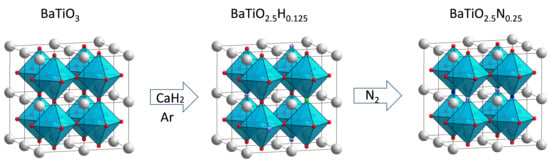
Barium Titanium Oxynitride from Ammonia-Free Nitridation of Reduced BaTiO3
Abstract
:1. Introduction
2. Results and Discussion
2.1. BTON Synthesis and Characterization
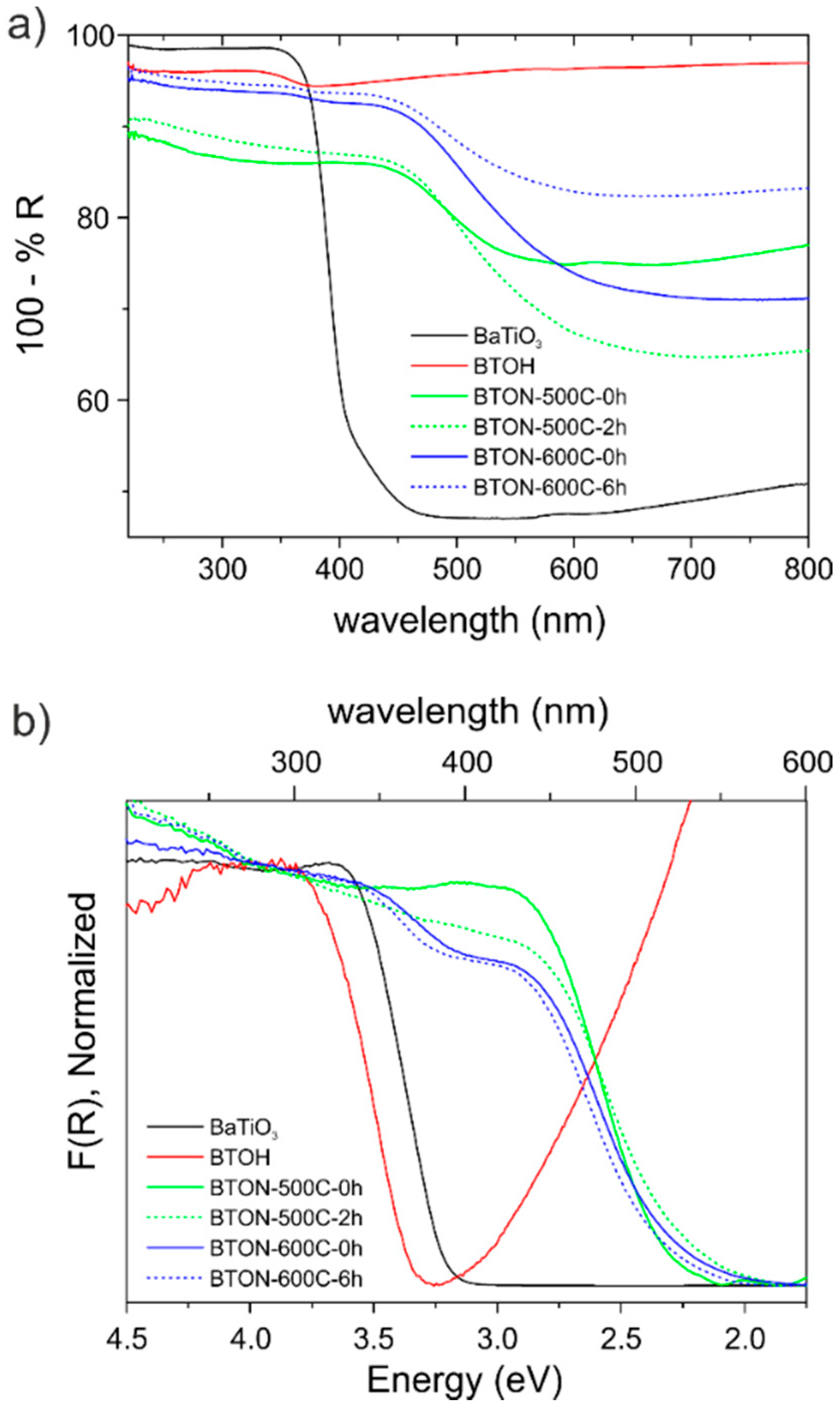
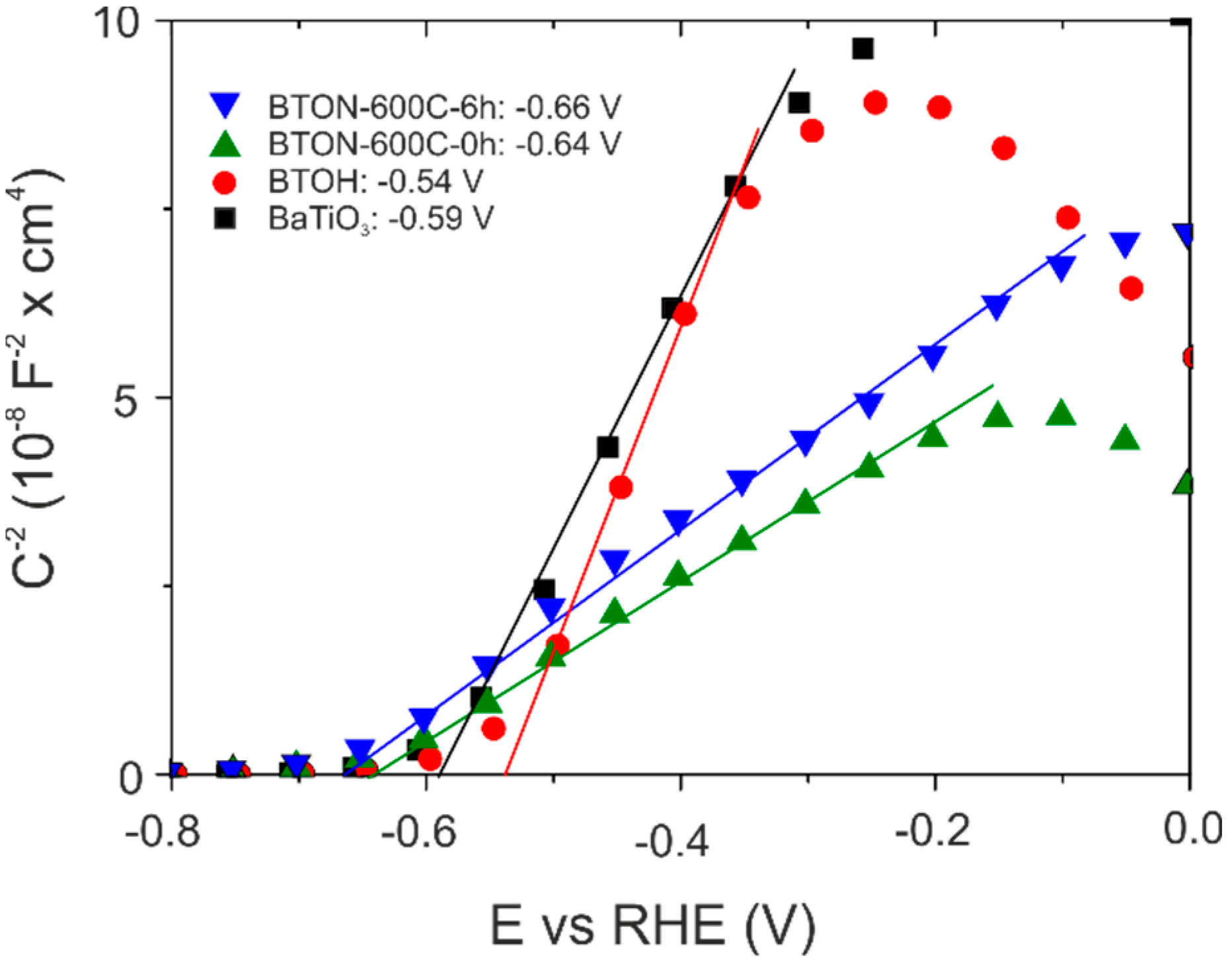
2.2. Spectroscopic and Mott-Schottky Analysis
3. Materials and Methods
3.1. Synthesis of the Materials
3.2. Powder X-ray Diffraction (PXRD) Analysis
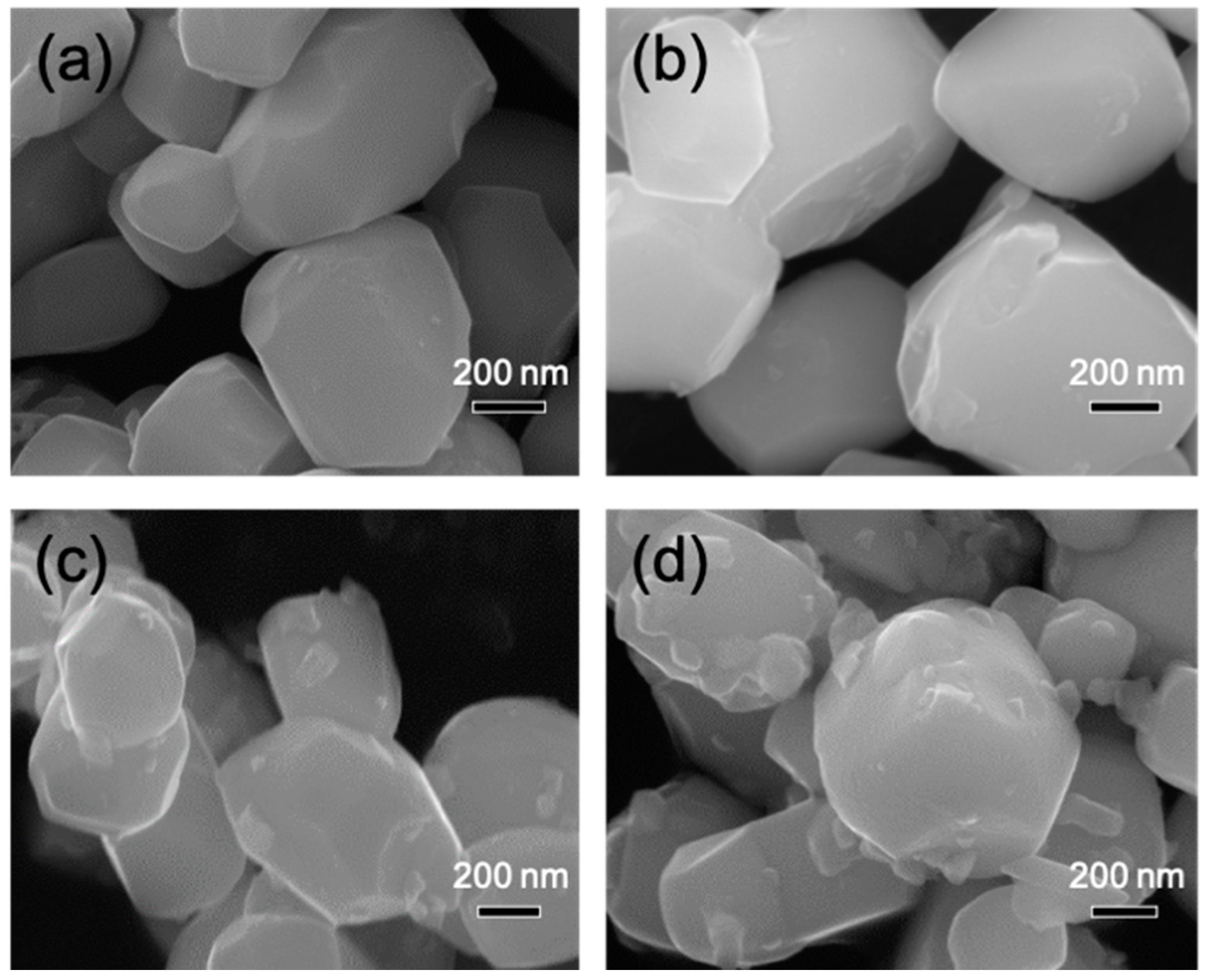
3.3. Scanning Electron Microscopy (SEM) Investigations
3.4. Raman Spectroscopy
3.5. Thermogravimetric Analysis (TGA)
3.6. Chemical Analysis
3.7. UV-VIS Diffuse Reflectance Spectroscopy
3.8. Magic Angle Spinning (MAS) NMR Spectroscopy
3.9. Mott–Schottky Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, Y.; Hernandez, O.J.; Sakaguchi, T.; Yajima, T.; Roisnel, T.; Tsujimoto, Y.; Morita, M.; Noda, Y.; Mogami, Y.; Kitada, A.; et al. An Oxyhydride of BaTiO3 Exhibiting Hydride Exchange and Electronic Conductivity. Nat. Mater. 2012, 11, 507–511. [Google Scholar] [CrossRef]
- Masuda, N.; Kobayashi, Y.; Hernandez, O.; Bataille, T.; Paofai, S.; Suzuki, H.; Ritter, C.; Ichijo, N.; Noda, Y.; Takegoshi, K.; et al. Hydride in BaTiO2.5H0.5: A Labile Ligand in Solid State Chemistry. J. Am. Chem. Soc. 2015, 137, 15315–15321. