Abstract
The Lucite Alpha process is the predominant technology for the preparation of acrylics. This two-stage process involves the palladium-catalysed formation of methyl propanoate from ethene, CO, and methanol, followed by the oxidative formylation of methyl propanoate into methyl methacrylate. A range of bis-1,2-disubstituted aminomethylferrocenes has been prepared and characterised. These complexes serve as precursors to a variety of bulky ferrocenylmethyldiphosphanes that, in turn, function as ligands in the palladium-catalysed process. We describe the crystal structures of five ligand precursors and provide a rationale for their design. In situ catalyst testing on palladium complexes derived from ferrocenylphosphanes demonstrates that these are highly selective (>99.5%) catalysts for the formation of methyl propanoate from ethene, CO, and methanol and have turnover numbers exceeding 50,000. This article credits those researchers who worked on this project in the early days, who received little or no credit for their achievements and endeavours.
1. Introduction
This paper documents early work between 1996 and 2006 on the synthesis of ferrocene-based ligands for the so-called Alpha process [1,2,3]. This work remained unpublished because of circumstances prevalent at the time of completion. There have been some work presentations; we published two short communications on ligand synthesis [4,5]. However, this is the story of the ligands’ synthetic development and use. This work brings together these reports [1,2,3,4,5] and adds previously unpublished material.
Figure 1 shows commercial routes to produce MMA (methyl methacrylate, methyl-2-methyl-2-propenoate). The first step in the two-step Lucite Alpha process [6] involves the palladium-catalysed production of methyl propanoate (MeP) from methanol, ethene, and carbon monoxide. In the second step, catalytic condensation of methyl propanoate and formaldehyde in a heterogeneous fixed-bed cerium oxide catalyst forms methyl methacrylate under anhydrous conditions [6,7].
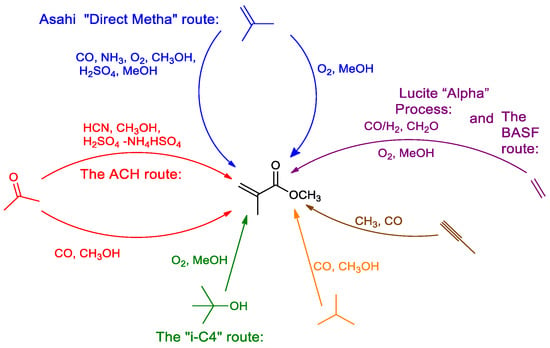
Figure 1.
Commercial routes to methyl methacrylate and related acrylics.
Tooze et al. developed the first step of the Lucite Alpha process [8,9,10,11]; their scheme is a refinement of a polymerisation process developed by Drent et al. at Shell [12,13,14,15]. The Drent process is mechanistically complex, and involves the reaction of ethene and carbon monoxide to furnish alternating polymers (Figure 2).
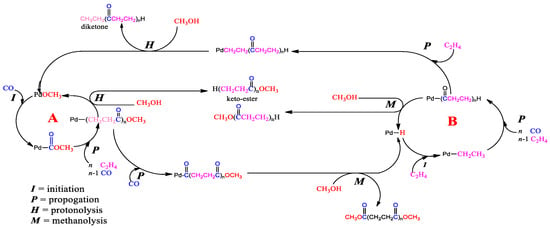
Figure 2.
Products obtained from the palladium-catalysed reaction of ethene, carbon monoxide, and methanol according to Drent et al.
Since the initial development work on ethene–CO polymerisation by Drent, the mechanism of this process has been well studied [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Tooze et al. modified the process to produce methyl propanoate, which is the most straightforward “polymer” obtained after quenching with methanol, i.e., the process operates for one cycle only (Figure 3).
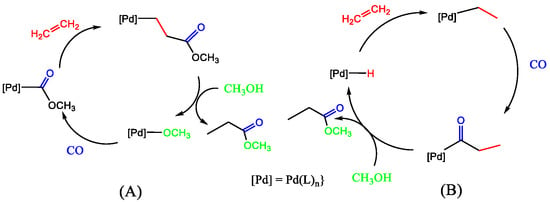
Figure 3.
(A) Simplified carbomethoxy cycle and (B) simplified hydride cycle to methyl propanoate formation [8,9,10].
Eastham and co-workers [32] performed further mechanistic investigations specific to the Lucite process, and their results support and clarify much of the work by Drent. Their research has led to a complete understanding of the reaction mechanisms of hydroalkoxycarbonylation and carboalkoxycarbonylation reactions under industrial operating conditions [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47].
Our group pioneered the preparation of ferrocene-based ligands for the methoxycarbonylation reaction of ethene, and we previously described the synthesis of ferrocene-based ligands—such as compound 2—that are similar in structure to the Alpha ligand, compound 1 [5,6]. Butler and Greenwell developed the ligand synthesis in 1998, and the work was further developed by Fortune in a programme partially funded by INEOS Acrylics/Lucite International. “Traditional” ferrocene-based ligands such as dppf [(1,1′-bis-(diphenylphosphino)ferrocene] [48], and its permethylated analogue octamethyl-1,1′-bis-(diphenylphosphino)ferrocene (omdppf) [49], have applications in the methoxycarbonylation and polymerisation of ethene and styrene. Subsequently, many studies have used ferrocene-based ligands [50,51,52,53,54,55,56]. Comparing the complexes of different metals in catalysis is difficult because of the other possible coordination modes of palladium when the metallocene metal centre is non-innocent. For example, dppf-type complexes feature a dative interaction between the palladium atom and the metallocene metal centre (Figure 4). Given all the accumulated evidence available from mechanistic studies, a bulky and highly basic ligand design should favour the formation of low-molecular-weight esters.
Figure 4.
Non-trivial coordination of ferrocenylphosphine ligands with palladium: the dative iron–palladium bond.
The complete mechanism of the Alpha process was unknown when performing this work; since then, there has been a steady flow of research articles on the subject, with key articles by Heaton and Iggo [34,35,36,37]. The number of research papers has increased substantially since performing the original work. For this reason, the reader is directed to the large number of articles cited in three key review papers [7,56,57]. The most recent paper [57] brings together much of the academic–industrial work in the research area. In this study, we focus on original work on our ligands, which predates these mechanistic studies.
2. Ferrocene-Derived Alpha Ligands: Results and Discussion
Our original communications highlighted the preparation of a ferrocene ligand for application in the palladium-catalysed reaction of carbon monoxide, ethene, and alcohols. This is essentially the ferrocenyl analogue, 2a, (butphos) of the Alpha ligand, 1,2-bis(di-tert-butylphosphinomethyl)benzene, 1 (Figure 5) [4].
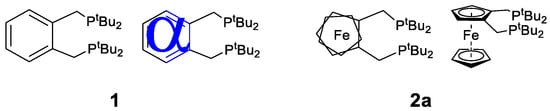
Figure 5.
The Alpha ligand, 1,2-bis (di-tert-butylphosphinomethyl)benzene (1), with a top view of butphos (2a), its ferrocene-based equivalent.
Figure 6 shows a synthetic route to the formation of the ferrocene ligand, 2a [2,4]. The 1,2-bis-dimethylaminomethylferrocene intermediate 5 was obtained in yields of approximately 90% and was purified via sublimation to provide a bright orange crystalline compound.
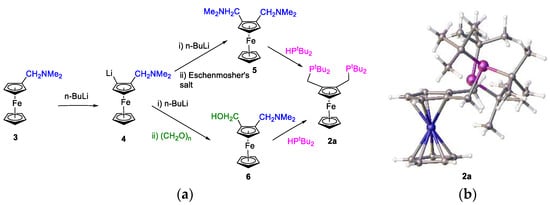
Figure 6.
(a) Synthetic route to 1,2-bis-[di-(tert-butyl)phosphano)] ferrocene, butphos, 2a, and (b) a view of the crystal structure of 2a.
In the 1H NMR spectrum of compound, 5, there is a characteristic singlet at 2.17 ppm due to the magnetically equivalent N-methyl groups. In the second step of the synthesis, we applied Ugi’s method to prepare the bis-phosphane 2a. In the 31P{1H} NMR spectrum of 2a, a single resonance at 23.80 ppm confirms that the two phosphane atoms are equivalent and are relatively basic.
In an alternative synthetic route, we used compound 7 as an intermediate to prepare 1,2,3-tris(tri-tbutylphosphinomethyl)ferrocene, 8, (Figure 7) [2,5]. Compound 8 was prepared as the first 1,2,3-trisubstituted ligand for use in the Alpha process; we felt that a third phosphorus donor group might benefit the catalytic lifetime by introducing steric effects below the plane of the substituted cyclopentadienyl ring.
