Abstract
Dental caries is one of the most common human diseases which can occur in both primary and permanent dentitions throughout the life of an individual. Hydroxyapatite is the major inorganic component of human teeth, consequently, nanosized hydroxyapatite (nHAP) has recently attracted researchers’ attention due to its unique properties and potential for caries management. This article provides a contemporary review of the potential beneficial effects of nHAP on caries lesions demonstrated in in vitro studies. Data showed that nHAP has potential to promote mineralization in initial caries, by being incorporated into the porous tooth structure, which resulted from the caries process, and subsequently increased mineral content and hardness. Notably, it is the particle size of nHAP which plays an important role in the mineralization process. Antimicrobial effects of nHAP can also be achieved by metal substitution in nHAP. Dual action property (mineralizing and antimicrobial) and enhanced chemical stability and bioactivity of nHAP can potentially be obtained using metal-substituted fluorhydroxyapatite nanoparticles. This provides a promising synergistic strategy which should be explored in further clinical research to enable the development of dental therapeutics for use in the treatment and management of caries.
1. Introduction
Dental caries is one of the most prevalent and ubiquitous non-communicable diseases worldwide. It is characterised by the progression of demineralization of the dental hard tissue, resulting in an imbalance in the equilibrium between the tooth mineral and biofilm [1]. Previously, the management of caries was driven by the understanding that it was a purely infectious disease and could only be managed invasively by removing all demineralized tissue. However, contemporary treatment strategies to manage caries have changed from the traditional surgical approach to a medical model, which now often includes dietary analysis and advice, oral hygiene instruction, placement of fissure sealants, and mineralization control by topical application of fluoride or other mineralising agents [2]. Among the available mineralization control strategies, fluoride-based treatments have the highest level of supporting evidence. Their widespread use is generally considered the main reason for reducing the incidence of dental caries in most populations. The released fluoride prevents enamel demineralization and reduces caries susceptibility by its incorporation into enamel hydroxyapatite, therefore, stabilizing the crystal structure by forming fluorapatite and lowering the solubility product constant (Ksp) [3]. Consequently, this increases resistance to acid demineralization. However, currently available fluoride therapies have limited efficacy in some individuals, and at the population level, the effect of fluoride in reducing dental caries prevalence is reaching a plateau [4]. More recently, investigators have been developing new mineralization approaches to close this gap in efficacy. Calcium and phosphate-based treatment systems are designed to ensure a constant supply of these ions and aim to directly increase mineral concentration in the environment around the caries lesions [5]. These systems include the use of amorphous calcium phosphate (ACP), functionalized tricalcium phosphate (fTCP), bioactive glass containing calcium sodium phosphosilicate and hydroxyapatite nanoparticles (nHAP) [6].
The enamel rod (prism) is the basic unit of human tooth enamel. Enamel rods are tightly packed hydroxyapatite (HAP) crystals structures which are hexagonal in shape, with sizes up to 70 nm in width and 30 nm in thickness. They provide rigidity to the enamel rod and provide significant strengthen to the enamel [7]. HAP is a mineral form of calcium apatite with the molecular formula Ca10(PO4)6(OH)2 and a calcium-to-phosphorus molar ratio of 1:67. It has a crystal lattice and its chemical composition is most similar to the apatite crystals of the human enamel. HAP also exhibits good biocompatibility and bioactivity [8]. Nanoparticles have a large surface area to volume ratio, which results in them exhibiting different biomedical activities compared with materials of increased size, furthermore, they can possess unique physical properties that make them desirable for use in biology and material science [9]. The synthesis of hydroxyapatite as nanosized particles has the potential to enable an improved physical and chemical characteristic by the enlargement of the reactive surface area. With respect to morphology and structure, the crystal lattice of nHAP is predicted to resemble the tooth enamel better and consequently would have beneficial effects for hard tissues repair.
Although there are a few previous reviews reporting nHAP in dentistry, they are either not contemporary [10] nor discuss its use within a broad scope of dental application [11,12]. One published systematic review reports on the efficacy of nHAP in caries prevention, however, no strong conclusions were made due to only a very limited number of in vivo and in situ studies being evaluated [13]. A significant number of in vitro studies have now investigated the effect of nHAP on dental hard tissues and cariogenic bacteria and biofilms have been conducted. Consequently, this review aims to provide recent novel information and reports on the action of nHAP in caries management, focussing on its effects on the dental hard tissues and cariogenic bacteria.
2. Materials and Methods
2.1. Literature Search Strategy
A literature search was undertaken in PubMed to identify English language publications from 2010 to 2022. The keywords included: (nano-HAP OR HAP-NPs OR nano-hydroxyapatite OR nanohydroxyapatite OR hydroxyapatite OR Hydroxyapatite nanoparticles) AND (caries OR carious OR cariogenic OR tooth decay).
2.2. Study Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
- (1)
- Laboratory studies.
- (2)
- Studies related to antimicrobial or biocompatibility effect of nHAP on cariogenic species.
