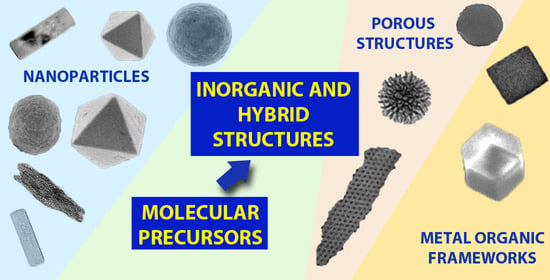
Molecular Bottom-Up Approaches for the Synthesis of Inorganic and Hybrid Nanostructures
Abstract
:1. Introduction
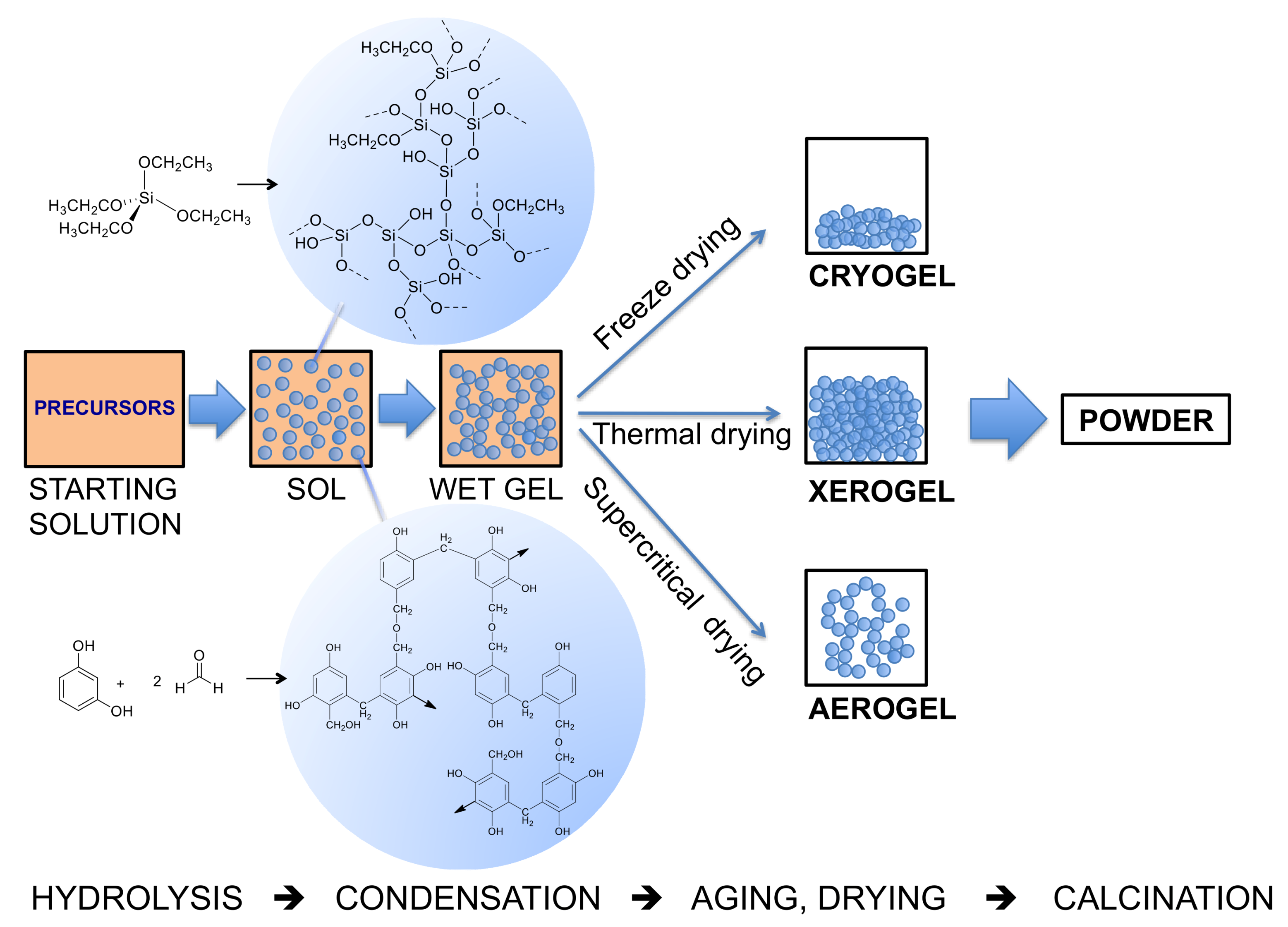
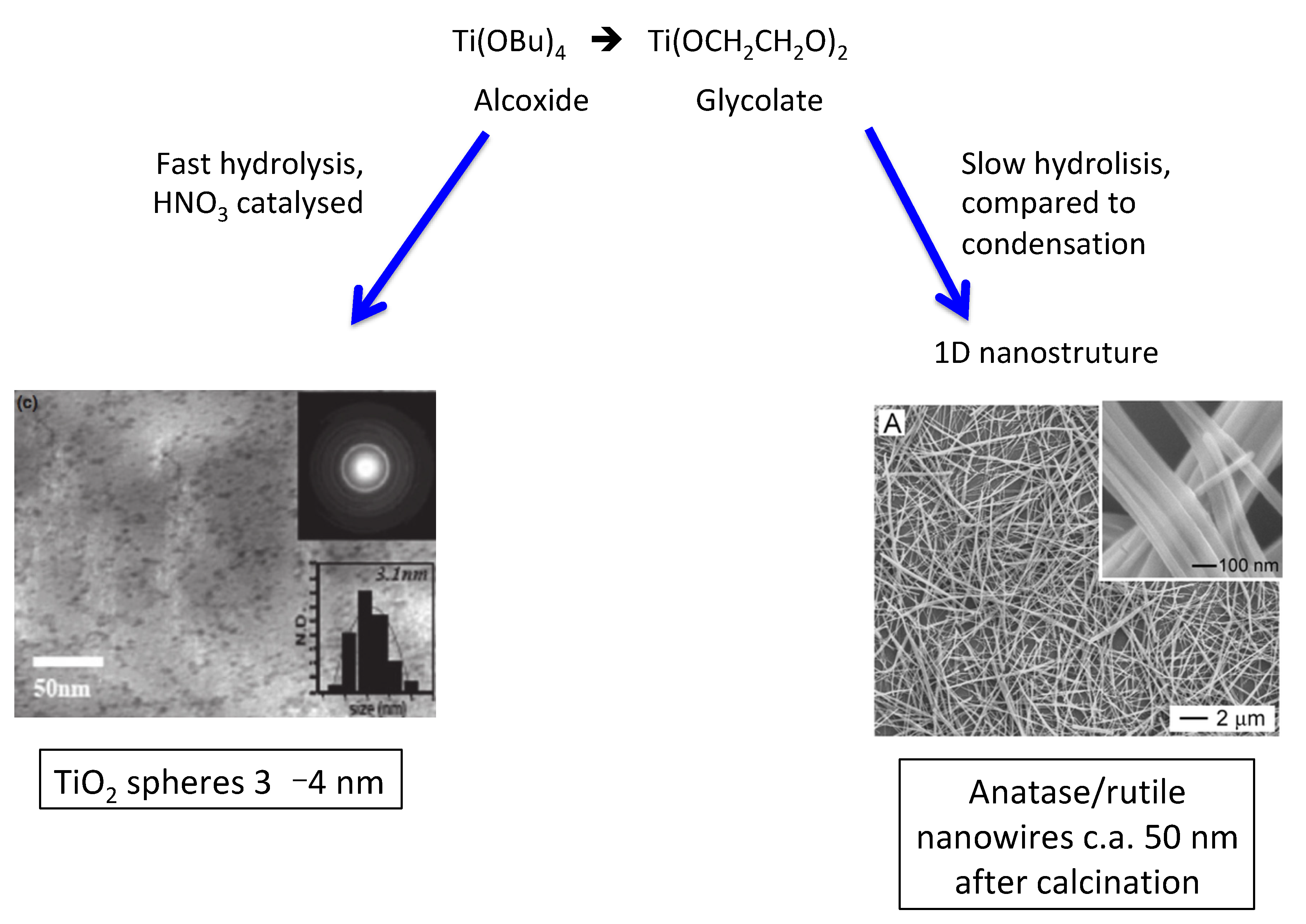
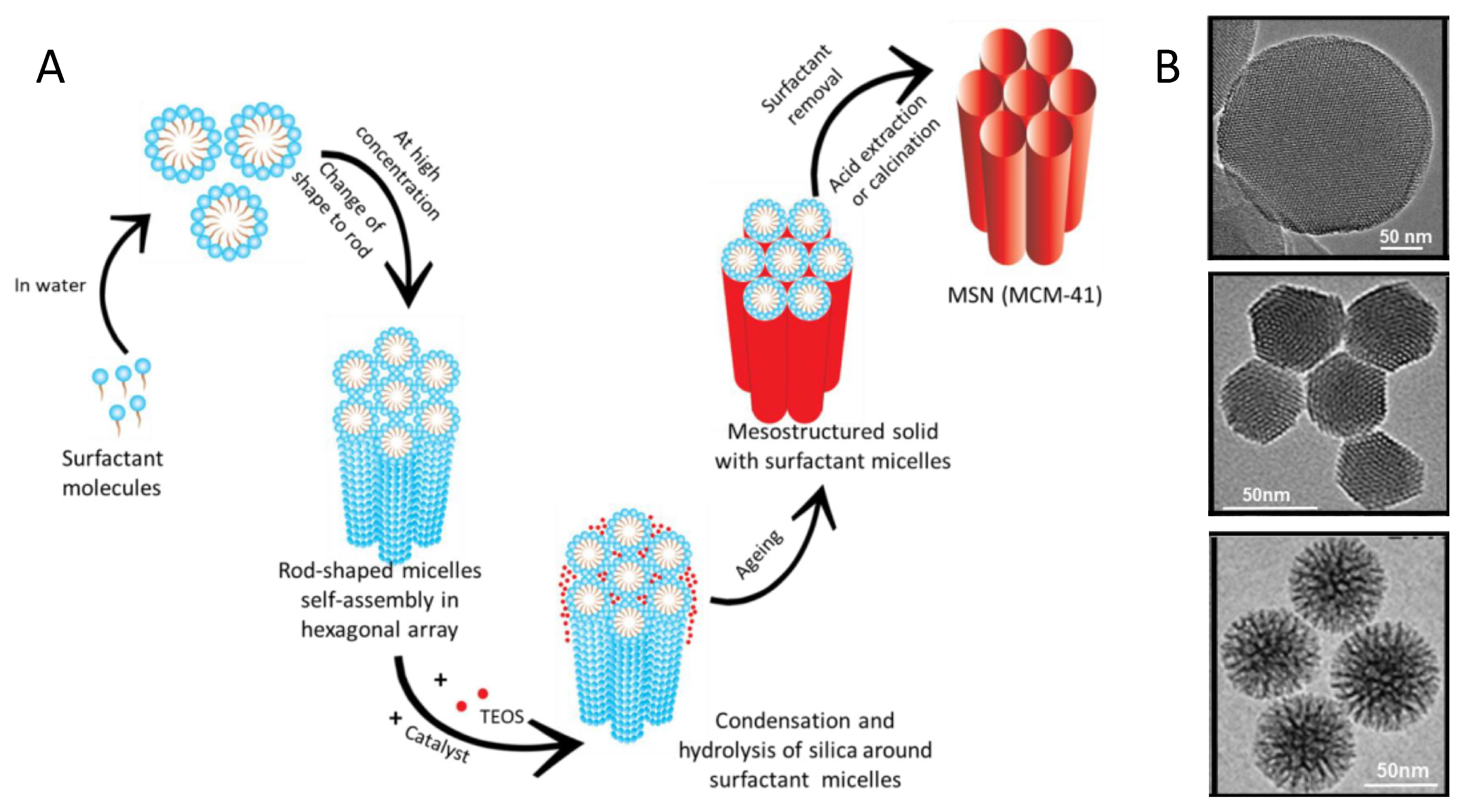
2. Condensation and Polymerization Reactions: Routes Based on the Sol–Gel Approach
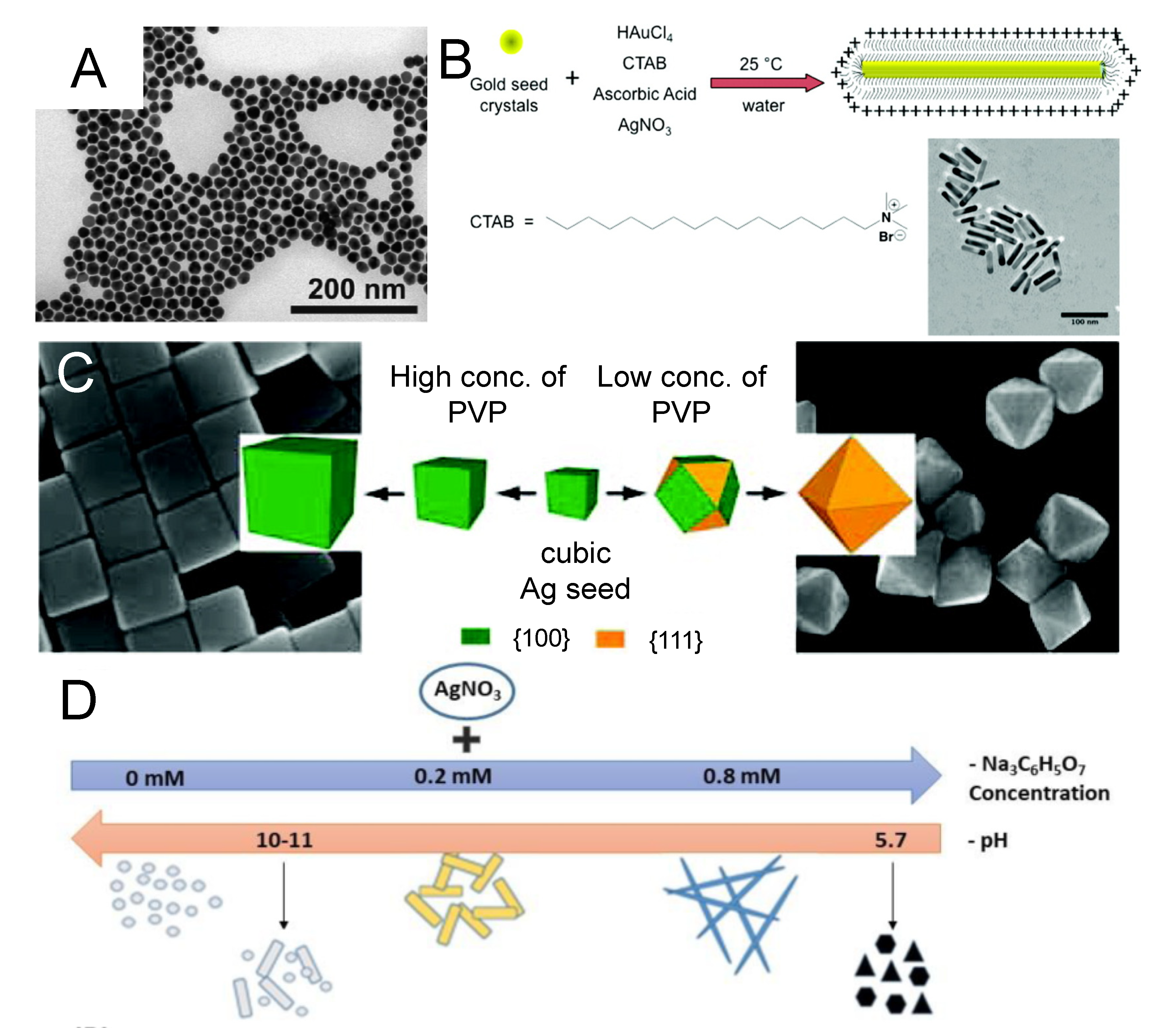
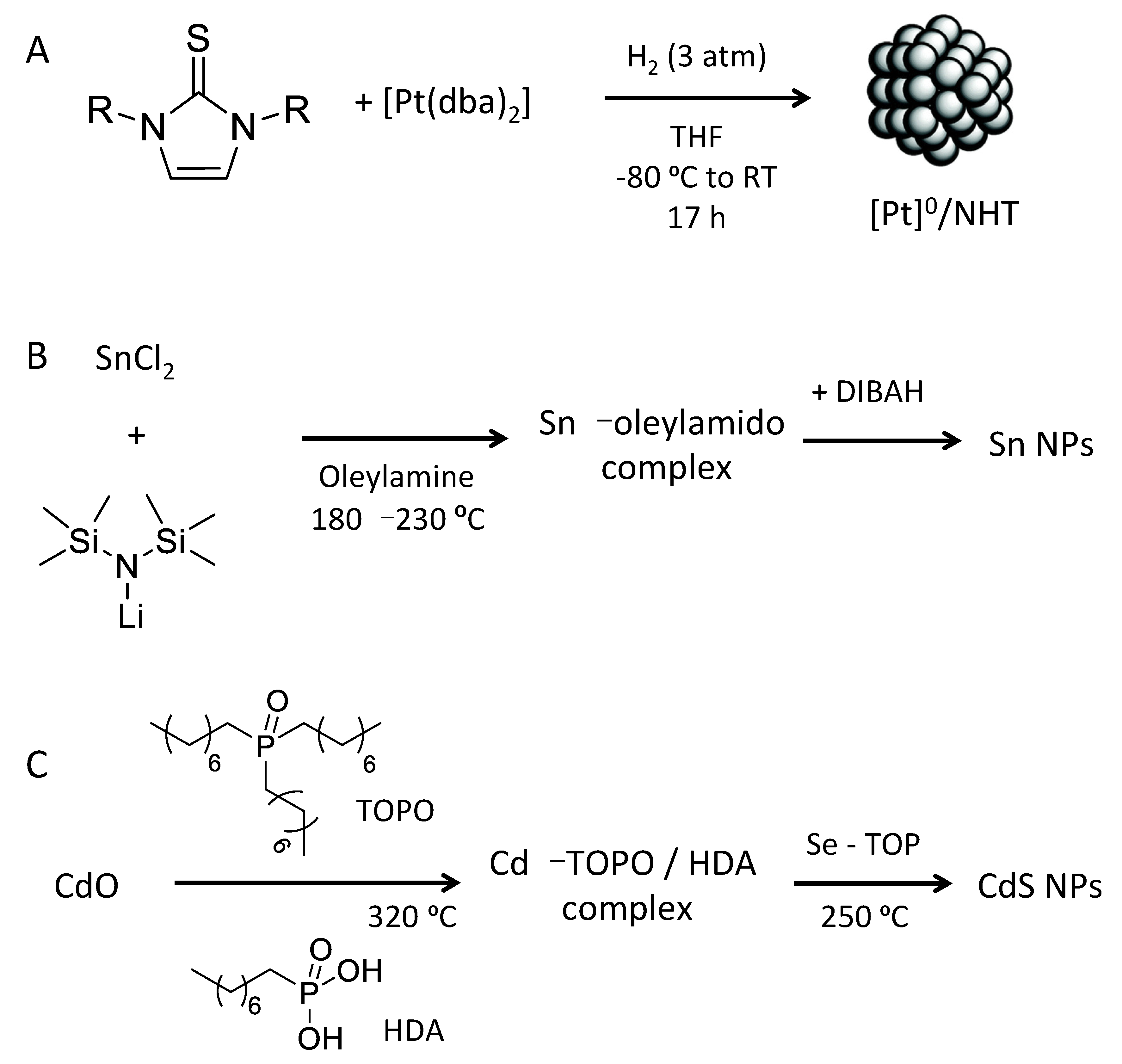
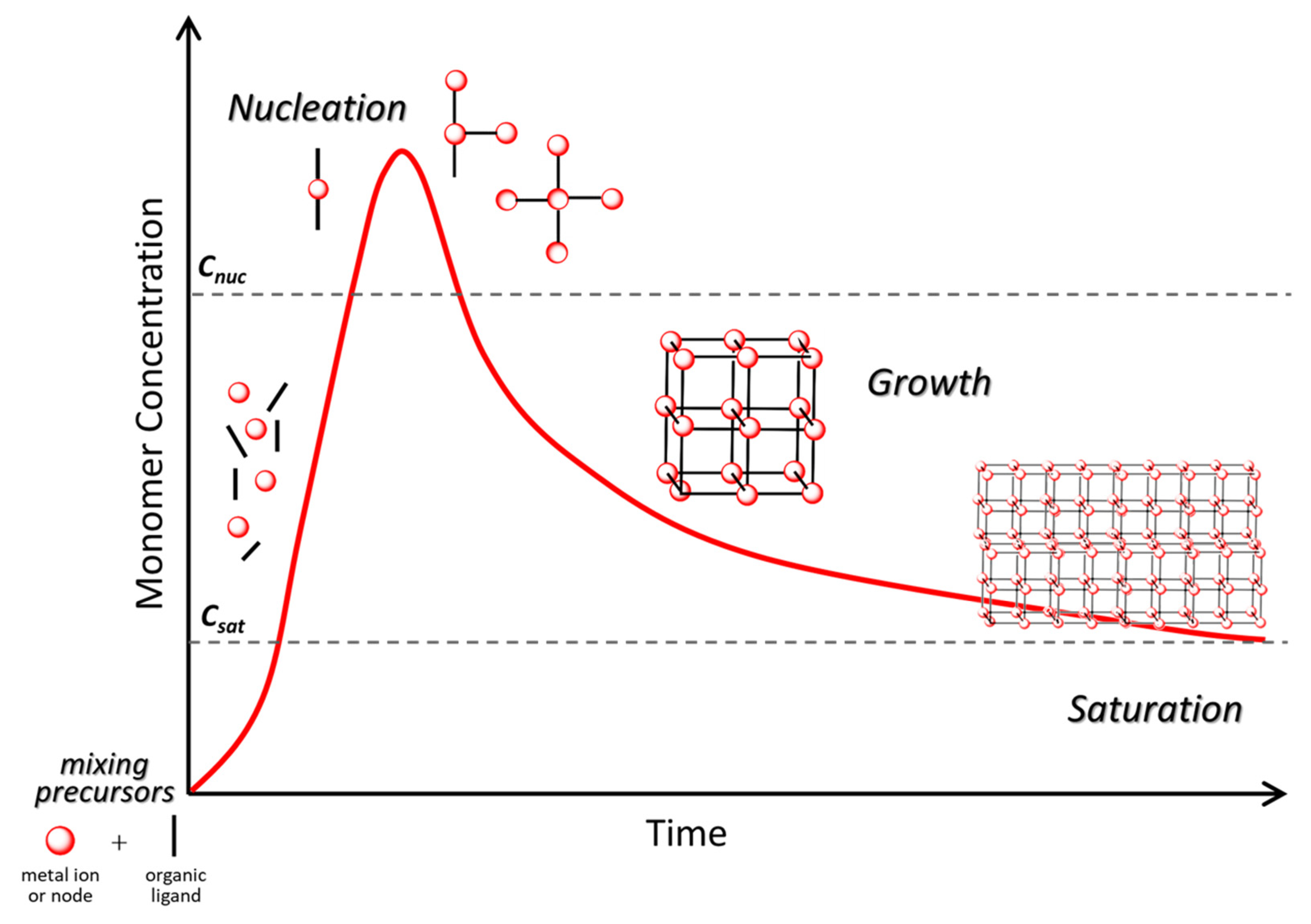
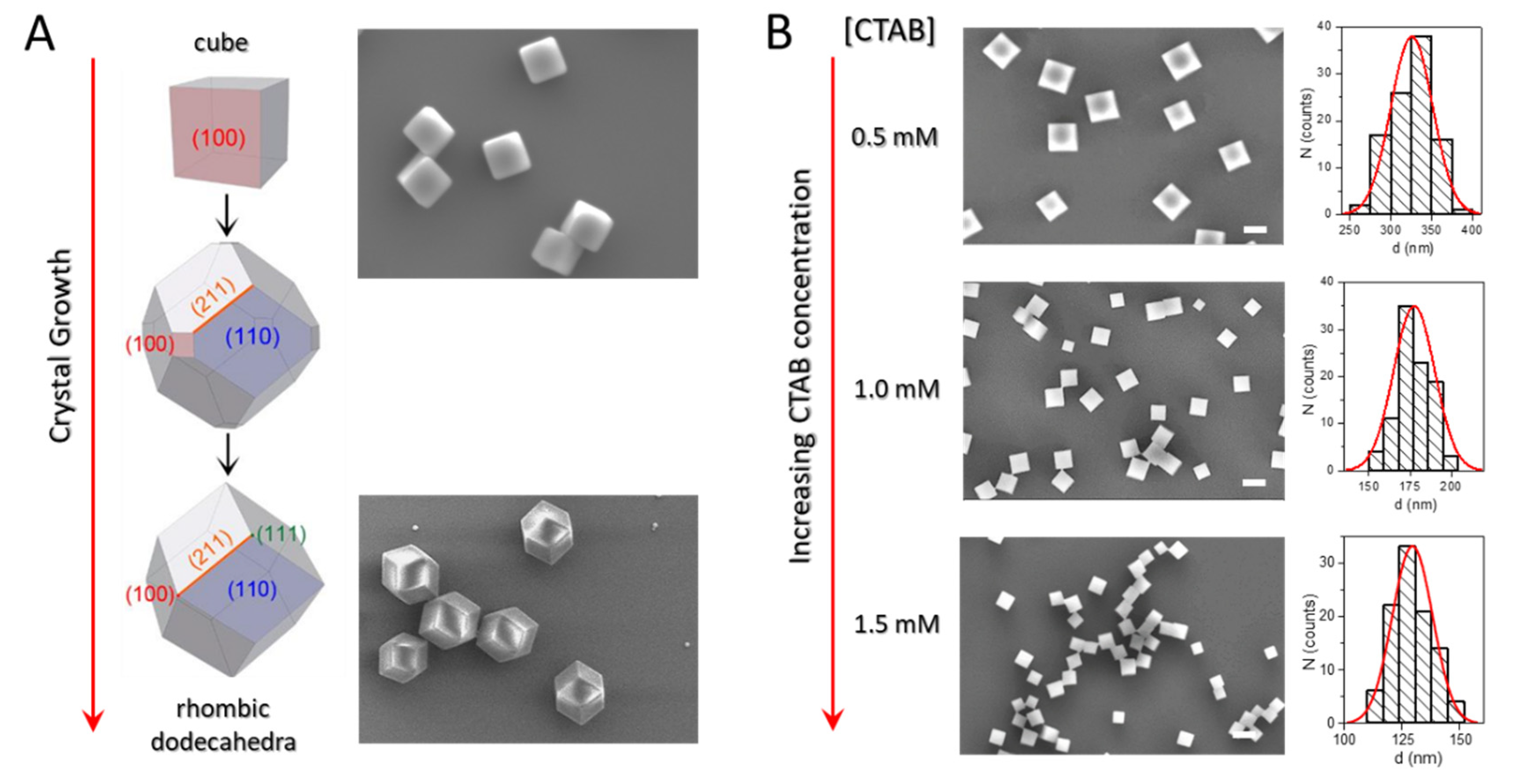
3. Colloidal Synthesis of Nanostructures
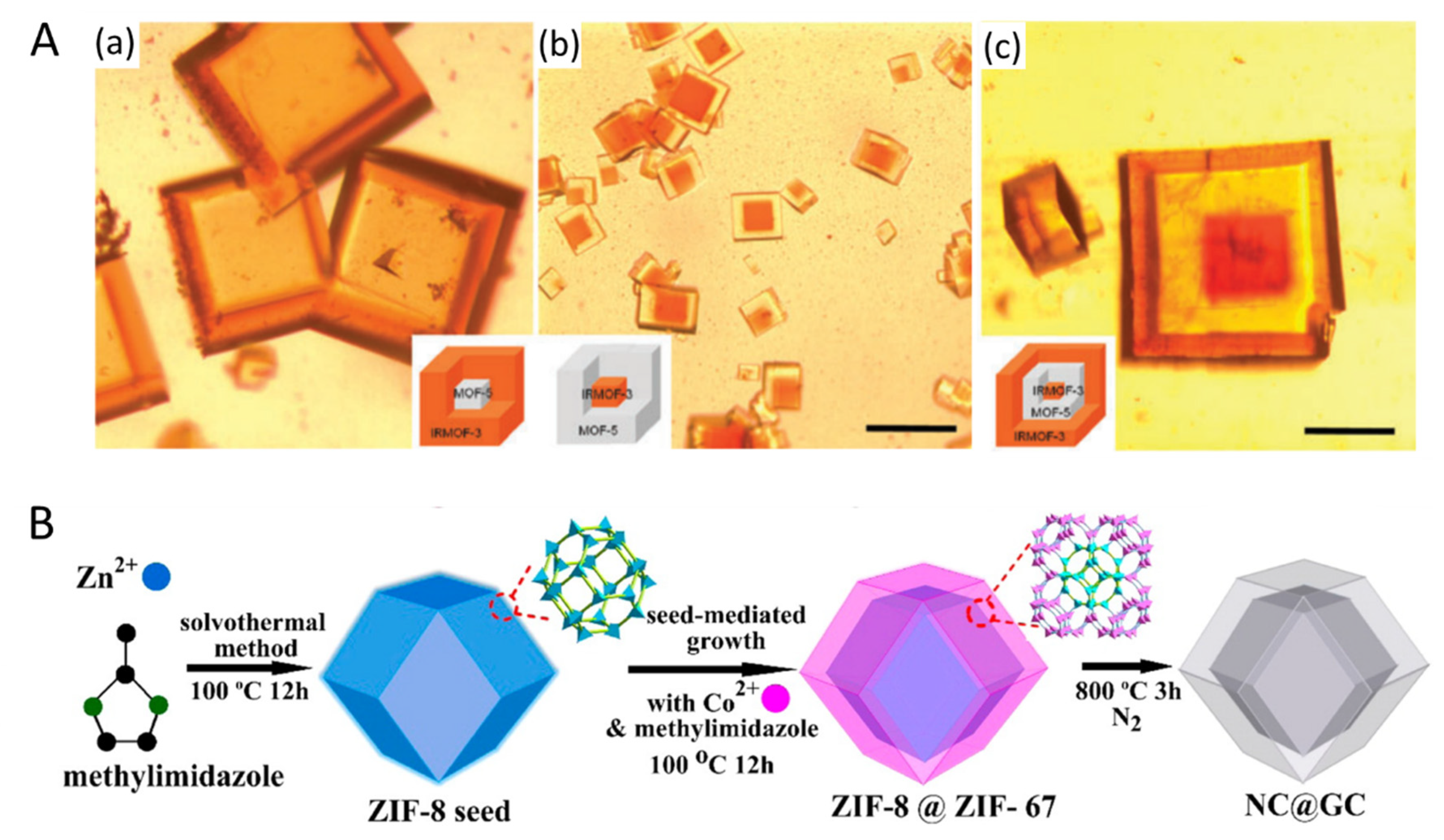
4. Synthesis of Metal–Organic Frameworks (MOF)-Based Nanomaterials
5. Evolution and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weller, M.; Overton, T.; Rourke, J.; Armstrong, F. Inorganic Chemistry, 7th ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhao, H.; Ma, D.; Rosei, F. Harnessing the properties of colloidal quantum dots in luminescent solar concentrators. Chem. Soc. Rev. 2018, 47, 5866–5890. [Google Scholar] [CrossRef]
- Argudo, P.G.; Carril, M.; Martín-Romero, M.T.; Giner-Casares, J.J.; Carrillo-Carrión, C. Surface-Active Fluorinated Quantum Dots for Enhanced Cellular Uptake. Chem. Eur. J. 2019, 25, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrión, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Bonatti, L.; Gil, G.; Giovannini, T.; Corni, S.; Cappelli, C. Plasmonic Resonances of Metal Nanoparticles: Atomistic vs. Continuum Approaches. Front. Chem. 2020, 8, 340. [Google Scholar] [CrossRef]
- Sundaresan, A.; Rao, C.N.R. Ferromagnetism as a universal feature of inorganic nanoparticles. Nano Today 2009, 4, 96–106. [Google Scholar] [CrossRef]
- Hötzer, B.; Medintz, I.L.; Hildebrandt, N. Fluorescence in Nanobiotechnology: Sophisticated Fluorophores for Novel Applications. Small 2012, 8, 2297–2326. [Google Scholar] [CrossRef]
- Taeho, K.; Taeghwan, H. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology 2014, 25, 012001. [Google Scholar]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [Green Version]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mat. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Martín-Palma, R.J.; Martínez-Duart, J.M. Nanotechnology for Microelectronics and Photonics; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Naseem, F.; Lu, P.; Zeng, J.; Lu, Z.; Ng, Y.H.; Zhao, H.; Du, Y.; Yin, Z. Solid Nanoporosity Governs Catalytic CO2 and N2 Reduction. ACS Nano 2020, 14, 7734–7759. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, X.; Li, L.; Miao, S.; Li, Y.; Li, Y.; Wang, X.; Huang, Y.; Zhang, T. Direct catalytic hydrogenation of CO2 to formate over a Schiff-base-mediated gold nanocatalyst. Nat. Commun. 2017, 8, 1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, H.Y.F.; Chen, J.R.T.; Koh, C.S.L.; Lee, H.K.; Han, X.; Phan-Quang, G.C.; Pang, J.Y.; Lay, C.L.; Pedireddy, S.; Phang, I.Y.; et al. ZIF-Induced d-Band Modification in a Bimetallic Nanocatalyst: Achieving Over 44% Efficiency in the Ambient Nitrogen Reduction Reaction. Angew. Chem. Int. Edit. 2020, 59, 16997–17003. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.; Fang, H.-T.; Eren, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat. Catal. 2019, 2, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Fu, S.; Song, J.; Zhu, C.; Xu, G.-L.; Amine, K.; Sun, C.; Li, X.; Engelhard, M.H.; Du, D.; Lin, Y. Ultrafine and highly disordered Ni2Fe1 nanofoams enabled highly efficient oxygen evolution reaction in alkaline electrolyte. Nano Energy 2018, 44, 319–326. [Google Scholar] [CrossRef]