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T.; Takeiri, F.; Aidzu, K.; Akamatsu, H.; Fujita, K.; Yoshimune, W.; Ohkura, M.; Lei, S.; Gopalan, V.; Tanaka, K.; et al. A Labile Hydride Strategy for the Synthesis of Heavily Nitridized BaTiO3. Nat. Chem. 2015, 7, 1017–1023. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tang, Y.; Kageyama, T.; Yamashita, H.; Masuda, N.; Hosokawa, S.; Kageyama, H. Titanium-Based Hydrides as Heterogeneous Catalysts for Ammonia Synthesis. J. Am. Chem. Soc. 2017, 139, 18240–18246. [Google Scholar] [CrossRef]
- Bräuniger, T.; Müller, T.; Pampel, A.; Abicht, H.-P. Study of Oxygen−Nitrogen Replacement in BaTiO3 by 14N Solid-State Nuclear Magnetic Resonance. Chem. Mater. 2005, 17, 4114–4117. [Google Scholar] [CrossRef]
- Müller, T.; Großmann, T.; Abicht, H.-P. Nitrogen Containing Barium Titanate: Preparation and Characterisation. J. Phys. Chem. Solids 2009, 70, 1093–1097. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kageyama, H. Hydride Reductions of Transition Metal Oxides. Chem. Lett. 2013, 42, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Hayward, M.A. Topochemical Reactions of Layered Transition-Metal Oxides. Semicond. Sci. Technol. 2014, 29, 29. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Li, Z.; Hirai, K.; Tassel, C.; Loyer, F.; Ichikawa, N.; Abe, N.; Yamamoto, T.; Shimakawa, Y.; Yoshimura, K.; et al. Gas Phase Contributions to Topochemical Hydride Reduction Reactions. J. Solid State Chem. 2013, 207, 190–193. [Google Scholar] [CrossRef]
