Testing the Efficacy of the Synthesis of Iron Antimony Sulfide Powders from Single Source Precursors
Abstract
Simple Summary
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Instrumentation
2.3. Synthesis of Antimony(III) Diethyldithiocarbamate, [Sb(S2CN(Et)2)3] (1)
2.4. Synthesis of Iron(III) Diethyldithiocarbamate, [Fe(S2CN(Et)2)3] (2)
2.5. Synthesis of the Sb2S3 System by Solvent-Less Thermolysis
2.6. Synthesis of Fe-Sb-S System by Solvent-Less Thermolysis Method
3. Results and Discussion
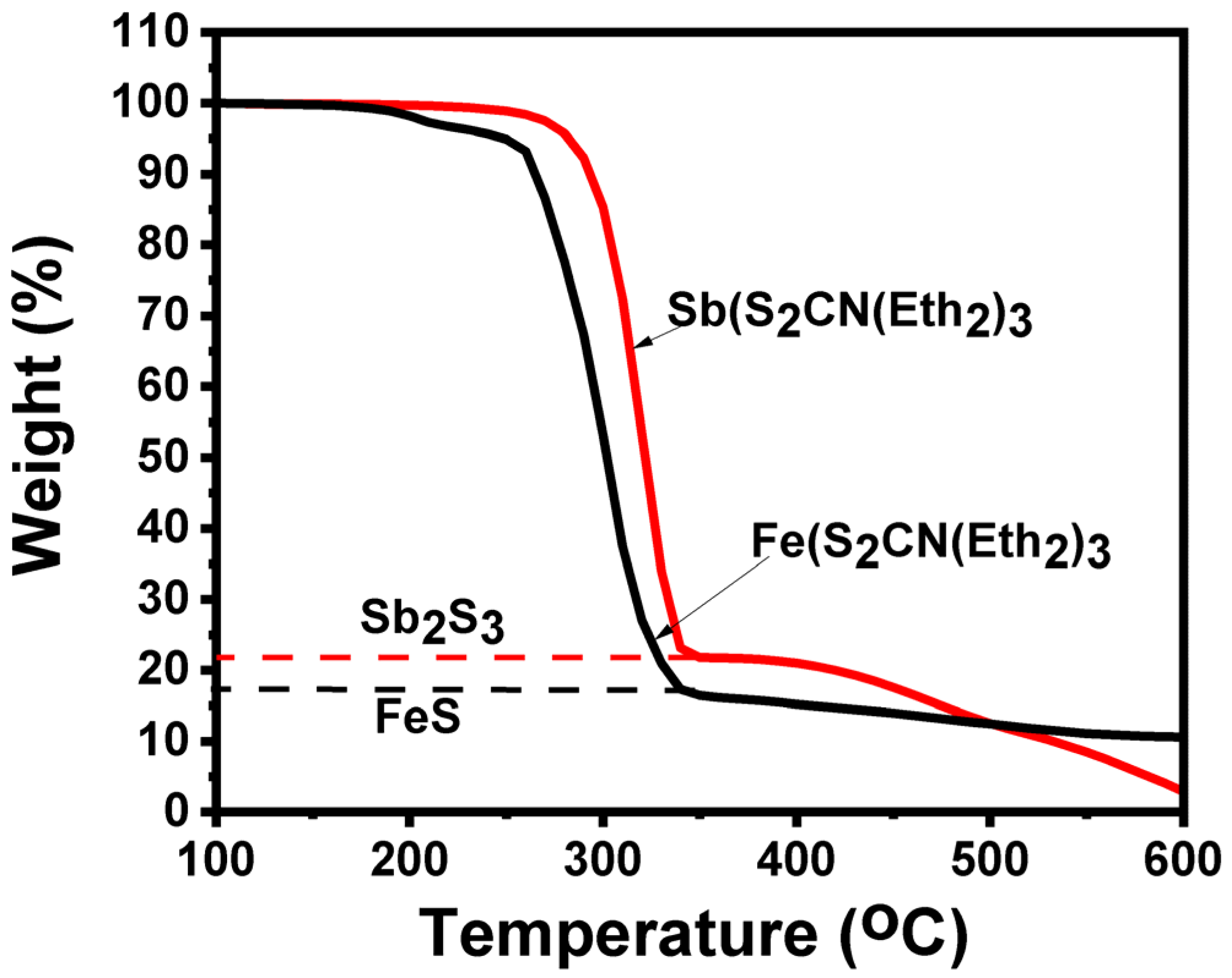
3.1. Thermogravimetric Analysis (TGA)
3.2. X-ray Diffraction (XRD)
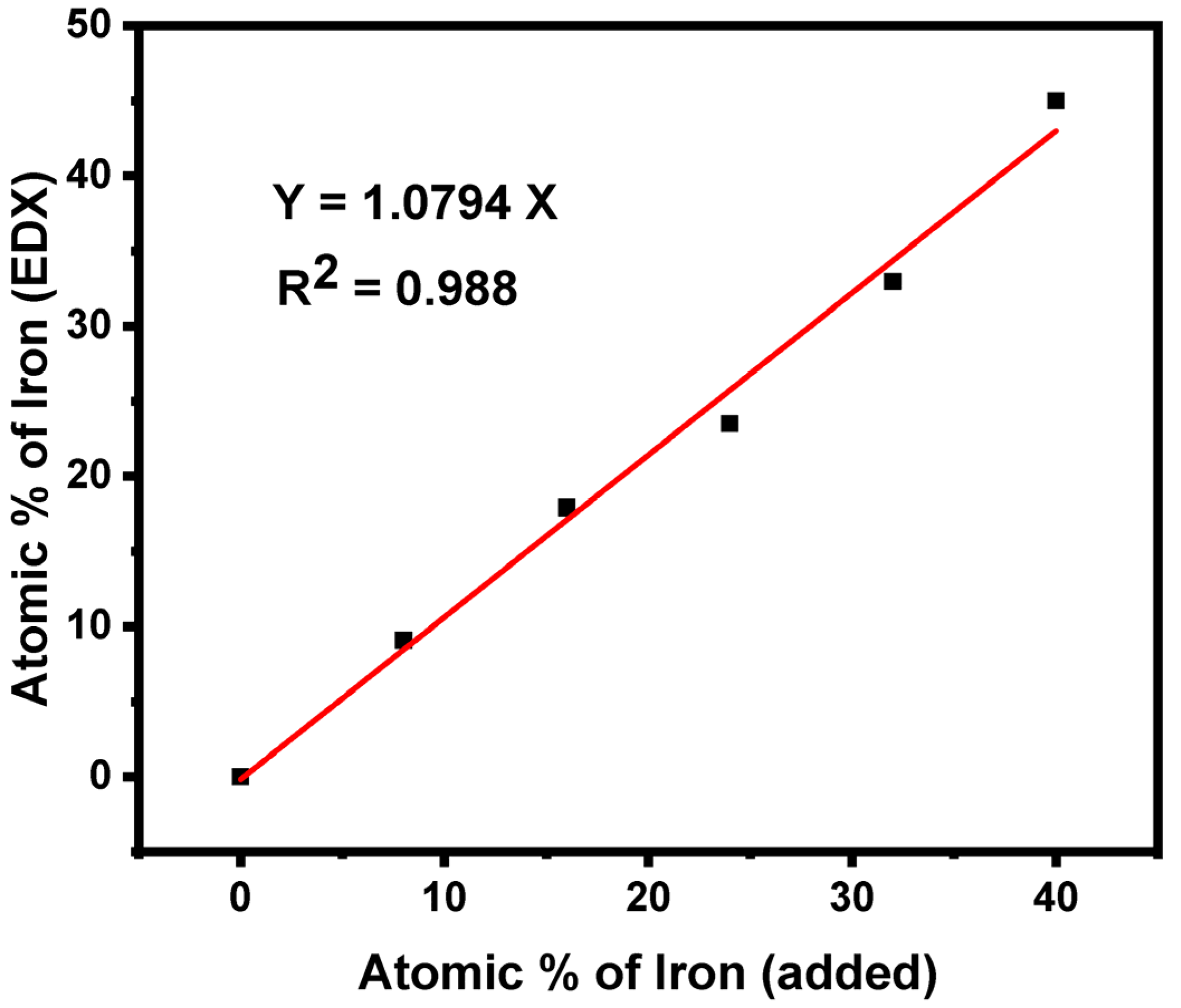
3.3. Energy Dispersive X-ray Spectroscopy
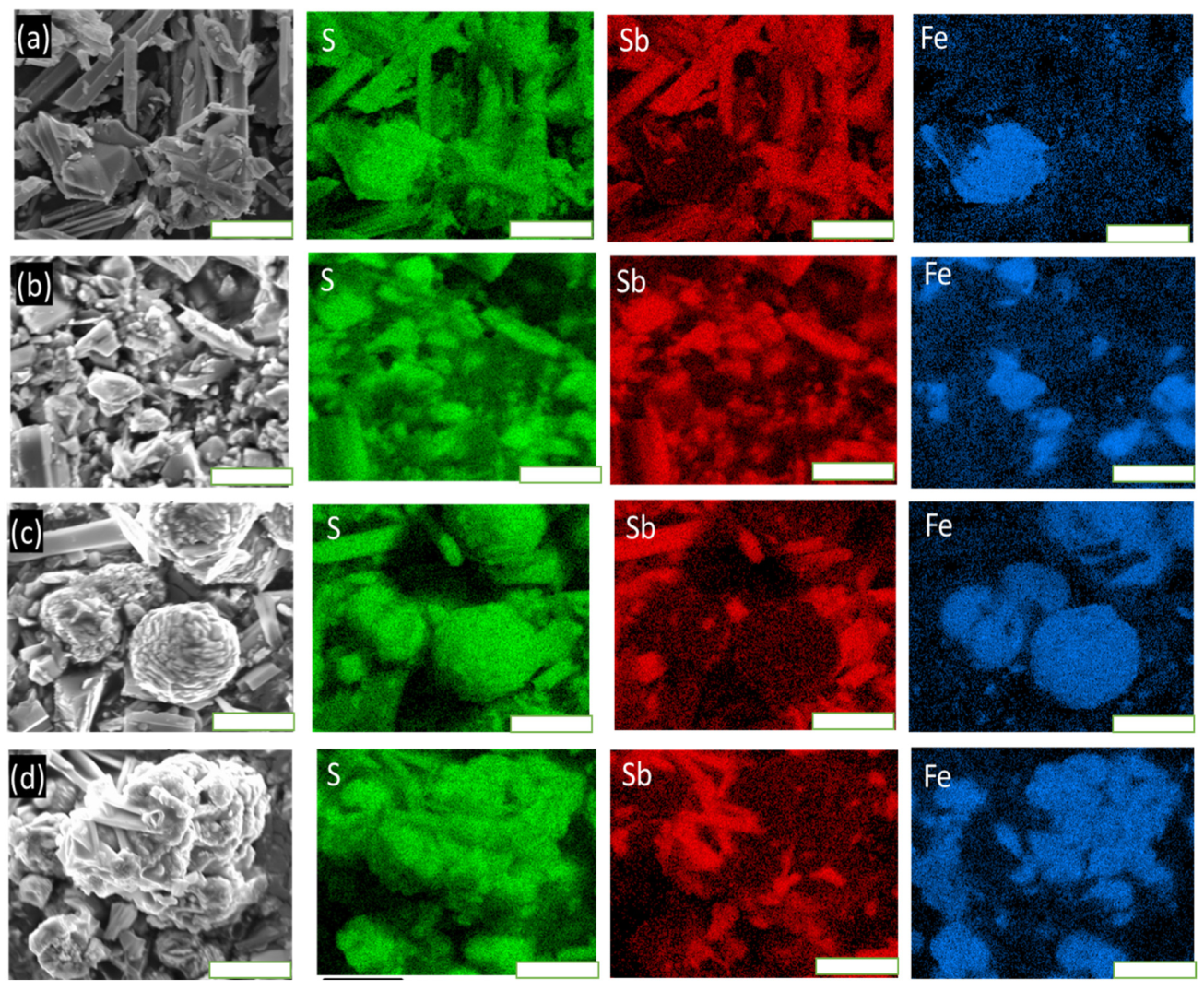
3.4. Scanning Electron Microscopy
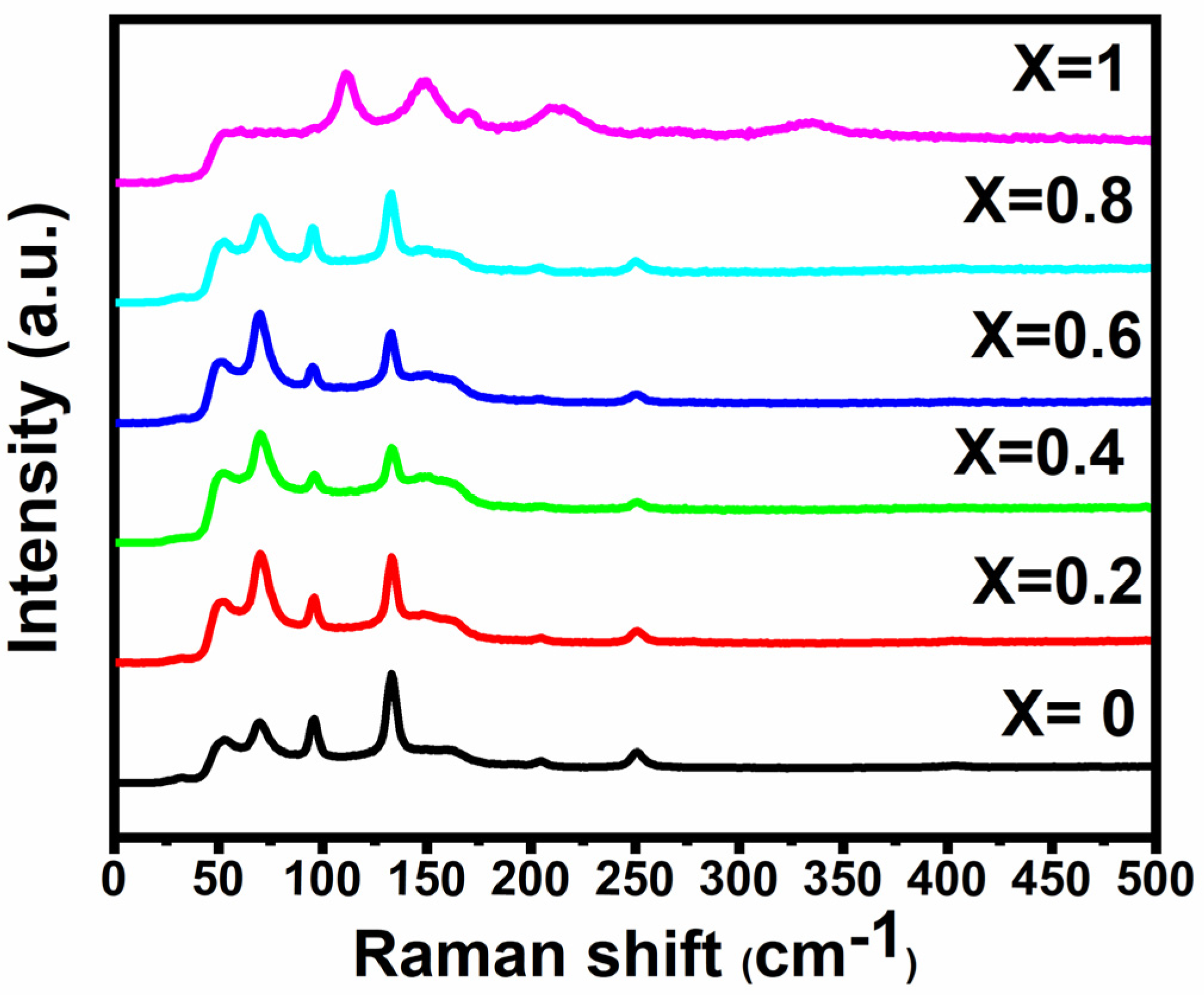
3.5. Raman Spectroscopy
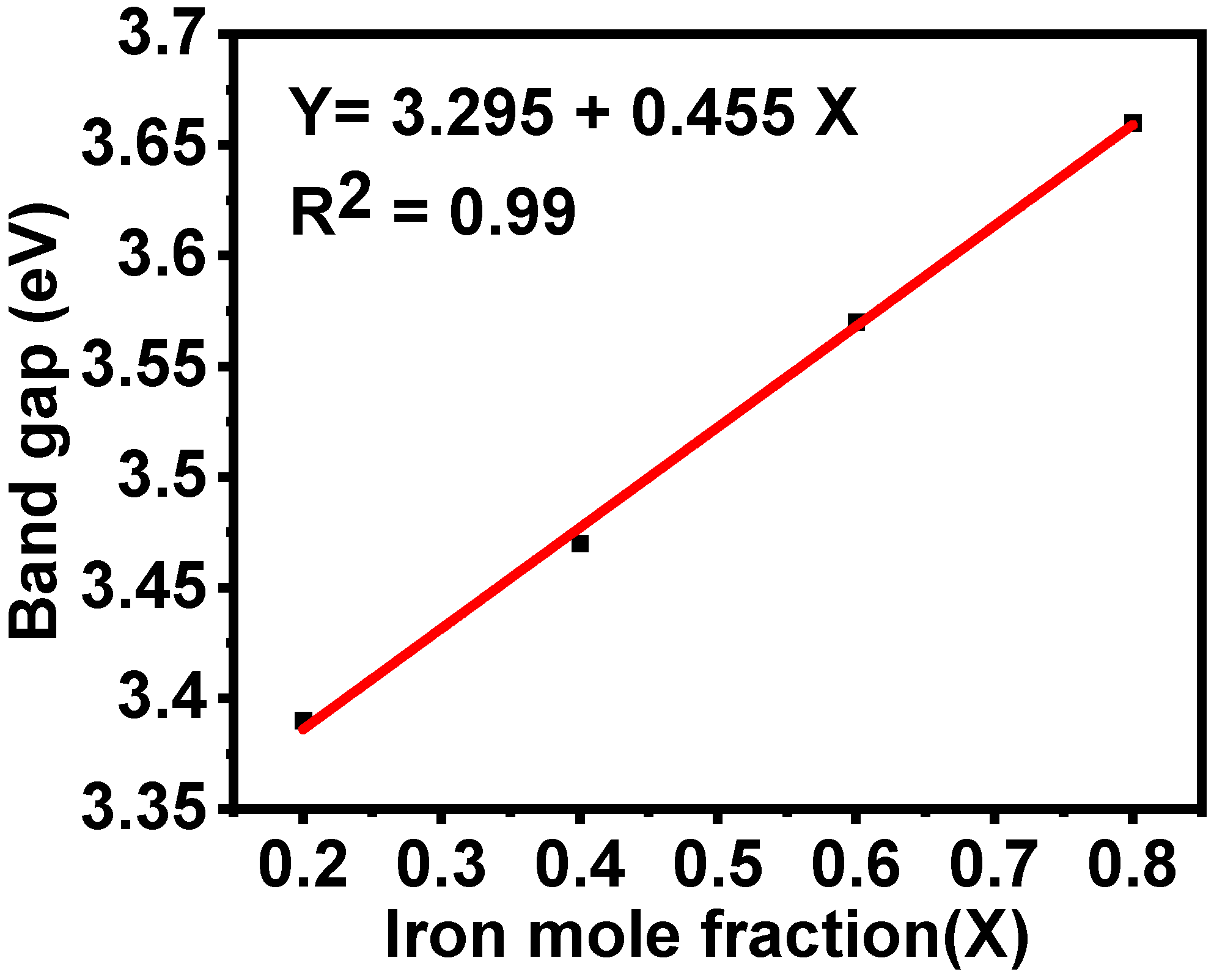
3.6. Optical Properties
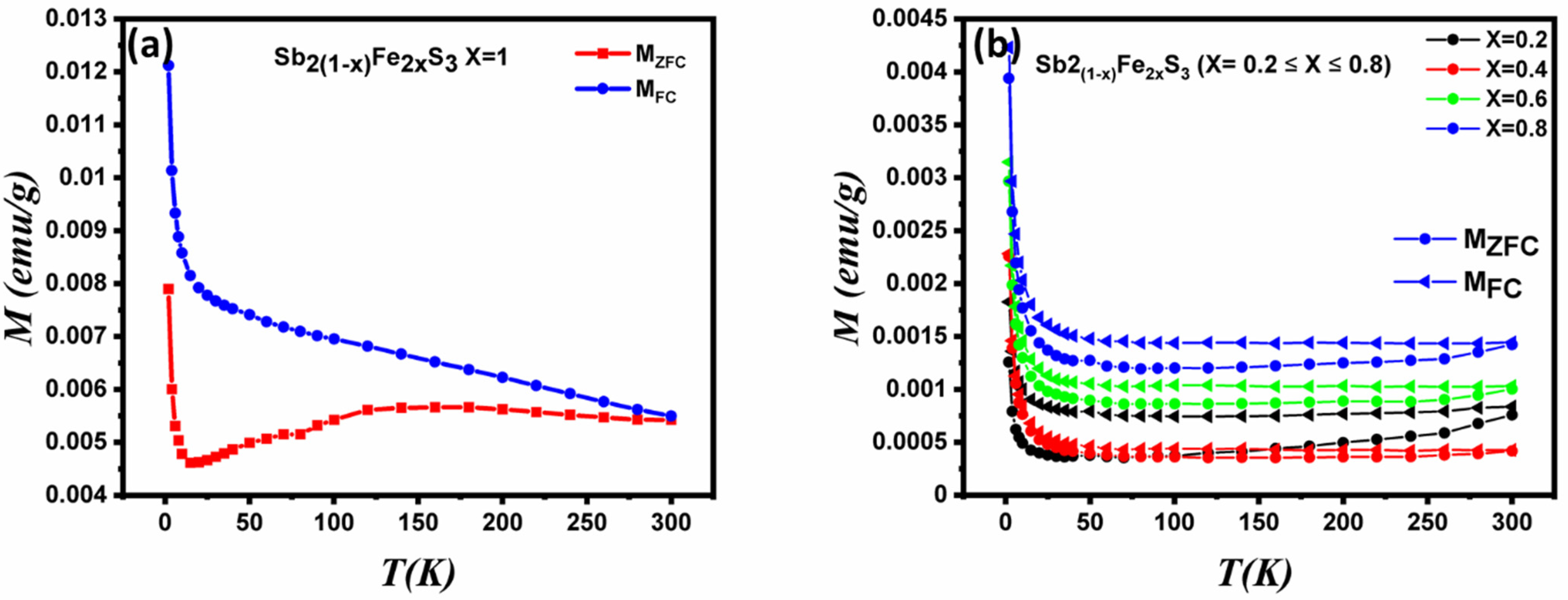
3.7. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Shen, Q.; Yu, D.; Shi, W.; Li, J.; Zhou, J.; Liu, X. A facile and green preparation of high-quality CdTe semiconductor nanocrystals at room temperature. Nanotechnology 2008, 19, 245601. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, J.; Yan, W.; Li, Z.; Wang, Y.; Yang, W.; Xie, Z.; Sun, T.; Fuchs, H. One-step controllable synthesis for high-quality ultrafine metal oxide semiconductor nanocrystals via a separated two-phase hydrolysis reaction. J. Am. Chem. Soc. 2008, 130, 2676–2680. [Google Scholar] [CrossRef]
- Zhu, Q.-A.; Gong, M.; Zhang, C.; Yong, G.-B.; Xiang, S. Preparation of Sb2S3 nanomaterials with different morphologies via a refluxing approach. J. Cryst. Growth 2009, 311, 3651–3655. [Google Scholar] [CrossRef]