- Müller, U. (Ed.) Nanostructures. In Inorganic Structural Chemistry; John WIlley & Sons: West Sussex, UK, 2006; pp. 241–245. [Google Scholar]
- Bellah, M.M.; Christensen, S.M.; Iqbal, S.M. Nanostructures for Medical Diagnostics. J. Nanomater. 2012, 2012, 486301. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvez Iqbal, J.A.P.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry: From Molecules to Nanomaterials; Gale, P.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Balzani, V. Nanoscience and Nanotechnology: A Personal View of a Chemist. Small 2005, 1, 278–283. [Google Scholar] [CrossRef]
- Pu, Y.; Cai, F.; Wang, D.; Wang, J.-X.; Chen, J.-F. Colloidal Synthesis of Semiconductor Quantum Dots toward Large-Scale Production: A Review. Ind. Eng. Chem. Res. 2018, 57, 1790–1802. [Google Scholar] [CrossRef]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet chemical synthesis of metal oxide nanoparticles: A review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mat. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [Green Version]
- Tranquillo, E.; Barrino, F.; Dal Poggetto, G.; Blanco, I. Sol–Gel Synthesis of Silica-Based Materials with Different Percentages of PEG or PCL and High Chlorogenic Acid Content. Materials 2019, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Schubert, U. 7.10-Sol–Gel Processing of Metal Compounds. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 629–656. [Google Scholar]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Feinle, A.; Elsaesser, M.S.; Hüsing, N. Sol–gel synthesis of monolithic materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3377–3399. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, Y.; Herricks, T.; Xia, Y. Ethylene glycol-mediated synthesis of metal oxide nanowires. J. Mater. Chem. 2004, 14, 695–703. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Yu, J. Facile Synthesis of Single-Phase TiO2 Nanocrystals with High Photocatalytic Performance. J. Am. Ceram. Soc. 2011, 94, 4112–4115. [Google Scholar] [CrossRef]
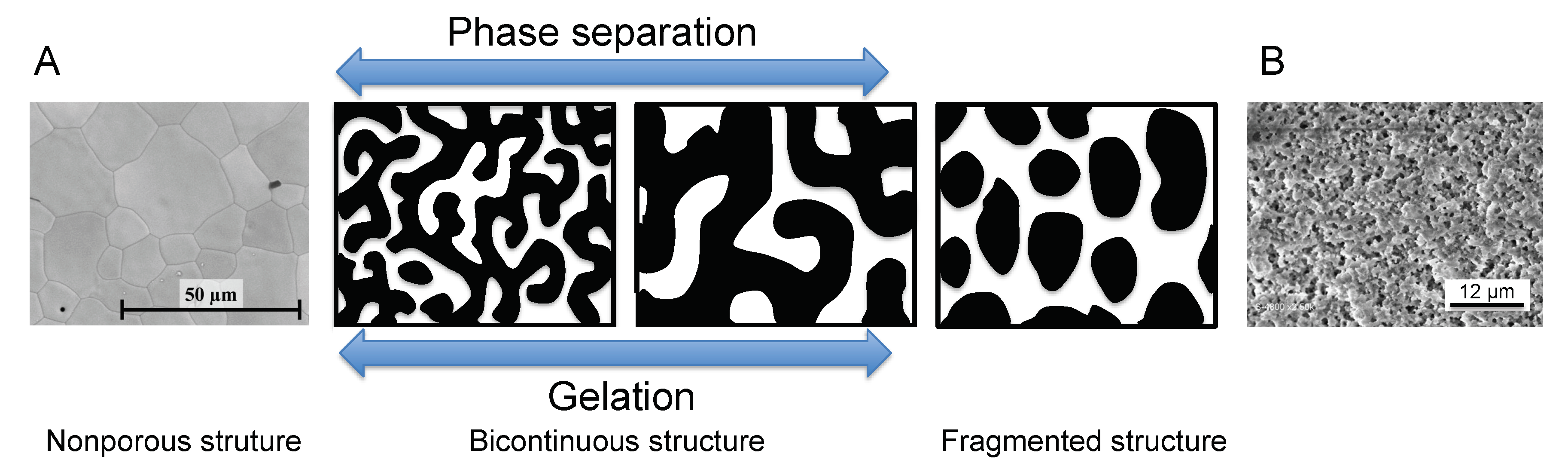
- Nakanishi, K.; Tanaka, N. Sol–Gel with Phase Separation. Hierarchically Porous Materials Optimized for High-Performance Liquid Chromatography Separations. Acc. Chem. Res. 2007, 40, 863–873. [Google Scholar] [CrossRef]
- Galarneau, A.; Abid, Z.; Said, B.; Didi, Y.; Szymanska, K.; Jarzębski, A.; Tancret, F.; Hamaizi, H.; Bengueddach, A.; Di Renzo, F.; et al. Synthesis and Textural Characterization of Mesoporous and Meso-/Macroporous Silica Monoliths Obtained by Spinodal Decomposition. Inorganics 2016, 4, 9. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Monolithic TiO2 with Controlled Multiscale Porosity via a Template-Free Sol−Gel Process Accompanied by Phase Separation. Chem. Mat. 2006, 18, 6069–6074. [Google Scholar] [CrossRef]