- Hernden, B.C.; Lussier, J.A.; Bieringer, M. Topotactic Solid-State Metal Hydride Reductions of Sr2MnO4. Inorg. Chem. 2015, 54, 4249–4256. [Google Scholar] [CrossRef] [PubMed]
- Nedumkandathil, R.; Jaworski, A.; Grins, J.; Bernin, D.; Karlsson, M.; Eklof-Osterberg, C.; Neagu, A.; Tai, C.-W.; Pell, A.J.; Haussermann, U. Hydride Reduction of BaTiO3-Oxyhydride Versus O Vacancy Formation. ACS Omega 2018, 3, 11426–11438. [Google Scholar] [CrossRef]
- Guo, H.; Jaworski, A.; Ma, Z.; Slabon, A.; Bacsik, Z.; Nedumkandathil, R.; Häussermann, U. Trapping of Different Stages of BaTiO3 Reduction with LiH. RSC Adv. 2020, 10, 35356–35365. [Google Scholar] [CrossRef]
- Adam, J.; Klein, G.; Lehnert, T. Hydroxyl Content of BaTiO3 Nanoparticles with Varied Size. J. Am. Ceram. Soc. 2013, 96, 2987–2993. [Google Scholar] [CrossRef]
- Atakan, V.; Chen, C.-W.; Paul, R.; Riman, R.E. Quantification of Hydroxyl Content in Ceramic Oxides: A Prompt Gamma Activation Analysis Study of BaTiO3. Anal. Chem. 2008, 80, 6626–6632. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, M.; Wemple, S.H.; Porto, S.P.S.; Bauman, R.P. Raman Spectrum of Single-Domain BaTiO3. Phys. Rev. 1968, 174, 522–530. [Google Scholar] [CrossRef]