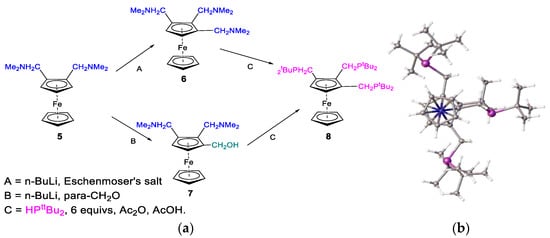
Figure 7.
(a) Synthetic route to 1,2,3-tris-(tri-tbutylphosphino)ferrocene, 8, and (b) crystal structure of compound 8 [4,5].
Compound 7, an orange oil, was converted to tris-phosphane 8 as analytically pure yellow/orange powder (Figure 7). In the 31P NMR spectrum of 8, there are two resonances with a 2:1 integration ratio: the two outer phosphanomethyl groups (Figure 8) at 20.58 ppm, and the inner phosphorus slightly more upfield at 21.35 ppm. The crystal structure of compound 8 revealed a high degree of steric crowding due to the proximity of the six tert-butyl groups. The molecular conformation exhibits the outer phosphane groups, with one t-butyl group above the substituted cp-ring plane, while the other is below it. The inner-arm methylenephosphine methyl groups are located above the cp-ring plane, thus minimising steric crowding.
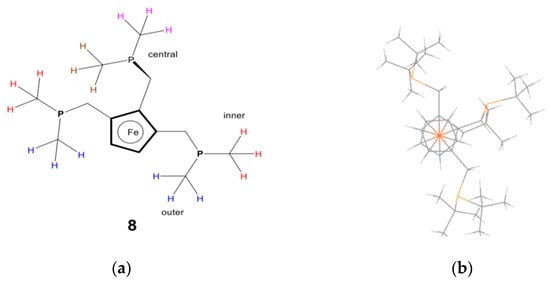
Figure 8.
Tris-phosphane 8 from above the cyclopentadienyl ferrocene ring plane: (a) schematic and (b) stick diagram, obtained from the crystal structure of 8.
One of the key targets in our ligand design process was the control of substitution of the unsubstituted ferrocenyl cyclopentadienyl ring. Greater control would allow changes in ligand basicity, and it would grant some additional steric control. There are several synthetic methods available to substitute the unsubstituted cyclopentadienyl ring. As reported by Hartwig et al., substitution at all positions on the cp ring is possible for simple mono-phosphino ferrocenes [58,59]. These authors modified some related ligands, including di-tert-butylphosphinoferrocene. We adopted this approach for the one-step synthesis of the less basic 6,7,8,9,10-penta-phenyl(diphenylphosphino)ferrocenyl, 10 (Figure 9); however, the yields were low. We also investigated the possible use of the N, N-dimethylaminomethylferrocene precursors in place of a phosphane, 9. Still, we could not isolate any product because the ortho-metallated palladium complex does not undergo self-catalysis. Attempts to obtain amine 11 were unsuccessful; on the attempted lithiation of 12 with t-butyllithium, a deep green/blue solution formed, which reverted to starting materials upon attempted quenching.
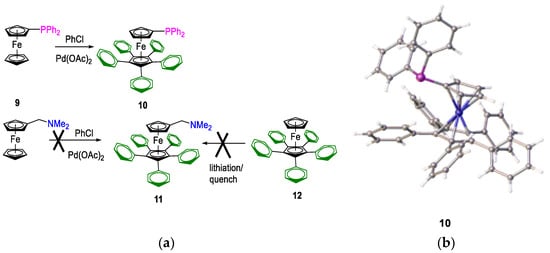
Figure 9.
(a) Preparation of per-aryl-substituted ferrocenylphosphine 10, from diphenylphosphinoferrocene 9, and the scheme showing the difficulty of preparing analogous ferrocenylethylamine, 11, from either N,N-dimethylaminomethylferrocene or compound 12 using n-BuLi lithiation followed by quenching with Eschenmoser’s salt. (b) crystal structure of compound 10.
Because of these disappointing results, the work moved on to examine the preparation of more easily prepared silyl-substituted ligands. These were chosen based on results obtained from the concurrent catalytic reaction trials using palladium complexes of substituted conventional benzene-based ligands (standard Alpha ligands). The synthetic route is shown in Figure 10, with the target precursors as silyl-substituted bis-(aminomethyl)ferrocenes, such as 17 and 20.
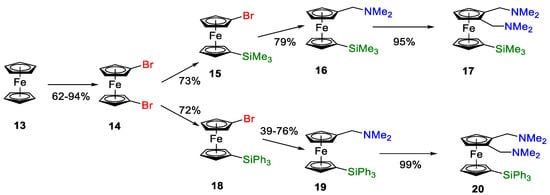
Figure 10.
Preparative route to bulky alkyl- or aryl-sily-ferrocenylmethylamine ligands.
The synthetic route uses 1,1′-dibromoferrocene, 14, as a key intermediate, as it is easy to prepare from ferrocene at high yields [60] Additionally, the tetra-substituted ferrocenes 22 and 24 (Figure 11a) were targeted as key precursors to bis-phosphanes. We had the requisite experience to make low-cost intermediates in this synthetic area [61,62].
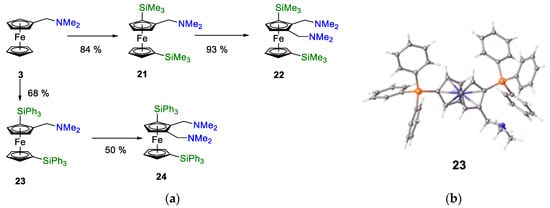
Figure 11.
(a) Synthetic route to multiply substituted ferrocenyl-alkylamines. Individual reaction steps involve lithiation, followed by quenching with ClSiPh3, ClSiMe3, or Eschenmoser’s Salt. (b) ORTEP diagram of 2-(N,N′-dimethylaminomethyl)-1,1′-bis-triphenylsilylferrocene, 23. Ellipsoids are at 50% probability, and hydrogen atoms are removed for clarity.
We characterised compounds 22 and 24 and structurally determined compounds 23 and 24. These compounds exhibit similar structures to the parent diamine, 5; the bulky triphenylsilyl groups on the cp rings lay in a staggered geometry (calculated torsion angles Si1-C(cp1)-C(cp2)-Si2 = 148.2° (23) and 123.1° (24)). Both amine groups lay above the cp plane, with the methyl groups orientating to limit the overall angle that the amine could sweep with respect to the cp ring. The crystal structure of 5 superimposes that of compound 24. The differences are only a few degrees of rotation of either amine group (Figure 12b) (data in Supplementary Materials).
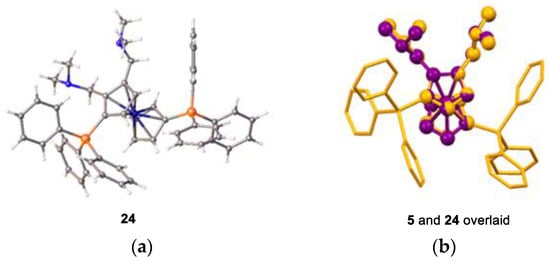
Figure 12.
(a) ORTEP diagram of bis-2,3-(N,N′-dimethylaminomethyl)-1,6-bis-(triphenylsilyl) ferrocene, 24 (showing ellipsoids at 50% probability and the hydrogen atoms removed for clarity) and (b) image showing the close overlap of compounds 5 (purple) and 24 (orange), with the similar areas highlighted by balls.
The amine ligands can be reacted with nickel(II) halide salts at room temperature to produce the corresponding complexes. Thus, for example, the complex of precursor 24 was prepared, and its structure was determined. However, the tetrahedral coordination mode as exemplified by the nickel dibromide complex—24-NiBr2 (Figure 13)—rules out bis-amine nickel complexes as potential catalysts. Nevertheless, the structural data are important, and crystallographic data are available in the Supplementary Materials.
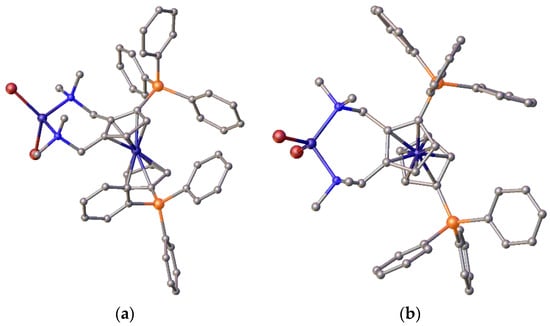
Figure 13.