- Ackermann, S.; Steinfeld, A. Spectral hemispherical reflectivity of nonstoichiometric cerium dioxide. Sol. Energy Mater. Sol. Cells 2017, 159, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Anwander, R.; Palm, C.; Stelzer, J.; Groeger, O.; Engelhardt, G. Silazane-silylation of mesoporous silicates: Towards tailor-made support materials. In Studies in Surface Science and Catalysis; Bonneviot, L., Béland, F., Danumah, C., Giasson, S., Kaliaguine, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 117, pp. 135–142. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Edit. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Wang, T.; Gao, L.; Hou, J.; Herou, S.J.A.; Griffiths, J.T.; Li, W.; Dong, J.; Gao, S.; Titirici, M.-M.; Kumar, R.V.; et al. Rational approach to guest confinement inside MOF cavities for low-temperature catalysis. Nat. Commun. 2019, 10, 1340. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Sanaeepur, H.; Omidkhah, M.; Kargari, A. “Ship-in-a-bottle”, a new synthesis strategy for preparing novel hybrid host–guest nanocomposites for highly selective membrane gas separation. J. Mater. Chem. A 2018, 6, 1751–1771. [Google Scholar] [CrossRef]
- Yu, Y.; Mai, J.; Wang, L.; Li, X.; Jiang, Z.; Wang, F. Ship-in-a-bottle synthesis of amine-functionalized ionic liquids in NaY zeolite for CO2 capture. Sci. Rep. 2014, 4, 5997. [Google Scholar] [CrossRef] [Green Version]
- Alkordi, M.H.; Liu, Y.; Larsen, R.W.; Eubank, J.F.; Eddaoudi, M. Zeolite-like Metal−Organic Frameworks as Platforms for Applications: On Metalloporphyrin-Based Catalysts. J. Am. Chem. Soc. 2008, 130, 12639–12641. [Google Scholar] [CrossRef] [PubMed]
- Juan-Alcañiz, J.; Gascon, J.; Kapteijn, F. Metal–organic frameworks as scaffolds for the encapsulation of active species: State of the art and future perspectives. J. Mater. Chem. 2012, 22, 10102–10118. [Google Scholar] [CrossRef]
- Zhan, B.-Z.; Li, X.-Y. A novel ‘build-bottle-around-ship’ method to encapsulate metalloporphyrins in zeolite-Y. An efficient biomimetic catalyst. Chem. Commun. 1998, 349–350. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Cao, J. A ‘build-bottle-around-ship’ method to encapsulate ammonium molybdophosphate in zeolite Y. An efficient adsorbent for cesium. J. Colloid Interface Sci. 2015, 455, 39–45. [Google Scholar] [CrossRef]
- Nakazawa, J.; Smith, B.J.; Stack, T.D.P. Discrete Complexes Immobilized onto Click-SBA-15 Silica: Controllable Loadings and the Impact of Surface Coverage on Catalysis. J. Am. Chem. Soc. 2012, 134, 2750–2759. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.R.; Park, J.-W.; Kim, H.; Ji, H.; Lim, S.H.; Jun, C.-H. A one-step co-condensation method for the synthesis of well-defined functionalized mesoporous SBA-15 using trimethallylsilanes as organosilane sources. Chem. Commun. 2015, 51, 17084–17087. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Zhu, D.; Parak, W.J.; Feliu, N.; Escudero, A. Development of Silica-Based Biodegradable Submicrometric Carriers and Investigating Their Characteristics as in Vitro Delivery Vehicles. Int. J. Mol. Sci. 2020, 21, 7563. [Google Scholar] [CrossRef]