- Gorer, S.; Hodes, G. Quantum size effects in the study of chemical solution deposition mechanisms of semiconductor films. J. Phys. Chem. 1994, 98, 5338–5346. [Google Scholar] [CrossRef]
- Moreels, I.Z.; Lambert, K.; De Muynck, D.; Vanhaecke, F.; Poelman, D.; Martins, J.C.; Allan, G.; Hens, Z. Composition and size-dependent extinction coefficient of colloidal PbSe quantum dots. Chem. Mater. 2007, 19, 6101–6106. [Google Scholar] [CrossRef]
- Zakutayev, A.; Major, J.D.; Hao, X.; Walsh, A.; Tang, J.; Todorov, T.K.; Wong, L.H.; Saucedo, E. Emerging inorganic solar cell efficiency tables (version 2). J. Phys. Energy 2021, 3, 032003. [Google Scholar] [CrossRef]
- Khalid, S.; Ahmed, E.; Khan, Y.; Riaz, K.N.; Malik, M.A. Nanocrystalline pyrite for photovoltaic applications. Chemistryselect 2018, 3, 6488–6524. [Google Scholar] [CrossRef]
- Luo, L.; Luan, W.; Yuan, B.; Zhang, C.; Jin, L. High efficient and stable solid solar cell: Based on FeS2 nanocrystals and P3HT: PCBM. Energy Procedia 2015, 75, 2181–2186. [Google Scholar] [CrossRef]
- Yang, R.X.; Butler, K.T.; Walsh, A. Assessment of hybrid organic–inorganic antimony sulfides for earth-abundant photovoltaic applications. J. Phys. Chem. Lett. 2015, 6, 5009–5014. [Google Scholar] [CrossRef]
- Roy, B.; Chakraborty, B.; Bhattacharya, R.; Dutta, A. Electrical and magnetic properties of antimony sulphide (Sb2S3) crystals and the mechanism of carrier transport in it. Solid State Commun. 1978, 25, 937–940. [Google Scholar] [CrossRef]
- Savadogo, O.; Mandal, K. Studies on new chemically deposited photoconducting antimony trisulphide thin films. Sol. Energy Mater. Sol. Cells 1992, 26, 117–136. [Google Scholar] [CrossRef]