(a) Side and (b) top views of the tetrahedral NiBr2 complex of the bis-methylamine precursor, 24. This tetrahedral coordination of nickel is considered disadvantageous for methoxycarbonylation catalysis.
Synthetic data for all ligand precursors including compounds 2a, 5, 6, 7, and 8 [2,3,4,5]—are available in the Supplementary Materials.
Early results from catalytic trial reactions of the palladium complexes derived from the benzene-based standard Alpha ligand, and those of ferrocene ligand complexes, indicated the dynamics of the metal–ligand complex; flipping can occur between conformers A, B, and C (Figure 14). The polymerisation reaction—i.e., alternating ethene and CO insertions—may propagate from intermediate stage B rather than from single ethene insertions at A or C (Figure 15). There is more space around the palladium to accommodate the geometry required by substrate coordination and migratory insertion reactions.
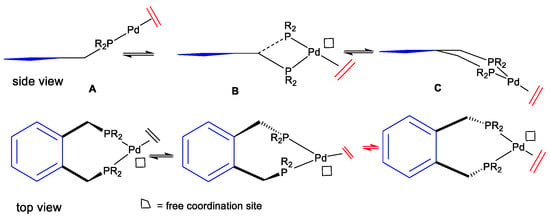
Figure 14.
Conformational scheme for the Alpha ligand palladium complex, showing conformational flipping.
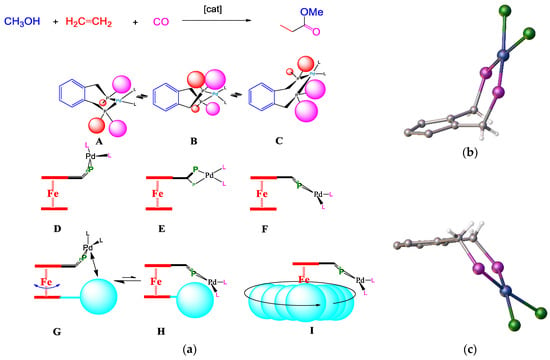
Figure 15.
(a) Conformational flipping of A–C with the Alpha catalyst, and D–I with a ferrocenyl phosphane complex. At conformer D, the palladium sits above the cp ring. E,F show the flipping process. G–I show the steric blocking effect of a substituent on the bottom non-P ring. (b) flipping of Pd centre above Cp plan and (c) flipping below cp plane.
Thus, as described in the ligand modification section, the single bulky silyl-substituent attached to the unsubstituted cp ring would increase the lower cp ring’s steric bulk. It is then possible to determine whether the rotation of the cyclopentadienyl ring would provide sufficient steric crowding (Figure 15a(G–I)). In summary, the rationale for using tri-alkyl- and tri-aryl-silyl substituents was because the synthesis of such compounds is straightforward and cheap, which is a prerequisite for an industrial-scale process. The silyl-substituted compounds 17 and 20, prepared according to the schematic (Figure 10), were ideal precursors.
2.1. Catalyst Testing
The structure of the palladium dichloride complex of ligand 2a was originally determined in 2004 from a crystal obtained via slow diffusion of petrol/ether into chloroform (Supplementary Materials). A more recent structure was crystallised from dichloromethane/petroleum ether (Figure 16, top and side views).
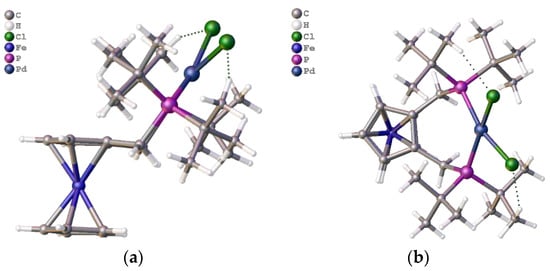
Figure 16.
(a) Side view and (b) top view of palladium dichloride complex of butphos, 2a [Pd(L2a)Cl2].
In addition to our report, a further study was also published later by Claver et al. [63]. These workers reported the preparation of the complex from a sample of the ligand donated without our knowledge. The catalyst testing was performed concurrently with benzene-based ligands at Lucite International Ltd., Cleveland, OH, USA. The synthetic procedure was as follows: reactions of 1,2-bis-(N,N-dimethylaminomethyl)ferrocene, 5, with a secondary phosphane were performed in acetic acid/acetic anhydride at 70 °C for 60 h. This methodology initially resulted in low (20–30%) yields of the bidentate phosphanes and, therefore, we developed an improved method. The improved method involved the reaction of a secondary phosphane with compound 5 at ca. 130 °C in a mixture of degassed acetic acid and acetic anhydride (9:1). The solvent was removed in vacuo, and the residue was stirred in methanol for 30 min. After removal of the methanol in vacuo, the residue was washed with ethanol. This preparation furnished a free-flowing solid of the adamantylphosphine-substituted derivative, with up to 83% yield. In Figure 17, drawings of bulky ferrocenylphosphanes, prepared in situ, are shown. These complexes were prepared and used under industrial conditions, and so complete characterisation data are unavailable. However, the synthetic methodology is available in the Supplementary Materials. The commercially available phosphane 1,3,5,7-tetramethyl-2,4,8-trioxa-6-phospha-adamantane was also reacted with 5 to provide the corresponding bidentate ferrocenylphosphane ligand, 2i (Figure 17).
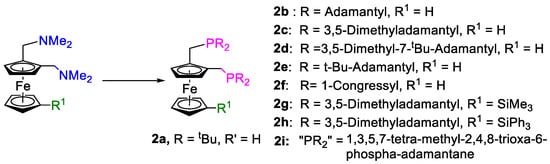
Figure 17.
Examples of the bulky ferrocenyldiphosphines obtained from precursor 5 and the secondary phosphanes, HPR2.
As examples, two of these bulky ligands 2c and 2f were characterised by crystallography, and are shown in Figure 18.
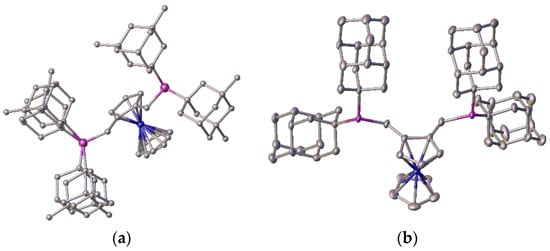
Figure 18.
Ferocenylbisphosphine ligands: (a) 2c (3,5-dimethyladamantyl, local disorder in one pendant arm) and (b) 2f (dicongressyl), showing the steric bulk of the phosphine substituents.
2.2. Catalyst Testing: Initial Evaluation of Ferrocene-Based Ligands vs. Alpha Ligand in the Palladium-Catalysed Formation of Methyl Propanoate from Ethene, CO, and Methanol
We continued catalysis development using the standard Alpha ligand palladium complexes in tandem with this work during the catalytic trials. We altered the experimental conditions throughout the study in order to accommodate improvements. Consequently, it is difficult to make direct comparisons except within individual datasets. The catalyst precursors generally were prepared in situ to reflect industrial conditions. We carried out the coordination of the ferrocenyl phosphine ligand to palladium to form the active catalyst according to the method reported for diphenylphosphinopropane [64,65].
In a typical run, [Pd(OAc)2] (1.44 × 10−5 mol) was added to an autoclave, together with ligand 2 (7.61 × 10−5 mol) and 70% w/w methyl propanoate/methanol (300 mL) and methanesulphonic acid (140 × Pd equivalent). The autoclave was heated to 100 °C and maintained at this operating temperature for the duration of the experiment. Then, we repeated the procedure for each ligand, 1, 2a, and 8. Table 1 shows the results from these initial trials over the first three hours.

Table 1.
First catalysis trials for methyl propanoate formation from ethene, CO, and methanol using palladium(II) complexes of bidentate phosphane ligands (1, 2a, and 8).
In all cases, the initial reaction rates for the benzene-based ligand (1) complex and the ferrocene-based complex (2a) are similar (circa 30,000 (mol cat.) (mol prod.)−1 h−1). However, turnover numbers (TONs) for the ferrocene-based complex are significantly higher than those for the benzene-based analogues. The selectivity for the formation of methyl propanoate for the catalyst derived from ligand 2a was always >99%; thus, these ligands are vastly superior to other ferrocene-based ligands, such as dppf (see [66] for an overview). The high turnover numbers for the ferrocenylphosphane-based catalysts resulted from the greater basicity of the ferrocenyl ligand and the difference in its bite angle. The fall in reaction rate, which occurred after three hours, was attributed to catalyst decomposition, possibly because of de-ligation of one of the phosphines from the metal centre. We used the palladium complex of ligand 8 to counter this; however, the turnover number was reduced, even though the maximum reaction rate was reached more quickly (8–10 min compared to 25 min). More comprehensive tests were carried out following these initial results, again, under the industrial operating conditions. Table 2 provides the results data as average weight gains for the complexes for standard Alpha 1 and butphos 2a. There was no evidence of metal deposition in any of these runs, and all reaction solutions from the autoclave were pale green/yellow.