- Ott, A.; Yu, X.; Hartmann, R.; Rejman, J.; Schütz, A.; Ochs, M.; Parak, W.J.; Carregal-Romero, S. Light-Addressable and Degradable Silica Capsules for Delivery of Molecular Cargo to the Cytosol of Cells. Chem. Mat. 2015, 27, 1929–1942. [Google Scholar] [CrossRef]
- El Kadib, A.; Bousmina, M. Chitosan Bio-Based Organic–Inorganic Hybrid Aerogel Microspheres. Chem. Eur. J. 2012, 18, 8264–8277. [Google Scholar] [CrossRef]
- El Kadib, A. Green and Functional Aerogels by Macromolecular and Textural Engineering of Chitosan Microspheres. Chem. Rec. 2020, 20, 753–772. [Google Scholar] [CrossRef]
- Rudisill, S.G.; Shaker, S.; Terzic, D.; Le Maire, R.; Su, B.-L.; Stein, A. Generalized Approach to the Microstructure Direction in Metal Oxide Ceramics via Polymerization-Induced Phase Separation. Inorg. Chem. 2015, 54, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, E.; de Mello Donegá, C. The Challenge of Colloidal Nanoparticle Synthesis. In Nanoparticles: Workhorses of Nanoscience; de Mello Donegá, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 145–189. [Google Scholar]
- Lee, K.J.; Yoon, J.; Lahann, J. Recent advances with anisotropic particles. Curr. Opin. Colloid Interface Sci. 2011, 16, 195–202. [Google Scholar] [CrossRef]
- Wichaita, W.; Polpanich, D.; Tangboriboonrat, P. Review on Synthesis of Colloidal Hollow Particles and Their Applications. Ind. Eng. Chem. Res. 2019, 58, 20880–20901. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; De Paula, J.; Keeler, J. Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [Green Version]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Pelaz, B.; del Pino, P.; Carril, M.; Escudero, A.; Parak, W.J.; Soliman, M.G.; Zhang, Q.; Carrillo-Carrion, C. Gold-Based Nanomaterials for Applications in Nanomedicine. In Light-Responsive Nanostructured Systems for Applications in Nanomedicine; Sortino, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 169–202. [Google Scholar]
- Niu, W.; Zhang, L.; Xu, G. Seed-mediated growth of noble metal nanocrystals: Crystal growth and shape control. Nanoscale 2013, 5, 3172–3181. [Google Scholar] [CrossRef]
- Murphy, C.J.; Thompson, L.B.; Chernak, D.J.; Yang, J.A.; Sivapalan, S.T.; Boulos, S.P.; Huang, J.; Alkilany, A.M.; Sisco, P.N. Gold nanorod crystal growth: From seed-mediated synthesis to nanoscale sculpting. Curr. Opin. Colloid Interface Sci. 2011, 16, 128–134. [Google Scholar] [CrossRef]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Pérez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: Controlling the morphology, composition and crystallinity. CrystEngComm 2015, 17, 3727–3762. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using Binary Surfactant Mixtures To Simultaneously Improve the Dimensional Tunability and Monodispersity in the Seeded Growth of Gold Nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Gold nanoparticle research before and after the Brust–Schiffrin method. Chem. Commun. 2013, 49, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Thompson, L.B.; Alkilany, A.M.; Sisco, P.N.; Boulos, S.P.; Sivapalan, S.T.; Yang, J.A.; Chernak, D.J.; Huang, J. The Many Faces of Gold Nanorods. J. Phys. Chem. Lett. 2010, 1, 2867–2875. [Google Scholar] [CrossRef]