- Savadogo, O. Chemically and electrochemically deposited thin films for solar energy materials. Sol. Energy Mater. Sol. Cells 1998, 52, 361–388. [Google Scholar] [CrossRef]
- George, J.; Radhakrishnan, M. Electrical conduction in coevaporated antimony trisulphide films. Solid State Commun. 1980, 33, 987–989. [Google Scholar] [CrossRef]
- Meshkauskas, J. Dielectric properties of Sb2S3 at microwave frequencies. Phys. Status Solid 1976, 39, 39–41. [Google Scholar]
- Biswal, J.B.; Garje, S.S.; Revaprasadu, N. A convenient synthesis of antimony sulfide and antimony phosphate nanorods using single source dithiolatoantimony (III) dialkyldithiophosphate precursors. Polyhedron 2014, 80, 216–222. [Google Scholar] [CrossRef]
- Lei, H.; Chen, J.; Tan, Z.; Fang, G. Review of recent progress in antimony chalcogenide-based solar cells: Materials and devices. Sol. RRL 2019, 3, 1900026. [Google Scholar] [CrossRef]
- Savadogo, O.; Mandal, K.C. Low-cost technique for preparing n-Sb2S3/p-Si heterojunction solar cells. Appl. Phys. Lett. 1993, 63, 228–230. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Chen, Y.; Zheng, Z.; Chen, Z.; Xu, W.; Liu, W.; Yang, Y.; Zhao, J.; Chen, T.; et al. Ultrafast self-trapping of photoexcited carriers sets the upper limit on antimony trisulfide photovoltaic devices. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Kondrotas, R.; Chen, C.; Tang, J. Sb2S3 solar cells. Joule 2018, 2, 857–878. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Wu, C.; Zhu, C.; Chen, T. Development of antimony sulfide–selenide Sb2(S, Se)3-based solar cells. J. Energy Chem. 2018, 27, 713–721. [Google Scholar] [CrossRef]