- Venkateswaran, U.D.; Naik, V.M.; Naik, R. High-Pressure Raman Studies of Polycrystalline BaTiO3. Phys. Rev. B 1998, 58, 14256–14260. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Zeng, L.-Z.; Wang, R.-P.; Zhu, Y.; Liu, Y.-L. Fundamental and Second-Order Raman Spectra of BaTiO3. J. Raman Spectrosc. 1996, 27, 31–34. [Google Scholar] [CrossRef]
- An, W.; Liu, T.H.; Wang, C.H.; Diao, C.L.; Luo, N.N.; Liu, Y.; Qi, Z.-M.; Shao, T.; Wang, Y.-Y.; Jiao, H. Assignment for Vibrational Spectra of BaTiO3 Ferroelectric Ceramic Based on the First-Principles Calculation. Acta Phys. Chim. Sin. 2015, 31, 1059–1068. [Google Scholar]
- Pokorný, J.; Pasha, U.M.; Ben, L.; Thakur, O.P.; Sinclair, D.C.; Reaney, I.M. Use of Raman Spectroscopy to Determine the Site Occupancy of Dopants in BaTiO3. J. Appl. Phys. 2011, 109, 114110. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakamura, T.; Ebina, T. In-Situ Raman Spectroscopy of BaTiO3 Particles for Tetragonal–Cubic Transformation. J. Phys. Chem. Solids 2013, 74, 957–962. [Google Scholar] [CrossRef]
- Chávez, E.; Fuentes, S.; Zarate, R.A.; Padilla-Campos, L. Structural Analysis of Nanocrystalline BaTiO3. J. Mol. Struct. 2010, 984, 131–136. [Google Scholar] [CrossRef]
- Cordes, N.; Bräuniger, T.; Schnick, W. Ammonothermal Synthesis of EAMO2N (EA = Sr, Ba; M = Nb, Ta) Perovskites and 14N Solid-State NMR Spectroscopic Investigations of AM(O,N)3 (A = Ca, Sr, Ba, La). Eur. J. Inorg. Chem. 2018, 2018, 5019–5026. [Google Scholar] [CrossRef]