- Xia, X.; Zeng, J.; Oetjen, L.K.; Li, Q.; Xia, Y. Quantitative Analysis of the Role Played by Poly(vinylpyrrolidone) in Seed-Mediated Growth of Ag Nanocrystals. J. Am. Chem. Soc. 2012, 134, 1793–1801. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matijevic, E. Preparation and properties of uniform size colloids. Chem. Mat. 1993, 5, 412–426. [Google Scholar] [CrossRef]
- Amiens, C.; Chaudret, B.; Ciuculescu-Pradines, D.; Collière, V.; Fajerwerg, K.; Fau, P.; Kahn, M.; Maisonnat, A.; Soulantica, K.; Philippot, K. Organometallic approach for the synthesis of nanostructures. New J. Chem. 2013, 37, 3374–3401. [Google Scholar] [CrossRef]
- Moraes, L.C.; Figueiredo, R.C.; Espinós, J.P.; Vattier, F.; Franconetti, A.; Jaime, C.; Lacroix, B.; Rojo, J.; Lara, P.; Conejero, S. Platinum nanoparticles stabilized by N-heterocyclic thiones. Synthesis and catalytic activity in mono- and di-hydroboration of alkynes. Nanoscale 2020, 12, 6821–6831. [Google Scholar] [CrossRef] [PubMed]
- Kravchyk, K.; Protesescu, L.; Bodnarchuk, M.I.; Krumeich, F.; Yarema, M.; Walter, M.; Guntlin, C.; Kovalenko, M.V. Monodisperse and Inorganically Capped Sn and Sn/SnO2 Nanocrystals for High-Performance Li-Ion Battery Anodes. J. Am. Chem. Soc. 2013, 135, 4199–4202. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mat. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.A.; Peng, X. Formation of High-Quality CdTe, CdSe, and CdS Nanocrystals Using CdO as Precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef]
- Núñez, N.; Sabek, J.; García-Sevillano, J.; Cantelar, E.; Escudero, A.; Ocaña, M. Solvent-Controlled Synthesis and Luminescence Properties of Uniform Eu:YVO4 Nanophosphors with Different Morphologies. Eur. J. Inorg. Chem. 2013, 2013, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Escudero, A.; Carrillo-Carrión, C.; Zyuzin, M.V.; Ashraf, S.; Hartmann, R.; Núñez, N.O.; Ocaña, M.; Parak, W.J. Synthesis and functionalization of monodisperse near-ultraviolet and visible excitable multifunctional Eu3+, Bi3+:REVO4 nanophosphors for bioimaging and biosensing applications. Nanoscale 2016, 8, 12221–12236. [Google Scholar] [CrossRef] [Green Version]
- González-Mancebo, D.; Becerro, A.I.; Genevois, C.; Allix, M.; Corral, A.; Parrado-Gallego, A.; Ocaña, M. Structural, optical and X-ray attenuation properties of Tb3+:BaxCe1−xF3−x (x = 0.18–0.48) nanospheres synthesized in polyol medium. Dalton Trans. 2018, 47, 8382–8391. [Google Scholar] [CrossRef]
- Jacob, D.S.; Bitton, L.; Grinblat, J.; Felner, I.; Koltypin, Y.; Gedanken, A. Are Ionic Liquids Really a Boon for the Synthesis of Inorganic Materials? A General Method for the Fabrication of Nanosized Metal Fluorides. Chem. Mat. 2006, 18, 3162–3168. [Google Scholar] [CrossRef]