- Escorcia-García, J.; Becerra, D.; Nair, M.; Nair, P. Heterojunction CdS/Sb2S3 solar cells using antimony sulfide thin films prepared by thermal evaporation. Thin Solid Film. 2014, 569, 28–34. [Google Scholar] [CrossRef]
- Choi, Y.C.; Seok, S.I. Efficient Sb2S3-sensitized solar cells via single-step deposition of Sb2S3 using S/Sb-ratio-controlled SbCl3-thiourea complex solution. Adv. Funct. Mater. 2015, 25, 2892–2898. [Google Scholar] [CrossRef]
- Cheong, S.; Ferguson, P.M.; Feindel, K.; Hermans, I.; Callaghan, P.T.; Meyer, C.; Slocombe, A.; Su, C.-H.; Cheng, F.-Y.; Yeh, C.-S.; et al. Simple synthesis and functionalization of iron nanoparticles for magnetic resonance imaging. Angew. Chem. Int. Ed. 2011, 50, 4206–4209. [Google Scholar] [CrossRef]
- Herman, D.A.J.; Ferguson, P.; Cheong, S.; Hermans, I.F.; Ruck, B.J.; Allan, K.M.; Prabakar, S.; Spencer, J.L.; Lendrum, C.D.; Tilley, R.D. Hot-injection synthesis of iron/iron oxide core/shell nanoparticles for T2 contrast enhancement in magnetic reso-nance imaging. Chem. Commun. 2011, 47, 9221–9223. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folksm, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Wintenberger, M.; André, G. Magnetic properties and spiral magnetic structure of berthierite FeSb2S4. Phys. B Condens. Matter 1990, 162, 5–12. [Google Scholar] [CrossRef]
- Yin, J.; Jin, J.; Lin, H.; Yin, Z.; Li, J.; Lu, M.; Guo, L.; Xi, P.; Tang, Y.; Yan, C. Optimized metal chalcogenides for boosting water splitting. Adv. Sci. 2020, 7, 1903070. [Google Scholar] [CrossRef] [PubMed]
- Azadar, R.; Hussain, I. Use of surfactants to tailor the morphologies and crystalline phases of thin films via aerosol assisted chemical vapor deposition. J. Solid State Chem. 2020, 288, 121429. [Google Scholar]