- Ma, Z.; Linnenberg, O.; Rokicinska, A.; Kustrowski, P.; Slabon, A. Augmenting the Photocurrent of CuWO4 Photoanodes by Heat Treatment in the Nitrogen Atmosphere. J. Phys. Chem. C 2018, 122, 19281–19288. [Google Scholar] [CrossRef]
- Ma, Z.; Lu, C.; Chen, J.; Rokicińska, A.; Kuśtrowski, P.; Coridan, R.; Dronskowski, R.; Slabon, A.; Jaworski, A. CeTiO2N Oxynitride Perovskite: Paramagnetic 14N MAS NMR without Paramagnetic Shifts. Z. Für Naturforschung B 2021, 76, 275–280. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Paik, Y. Bond Covalency in Perovskite Oxynitrides ATaO2N (A = Ca, Sr, Ba) Studied by 14N NMR Spectroscopy. Solid State Sci. 2012, 14, 580–582. [Google Scholar] [CrossRef]
- Wemple, S. Polarization fluctuations and optical-absorption edge in BaTiO3. Phys. Rev. B 1970, 2, 2679. [Google Scholar] [CrossRef]
- Suzuki, K.; Kijima, K. Optical Band Gap of Barium Titanate Nanoparticles Prepared by RF-Plasma Chemical Vapor Deposition. Jpn. J. Appl. Phys. PART 1-Regul. Pap. BRIEF Commun. Rev. Pap. 2005, 44, 2081–2082. [Google Scholar] [CrossRef]
- Sanna, S.; Thierfelder, C.; Wippermann, S.; Sinha, T.P.; Schmidt, W.G. Barium Titanate Ground- and Excited-State Properties from First-Principles Calculations. Phys. Rev. B 2011, 83, 054112. [Google Scholar] [CrossRef] [Green Version]