- (3)
- Studies related to the mineralization effect of nHAP on tooth hard tissue (enamel, dentine, cementum) (this includes remineralization and modulation of demineralization on hard tissue).
2.2.2. Exclusion Criteria
- (1)
- Studies not reported using the English language.
- (2)
- Studies on HAP that were not in the nano-scale range.
- (3)
- Studies not related to dental caries and abstracts without the associated full papers.
- (4)
- Case reports, conference papers, book chapters, patents, letters to the editor, systematic reviews, meta-analyses, and literature review papers.
3. Results
A total of 1052 potentially eligible articles published up to February 2022 were identified using the search terms described (Figure 1). After screening titles and abstracts, 125 articles remained for further analysis. Full-text reading was undertaken and 38 articles were finalized for inclusion in this review. Among these articles, twenty-nine studies investigated the effects of nHAP on enamel mineralization (Table 1), five studies were reported on dentine remineralization and one study on cementum (Table 2), and three studies investigated the antimicrobial effect of nHAP on cariogenic bacteria (Table 3). Twenty-two studies used toothpaste containing nHAP, nine studies used an aqueous slurry of nHAP; nHAP was also incorporated in other formats such as tooth cream, tooth gel, and resin infiltration in three studies.
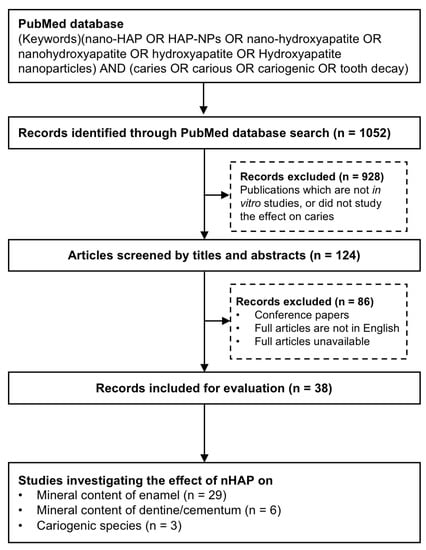
Figure 1.
Flowchart of study selection process for final review.
3.1. Effect of nHAP on Mineralization of Enamel
Table 1 presents the main findings of the studies which investigated the role of nHAP on enamel mineralization. Enamel specimens were prepared using either bovine or human teeth; the majority (28 out of 29) of studies generated early artificial caries lesions by chemical means on enamel specimens prior to treatment to mimic incipient enamel caries. These caries lesions were then treated with a range of treatments including nHAP formulations. The format of the nHAP containing products included toothpastes (18 studies) [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31], slurries prepared with nHAP powder (eight studies) [26,32,33,34,35,36,37,38], resin infiltration (one study) [39], cream (one study) [40], and gel (one study) [41]. Nineteen studies employed microhardness to evaluate the effect of nHAP on remineralization. Eighteen of these studies found that products with a nHAP component demonstrated increased enamel surface microhardness when compared with control groups. Five studies measured the mineral recovery from the enamel lesion after nHAP treatment, with all studies reporting that the nHAP treatment groups exhibited higher mineral recovery when compared with negative controls. Other studies also demonstrated the repair of surface defects and a smoother uniform distribution of Ca/P particles in the surface area exposed to nHAP when compared with other treatment groups [14,15,16]. A few studies reported nHAP used in combination with fluoride revealed a significant reduction in lesion depth in contrast to other agents [16,17].
3.2. Effect of nHAP on Mineralization of Dentine/Cementum
Table 2 presents the key findings from the six studies which investigated the role of nHAP on dentine or cementum mineralization, with inconsistent findings being reported. The format of the nHAP products used were toothpaste (four studies) [19,20,42,43], slurry prepared using nHAP powder (one study) [44], and gel (one study) [41]. One study identified a significant increase in surface microhardness following nHAP treatment of cementum when compared with fluoride treatment [41]. While another study demonstrated formation of a partially filled remineralized layer after nHAP treatment in contrast to the group treated with 30% pre-reacted glass ionomer (S-PRG) filler, which resulted in formation of a fully remineralized layer on dentine [42]. Similarly, a further study showed reduction in lesion depth when nHAP was used in combination with fluoride [18]. Notably, one study claimed that nHAP was unable to reduce microhardness loss [19].
3.3. Effect of Hydroxyapatite Nanoparticles on Cariogenic Bacteria
Table 3 presents the key findings of studies which investigated the effect of nHAP on cariogenic bacteria and biofilms. One study showed that pure nHAP tends to enhance Streptococcus mutans biofilm formation; the mechanisms behind could be that nHAP was capable to increase glucosyltransferase transcription which resulted in an increase in production of insoluble glucans [45]. However, there were different results when calcium ions were substituted partially with metal ions. A study investigated the effect of loading zinc ions with nHAP in combination with alendronate grafted poly-acrylic acid, concluding that zinc ions significantly enhanced the materials’ antibacterial effect against S. mutans in contrast to samples where nHAP was used alone [32]. In another study, toothpaste with zinc substituted nHAP was also found to be less antimicrobial against S. mutans when compared to the one with strontium (Sr), magnesium (Mg), and fluoride (F) substituted nHAP [46].