- Park, J.-H.; Kang, S.G.; Lee, Y.K.; Chung, T.-M.; Park, B.K.; Kim, C.G. Tin (II) aminothiolate and Tin (IV) aminothiolate selenide compounds as single-source precursors for tin chalcogenide materials. Inorg. Chem. 2020, 59, 3513–3517. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.D.; Revaprasadu, N. Metal-organic precursors for ternary and quaternary metal chalcogenide nanoparticles and thin films. R. Soc. Chem. 2020, 1–31. [Google Scholar] [CrossRef]
- Lou, W.; Chen, M.; Wang, X.; Liu, W. Novel single-source precursors approach to prepare highly uniform Bi2S3 and Sb2S3 nanorods via a solvothermal treatment. Chem. Mater. 2007, 19, 872–878. [Google Scholar] [CrossRef]
- Pickett, N.L.; O’Brien, P. Syntheses of semiconductor nanoparticles using single-molecular precursors. Chem. Rec. 2001, 1, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, J.O.; Onwudiwe, D.C. Organotin (IV) dithiocarbamate complexes: Chemistry and biological activity. Molecules 2018, 23, 2571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, Y.; Xu, H.; Wang, Q. Diverse-shaped iron sulfide nanostructures synthesized from a single source precursor approach. CrystEngComm 2010, 12, 3658–3663. [Google Scholar] [CrossRef]
- Xie, G.; Qiao, Z.-P.; Zeng, M.-H.; Chen, X.-M.; Gao, S.-L. A single-source approach to Bi2S3 and Sb2S3 nanorods via a hydrothermal treatment. Cryst. Growth Des. 2004, 4, 513–516. [Google Scholar] [CrossRef]
- Sun, J.; Shen, X.; Guo, L.; Wang, G.; Park, J.; Wang, K. Solvothermal synthesis of ternary sulfides of Sb2−xBixS3 (x = 0.4, 1) with 3D flower-like architectures. Nanoscale Res. Lett. 2010, 5, 364–369. [Google Scholar] [CrossRef][Green Version]