Table 2.
The methoxycarbonylation of ethene using the alpha ligand, 1, and butphos, 2a *.
In this set of experiments, 0.1mmol of the palladium complex was used in neat methanol. The gas composition was 50:50 CO:ethene for all experiments. The use of neat methanol and relatively high palladium concentrations is generally optimal for high initial rates but accelerated catalyst decay can occur under these conditions. The palladium complex of the Alpha 1 standard gave rise to an average productivity after 3 h of approximately 257.3 g—or an average TON of 45,932. Independent research carried out by Lucite International has shown that average productivity is enhanced when bulky groups, such as tert-butyl, are attached to the 4-position, and increase the steric bulk of alpha ligands [64]. The disadvantage in benzene-derived Alpha ligand design (Figure 10), identified from crystallographic analysis of their palladium complexes, is that the Pd, P, and methylene atoms in the ligands lie approximately in the same plane of a square planar palladium(II) complex [65]. The benzene ring then tilts at 60–80 degrees to this plane, suggesting that the benzene ring can flip between three different positions: up, down, or one up and one down. This ring flipping can, in some cases, lead to ligand dissociation and, hence, catalyst decay. A study of complexes like [(P-P)PdCl2] supports this, and the exchange of the CH2 proton has been observed [65]. For the Alpha ligand, by adding steric bulk to the benzene ring, it is possible to influence the rate of the ring flipping and, consequently, retard the rate of catalyst decay. In the butphos ligand family 2a, the average productivity after 3 h outperforms the Alpha standard, with an average weight gain of 300.9 g, or an average TON of 53,731.
Additionally, variable-temperature NMR analysis of 1,2-di-tert-butylphosphinomethyl ferrocene palladium complexes shows that the chemical shifts of CH2 protons are essentially static at room temperature (see supporting information). Thus, the ferrocenyl ring is not flipping on the NMR timescale at room temperature. Finally, the conformation of the complexed ligand is confirmed by crystal structural data obtained from complexes of methanesulphonic acid salts of the ferrocenylphosphines (isolated during catalyst trials), as shown in Figure 19.
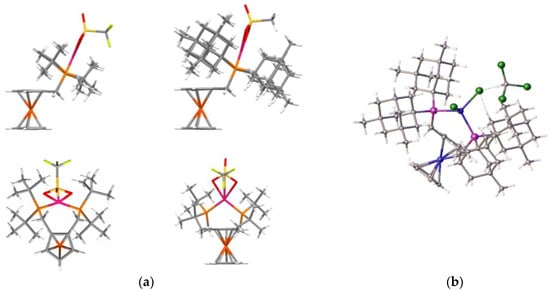
Figure 19.
(a) Typical “above the plane” coordination of palladium (there is a methylsulphonate bound to the palladium); (b) NiCl2 complex of ligand 2c. Figures supplied by I.R.Butler and P.N.Horton (data to be published).
The graphics in Figure 19a, which were prepared from crystal structural data, clearly show the palladium atom positioned above the plane of the ferrocene cyclopentadienyl rings. This positioning may be one reason for the improved activity over the standard Alpha ligand system and allows better steric control of the catalyst system. Even in the nickel complex of ligand 2c, the nickel lies above the cp plane (Figure 19b). However, in this complex, it was also clear that the steric crowding required for optimal catalytic performance was not present in the tetrahedral coordination mode. Finally, data were collected from the silyl-substituted ligand complexes and run again, compared with the Alpha standard. Table 3 presents the results from these experiments in tabular form.

Table 3.
Pd-based catalysis with ferrocenyl phosphine ligand substituted on the lower ring with silyl groups. The graphic shows the palladium dichloride complex, 1′-triphenylsilyl-bis-1,2-(di-tbutylphosphinomethyl)ferrocene, and catalyst precursor.
The average weight gains achieved with the trimethylsilyl-substituted ligand 2g were low and did not significantly improve catalytic performance. However, the triphenyl-substituted ligand, 2h, did show the expected improvement. In addition, the effect of increasing the steric bulk of the phosphine substituents on the catalysis rate did occur for the diadamantyl-substituted ligand 2b and the bulky dimethyladamantylphosphinoferrocene 2c (Table 4).

Table 4.
Comparison of the Alpha ligand, 1, and bulky adamantyl ferrocene ligands—2b, and 2c—in the palladium-catalysed formation of methyl propanoate.
These data show that the steric bulk on the ferrocenylphosphine increases the reaction rate and turnover numbers. In addition, the catalysts are recyclable, and give highly cumulative turnover numbers. In the case of ligand 2c, the fall in reaction rate from the initial rate of 52,700 to 34,125 (approx. 58% of original activity) occurred on its fourth run with the same catalyst, making this a very stable catalyst. The palladium catalyst derived from the standard Alpha ligand had lost approximately 95% of its activity after two recycles. The catalyst derived from ligand 2b also retained about 50% of its activity after two cycles. The adamantylphosphinoferrocene complexes had a much higher TON (approximately 40% increase) when compared to Alpha standards. We also compared some of the more sterically bulky ligands under identical catalytic conditions (Table 5).

Table 5.
Comparison of the lifetimes of catalysts derived from bulky ligands 2b, 2c, 2f, and 2i, with prototypic palladium dichloride complexes of ligands 1 and 2a, under optimised conditions.
From these data, the catalysts prepared from less bulky phosphines show high initial rates; however, the weight gain data show that the metal complexes with more bulky ligands perform better after 3 h. Finally, for completeness, the ruthenocenyl bis-amine analogue of compound 5—compound 26—was prepared and crystallographically characterised (Figure 20).
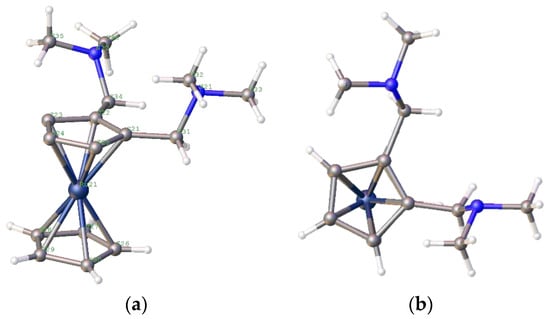
Figure 20.
(a) Side and (b) top views of the crystal structure of the bis-methylamine of ruthenocene, compound 26.
Although the basic structure of compound 26 is remarkably similar to that of compound 5, with the amines both sitting above the cp ring, the actual macrostructures of the crystals used had rather different characteristics. Compound 25 was the starting compound [66,67,68,69]; it was only possible to obtain crystals of 26 as fine needles, generally clumped together. This clumping made the crystallography difficult, and the structure obtained indicated two molecules—the second with 15% occupancy—at a 53° angle to the first, and the second Ru atom sitting 1.4 Å away from the first (Supplementary Materials). However, the ligand precursor is isomorphic; we would expect similar reactivity of the metal complexes, with any differences caused by the relative stabilities of the complexes and the different electron donation properties of ruthenocene compared to ferrocene. The complex 27.PdCl2 (Figure 21) was prepared in situ, and we compare its performance in the catalytic preparation of methyl propanoate to its ferrocenyl analogue 2a.PdCl2.
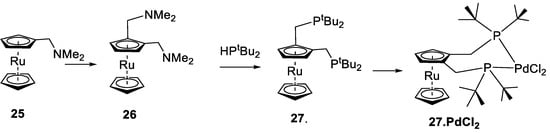
Figure 21.
Schematic of the preparation of the 1,2-bis-(N,N-dimethylaminomethyl)ruthenocene, 26, 1,2-bis-[di-(tert-butyl)phosphano)]ruthenocene, rubutphos, 27, and the complex 27.PdCl2 [69].
These data (Table 6) indicate that the average catalyst activity of the ruthenocene analogue of the catalyst again outperforms both catalysts 1.PdCl2 and 2a.PdCl2 under the reaction conditions used. The rationale for this is the improved stability of the ligand and its palladium complex under the operating conditions. These data were all produced pre-2006, and it is necessary to update the reader on more recent progress with ferrocene-based ligands. We briefly discuss the reaction mechanism studies of the hydride and methoxy routes (Figure 22). Several reversible steps account for the incorporation of deuterium into the methyl propanoate part when d-methanol is a substrate for both cycles. Maintaining a strict square planar coordination leads to a congested environment around palladium when bulky ligands are present. In these mechanisms, there are many intermediates where there is a free coordination site where stabilisation by solvent will play a significant role. It would be interesting to see whether any deuterium ends up in the ligand because of the possible stabilisation of the metal centre by protons on the bulky ligands. The mechanism for our catalyst is more likely to follow the protonation route, given the strongly acidic conditions of our reaction environment.