- Rodríguez-Liviano, S.; Núñez, N.O.; Rivera-Fernández, S.; de la Fuente, J.M.; Ocaña, M. Ionic Liquid Mediated Synthesis and Surface Modification of Multifunctional Mesoporous Eu:GdF3 Nanoparticles for Biomedical Applications. Langmuir 2013, 29, 3411–3418. [Google Scholar] [CrossRef]
- Tan, J.; Jin, X. Monodisperse, colloidal and luminescent calcium fluoride nanoparticles via a citrate-assisted hydrothermal route. J. Colloid Interface Sci. 2018, 531, 444–450. [Google Scholar] [CrossRef]
- Laguna, M.; Escudero, A.; Núñez, N.O.; Becerro, A.I.; Ocaña, M. Europium-doped NaGd(WO4)2 nanophosphors: Synthesis, luminescence and their coating with fluorescein for pH sensing. Dalton Trans. 2017, 46, 11575–11583. [Google Scholar] [CrossRef] [PubMed]
- Laguna, M.; Núñez, N.O.; Rodríguez, V.; Cantelar, E.; Stepien, G.; García, M.L.; de la Fuente, J.M.; Ocaña, M. Multifunctional Eu-doped NaGd(MoO4)2 nanoparticles functionalized with poly(l-lysine) for optical and MRI imaging. Dalton Trans. 2016, 45, 16354–16365. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.; Will, J.; Hoppe, A.; Roether, J.A.; Boccaccini, A.R. Crystallization and Melting Behavior of Poly(Butylene Succinate) Nanocomposites Containing Silica-Nanotubes and Strontium Hydroxyapatite Nanorods. Ind. Eng. Chem. Res. 2014, 53, 678–692. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Roumeli, E.; Chrissafis, K.; Lioutas, C.; Triantafyllidis, K.; Bikiaris, D.; Boccaccini, A.R. Thermal degradation kinetics and decomposition mechanism of PBSu nanocomposites with silica-nanotubes and strontium hydroxyapatite nanorods. Phys. Chem. Chem. Phys. 2014, 16, 4830–4842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippousi, M.; Siafaka, P.I.; Amanatiadou, E.P.; Nanaki, S.G.; Nerantzaki, M.; Bikiaris, D.N.; Vizirianakis, I.S.; Van Tendeloo, G. Modified chitosan coated mesoporous strontium hydroxyapatite nanorods as drug carriers. J. Mater. Chem. B 2015, 3, 5991–6000. [Google Scholar] [CrossRef]
- Escudero, A.; Calvo, M.E.; Rivera-Fernández, S.; de la Fuente, J.M.; Ocaña, M. Microwave-Assisted Synthesis of Biocompatible Europium-Doped Calcium Hydroxyapatite and Fluoroapatite Luminescent Nanospindles Functionalized with Poly(acrylic acid). Langmuir 2013, 29, 1985–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudero, A.; Carrillo-Carrión, C.; Zyuzin, M.V.; Parak, W.J. Luminescent Rare-earth-based Nanoparticles: A Summarized Overview of their Synthesis, Functionalization, and Applications. Top. Curr. Chem. 2016, 374, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudero, A.; Becerro, A.I.; Carrillo-Carrion, C.; Núñez, N.; Zyuzin, M.V.; Laguna, M.; González-Mancebo, D.; Ocaña, M.; Parak, W.J. Rare earth based nanostructured materials: Synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics 2017, 6, 881–921. [Google Scholar] [CrossRef]
- Escudero, A.; Moretti, E.; Ocaña, M. Synthesis and luminescence of uniform europium-doped bismuth fluoride and bismuth oxyfluoride particles with different morphologies. CrystEngComm 2014, 16, 3274–3283. [Google Scholar] [CrossRef] [Green Version]