- Xu, D.; Shen, S.; Zhang, Y.; Gu, H.; Wang, Q. Selective synthesis of ternary copper–antimony sulfide nanocrystals. Inorg. Chem. 2013, 52, 12958–12962. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, A.; Xiao, Y.; Gong, F.; Jia, D.; Li, F. Effect of solid inorganic salts on the formation of cubic-like aggregates of ZnSnO3 nanoparticles in solventless, organic-free reactions and their gas sensing behaviors. Mater. Sci. Eng. B 2012, 177, 942–948. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Chen, L.; Wu, L.-M. Structure-controlled solventless thermolytic synthesis of uniform silver nanodisks. Inorg. Chem. 2005, 44, 9817–9822. [Google Scholar] [CrossRef]
- García-Peña, N.G.; Caminade, A.-M.; Ouali, A.; Redón, R.; Turrin, C.-O. Solventless synthesis of Ru (0) composites stabilized with polyphosphorhydrazone (PPH) dendrons and their use in catalysis. RSC Adv. 2016, 6, 64557–64567. [Google Scholar] [CrossRef]
- Lewis, E.; Haigh, S.; O’Brien, P. The synthesis of metallic and semiconducting nanoparticles from reactive melts of precursors. J. Mater. Chem. A 2014, 2, 570–580. [Google Scholar] [CrossRef]
- Zeng, N.; Wang, Y.-C.; Neilson, J.; Fairclough, S.M.; Zou, Y.; Thomas, A.G.; Cernik, R.J.; Haigh, S.J.; Lewis, D.J. Rapid and low-temperature molecular precursor approach toward ternary layered metal chalcogenides and oxides: Mo1−xWxS2 and Mo1−xWxO3 Alloys (0 ≤ x ≤ 1). Chem. Mater. 2020, 32, 7895–7907. [Google Scholar] [CrossRef] [PubMed]
- Urazov, G.G.; Bolshakov, K.A.; Federov, P.I.; Vasilevskaya, I.I. The antimony-iron-sulphur ternary system. A contribution to the theory of precipitation smelting of antimony. Russ. J. Inorg. Chem. 1960, 5, 214–218. [Google Scholar]