- Balaz, S.; Porter, S.H.; Woodward, P.M.; Brillson, L.J. Electronic Structure of Tantalum Oxynitride Perovskite Photocatalysts. Chem. Mater. 2013, 25, 3337–3343. [Google Scholar] [CrossRef]
- Ebbinghaus, S.G.; Abicht, H.-P.; Dronskowski, R.; Müller, T.; Reller, A.; Weidenkaff, A. Perovskite-Related Oxynitrides—Recent Developments in Synthesis, Characterisation and Investigations of Physical Properties. Ion. Solid Solut.—Mix. Solid State 2009, 37, 173–205. [Google Scholar] [CrossRef] [Green Version]
- Fuertes, A. Chemistry and Applications of Oxynitride Perovskites. J. Mater. Chem. 2012, 22, 3293–3299. [Google Scholar] [CrossRef]
- Fuertes, A. Nitride Tuning of Transition Metal Perovskites. APL Mater. 2020, 8, 020903. [Google Scholar] [CrossRef] [Green Version]
- Grundmann, M. The Physics of Semiconductors; Chapter 9; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Amano, F.; Nakata, M.; Yamamoto, A.; Tanaka, T. Effect of Ti3+ Ions and Conduction Band Electrons on Photocatalytic and Photoelectrochemical Activity of Rutile Titania for Water Oxidation. J. Phys. Chem. C 2016, 120, 6467–6474. [Google Scholar] [CrossRef]
- Granhed, E.J.; Lindman, A.; Eklof-Osterberg, C.; Karlsson, M.; Parker, S.F.; Wahnstrom, G. Band vs. Polaron: Vibrational Motion and Chemical Expansion of Hydride Ions as Signatures for the Electronic Character in Oxyhydride Barium Titanate. J. Mater. Chem. A 2019, 7, 16211–16221. [Google Scholar] [CrossRef] [Green Version]