Table 6.
The individual and average weight gains and catalytic testing results for the palladium dichloride complex of 1,2-di-tert-butylphosphinomethyl ruthenocene, 27. These are the same conditions used for 2aPdCl2 (see Table 2).
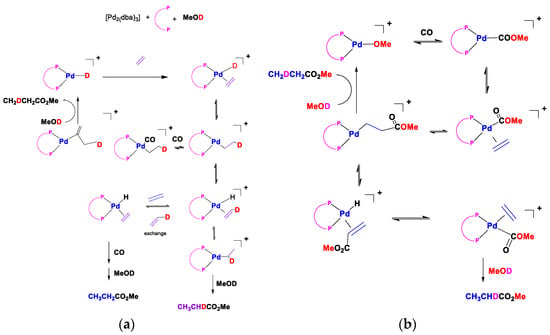
Figure 22.
(a) The proposed hydride (left) and (b) carbomethoxy (right) mechanisms for the formation of methyl propanoate. The palladium complex of ligand 1 catalyses the CO, ethene, and MeOD. (amended from [44,57]).
Finally, it is prudent to update the reader on the work of Beller et al. [70,71,72,73,74,75] in this field, which is much more recent and detailed. These researchers have examined methoxycarbonylation using metal complexes of several adapted ferrocene-based ligands. These ligands generally contain phosphines substituted with t-butyl groups, such as compound 29 shown in Figure 23. These ligand types are essentially variants of those developed by Cullen et al. [76,77]. We had trialled some of these in early our work but discounted them because of their poorer selectivity. These tend to be sterically less congested around the metal centre in comparison to our ligands; however, they incorporate additional features due to the nature of additional functional groups, such as coordinating and protonation sites. However, clearly Beller et al. have championed these ligands and have reported excellent product yields. They have also incorporated adamantyl groups on the phosphorus similar to those in our ligand family thus adding additional steric bulk.

Figure 23.
A small selection of the ferrocene-based ligands used in methoxycarbonylation, from the ligands developed by Cullen et al. (28: T-J. Kim; 29: Butler), 30, general ligand design; and 31, 32, 33, ligands developed by Beller et al.
The inclusion of pyridyl substituents in the ligand design is a useful innovation. However, these would probably become protonated during catalysis, decreasing their solubility. It would be interesting to use the palladium complex of these ligands to isolate critical intermediates. In summary, they have shown that ferrocene-based ligands are extremely valuable in this research area, and numerous patents have come from their work. Thus, we have even more confidence that the ligands described in this paper are worthy of much deeper investigation, as are the new ligands under development within our group. A recent DFT study may help advance the mechanistic insights of this process [78]. Further synthetic details of in situ methods for phosphane preparation can be found in the Supplementary Materials.
3. Conclusions
This review/report covers the historical synthesis of ferrocenylmethylphosphines, which are important ligands in the preparation of methyl propanoate from ethene, CO, and methanol using the palladium-catalysed process, with >99% product selectivity. The research work was internationally leading at the time, but deemed less important in-house. Given that the Lucite Alpha process now dominates the acrylics industry, one can only speculate how this was allowed to happen, and how far this would have progressed in the interim if this had not occurred. This review credits those individuals who worked on the project but have received little or no recognition—in particular, the work of Dr. Kevin Morris, whose Ph.D. work appears in this report. Previously undisclosed catalysis results and structural data are contained in the present work. Further mechanistic work on the ferrocene-based catalysts has been carried out by Dr. Ian Butler (IRB), Professor Brian Heaton, Dr. Jonathan Iggo, and Dr. F. Zacchini; this has yet to be reported.
4. Patents
- Butler, I.; Eastham, G. EP1554039A2 a catalyst system comprising a 1,2-bis-(phosphino)metallocene ligand1, novel carbonylation ligands, and their use in the carbonylation of ethylenically unsaturated compounds. US2010113255A1 (B2) • 6 May 2010 • Earliest priority: 2 December 2006 • Earliest publication: 5 June 2008;
- Butler, I.; Eastham, G. a catalyst system comprising a 1,2-bis-(phosphino)metallocene ligand. EP1554039A2 (B1) • 20 July 2005 • LUCITE INT UK LTD [GB] Earliest priority: 12 September 2002 • Earliest publication: 25 March 2004;
- Butler, I.; Eastham, G.R. Novel carbonylation ligands and their use in the carbonylation of ethylenically unsaturated compounds. ZA200903063B • 28 April 2010 • LUCITE INT UK LTD. Earliest priority: 2 December 2006 • Earliest publication: 7 October 2009;
- Butler, I.R.; Eastham, G.; Fortune, K. a catalyst system comprising a 1,2-bis-(phosphinoalkyl) ferrocene ligand. CA2498293A1 (C) • 25 March 2004 • LUCITE INT UK LTD [GB]Earliest priority: 12 September 2002 • Earliest publication: 25 March 2004.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/inorganics9070057/s1: Crystal structural data for compounds included with deposition codes; experimental data for ligand preparation including previously reported compounds, and details of catalyst preparation (57 pages).
Author Contributions
The following lists the contributions made by each of the authors: K.M.F., performing synthetic experiments, data analysis, proofreading, and editing; C.M.R., crystallographic investigation; C.C. (nee Bünzli), ligand synthesis work; P.N.H., multiple crystallographic investigations, proofreading and data write-ups, depositions; M.E.L., crystallographic investigations; S.J.C., crystallographic investigation; M.W., catalyst formation and testing; W.C., crystallographic investigations and proofreading; R.W.H., crystallographic investigations; I.R.B., project conception, supervision, and planning, performing synthetic experiments, data analysis, manuscript writing, and proofing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded (ca. 15%) by Lucite International plc. The remainder of the funding was from Bangor University (ca. 30%), funding by the authors (ca. 35%), and National Research Services (20%).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Partial data is available in the thesis work reported as refs [2,3].
Acknowledgments
We thank Kevin Morris for his hard work, and he should be a co-author of this work, but sadly, we have been unable to obtain the current contact information required for authorship. We thank Bob Tooze for getting this work started; and especially Graham Eastham, Senior Research Scientist at Lucite International at the time, for taking over the project guidance and seeing it through. We thank David Hughes and Denis Williams, both of Bangor University, for additional support with spectroscopic experiments; the National Mass Spectrometry Service, and its staff, based in Swansea University, for mass spectroscopic results; and the National Crystallography Service (laboratory facilities in Southampton and synchrotron facilities at Daresbury Laboratory SRS operated by Newcastle researchers) for crystallographic support and provision of services. Many students and past researchers have aided this work: Chris Greenwell, Mark Parry, Richard Dickinson, Emily Dickinson, and Dafydd Thomas. We thank Jonathon Iggo and Brian Heaton of Liverpool University for continuing this work. Finally, IRB thanks Heinrich Lang for the kind invitation to present an overview of this work at the 9th Ferrocene Colloquium, 2011 in Chemnitz, Germany (https://www.tu-chemnitz.de/chemie/anorg/files/tagungen/fc9.pdf, accessed on 16 July 2021). We understand these acknowledgements do not constitute endorsement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Butler, I.R. The Simple Synthesis of Ferrocene Ligands from a Practitioner’s Perspective. Eur. J. Inorg. Chem. 2012, 2012, 4387–4406. [Google Scholar] [CrossRef]
- Fortune, K.M. Nitrogen Donor Complexes of Molybdenum and Tungsten and New Routes to bis-1,2 & tris-1,2,3 Substituted Ferrocenes. Ph.D. Thesis, Bangor University, Gwynedd, UK, 2004. [Google Scholar]
- Morris, K. An Investigation into the Synthesis of Phosphine-Based Ligands and Their Application in Pd-Catalysed Processes in the Production of Polymethylmethacrylate. Ph.D. Thesis, Bangor University, Bangor, UK, 2008. [Google Scholar]
- Butler, I.R.; Baker, P.K.; Eastham, G.R.; Fortune, K.M.; Horton, P.N.; Hursthouse, M.B. Ferrocenylmethylphosphines ligands in the palladium-catalysed synthesis of methyl propionate. Inorg. Chem. Commun. 2004, 7, 1049–1052. [Google Scholar] [CrossRef]
- Butler, I.R.; Horton, P.N.; Fortune, K.M.; Morris, K.; Greenwell, C.; Eastham, G.R.; Hursthouse, M.B. The first 1,2,3-tris(phosphinomethyl)ferrocene. Inorg. Chem. Commun. 2004, 7, 923–928. [Google Scholar] [CrossRef]
- Harris, B. Acrylics for the Future. Ingenia. Issue 45. December 2010. Available online: https://www.ingenia.org.uk/Ingenia/Issue-45/Acrylics-for-the-future (accessed on 16 July 2021).