Table 1.
Summary of the effect of hydroxyapatite nanoparticles on enamel mineralization.
Table 1.
Summary of the effect of hydroxyapatite nanoparticles on enamel mineralization.
| Reference | Experimental Groups | Study Model and Design | nHAP Details | Main Findings |
|---|---|---|---|---|
| Huang et al. 2010 [33] |
|
|
|
|
| Huang et al. 2011 [34] |
|
|
|
|
| Tschoppe et al. 2011 [20] |
|
|
|
|
| Swarup et al. 2012 [35] |
|
|
|
|
| Comar et al., 2013 [19] |
|
|
|
|
| Mielczarek 2014 [21] |
|
|
|
|
| Haghgoo et al. 2014 [22] |
|
|
|
|
| de Carvalho et al. 2014 [23] |
|
|
|
|
| Vyavhare et al. 2015 [24] |
|
|
|
|
| Haghgoo et al. 2016 [25] |
|
|
|
|
| Krishnan et al., 2016 [26] |
|
|
|
|
| Andrade Neto et al. 2016 [39] |
|
|
|
|
| Ebadifar et al. 2017 [27] |
|
|
|
|
| Kamath et al. 2017 [40] |
|
|
|
|
| Sharma et al. 2017 [28] |
|
|
|
|
| Daas et al. 2017 [18] |
|
|
|
|
| Alsherif et al. 2017 [36] |
|
|
|
|
| Talaat et al. 2018 [16] |
|
|
|
|
| Juntavee et al., 2018 [41] |
|
|
|
|
| Madhusudanan et al. 2018 [29] |
|
|
|
|
| Memarpour et al. 2019 [37] |
|
|
|
|
| Thimmaiah et al. 2019 [30] |
|
|
|
|
| Hanafay et al. 2019 [47] |
|
|
|
|
| Vijayasankari et al. 2019 [15] |
|
|
|
|
| Manchery et al. 2019 [17] |
|
|
|
|
| Bossu et al. 2019 [14] |
|
|
|
|
| Joshi et al. 2019 [31] |
|
|
|
|
| Konagala et al., 2020 [38] |
|
|
|
|
| Xu et al. 2020 [32] |
|
|
|
|
AFM: Atomic force microscopy; APF: Acidulated phosphate fluoride; CPP-ACP: Casein Phosphopeptide-amorphous Calcium Phosphate; Ca: Calcium; nHAP: Hydroxyapatite nanoparticles; D%: Demineralization %, R%: Remineralization; FTIR: Fourier Transform Infrared Spectroscopy; NaF: Sodium Fluoride; P: Phosphorus; SEM: Scanning Electron Microscope; SEM-EDX: Scanning Electron Microscope and Energy dispersive X-ray analysis; TCP: Tri-calcium phosphate; f-TCP: Functionalized tri-calcium phosphate; Sr: Strontium; TEM: Transmission electron microscopy; XRD: X-ray diffraction; Zn: Zinc.

Table 2.
Summary of the effect of hydroxyapatite nanoparticles on dentine/cementum mineralization.
Table 2.
Summary of the effect of hydroxyapatite nanoparticles on dentine/cementum mineralization.
| Reference | Experimental Groups | Study Model and Design | nHAP Details | Main Findings on nHAP |
|---|---|---|---|---|
| Tschoppe et al., 2011 [20] |
|
|
|
|
| Comar et al., 2013 [19] |
|
|
|
|
| Besinis et al., 2014 [44] |
|
|
|
|
| Iijma et al., 2019 [42] |
|
|
|
|
| Juntavee et al., 2018 [41] |
|
|
|
|
| Leal et al., 2020 [43] |
|
|
|
|
Ca: Calcium; CPP-ACP: Casein phosphopeptide-amorphous calcium phosphate; EDX: Energy-dispersive X-ray spectroscopy; F: Fluoride; micro-CT: Micro-Computed tomography; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assay; NaF: Sodium fluoride; nHAP: Hydroxyapatite nanoparticles; P: Phosphorus; SEM: Scanning electron microscope; SiO2: Silicon dioxide; S-PRG: Surface reaction type pre-reacted glass ionomer filler; TEM: Transmission electron microscopy.

Table 3.
Summary of the antimicrobial effect of hydroxyapatite nanoparticles.
Table 3.
Summary of the antimicrobial effect of hydroxyapatite nanoparticles.
| Reference | Experimental Groups | Study Model and Design | nHAP Details | Main Findings on nHAP |
|---|---|---|---|---|
| Park et al. 2019 [45] |
|
|
|
|
| Xu et al. 2020 [32] |
|
|
|
|
| Ionescu et al. 2020 [46] |
|
|
|
|
F: Fluoride; IR: Inhibition ratio; Mg: Magnesium; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide Cell viability assay; nHAP: Hydroxyapatite nanoparticles; Sr: Strontium; Zn: Zinc.