- Schnabel, C.; Worner, M.; Gonzalez, B.; del Olmo, I.; Braun, A. Photoelectrochemical Characterization of p- and n-Doped Single Crystalline Silicon Carbide and Photoinduced Reductive Dehalogenation of Organic Pollutants at p-Doped Silicon Carbide. Electrochim. Acta 2001, 47, 719–727. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis. In Proceedings of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, Toulouse, France, 19–28 July 1990; Volume 127. [Google Scholar]
- Hwang, T.; van Zijl, P.; Garwood, M. Fast Broadband Inversion by Adiabatic Pulses. J. Magn. Reson. 1998, 133, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Kervern, G.; Pintacuda, G.; Emsley, L. Fast Adiabatic Pulses for Solid-State NMR of Paramagnetic Systems. Chem. Phys. Lett. 2007, 435, 157–162. [Google Scholar] [CrossRef]
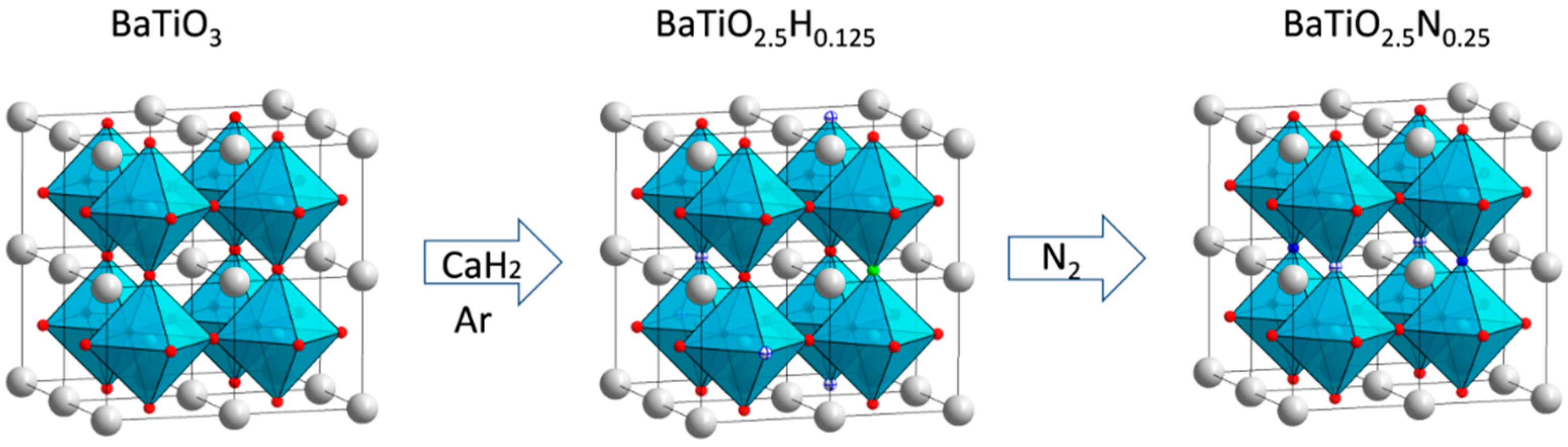

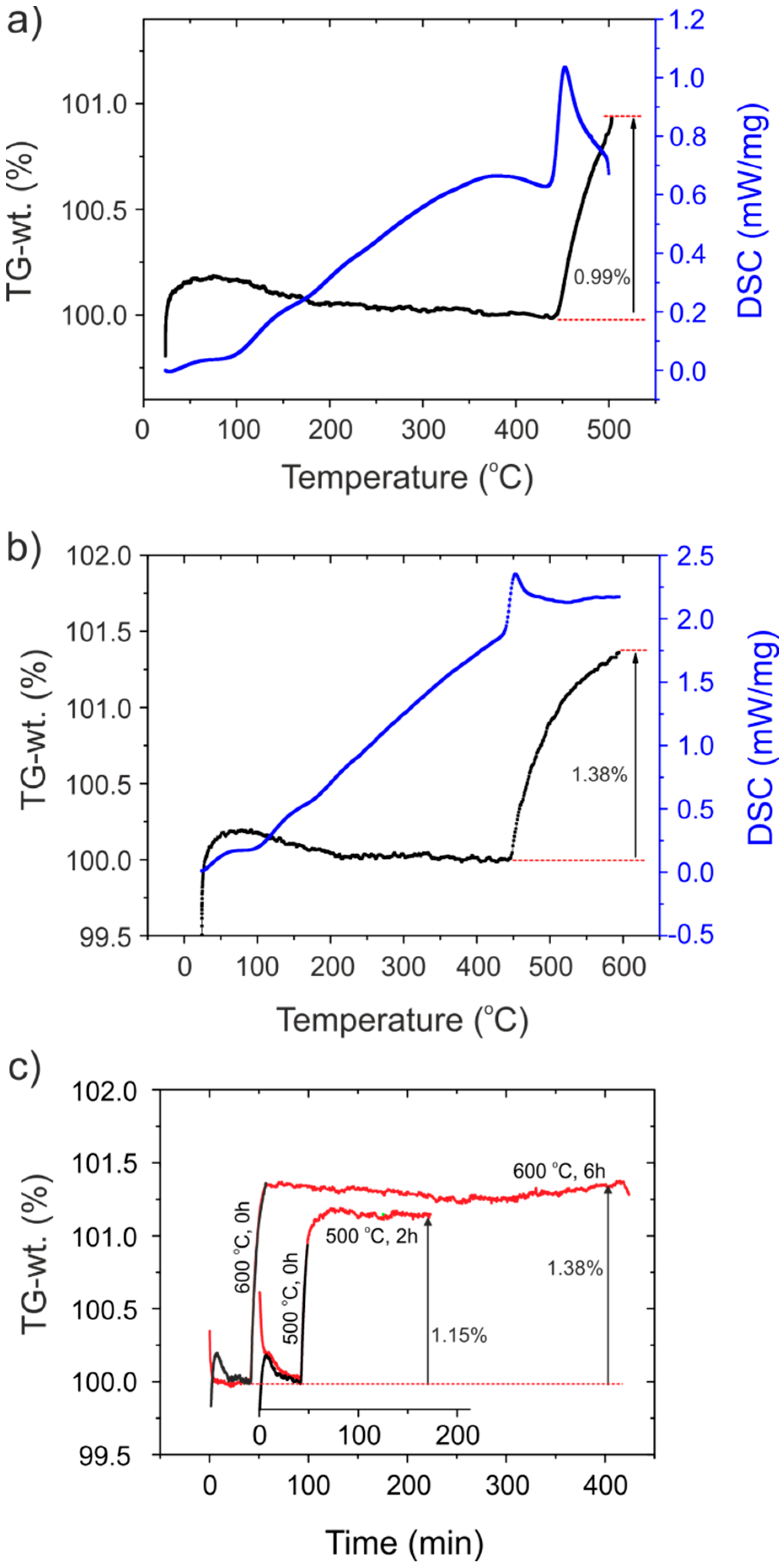
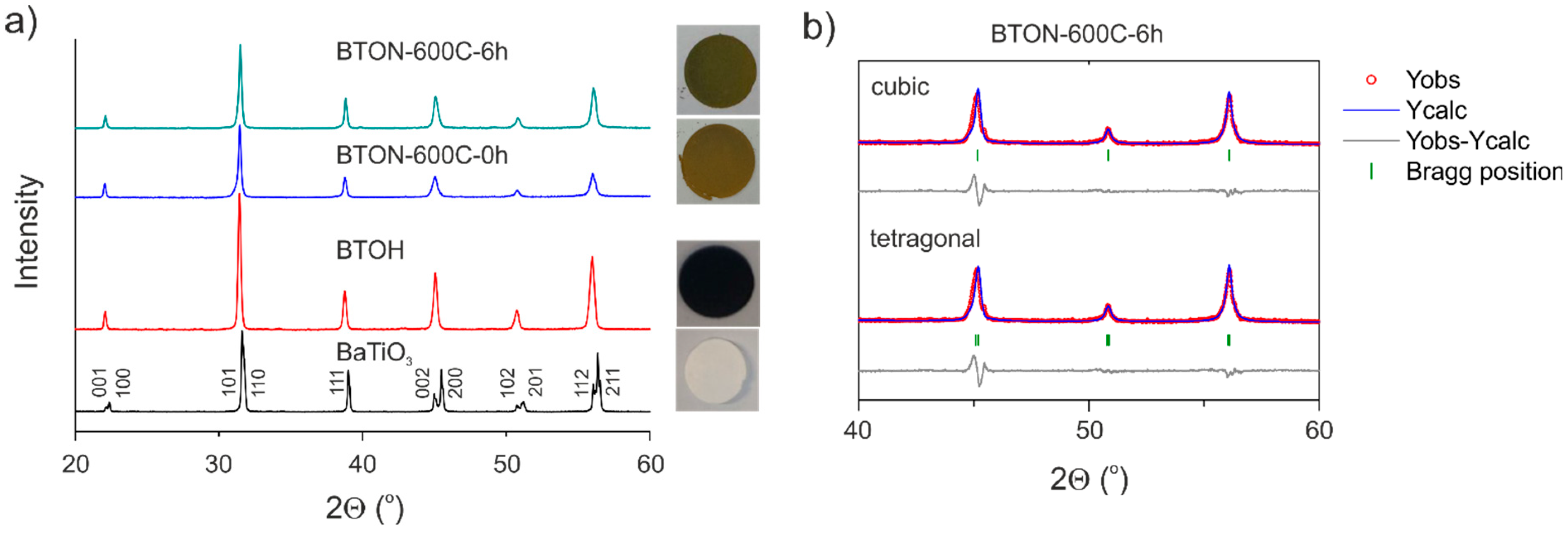

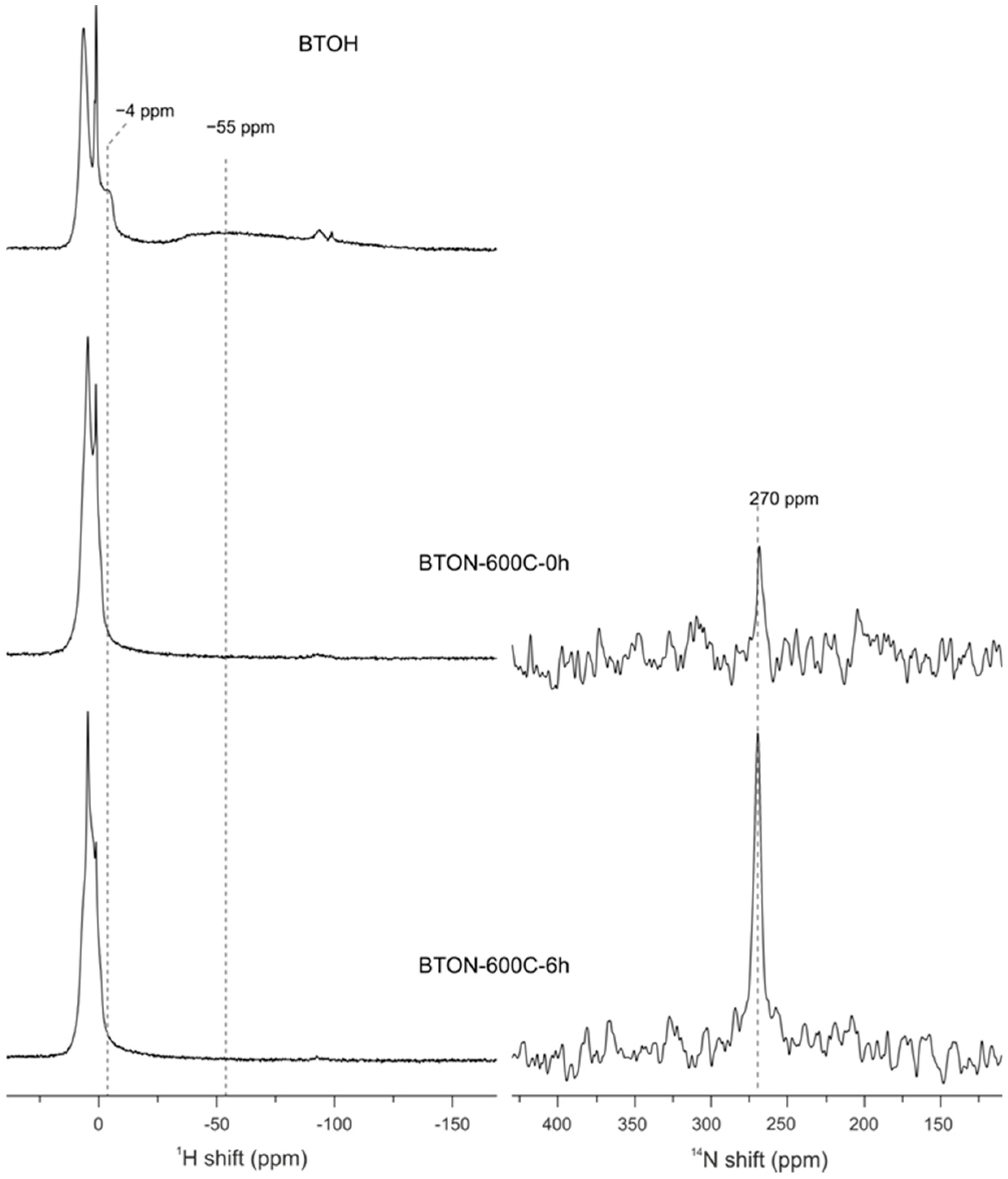
| Sample | Lattice Parameters (Å) | Volume (Å3) | XTG,H 1 | XTG,◻ 2 | XNMR,H | X◻ | Formula |
|---|---|---|---|---|---|---|---|
| BaTiO3 | a = 3.9964(1) c = 4.0310(2) | 64.379(2) | |||||
| BTOH | 4.0220(2) | 65.062(1) | 0.422 | 0.396 | 0.08 | 0.32 | BaTiO2.60H0.08 |
| Sample | Lattice Parameters (Å) | Volume (Å3) | XTG,N | Xchem.anal.,N | Formula |
