of Care Technologies, HI-POCT 2019, Bethesda, Maryland, 20–22 November 2019; Available online: https://ieeexplore.ieee.org/document/8962638 (accessed on 25 November 2022).
- Joo, J.; Jeong, S.; Jin, H.; Lee, U.; Yoon, J.Y.; Kim, S.C. (Eds.) Periodontal disease detection using convolutional neural networks. In Proceedings of the 2019 International Conference on Artificial Intelligence in Information and Communication (ICAIIC), Okinawa, Japan, 11–13 February 2019. [Google Scholar]
- Kim, J.; Lee, H.-S.; Song, I.-S.; Jung, K.-H. DeNTNet: Deep Neural Transfer Network for the detection of periodontal bone loss using panoramic dental radiographs. Sci. Rep. 2019, 9, 17615. [Google Scholar] [CrossRef] [PubMed]
- Krois, J.; Ekert, T.; Meinhold, L.; Golla, T.; Kharbot, B.; Wittemeier, A.; Dörfer, C.; Schwendicke, F. Deep Learning for the Radiographic Detection of Periodontal Bone Loss. Sci. Rep. 2019, 9, 8495. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Lee, C.; Date, S.; Kashiwagi, Y.; Narukawa, Y.; Nozaki, K.; Murakami, S. Evaluation of dental image augmentation for the severity assessment of periodontal disease. In Proceedings of the 6th Annual Conference on Computational Science and Computational Intelligence, CSCI 2019, Las Vegas, NV, USA, 5–7 December 2019. [Google Scholar]
- Yauney, G.; Rana, A.; Wong, L.C.; Javia, P.; Muftu, A.; Shah, P. Automated process incorporating machine learning segmentation and correlation of oral diseases with systemic health. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference, Berlin, Germany, 23–27 July 2019; pp. 3387–3393. [Google Scholar]
- Alalharith, D.M.; Alharthi, H.M.; Alghamdi, W.M.; Alsenbel, Y.M.; Aslam, N.; Khan, I.U.; Shahin, S.Y.; Dianišková, S.; Alhareky, M.S.; Barouch, K.K. A Deep Learning-Based Approach for the Detection of Early Signs of Gingivitis in Orthodontic Patients Using Faster Region-Based Convolutional Neural Networks. Int. J. Environ. Res. Public Heal. 2020, 17, 8447. [Google Scholar] [CrossRef]
- Bayrakdar, S.K.; Ҫelik, Ö.; Bayrakdar, I.S.; Orhan, K.; Bilgir, E.; Odabaş, A.; Aslan, A.F. Success Of Artificial Intelligence System In Determining Alveolar Bone Loss From Dental Panoramic Radiography Images. Cumhur. Dent. J. 2020, 23, 318–324. [Google Scholar]
- Chang, H.-J.; Lee, S.-J.; Yong, T.-H.; Shin, N.-Y.; Jang, B.-G.; Kim, J.-E.; Huh, K.-H.; Lee, S.-S.; Heo, M.-S.; Choi, S.-C.; et al. Deep Learning Hybrid Method to Automatically Diagnose Periodontal Bone Loss and Stage Periodontitis. Sci. Rep. 2020, 10, 7531. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X. Gingivitis identification via GLCM and artificial neural network. In Medical Imaging and Computer-Aided Diagnosis: Proceeding of 2020 International Conference on Medical Imaging and Computer-Aided Diagnosis (MICAD 2020); Springer: Singapore, 2020; Volume 633, pp. 95–106. [Google Scholar] [CrossRef]
- Lee, J.-H.D.; Jeong, S.-N. Efficacy of deep convolutional neural network algorithm for the identification and classification of dental implant systems, using panoramic and periapical radiographs. Medicine 2020, 99, e20787. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhou, Y.; Chen, J.; Gao, F.; Xu, Y.; Gao, X. Automatic and interpretable model for periodontitis diagnosis in panoramic radiographs. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2020: 23rd International Conference, Lima, Peru, 4–8 October 2020; pp. 454–463. Available online: https://dl.acm.org/doi/abs/10.1007/978-3-030-59713-9_44 (accessed on 25 November 2022).