- Cavinato, G.; Toniolo, L. Carbonylation of Ethene Catalysed by Pd(II)-Phosphine Complexes. Molecules 2014, 19, 15116–15161. [Google Scholar] [CrossRef] [Green Version]
- Clegg, W.; Elsegood, M.; Eastham, G.R.; Tooze, R.P.; Wang, X.L.; Whiston, K. Highly active and selective catalysts for the production of methyl propanoate via the methoxycarbonylation of ethene. Chem. Commun. 1999, 1877–1878. [Google Scholar] [CrossRef]
- Knight, J.G.; Doherty, S.; Harriman, A.; Robins, E.G.; Betham, M.; Eastham, G.R.; Tooze, R.P.; Elsegood, M.R.J.; Champkin, P.; Clegg, W. Remarkable Differences in Catalyst Activity and Selectivity for the Production of Methyl Propanoate versus CO−Ethylene Copolymer by a Series of Palladium Complexes of Related C4-Bridged Diphosphines. Organometallics 2000, 19, 4957–4967. [Google Scholar] [CrossRef]
- Frew, J.J.R.; Damian, K.; Van Rensburg, H.; Slawin, A.M.Z.; Tooze, R.P.; Clarke, M. Palladium(II) Complexes of New Bulky Bidentate Phosphanes: Active and Highly Regioselective Catalysts for the Hydroxycarbonylation of Styrene. Chem. Eur. J. 2009, 15, 10504–10513. [Google Scholar] [CrossRef]
- De La Fuente, V.; Waugh, M.; Eastham, G.R.; Iggo, J.A.; Castillon, S.; Claver, C. Phosphine Ligands in the Palladium-Catalysed Methoxycarbonylation of Ethene: Insights into the Catalytic Cycle through an HP NMR Spectroscopic Study. Chem. Eur. J. 2010, 16, 6919–6932. [Google Scholar] [CrossRef]
- Drent, E.; Van Broekhoven, J.; Doyle, M. Efficient palladium catalysts for the copolymerization of carbon monoxide with olefins to produce perfectly alternating polyketones. J. Organomet. Chem. 1991, 417, 235–251. [Google Scholar] [CrossRef]
- Wong, P.K.; Van Doorn, J.A.; Drent, E.; Sudmeijer, O.; Stil, H.A. Palladium-catalyzed alternating copolymerization of propylene and carbon monoxide. Formation of poly(spiroketal/ketone). Ind. Eng. Chem. Res. 1993, 32, 986–988. [Google Scholar] [CrossRef]
- Drent, E.; Budzelaar, P.H.M. Palladium-Catalyzed Alternating Copolymerization of Alkenes and Carbon Monoxide. Chem. Rev. 1996, 96, 663–682. [Google Scholar] [CrossRef]
- Baya, M.; Houghton, J.; Konya, D.; Champouret, Y.; Daran, J.-C.; Leñero, K.Q.A.; Schoon, L.; Mul, W.P.; van Oort, A.B.; Meijboom, N.; et al. Pd(I) Phosphine Carbonyl and Hydride Complexes Implicated in the Palladium-Catalyzed Oxo Process. J. Am. Chem. Soc. 2008, 130, 10612–10624. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A. Alternating copolymerization of carbon monoxide and olefins by single-site metal catalysis. Coord. Chem. Rev. 2002, 225, 35–66. [Google Scholar] [CrossRef]
- Robertson, R.A.; Cole-Hamilton, D.J. The production of low molecular weight oxygenates from carbon monoxide and ethene. Coord. Chem. Rev. 2002, 225, 67–90. [Google Scholar] [CrossRef]
- Sen, A. Mechanistic aspects of metal-catalyzed alternating copolymerization of olefins with carbon monoxide. Acc. Chem. Res. 1993, 26, 303–310. [Google Scholar] [CrossRef]
- Cavinato, G.; Tonioli, L.; Vavasori, A. Carbonylation of Ethene in Methanol Catalysed by Cationic Phosphine Complexes of Pd(II): From Polyketones to Monocarbonylated Products. Top. Organomet. Chem. 2006, 18, 125–164. [Google Scholar] [CrossRef]
- Garcia-Suarez, E.J.; Godard, C.; Ruiz, A.; Claver, C. Alternating and Non-Alternating Pd-Catalysed Co- and Terpolymerisation of Carbon Monoxide and Alkenes. Eur. J. Inorg. Chem. 2007, 2007, 2582–2593. [Google Scholar] [CrossRef]
- Pascu, S.I. CO/alkene copolymerisation reactions catalysed by chelating diphosphine, diimine and hemilabile N/O, P/O and P/N late transition metal complexes revisited. Rev. Roum. Chim. 2009, 54, 477–500. Available online: https://revroum.lew.ro/wp-content/uploads/2009/RRCh_6_2009/Art%2008.pdf (accessed on 22 June 2021).
- Consiglio, G.; Milani, B. Stereochemical Aspects of Cooligomerization and Copolymerization. In Catalysis by Metal Complexes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; pp. 189–215. [Google Scholar]
- Bianchini, C.; Meli, A.; Oberhauser, W. Catalyst design and mechanistic aspects of the alternating copolymerisation of ethene and carbon monoxide by diphosphine-modified palladium catalysisThe illustration of John Dalton (reproduced courtesy of the Library and Information Centre, Royal Society of Chemistry) marks the 200th anniversary of his investigations which led to the determination of atomic weights for hydrogen, nitrogen, carbon, oxygen, phosphorus and sulfur. Dalton Trans. 2003, 2627–2635. [Google Scholar] [CrossRef]
- Sen, A. Chain Propagation Mechanisms; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; Volume 27, pp. 237–263. [Google Scholar]
- Belov, G.P.; Novikova, E.V. Polyketones as alternating copolymers of carbon monoxide. Russ. Chem. Rev. 2004, 73, 267–291. [Google Scholar] [CrossRef]
- Keim, W.; Maas, H. Copolymerization of ethylene and carbon monoxide by phosphinite-modified palladium catalysts. J. Organomet. Chem. 1996, 514, 271–276. [Google Scholar] [CrossRef]
- Cavinato, G.; Vavasori, A.; Amadio, E.; Tonioli, L. CO–ethene copolymerisation catalysed by [PdCl2(PPh3)2]/PPh3/HCl in MeOH. J. Mol. Catal. A Chem. 2007, 278, 251–257. [Google Scholar] [CrossRef]
- Margl, P.; Michalak, A.; Ziegler, T. Theoretical Studies on Copolymerization of Polar Monomers. In Catalysis by Metal Complexes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; Volume 27, pp. 265–307. [Google Scholar]
- Nozaki, K. Synthesis of Chiral, Optically Active Copolymers. In Catalysis by Metal Complexes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; Volume 27, pp. 217–235. [Google Scholar]
- Schwarz, J.; Herdtweck, A.E.; Herrmann, W.A.; Gardiner, M. Highly Efficient Monocationic Palladacycles of Chelating Diphosphines in C2H4/CO Copolymerization. Organometallics 2000, 19, 3154–3160. [Google Scholar] [CrossRef]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-Insertion Copolymerization of Fundamental Polar Monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef]
- Eastham, G.R.; Tooze, R.P.; Heaton, B.T.; Iggo, J.A.; Whyman, R.; Zacchini, S. Synthesis and spectroscopic characterisation of all the intermediates in the Pd-catalysed methoxycarbonylation of ethene. Chem. Commun. 2000, 609–610. [Google Scholar] [CrossRef]
- Del Rio, I.; Claver, C.; van Leeuwen, P.W.N.M. On the Mechanism of the Hydroxycarbonylation of Styrene with Palladium Systems. Eur. J. Inorg. Chem. 2001, 2719–2738. [Google Scholar] [CrossRef]