|---|---|---|---|---|---|
| BTON-600C-0 h | a = 4.0086(3) c = 4.0180(5) | 64.630(2) | 0.22 | 0.20 | BaTiO2.60N0.20◻0.20 |
| BTON-600C-6 h | a = 4.0085(3) c = 4.0184(2) | 64.533(2) | 0.21 | 0.18 | BaTiO2.60N0.18◻0.22 |
| Sample | Bandgap (eV) (Direct) | Flat Band Potential (vs. RHE, V) |
|---|---|---|
| BaTiO3 | 3.18 | −0.59 |
| BTOH | 3.33 | −0.54 |
| BTON-600-0 h | 2.45 | −0.64 |
| BTON-600-6 h | 2.46 | −0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Jaworski, A.; Chen, Z.; Lu, C.; Slabon, A.; Häussermann, U. Barium Titanium Oxynitride from Ammonia-Free Nitridation of Reduced BaTiO3. Inorganics 2021, 9, 62. https://doi.org/10.3390/inorganics9080062
Guo H, Jaworski A, Chen Z, Lu C, Slabon A, Häussermann U. Barium Titanium Oxynitride from Ammonia-Free Nitridation of Reduced BaTiO3. Inorganics. 2021; 9(8):62. https://doi.org/10.3390/inorganics9080062
Chicago/Turabian StyleGuo, Hua, Aleksander Jaworski, Zheng Chen, Can Lu, Adam Slabon, and Ulrich Häussermann. 2021. "Barium Titanium Oxynitride from Ammonia-Free Nitridation of Reduced BaTiO3" Inorganics 9, no. 8: 62. https://doi.org/10.3390/inorganics9080062
APA StyleGuo, H., Jaworski, A., Chen, Z., Lu, C., Slabon, A., & Häussermann, U. (2021). Barium Titanium Oxynitride from Ammonia-Free Nitridation of Reduced BaTiO3. Inorganics, 9(8), 62. https://doi.org/10.3390/inorganics9080062