4. Discussion
While a recent systematic review, which was based on in vivo and in situ studies of nHAP in caries prevention, has been undertaken with a proper risk of bias evaluation [10], only four studies were included in the analysis. The major reasons for exclusion of studies were lack of a control group, a short study duration (less than 1 month), and not reporting an Institutional Review Board (IRB) approval. This very low number of included studies resulted in inconclusive evidence for the efficacy of nHAP. This highlights the need for more well controlled clinical trials to be undertaken in the future. Ideally, a risk of bias of the included studies should also be provided in the current review. In particular, studies that provide no details of the nHAP particle size and concentrations compromise the quality of the review.
A wide range of nHAP structures, such as nano-rods, microspheres, or hierarchically nano-structures, are reported in the articles reviewed here, and these potentially influence the effects exerted by nHAP. Indeed, it has been previously demonstrated that the morphology of the nanoparticles will affect the speed of Ca2+ release, as Ca2+ in microspheres appears to release more rapidly than hierarchically nano-structures [48]. The synthesized nHAP in the reviewed studies was prepared by using several different techniques, including a hydrothermal method, a sol-gel method, and a wet-chemistry method, which are believed to be able to provide high phase purity and grain sizes of between 20–50 nm [49]. Aqueous nHAP slurry in distilled water provides a relatively simple approach to assess the effect of the nanoparticles on the caries lesion. However, it may not be highly clinically relevant. Furthermore, nHAP was incorporated into several different formats of dental products, including toothpastes, creams, and restorative materials. Studies used either commercialized dental products, which contained nHAP, or incorporated nHAP into existing toothpastes by a certain weight percentage. One study attempted to incorporate nHAP into the resin infiltration, and they found that this test material caused higher enamel resistance against demineralization compared with the control nHAP-free infiltration [39].
The most common substrate used in the studies was enamel with chemically-induced artificial early caries to mimic the clinical situation of incipient caries (non-cavitied) presented on the coronal side of the tooth. The human enamel includes morphologically aligned, parallel, up to 70 nm in width and 30 nm in thickness, micron-long carbonated hydroxyapatite nanocrystals, bundled either into 5-μm-wide rods (prisms) or a space-filling interrod arrangement [50]. When caries (demineralization) are initiated, the enamel prism junctions are enlarged and destruction of the interprismatic structure is observed [51], while the size of nHAP enhances its penetration into the interprismatic enamel space [52]. Moreover, a few matrix proteins remain embedded in the enamel’s organic component and these proteins operate as a scaffold for conduction of nHAPs so they can be easily deposited within the nano-gaps [53]. These proteins enable the capture of the minerals and subsequently facilitate mineral apatite formation [52]. In comparison, fluoride has been shown to predominately integrate into the surface of the initial caries lesion, while being less effective in deep sub-surface lesions and this effect has been found alongside both high fluoride and low fluoride treatment [54]. This lamination effect results in a limitation in the dose-dependent effectiveness of fluoride products, therefore, creating a negative effect for complete remineralization into the deeper zones and subsequently limiting application [55]. An in situ study, which compared the effectiveness of two toothpastes containing nHAP or fluoride in promoting remineralization of initial enamel caries, found that while nHAP achieved comparable efficacy with 500 ppm fluoride, it enabled a more homogenous lesion of remineralization when compared with the fluoride induced lesion surface lamination following transverse microradiography analysis [56]. Therefore, nHAP should be developed and applied to enhance the effect of existing fluoride therapies rather than to replace them, but it still provides an alternative option to those who are reluctant to use fluoride.
Root dentine rather than cementum is the most frequently used substrate when investigating root caries. This is due to the cementum being relatively thin (20 to 50 microns near the cemento-enamel junction) [57]. Therefore, the dentine beneath the cementum is considered the major component in root caries progression. The results of the studies on dentine are somewhat inconsistent, which might be due to the different experimental conditions and different particle sizes used. Comar et al. [19] found that an nHAP paste was unable to reduce the loss of dentine surface hardness, which may be due to the relatively large size of the nHAP particle used in their study, which was 100 nm and already reached the maximum size due to the definition of a nanomaterial. It is plausible that a mechanical imbrication of the paste into the inter-tubular dentine space due to the size of the particles occurred. Besinis et al. [44] reduced fully demineralized dentine blocks to a collagen matrix prior to the nHAP treatment. They found that the nHAP with a particles size of ~20 nm was able to locally infiltrate into the scaffolds with increased levels of calcium and phosphate deposition. Both Tschoppe et al. [20] and Leal et al. [43] demonstrated an increased remineralizing effect on dentine when nHAP was used alone or with fluoride; the particles sizes of their studies were less than 20 nm. Juntavee et al. [41] showed that nHAP containing gels had a higher capability for the remineralization of cementum when compared with fluoride varnish, which was possibly associated with the nanoparticle size as it was capable of constructive interdigitation with the cementum structure. Future studies should focus on the mechanisms of nHAP-induced remineralization at a more in-depth level to determine the role of nHAP in the calcium phosphate formation process and to determine how the size of the nHAP affects this process.