- Doherty, S.; Eastham, G.R.; Tooze, R.P.; Scanlan, T.H.; Williams, D.; Elsegood, M.R.J.; Clegg, W. Palladium Complexes of C2-, C3-, and C4-Bridged Bis(phospholyl) Ligands: Remarkably Active Catalysts for the Copolymerization of Ethylene and Carbon Monoxide. Organometallics 1999, 18, 3558–3560. [Google Scholar] [CrossRef]
- Liu, J.; Heaton, B.T.; Iggo, J.A.; Whyman, R. The Complete Delineation of the Initiation, Propagation, and Termination Steps of the Carbomethoxy Cycle for the Carboalkoxylation of Ethene by Pd–Diphosphane Catalysts. Angew. Chem. Int. Ed. 2004, 43, 90–94. [Google Scholar] [CrossRef]
- Liu, J.; Heaton, B.T.; Iggo, J.A.; Whyman, R.; Bickley, J.F.; Steiner, A. The Mechanism of the Hydroalkoxycarbonylation of Ethene and Alkene–CO Copolymerization Catalyzed by PdII–Diphosphine Cations. Chem. Eur. J. 2006, 12, 4417–4430. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Heaton, B.T.; Iggo, J.A.; Whyman, R. Methanolysis of acyl–Pd(ii) complexes relevant to CO/ethene coupling reactions. Chem. Commun. 2004, 1326–1327. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Zuideveld, M.A.; Swennenhuis, B.H.G.; Freixa, Z.; Kamer, P.C.J.; Goubitz, K.; Fraanje, J.; Lutz, A.M.; Spek‡, A.L. Alcoholysis of Acylpalladium(II) Complexes Relevant to the Alternating Copolymerization of Ethene and Carbon Monoxide and the Alkoxycarbonylation of Alkenes: The Importance of Cis-Coordinating Phosphines. J. Am. Chem. Soc. 2003, 125, 5523–5539. [Google Scholar] [CrossRef]
- Mul, W.P.; Oosterbeek, H.; Beitel, G.A.; Kramer, G.J.; Drent, E. In Situ Monitoring of a Heterogeneus Palladium-Based Polyketone Catalyst. Angew. Chem. Int. Ed. 2000, 39, 1848–1851. [Google Scholar] [CrossRef]
- Shultz, C.S.; Ledford, J.; DeSimone, J.M.; Brookhart, M. Kinetic Studies of Migratory Insertion Reactions at the (1,3-Bis(diphenylphosphino)propane)Pd(II) Center and Their Relationship to the Alternating Copolymerization of Ethylene and Carbon Monoxide. J. Am. Chem. Soc. 2000, 122, 6351–6356. [Google Scholar] [CrossRef]
- Zuideveld, M.A.; Kamer, P.C.J.; Van Leeuwen, P.W.N.M.; Klusener, P.A.A.; Stil, H.A.; Roobeek, C.F. Chain-Transfer Mechanisms of the Alternating Copolymerization of Carbon Monoxide and Ethene Catalyzed by Palladium(II) Complexes: Rearrangement to Highly Reactive Enolates. J. Am. Chem. Soc. 1998, 120, 7977–7978. [Google Scholar] [CrossRef]
- Ledford, J.; Shultz, C.S.; Gates, D.P.; White, P.S.; DeSimone, J.M.; Brookhart, M. Bond Angle Effects on the Migratory Insertion of Ethylene and Carbon Monoxide into Palladium(II)−Methyl Bonds in Complexes Bearing Bidentate Phosphine Ligands. Organometallics 2001, 20, 5266–5276. [Google Scholar] [CrossRef]
- Zuidema, E.; Bo, C.; Van Leeuwen, P.W.N.M. Ester versus Polyketone Formation in the Palladium−Diphosphine Catalyzed Carbonylation of Ethene. J. Am. Chem. Soc. 2007, 129, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Eastham, G.R.; Tooze, R.P.; Kilner, M.; Foster, D.F.; Cole-Hamilton, D.J. Deuterium labelling evidence for a hydride mechanism in the formation of methyl propanoate from carbon monoxide, ethene and methanol catalysed by a palladium complex. J. Chem. Soc. Dalton Trans. 2002, 1613–1617. [Google Scholar] [CrossRef]
- Clegg, W.; Eastham, G.R.; Elsegood, M.R.J.; Heaton, B.T.; Iggo, J.A.; Tooze, R.P.; Whyman, R.; Zacchini, S. Synthesis and reactivity of palladium hydrido-solvento complexes, including a key intermediate in the catalytic methoxycarbonylation of ethene to methyl propanoate. J. Chem. Soc. Dalton Trans. 2002, 3300–3308. [Google Scholar] [CrossRef]
- Kalsin, A.; Vologdin, N.V.; Peganova, T.A.; Petrovskii, P.V.; Lyssenko, K.; Dolgushin, F.M.; Gusev, O.V. Palladium(II) complexes with o-aryl substituted 1,1′-bis(phosphino)ferrocenes [Fe(η5-C5H4PR2)2Pd(NCMe)n](OTf)2 (R=o-MeOC6H4, o-MeC6H4, o-PriC6H4, C6F5): Synthesis, structure and catalytic properties in methoxycarbonylation of ethylene. J. Organomet. Chem. 2006, 691, 921–927. [Google Scholar] [CrossRef]
- Gusev, O.V.; Kalsin, A.; Peterleitner, M.G.; Petrovskii, P.V.; Lyssenko, K.; Akhmedov, N.G.; Bianchini, C.; Meli, A.; Oberhauser, W. Palladium(II) Complexes with 1,1′−Bis(diphenylphosphino)ferrocenes [Fe(η5-C5R4PPh2)2]n+ (dppf, R = H, n = 0; dppomf, R = Me, n = 0; dppomf+, R = Me, n = 1). Synthesis, Characterization, and Catalytic Activity in Ethene Methoxycarbonylation. Organometallics 2002, 21, 3637–3649. [Google Scholar] [CrossRef]
- Zúñiga, C.; Moya, S.A.; Aguirre, P. Methoxycarbonylation of Styrene Catalyzed by Palladium Complexes with Ferrocene Derivatives Containing Nitrogen and Phosphine Ligands. Catal. Lett. 2009, 130, 373–379. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Parisel, S.; Gusev, O.V.; Kalsin, A.; Vologdin, N.V.; Dolgushin, F.M. Methoxycarbonylation of styrene to methyl arylpropanoates catalyzed by palladium(II) precursors with 1,1′-bis(diphenylphosphino)metallocenes. J. Mol. Catal. A Chem. 2004, 224, 35–49. [Google Scholar] [CrossRef]
- Gusev, O.V.; Kalsin, A.; Petrovskii, P.V.; Lyssenko, K.; Oprunenko, Y.F.; Bianchini, C.; Meli, A.; Oberhauser, W. Synthesis, Characterization, and Reactivity of 1,1′-Bis(diphenylphosphino)osmocene: Palladium(II) Complexes and Their Use as Catalysts in the Methoxycarbonylation of Olefins. Organometallics 2003, 22, 913–915. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A.A.; Oberhauser, W.; Zuideveld, M.A.; Freixa, Z.; Kamer, P.C.J.; Spek, A.L.; Gusev, O.V.; Kal’Sin, A.M. Methoxycarbonylation of Ethene by Palladium(II) Complexes with 1,1′-Bis(diphenylphosphino)ferrocene (dppf) and 1,1′-Bis(diphenylphosphino)octamethylferrocene (dppomf). Organometallics 2003, 22, 2409–2421. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Parisel, S.; Passaglia, E.; Ciardelli, F.; Gusev, O.V.; Kal’Si, A.M.; Vologdin, N.V. Ethylene Carbonylation in Methanol and in Aqueous Media by Palladium(II) Catalysts Modified with 1,1′-Bis(dialkylphosphino)ferrocenes. Organometallics 2005, 24, 1018–1030. [Google Scholar] [CrossRef]
- Liptau, P.; Seki, T.; Kehr, G.; Abele, A.; Froehlich, R.; Erker, G.; Grimme, S. Formation of a Chelate Bis(phosphino)[3]ferrocenophane Ligand and Its Use in Palladium-Catalyzed Alternating CO/Ethene Copolymerization. Organometallics 2003, 22, 2226–2232. [Google Scholar] [CrossRef]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Segarra, A.M.; Passaglia, E.; Lamac, M.; Štěpnička, P. Palladium(II) Complexes with Phosphanylferrocenecarboxylate Ligands and Their Use as Catalyst Precursors for Semialternating CO–Ethylene Copolymerization. Eur. J. Inorg. Chem. 2008, 2008, 441–452. [Google Scholar] [CrossRef]