- Silvert, P.-Y.; Herrera-Urbina, R.; Duvauchelle, N.; Vijayakrishnan, V.; Elhsissen, K.T. Preparation of colloidal silver dispersions by the polyol process. Part 1—Synthesis and characterization. J. Mater. Chem. 1996, 6, 573–577. [Google Scholar] [CrossRef]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Schäf, O.; Ghobarkar, H.; Knauth, P. Hydrothermal Synthesis of Nanomaterials. In Nanostructured Materials: Selected Synthesis Methods Properties and Applications; Knauth, P., Schoonman, J., Eds.; Springer: Boston, MA, USA, 2002; pp. 23–41. [Google Scholar]
- Morris, R.E. Ionothermal synthesis—ionic liquids as functional solvents in the preparation of crystalline materials. Chem. Commun. 2009, 2990–2998. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. Ionothermal Synthesis of Zeolites, Metal–Organic Frameworks, and Inorganic—Organic Hybrids. Acc. Chem. Res. 2007, 40, 1005–1013. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Zhou, H.-C.J.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Lin, R.-B.; Zhou, W.; Zhang, Z.; Xiang, S.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. EnergyChem 2019, 1, 100006. [Google Scholar] [CrossRef]
- Liu, G.; Chernikova, V.; Liu, Y.; Zhang, K.; Belmabkhout, Y.; Shekhah, O.; Zhang, C.; Yi, S.; Eddaoudi, M.; Koros, W.J. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat. Mater. 2018, 17, 283–289. [Google Scholar] [CrossRef]
- Qiu, T.; Liang, Z.; Guo, W.; Tabassum, H.; Gao, S.; Zou, R. Metal–Organic Framework-Based Materials for Energy Conversion and Storage. ACS Energy Lett. 2020, 5, 520–532. [Google Scholar] [CrossRef] [Green Version]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Wuttke, S.; Lismont, M.; Escudero, A.; Rungtaweevoranit, B.; Parak, W.J. Positioning metal-organic framework nanoparticles within the context of drug delivery—A comparison with mesoporous silica nanoparticles and dendrimers. Biomaterials 2017, 123, 172–183. [Google Scholar] [CrossRef]
- Anstoetz, M.; Rose, T.J.; Clark, M.W.; Yee, L.H.; Raymond, C.A.; Vancov, T. Novel Applications for Oxalate-Phosphate-Amine Metal-Organic-Frameworks (OPA-MOFs): Can an Iron-Based OPA-MOF Be Used as Slow-Release Fertilizer? PLoS ONE 2015, 10, e0144169. [Google Scholar] [CrossRef]
- Forgan, R.S. Modulated self-assembly of metal–organic frameworks. Chem. Sci. 2020, 11, 4546–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.G.; Haldoupis, E.; Bucior, B.J.; Haranczyk, M.; Lee, S.; Zhang, H.; Vogiatzis, K.D.; Milisavljevic, M.; Ling, S.; Camp, J.S.; et al. Advances, Updates, and Analytics for the Computation-Ready, Experimental Metal–Organic Framework Database: CoRE MOF 2019. J. Chem. Eng. Data 2019, 64, 5985–5998. [Google Scholar] [CrossRef]
- Férey, G.; Haouas, M.; Loiseau, T.; Taulelle, F. Nanoporous Solids: How Do They Form? An In Situ Approach. Chem. Mat. 2014, 26, 299–309. [Google Scholar] [CrossRef]
- Biswal, D.; Kusalik, P.G. Probing Molecular Mechanisms of Self-Assembly in Metal–Organic Frameworks. ACS Nano 2017, 11, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Kollias, L.; Cantu, D.C.; Tubbs, M.A.; Rousseau, R.; Glezakou, V.-A.; Salvalaglio, M. Molecular Level Understanding of the Free Energy Landscape in Early Stages of Metal–Organic Framework Nucleation. J. Am. Chem. Soc. 2019, 141, 6073–6081. [Google Scholar] [CrossRef]