The antimicrobial effect of nHAP was negligeable given its high biocompatibility and low toxicity. In addition, it was also found that the formation of S. mutan biofilm was enhanced when S. mutans was co-cultured with the presence of 5% nHAP suspension [45]. Caries result from an ecological imbalance in the physiological equilibrium between oral microbial biofilms and tooth minerals. Maintaining tooth minerals and controlling oral microbial biofilms are essential for the control of caries [58]. Aimed at a dual action effect nHAP in both mineralization and antimicrobial effect, studies attempted to develop a hybrid nHAP by substituting calcium ions with metallic ions. Silver is a well-known antimicrobial agent due to its broad spectrum, low toxicity, and lack of cross-spectrum bacterial resistance [59]. Silver can be incorporated into the crystal structure of hydroxyapatite to produce silver-containing hydroxyapatite [60] to give a formula of Ca10−xAgx(PO4)6(OH)2 with 0.0 ≤ x ≤ 0.5. Notably, a relatively small number of calcium ions are substituted with silver ions [61,62]. This silver-containing hydroxyapatite has been shown to reduce bacterial adhesion and to have minimal tissue cytotoxicity [60], however, aesthetic effects may be compromised due to the fact that silver has a black coloration when it is oxidized, which readily occurs in air [63]. Zinc is another commonly used metal which can substitute for the calcium ion in nHAP. In the orthopaedic field, it is regarded as an essential trace element that promotes bone formation and exhibits antimicrobial effects [3]. Several studies involving Zn-substituted apatite containing a low amount of zinc (<1 wt%) have been reported, and demonstrate the material to exhibit good bioactivity with antibacterial properties [64,65,66]. Indeed, Chung et al. [67] and Stanic et al. [66] observed a reduction of bacterial strains Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Candida albicans (C. albicans), and S. mutans cultured on Zn-substituted HAP. Furthermore, strontium is a divalent cation that is located in the same column of the periodic table as calcium. Consequently, strontium has chemical properties that are similar to those of calcium, and it can partly substitute for calcium and be incorporated into the crystal lattice structure of hydroxyapatite. Sr-substituted HAP also demonstrates good bioactivity and can directly bond to bony tissue under non-weight-bearing conditions [68]. A synergistic effect of strontium and fluoride on hydroxyapatite formation has been demonstrated in previous studies, and strontium was able to aid remineralization of a carious lesion in the presence of fluoride [69,70]. Sr-substituted nHAP has been shown to display enhanced biocompatibility compared with pure nHAP [26]. However, reports on the application of nanosized metal-substituted HAP in caries management are limited. One previous study found that the inhibition ratio of synthesized Zn-substituted nHAP against S. mutans was significantly higher compared with the pure nHAP group [32]. A further study used a toothpaste containing Mg-Sr-F substituted hydroxyapatite and demonstrated that it exerted a decreased rate of early colonisation of S. mutans in comparison with those containing Zn-substituted nHAP after 12 h. This may be due to the synergistic effect of fluoride and strontium in caries management [46,69,70]. Notably, instead of calcium being substituted by metal cations, fluorohydroxyapatite (Ca5(PO4)3(OH)1−xFx) could be formed by exchanging fluoride ions for the hydroxyl group in the HAP; the isotropic distribution of the charge on fluoride anions allows for a better fit in the lattice structure compared with the larger asymmetric hydroxyl ions. This produces a more ordered apatite structure, which is characterized by increased thermal and chemical stability compared with HAP [63,71]. Future studies on metal-substituted fluorohydroxyapatite nanoparticles could identify this as a promising synergistic strategy for caries management.
5. Conclusions
This review of the current literature concludes that nHAP can promote mineralization in early caries lesions, potentially by being incorporated into the porous tooth structure caused by the caries process and thereby increasing its mineral content and hardness. The particle size of nHAP plays an important role in this remineralization process. The antimicrobial effect of nHAP can be potentially achieved by using metal substituted nHAP. All the above properties highlight nHAP as a promising bioactive material for potential use in the management of dental caries, which should be explored in further clinical research.