- Malik, A. Deposition of iron sulfide thin films by AACVD from single source precursors. J. Cryst. Growth 2011, 346, 106–112. [Google Scholar]
- Shavel, A.; Ibáñez, M.; Luo, Z.; De Roo, J.; Carrete, A.; Dimitrievska, M.; Gencì, A.; Meyns, M.; Pérez-Rodríguez, A.; Kovalenko, M.V.; et al. Scalable heating-up synthesis of monodisperse Cu2ZnSnS4 nanocrystals. Chem. Mater. 2016, 28, 720–726. [Google Scholar] [CrossRef]
- Inagawa, I.; Morimoto, S.; Yamashita, T.; Shirotani, I. Temperature dependence of transmission loss of chalcogenide glass fibers. Jpn. J. Appl. Phys. 1997, 36, 2229–2235. [Google Scholar] [CrossRef]
- Almalki, R.; Mkawi, E.M.; Al-Hadeethi, Y. Fabricating antimony sulfide Sb2S3 microbars using solvothermal synthesis: Effect of the solvents used on the optical, structural, and morphological properties. J. Mater. Sci. Mater. Electron. 2020, 31, 9203–9211. [Google Scholar] [CrossRef]
- Remazeilles, C.; Tran, K.; Guilminot, E.; Conforto, E.; Refait, P. Study of Iron(II) Sulphides by Environmental Scanning Electron Microscopy (ESEM) and Micro-Raman Spectroscopy in Waterlogged Archaeological Woods. In Proceedings of the 10th International Conference on Non-Destructive Investigations and Microanalysis for the Diagnostics and Conservation of Cultural and Environmental Heritage, Florence, Italy, 13–15 April 2011. [Google Scholar] [CrossRef]
- Sultan, M.; Khan, Y.; Hussain, S.T.; Shafiq, M. Growth and optical properties of antimony sulfide decorated ZnO nano-rod heterojunctions. Mater. Lett. 2013, 104, 44–47. [Google Scholar] [CrossRef]