- Moran, M.B.H.; Faria, M.; Giraldi, G.; Bastos, L.; Inacio, B.D.S.; Conci, A. On using convolutional neural networks to classify periodontal bone destruction in periapical radiographs. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Republic of Korea, 16–19 December 2020; pp. 2036–2039. [Google Scholar] [CrossRef]
- Romm, E.; Li, J.; Kouznetsova, V.L.; Tsigelny, I.F. Machine Learning Strategies to Distinguish Oral Cancer from Periodontitis Using Salivary Metabolites. Adv. Intell. Syst. Comput. 2020, 1252, 511–526. [Google Scholar] [CrossRef]
- Thanathornwong, B.; Suebnukarn, S. Automatic detection of periodontal compromised teeth in digital panoramic radiographs using faster regional convolutional neural networks. Imaging Sci. Dent. 2020, 50, 169–174. [Google Scholar] [CrossRef]
- You, W.; Hao, A.; Li, S.; Wang, Y.; Xia, B. Deep learning-based dental plaque detection on primary teeth: A comparison with clinical assessments. BMC Oral Heal. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Cetiner, D.; Isler, S.; Bakirarar, B.; Uraz, A. Identification of a Predictive Decision Model Using Different Data Mining Algorithms for Diagnosing Peri-implant Health and Disease: A Cross-Sectional Study. Int. J. Oral Maxillofac. Implant. 2021, 36, 952–965. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Zhao, Y.; Zhao, J.; Wang, Y. Dental disease detection on periapical radiographs based on deep convolutional neural networks. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Danks, R.P.; Bano, S.; Orishko, A.; Tan, H.J.; Sancho, F.M.; D’Aiuto, F.; Stoyanov, D. Automating Periodontal bone loss measurement via dental landmark localisation. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 1189–1199. [Google Scholar] [CrossRef]
- Khaleel, B.I.; Aziz, M.S. Using Artificial Intelligence Methods For Diagnosis Of Gingivitis Diseases. J. Phys. Conf. Ser. 2021, 1897, 012027. [Google Scholar] [CrossRef]
- Kouznetsova, V.L.; Li, J.; Romm, E.; Tsigelny, I.F. Finding distinctions between oral cancer and periodontitis using saliva metabolites and machine learning. Oral Dis. 2020, 27, 484–493. [Google Scholar] [CrossRef]
- Li, W.; Liang, Y.; Zhang, X.; Liu, C.; He, L.; Miao, L.; Sun, W. A deep learning approach to automatic gingivitis screening based on classification and localization in RGB photos. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Ma, K.S.-K.; Liou, Y.-J.; Huang, P.-H.; Lin, P.-S.; Chen, Y.-W.; Chang, R.-F. Identifying medically-compromised patients with periodontitis-associated cardiovascular diseases using convolutional neural network-facilitated multilabel classification of panoramic radiographs. In Proceedings of the 2021 International Conference on Applied Artificial Intelligence (ICAPAI), Halden, Norway, 19–21 May 2021. [Google Scholar]
- Moran, M.; Faria, M.; Giraldi, G.; Bastos, L.; Conci, A. Do Radiographic Assessments of Periodontal Bone Loss Improve with Deep Learning Methods for Enhanced Image Resolution? Sensors 2021, 21, 2013. [Google Scholar] [CrossRef]
- Ning, W.; Acharya, A.; Sun, Z.; Ogbuehi, A.C.; Li, C.; Hua, S.; Ou, Q.; Zeng, M.; Liu, X.; Deng, Y.; et al. Deep Learning Reveals Key Immunosuppression Genes and Distinct Immunotypes in Periodontitis. Front. Genet. 2021, 12. [Google Scholar] [CrossRef]
- Wang, C.-W.; Hao, Y.; Di Gianfilippo, R.; Sugai, J.; Li, J.; Gong, W.; Kornman, K.S.; Wang, H.-L.; Kamada, N.; Xie, Y.; et al. Machine learning-assisted immune profiling stratifies peri-implantitis patients with unique microbial colonization and clinical outcomes. Theranostics 2021, 11, 6703–6716. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.; Chen, H.; Liu, Y. A pilot study of a deep learning approach to detect marginal bone loss around implants. BMC Oral Heal. 2022, 22, 1–8. [Google Scholar] [CrossRef]
- Celi, L.A.; Cellini, J.; Charpignon, M.-L.; Dee, E.C.; Dernoncourt, F.; Eber, R.; Mitchell, W.G.; Moukheiber, L.; Schirmer, J.; Situ, J.; et al. Sources of bias in artificial intelligence that perpetuate healthcare disparities—A global review. PLOS Digit. Heal. 2022, 1, e0000022. [Google Scholar] [CrossRef]
- Schwendicke, F.; Golla, T.; Dreher, M.; Krois, J. Convolutional neural networks for dental image diagnostics: A scoping review. J. Dent. 2019, 91, 103226. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Montalvao, C.; Tanaka, R.; Kawai, T.; Bornstein, M.M. The use and performance of artificial intelligence applications in dental and maxillofacial radiology: A systematic review. Dentomaxillofacial Radiol. 2020, 49, 20190107. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Schwendicke, F.; Singh, T.; Lee, J.-H.; Gaudin, R.; Chaurasia, A.; Wiegand, T.; Uribe, S.; Krois, J. Artificial intelligence in dental research: Checklist for authors, reviewers, readers. J. Dent. 2021, 107, 103610. [Google Scholar] [CrossRef] [PubMed]
- Mongan, J.; Moy, L.; Kahn, C.E., Jr. Checklist for artificial intelligence in medical imaging (CLAIM): A guide for authors and reviewers. Radiol. Artif. Intell. 2020, 2. [Google Scholar]
- Rother, C.; Kolmogorov, V.; Blake, A. “GrabCut” interactive foreground extraction using iterated graph cuts. ACM Trans. Graph. (TOG) 2004, 23, 309–314. [Google Scholar]
- Kuhn, M.; Johnson, K. Applied Predictive Modelling; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, J.; Biancardi, A.M.; Jones, O.; Andrew, D. Artificial Intelligence in Periodontology: A Scoping Review. Dent. J. 2023, 11, 43. https://doi.org/10.3390/dj11020043
Scott J, Biancardi AM, Jones O, Andrew D. Artificial Intelligence in Periodontology: A Scoping Review. Dentistry Journal. 2023; 11(2):43. https://doi.org/10.3390/dj11020043
Chicago/Turabian StyleScott, James, Alberto M. Biancardi, Oliver Jones, and David Andrew. 2023. "Artificial Intelligence in Periodontology: A Scoping Review" Dentistry Journal 11, no. 2: 43. https://doi.org/10.3390/dj11020043
APA StyleScott, J., Biancardi, A. M., Jones, O., & Andrew, D. (2023). Artificial Intelligence in Periodontology: A Scoping Review. Dentistry Journal, 11(2), 43. https://doi.org/10.3390/dj11020043