Author Contributions
Conceptualization, P.R.C. and M.L.M.; writing—original draft preparation, E.I.; writing—review and editing, J.R., M.E., P.R.C. and M.L.M.; funding acquisition, J.R., P.R.C. and M.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by New Zealand Dental Research Foundation (RF8.02 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Featherstone, J.D. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Mei, M.L.; Lo, E.C. Use of fluorides in dental caries management. Gen. Dent. 2010, 58, 37–43. [Google Scholar] [PubMed]
- Ionescu, A.C.; Degli Esposti, L.; Iafisco, M.; Brambilla, E. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci. Rep. 2022, 12, 5994. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cabezas, C.; Fernandez, C.E. Recent Advances in Remineralization Therapies for Caries Lesions. Adv. Dent. Res. 2018, 29, 55–59. [Google Scholar] [CrossRef]
- Ekambaram, M.; Mohd Said, S.N.B.; Yiu, C.K.Y. A Review of Enamel Remineralisation Potential of Calcium- and Phosphate-based Remineralisation Systems. Oral Health Prev. Dent. 2017, 15, 415–420. [Google Scholar]
- Yong, D.; Choi, J.J.E.; Cathro, P.; Cooper, P.R.; Dias, G.; Huang, J.; Ratnayake, J. Development and Analysis of a Hydroxyapatite Supplemented Calcium Silicate Cement for Endodontic Treatment. Materials 2022, 15, 1176. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 2009, 30, 478–483. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhao, I.S.; Mei, M.L.; Li, Q.; Yu, O.Y.; Chu, C.H. Use of Silver Nanomaterials for Caries Prevention: A Concise Review. Int. J. Nanomed. 2020, 15, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanotechnology and its role in caries therapy. Adv. Dent. Res. 2012, 24, 53–57. [Google Scholar] [CrossRef]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental Applications of Systems Based on Hydroxyapatite Nanoparticles-An Evidence-Based Update. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Baciut, M.; Baciut, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Wolf, T.G.; Campus, G.; Carvalho, T.S. Efficacy of nano-hydroxyapatite on caries prevention-a systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 3373–3381. [Google Scholar] [CrossRef]
- Bossù, M.; Saccucci, M.; Salucci, A.; Di Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnol. 2019, 17, 17. [Google Scholar] [CrossRef]
- Vijayasankari, V.; Asokan, S.; GeethaPriya, P.R. Evaluation of remineralisation potential of experimental nano hydroxyapatite pastes using scanning electron microscope with energy dispersive X-ray analysis: An in-vitro trial. Eur. Arch. Paediatr. Dent. 2019, 20, 529–536. [Google Scholar] [CrossRef]
- Talaat, D.M.; Abdelrahman, A.A.M.; Abdelaziz, R.H.; Nagy, D. Effect of two remineralizing agents on initial caries-like lesions in young permanent teeth: An in vitro study. Dent. Res. J. 2018, 19, 1181–1188. [Google Scholar] [CrossRef]
- Manchery, N.; John, J.; Nagappan, N.; Subbiah, G.K.; Premnath, P. Remineralization potential of dentifrice containing nanohydroxyapatite on artificial carious lesions of enamel: A comparative in vitro study. Dent. Res. J. 2019, 16, 310. [Google Scholar] [CrossRef]
- Daas, I.; Badr, S.; Osman, E. Comparison between fluoride and nano-hydroxyapatite in remineralizing initial enamel lesion: An in vitro study. J. Contemp. Dent. Pract. 2018, 19, 306–312. [Google Scholar] [CrossRef]
- Comar, L.P.; Souza, B.M.; Gracindo, L.F.; Buzalaf, M.A.R.; Magalhães, A.C. Impact of experimental nano-HAP pastes on bovine enamel and dentin submitted to a pH cycling model. Braz. Dent. J. 2013, 24, 273–278. [Google Scholar] [CrossRef]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef]
- Mielczarek, A.; Michalik, J. The effect of nano-hydroxyapatite toothpaste on enamel surface remineralization. An in vitro study New nano-formulations for oral and maxillo-facial implants View project The effect of nano-hydroxyapatite toothpaste on enamel surface remineralization. An in vitro study. Am. J. Dent. 2014, 27, 287–290. [Google Scholar]
- Haghgoo, R.; Rezvani, M.B.; Zeinabadi, M.S.; Zeinabadi, M.S. Comparison of Nano-Hydroxyapatite and Sodium Fluoride Mouthrinse for Remineralization of Incipient Carious Lesions. J. Dent. 2014, 11, 406–410. [Google Scholar]
- de Carvalho, F.G.; Vieira, B.R.; Santos, R.L.D.; Carlo, H.L.; Lopes, P.Q.; de Lima, B. In Vitro Effects of Nano-hydroxyapatite Paste on Initial Enamel Carious Lesions. Pediatr. Dent. 2014, 36, 85–89. [Google Scholar]
- Vyavhare, S.; Sharma, D.S.; Kulkarni, V.K. Effect of three different pastes on remineralization of initial enamel lesion: An in vitro study. J. Clin. Pediatr. Dent. 2015, 39, 149–160. [Google Scholar] [CrossRef]
- Haghgoo, R.; Ahmadvand, M.; Moshaverinia, S. Remineralizing effect of topical novamin and nanohydroxyapatite on caries-like lesions in primary teeth. J. Contemp. Dent. Pract. 2016, 17, 645–649. [Google Scholar] [CrossRef]