- Bhowmik, R. Ferromagnetism in lead graphite-pencils and magnetic composite with CoFe2O4 particles. Compos. Part B Eng. 2012, 43, 503–509. [Google Scholar] [CrossRef]


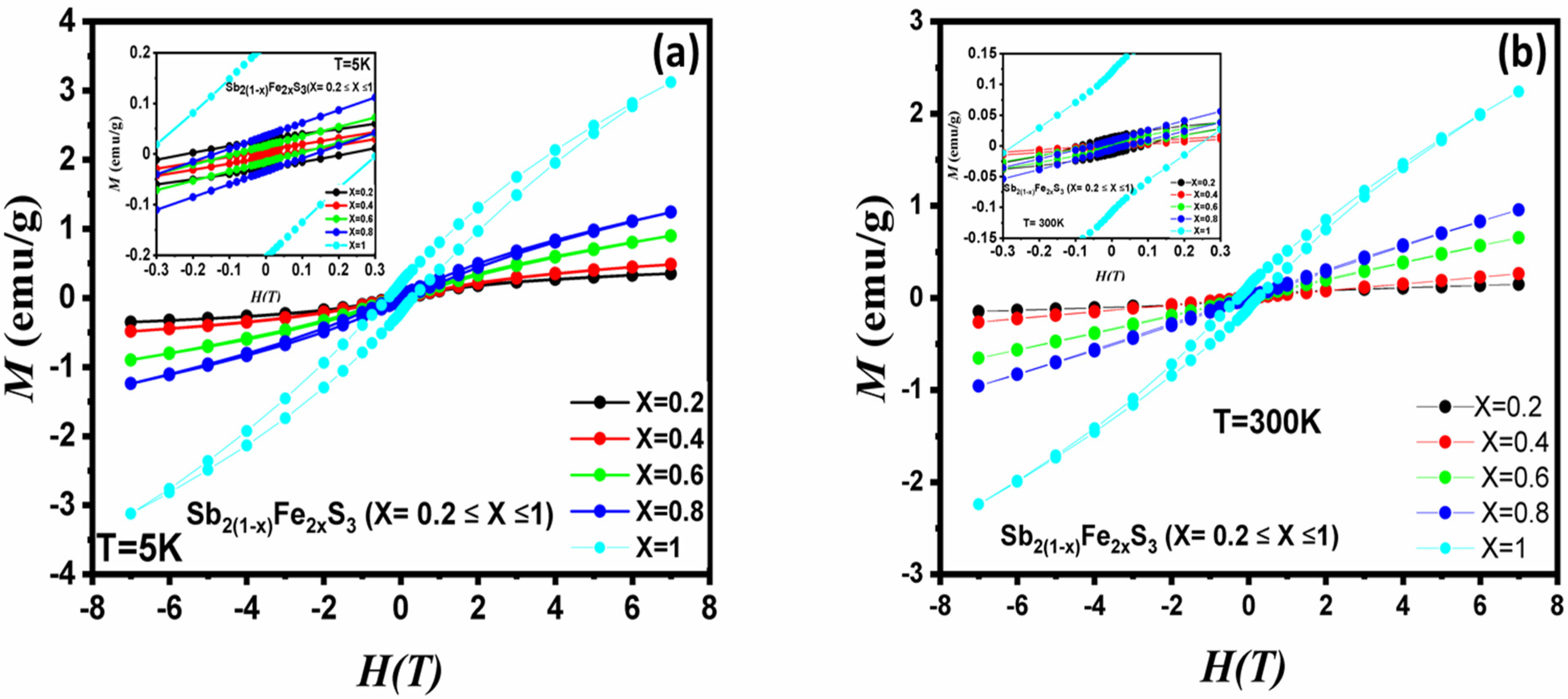
| Sample | T (K) | Hc (Oe) | Mr (emu/g) | Mmax (emu/g) |
|---|---|---|---|---|
| x = 0.2 | 5 | 2220.60 | 0.028 | 0.35 |
| 300 | 867.85 | 0.014 | 0.147 | |
| x = 0.4 | 5 | 575.04 | 0.006 | 0.48 |
| 300 | 352.04 | 0.002 | 0.26 | |
| x = 0.6 | 5 | 852.40 | 0.016 | 0.89 |
| 300 | 376.64 | 0.0059 | 0.65 | |
| x = 0.8 | 5 | 1327.64 | 0.034 | 1.24 |
| 300 | 396.41 | 0.0063 | 0.95 | |
| x = 1 | 5 | 3110.58 | 0.22 | 3.12 |
| 300 | 2374.44 | 0.12 | 2.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makin, F.; Alzahrani, D.; Alam, F.; Tuna, F.; Lewis, D.J. Testing the Efficacy of the Synthesis of Iron Antimony Sulfide Powders from Single Source Precursors. Inorganics 2021, 9, 61. https://doi.org/10.3390/inorganics9080061
Makin F, Alzahrani D, Alam F, Tuna F, Lewis DJ. Testing the Efficacy of the Synthesis of Iron Antimony Sulfide Powders from Single Source Precursors. Inorganics. 2021; 9(8):61. https://doi.org/10.3390/inorganics9080061
Chicago/Turabian StyleMakin, Fadiyah, Dalal Alzahrani, Firoz Alam, Floriana Tuna, and David J. Lewis. 2021. "Testing the Efficacy of the Synthesis of Iron Antimony Sulfide Powders from Single Source Precursors" Inorganics 9, no. 8: 61. https://doi.org/10.3390/inorganics9080061
APA StyleMakin, F., Alzahrani, D., Alam, F., Tuna, F., & Lewis, D. J. (2021). Testing the Efficacy of the Synthesis of Iron Antimony Sulfide Powders from Single Source Precursors. Inorganics, 9(8), 61. https://doi.org/10.3390/inorganics9080061