- Chen, C.; Anselment, T.M.J.; Fröhlich, R.; Rieger, B.; Kehr, G.; Erker, G. o-Diarylphosphinoferrocene Sulfonate Palladium Systems for Nonalternating Ethene–Carbon Monoxide Copolymerization. Organometallics 2011, 30, 5248–5257. [Google Scholar] [CrossRef]
- Fanjul, T.; Eastham, G.; Fey, N.; Hamilton, A.; Orpen, A.G.; Pringle, P.G.; Waugh, M. Palladium Complexes of the Heterodiphosphine o-C6H4(CH2PtBu2)(CH2PPh2) Are Highly Selective and Robust Catalysts for the Hydromethoxycarbonylation of Ethene. Organometallics 2010, 29, 2292–2305. [Google Scholar] [CrossRef]
- Vondran, J.; Furst, M.R.L.; Eastham, G.R.; Seidensticker, T.; Cole-Hamilton, D.J. Magic of Alpha: The Chemistry of a Remarkable Bidentate Phosphine, 1,2-Bis(di-tert-butylphosphinomethyl)benzene. Chem. Rev. 2021, 121, 6610–6653. [Google Scholar] [CrossRef]
- Hamann, B.C.; Hartwig, J.F. Sterically Hindered Chelating Alkyl Phosphines Provide Large Rate Accelerations in Palladium-Catalyzed Amination of Aryl Iodides, Bromides, and Chlorides, and the First Amination of Aryl Tosylates. J. Am. Chem. Soc. 1998, 120, 7369–7370. [Google Scholar] [CrossRef]
- Kataoka, N.; Shelby, Q.; Stambuli, A.J.P.; Hartwig, J.F. Air Stable, Sterically Hindered Ferrocenyl Dialkylphosphines for Palladium-Catalyzed C−C, C−N, and C−O Bond-Forming Cross-Couplings. J. Org. Chem. 2002, 67, 5553–5566. [Google Scholar] [CrossRef]
- Hnetinka, C.A.; Hunter, A.D.; Zeller, M.; Lesley, M.J.G. 1,1′-Dibromoferrocene. Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m1806–m1807. [Google Scholar] [CrossRef]
- Butler, I.R.; Cullen, W.R.; Rettig, S.J. Synthesis of derivatives of [.alpha.(dimethylamino)ethyl]ferrocene via lithiation reactions and the structure of 2-[.alpha.-(dimethylamino)ethyl]-1,1’,3-tris(trimethylsilyl)ferrocene. Organometallics 1986, 5, 1320–1328. [Google Scholar] [CrossRef]
- Butler, I.R.; Cullen, W.R.; Rettig, S.J. Reaction of phosphorus-bridged ferrocenophane Fe(.eta.5-C5H4PPh)(.eta.5-C5H4) with LiC5H5 and NaFe(CO)2(.eta.5-C5H5). Structures of {Fe[(.eta.5-C5H4)2]P(C6H5)-P}Fe(H)(.eta.5-C5H5)(CO) and {(C6H5)[Fe(.eta.5-C5H5)(.eta.5-C5H4)][Fe(.eta.5-C5H5)(.eta.5-C5H3C(O))]P-P,C}Fe(.eta.5-C5H5)(CO).cntdot.CHCl3. Organometallics 1987, 6, 872–880. [Google Scholar] [CrossRef]
- Muñoz, B.K.; Garcia, E.S.; Godard, C.; Zangrando, E.; Bo, C.; Ruiz, A.; Claver, C. HP-NMR Study of the Pd-Catalyzed Methoxycarbonylation of Styrene Using Monodentate and Bidentate Phosphane-Modified Systems. Eur. J. Inorg. Chem. 2008, 2008, 4625–4637. [Google Scholar] [CrossRef]
- Eastham, G.; Scanlan, T.; Barclay, C. MeP Synthesis–Technical Report 138. Lucite International—Project Alpha. Private Communication; Lucite International Inc.: Memphis, TN, USA, 2005. [Google Scholar]
- Eastham, G.; Wright, W.; Scanlan, T.; Barcley, C. MeP Synthesis–Technical Report 139. Lucite International—Project Alpha. Private Communication; Lucite International Inc.: Memphis, TN, USA, 2005. [Google Scholar]
- Abbenhuis, H.C.L.; Burckhardt, U.; Gramlich, V.; Martelletti, A.; Spencer, J.; Steiner, I.; Togni, A. Comparing Chiral Ferrocenyl and Ruthenocenyl Ligands: How Subtle Structural Changes Influence Their Performance in Asymmetric Catalysis. Organometallics 1996, 15, 1614–1621. [Google Scholar] [CrossRef] [Green Version]
- Glidewell, C.; Royles, B.J.; Smith, D.M. A simple high-yielding synthesis of ferrocene-1,1′-diylbis-(methyltrimethylammonium iodide). J. Organomet. Chem. 1997, 527, 259–261. [Google Scholar] [CrossRef]
- Hadlington, M.; Rockett, B.W.; Nelhans, A. Unsymmetrically disubstituted ferrocenes. Part I. Synthesis of 1,2-disubstituted ferrocenes by metallation and nucleophilic substitution reactions. J. Chem. Soc. C 1967, 1436–1440. [Google Scholar] [CrossRef]
- Kamiyama, S.-I.; Kimura, T.; Kasahara, A.; Izumi, T.; Maemura, M. The σ-Bonded Palladium(II) Complex of (Dimethylaminomethyl)ruthenocene. Bull. Chem. Soc. Jpn. 1979, 52, 142–145. [Google Scholar] [CrossRef]
- Sang, R.; Hu, Y.; Razzaq, R.; Jackstell, R.; Franke, R.; Beller, M. State-of-the-art palladium-catalyzed alkoxycarbonylations. Org. Chem. Front. 2021, 8, 799–811. [Google Scholar] [CrossRef]
- Liu, J.; Dong, K.; Franke, R.; Neumann, H.; Jackstell, R.; Beller, M. Development of efficient palladium catalysts for alkoxycarbonylation of alkenes. Chem. Commun. 2018, 54, 12238–12241. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Sang, R.; Fang, X.; Franke, R.; Spannenberg, A.; Neumann, H.; Jackstell, R.; Beller, M. Efficient Palladium-Catalyzed Alkoxycarbonylation of Bulk Industrial Olefins Using Ferrocenyl Phosphine Ligands. Angew. Chem. Int. Ed. 2017, 56, 5267–5271. [Google Scholar] [CrossRef] [PubMed]
- EVONIK DEGUSSA GMBH; Dong, K.; Neumann, H.; Jackstell, R.; Beller, M.; Franke, R.; Hess, D.; Dyballa, K.M.; Fridag, D.; Geilen, F. Ferrocene-Based Compounds and Palladium Catalysts Based Thereon for the Alkoxycarbonylation of Ethylenically Unsaturated Compounds. Patent: 10077228, 18 September 2018. Available online: https://patents.justia.com/patent/10077228 (accessed on 15 July 2021).
- EVONIK DEGUSSA GMBH; Dong, K.; Neumann, H.; Jackstell, R.; Beller, M.; Franke, R.; Hess, D.; Dyballa, K.M.; Fridag, D.; Geilen, F. Ferrocene-Based Compounds and Palladium Catalysts Based Thereon for the Alkoxycarbonylation of Ethylenically Unsaturated Compounds. Patent: 10202329, 12 February 2019. Available online: https://patents.justia.com/patent/10202329 (accessed on 15 July 2021).
- EVONIK DEGUSSA GMBH; Dong, K.; Neumann, H.; Jackstell, R.; Beller, M.; Fridag, D.; Hess, D.; Dyballa, K.M.; Geilen, F.; Franke, R. 1,1′-Bis(phosphino)ferrocene Ligands for Alkoxycarbonylation. Patent: 9938310, 10 April 2018. Available online: https://patents.justia.com/patent/20180022773 (accessed on 16 July 2021).
- Butler, I.R.; Cullen, W.R.; Kim, T.J.; Rettig, S.J.; Trotter, J. 1,1′-Bis(alkylarylphosphino)ferrocenes: Synthesis, metal complex formation, and crystal structure of three metal complexes of Fe(.eta.5-C5H4PPh2)2. Organometallics 1985, 4, 972–980. [Google Scholar] [CrossRef]
- Butler, I.R.; Cullen, W.R.; Einstein, F.W.B.; Rettig, S.J.; Willis, A.J. Synthesis of some ring-substituted [1]ferrocenophanes and the structure of four representative examples. Organometallics 1983, 2, 128–135. [Google Scholar] [CrossRef]
- Ahmad, S.; Crawford, L.E.; Bühl, M. Palladium-catalysed methoxycarbonylation of ethene with bidentate diphosphine ligands: A density functional theory study. Phys. Chem. Chem. Phys. 2020, 22, 24330–24336. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).