- Krishnan, V.; Bhatia, A.; Varma, H. Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration. Dent. Mater. 2016, 32, 646–659. [Google Scholar] [CrossRef]
- Ebadifar, A.; Nomani, M.; Fatemi, S.A. Effect of nano-hydroxyapatite toothpaste on microhardness of artificial carious lesions created on extracted teeth. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 14–17. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, A.; Shenoy, R.; Suprabha, B.S. Comparative evaluation of Nano-hydroxyapatite and casein Phosphopeptide-amorphous calcium phosphate on the remineralization potential of early enamel lesions: An in vitro study. J. Orofac. Sci. 2017, 9, 28–33. [Google Scholar]
- Madhusudanan, P.; Praveena, S.V. Comparative Evaluation of Surface Microhardness of Artificially Demineralized Human Enamel with Nano Hydroxyapatite, Calcium Phosphate, and Potassium Nitrate Remineralizing Agents: An In Vitro Study. Cons. Dent. Endo J. 2018, 3, 50–55. [Google Scholar] [CrossRef]
- Thimmaiah, C.; Shetty, P.; Shetty, S.B.; Natarajan, S.; Thomas, N.A. Comparative analysis of the remineralization potential of cpp-acp with fluoride, tri-calcium phosphate and nano hydroxyapatite using SEM/EDx-an in vitro study. J. Clin. Exp. Dent. 2019, 11, e1120–e1126. [Google Scholar] [CrossRef]
- Joshi, C.; Gohil, U.; Parekh, V.; Joshi, S. Comparative evaluation of the remineralizing potential of commercially available agents on artificially demineralized human enamel: An in vitro study. Contemp. Clin. Dent. 2019, 10, 605–613. [Google Scholar] [CrossRef]
- Xu, X.; Wang, N.; Wu, M.; Wang, J.; Wang, D.; Chen, Z.; Xie, J.; Ding, C.; Li, J. Programmed antibacterial and mineralization therapy for dental caries based on zinc-substituted hydroxyapatite/ alendronate-grafted polyacrylic acid hybrid material. Colloids Surf. B Biointerfaces 2020, 194, 111206. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Combined effects of nano-hydroxyapatite and Galla chinensis on remineralisation of initial enamel lesion in vitro. J. Dent. 2010, 38, 811–819. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Swarup, J.; Rao, A. Enamel surface remineralization: Using synthetic nanohydroxyapatite. Contemp. Clin. Dent. 2012, 3, 433–436. [Google Scholar]
- Alsherif, A.A.; Mohammed Elbardisy, D.; Ameen Taiema, D. Efficacy of nanohydroxyapatite versus acidulated phosphate fluoride on initial demineralized enamel surface (in vitro study). Egypt. Dent. J. 2017, 63, 790. [Google Scholar]
- Memarpour, M.; Shafiei, F.; Rafiee, A.; Soltani, M.; Dashti, M.H. Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: An in vitro study. BMC Oral Health 2019, 19, 92. [Google Scholar] [CrossRef]
- Konagala, R.K.; Mandava, J.; Anwarullah, A.; Uppalapati, L.V.; Karumuri, S.; Angadala, P.L. Synergistic Effect of Arginine on Remineralization Potential of Fluoride Varnish and Nanohydroxyapatite on Artificial Caries Lesions: An In Vitro Study. J. Contemp. Dent. Pract. 2020, 21, 1048–1053. [Google Scholar] [CrossRef]
- Andrade Neto, D.M.; Carvalho, E.V.; Rodrigues, E.A.; Feitosa, V.P.; Sauro, S.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Rodrigues, L.K.; Fechine, P.B. Novel hydroxyapatite nanorods improve anti-caries efficacy of enamel infiltrants. Dent. Mater. 2016, 32, 784–793. [Google Scholar] [CrossRef]
- Kamath, P.; Nayak, R.; Kamath, S.U.; Pai, D. A comparative evaluation of the remineralization potential of three commercially available remineralizing agents on white spot lesions in primary teeth: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2017, 35, 229–237. [Google Scholar]
- Juntavee, N.; Juntavee, A.; Plongniras, P. Remineralization potential of nano-hydroxyapatite on enamel and cementum surrounding margin of computer-aided design and computer-aided manufacturing ceramic restoration. Int. J. Nanomed. 2018, 13, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Ishikawa, R.; Kawaguchi, K.; Ito, S.; Saito, T.; Mizoguchi, I. Effects of pastes containing ion-releasing particles on dentin remineralization. Dent. Mater. J. 2019, 38, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.M.C.; Vinícius, M.; Dos Santos, B.; da Silva Filho, E.C.; de Carvalho, A.L.M.; Tabchoury, C.P.M.; Vale, G.C. Development of an experimental dentifrice with hydroxyapatite nanoparticles and high fluoride concentration to manage root dentin demineralization. Int. J. Nanomed. 2020, 15, 7469–7479. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Van Noort, R.; Martin, N. Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent. Mater. 2014, 30, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Sutherland, J.B.; Rafii, F. Effects of nano-hydroxyapatite on the formation of biofilms by Streptococcus mutans in two different media. Arch. Oral Biol. 2019, 107, 104484. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Garcia-Godoy, F.; Brambilla, E. Substituted nano-hydroxyapatite toothpastes reduce biofilm formation on enamel and resin-based composite surfaces. J. Funct. Biomater. 2020, 11, 36. [Google Scholar] [CrossRef]
- Hanafy, R.A.; El-Fattah, A.A.; Mostafa, D.; Kandil, S. Biomimetic chitosan against bioinspired nanohydroxyapatite for repairing enamel surfaces. Bioinspire Biomim. Nanobiomater. 2020, 9, 85–94. [Google Scholar] [CrossRef]
- Carmona, F.J.; Dal Sasso, G.; Bertolotti, F.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. The role of nanoparticle structure and morphology in the dissolution kinetics and nutrient release of nitrate-doped calcium phosphate nanofertilizers. Sci. Rep. 2020, 10, 12396. [Google Scholar] [CrossRef]
- Sanosh, K.P.; Chu, M.C.; Balakrishnan, A.; Kim, T.N.; Cho, S.J. Preparation and characterization of nano-hydroxyapatite powder using sol-gel technique. Bull. Mater. Sci. 2009, 32, 465–470. [Google Scholar] [CrossRef]
- Beniash, E.; Stifler, C.A.; Sun, C.Y.; Jung, G.S.; Qin, Z.; Buehler, M.J.; Gilbert, P. The hidden structure of human enamel. Nat. Commun. 2019, 10, 4383. [Google Scholar] [CrossRef]
- Frank, R.M. Structural events in the caries process in enamel, cementum, and dentin. J. Dent. Res. 1990, 69, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kunin, A.A.; Evdokimova, A.Y.; Moiseeva, N.S. Age-related differences of tooth enamel morphochemistry in health and dental caries. EPMA J. 2015, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Lubarsky, G.V.; Lemoine, P.; Meenan, B.J.; Deb, S.; Mutreja, I.; Carolan, P.; Petkov, N. Enamel proteins mitigate mechanical and structural degradations in mature human enamel during acid attack. Mater. Res. Express. 2014, 1, 025404. [Google Scholar] [CrossRef]
- Hellwig, E.; Altenburger, M.; Attin, T.; Lussi, A.; Buchalla, W. Remineralization of initial carious lesions in deciduous enamel after application of dentifrices of different fluoride concentrations. Clin. Oral Investig. 2010, 14, 265–269. [Google Scholar] [CrossRef]
- Marinho, V.C.; Chong, L.Y.; Worthington, H.V.; Walsh, T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2016, 7, CD002284. [Google Scholar]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.d.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef]
- Nanci, A. Ten Cate’s Oral Histology Development, Structure, and Function. Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. A novel dual-action antimicrobial peptide for caries management. J. Dent. 2021, 111, 103729. [Google Scholar] [CrossRef]
- Peng, J.J.; Botelho, M.G.; Matinlinna, J.P. Silver compounds used in dentistry for caries management: A review. J. Dent. 2012, 40, 531–541. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Singh, B.; Dubey, A.K.; Kumar, S.; Saha, N.; Basu, B.; Gupta, R. In vitro biocompatibility and antimicrobial activity of wet chemically prepared Ca10−xAgx(PO4)6(OH)2 (0.0 ≤ x ≤ 0.5) hydroxyapatites. Mat. Sci. Eng. C-Mater. 2011, 31, 1320–1329. [Google Scholar] [CrossRef]
- Feng, Q.L.; Kim, T.N.; Wu, J.; Park, E.S.; Kim, J.O.; Lim, D.Y.; Cui, F.Z. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Film. 1998, 335, 214–219. [Google Scholar] [CrossRef]
- Mei, M.L.; Lo, E.C.M.; Chu, C.H. Arresting Dentine Caries with Silver Diamine Fluoride: What’s Behind It? J. Dent. Res. 2018, 97, 751–758. [Google Scholar] [CrossRef]
- Wang, X.; Ito, A.; Sogo, Y.; Li, X.; Oyane, A. Zinc-containing apatite layers on external fixation rods promoting cell activity. Acta Biomater. 2010, 6, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ojima, K.; Naito, H.; Ichinose, N.; Tateishi, T. Preparation, solubility, and cytocompatibility of zinc-releasing calcium phosphate ceramics. J. Biomed. Mater. Res. 2000, 50, 178–183. [Google Scholar] [CrossRef]
- Stanic, V.; Dimitrijevic, S.; Antic-Stankovic, J.; Mitric, M.; Jokic, B.; Plecas, I.B.; Raicevic, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Chung, R.J.; Hsieh, M.F.; Huang, C.W.; Perng, L.H.; Wen, H.W.; Chin, T.S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J. Biomed. Mater. Res. B 2006, 76B, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.X.; Chiu, K.Y.; Lu, W.W.; Wang, Y.; Zhang, Y.G.; Hao, L.B.; Li, Z.Y.; Lam, W.M.; Lu, S.B.; Luk, K.D. Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials 2006, 27, 4348–4355. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. J. Dent. 2021, 108, 103633. [Google Scholar] [CrossRef]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. The effects of strontium-doped bioactive glass and fluoride on hydroxyapatite crystallization. J. Dent. 2021, 105, 103581. [Google Scholar] [CrossRef]
- Sipert, C.R.; Hussne, R.P.; Nishiyama, C.K.; Torres, S.A. In vitro antimicrobial activity of Fill Canal, Sealapex, Mineral Trioxide Aggregate, Portland cement and EndoRez. Int. Endod. J. 2005, 38, 539–543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).