- Xing, J.; Schweighauser, L.; Okada, S.; Harano, K.; Nakamura, E. Atomistic structures and dynamics of prenucleation clusters in MOF-2 and MOF-5 syntheses. Nat. Commun. 2019, 10, 3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Powell, J.; Zhou, H.-C. Controllable Synthesis of Metal-Organic Frameworks and Their Hierarchical Assemblies. Matter 2019, 1, 801–824. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; McGuirk, C.M.; d’Aquino, A.; Mason, J.A.; Mirkin, C.A. Metal–Organic Framework Nanoparticles. Adv. Mater. 2018, 30, 1800202. [Google Scholar] [CrossRef] [PubMed]
- Carné, A.; Carbonell, C.; Imaz, I.; Maspoch, D. Nanoscale metal–organic materials. Chem. Soc. Rev. 2011, 40, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Heryadi, D.; Zhou, F.; Zhao, L.; Lestari, G.; Su, H.; Lai, Z. Tuning the crystal morphology and size of zeolitic imidazolate framework-8 in aqueous solution by surfactants. CrystEngComm 2011, 13, 6937–6940. [Google Scholar] [CrossRef]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations. CrystEngComm 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Umemura, A.; Diring, S.; Furukawa, S.; Uehara, H.; Tsuruoka, T.; Kitagawa, S. Morphology Design of Porous Coordination Polymer Crystals by Coordination Modulation. J. Am. Chem. Soc. 2011, 133, 15506–15513. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. MOF@MOF: Microporous core–shell architectures. Chem. Commun. 2009, 6162–6164. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal Conversion of Core–Shell Metal–Organic Frameworks: A New Method for Selectively Functionalized Nanoporous Hybrid Carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef]
- Avci, C.; Ariñez-Soriano, J.; Carné-Sánchez, A.; Guillerm, V.; Carbonell, C.; Imaz, I.; Maspoch, D. Post-Synthetic Anisotropic Wet-Chemical Etching of Colloidal Sodalite ZIF Crystals. Angew. Chem. Int. Edit. 2015, 54, 14417–14421. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Jhung, S.H. Synthesis of metal-organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Friščić, T. Metal-Organic Frameworks: Mechanochemical Synthesis Strategies. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–19. [Google Scholar]
- Liu, Z.; Zhu, J.; Peng, C.; Wakihara, T.; Okubo, T. Continuous flow synthesis of ordered porous materials: From zeolites to metal–organic frameworks and mesoporous silica. React. Chem. Eng. 2019, 4, 1699–1720. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Debecker, D.P.; Mutin, P.H. Non-hydrolytic sol–gel routes to heterogeneous catalysts. Chem. Soc. Rev. 2012, 41, 3624–3650. [Google Scholar] [CrossRef] [PubMed]
- Abutbul, R.E.; Golan, Y. ‘Beneficial impurities’ in colloidal synthesis of surfactant coated inorganic nanoparticles. Nanotechnology 2021, 32, 102001. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero, A.; Carrillo-Carrión, C.; Romero-Ben, E.; Franco, A.; Rosales-Barrios, C.; Castillejos, M.C.; Khiar, N. Molecular Bottom-Up Approaches for the Synthesis of Inorganic and Hybrid Nanostructures. Inorganics 2021, 9, 58. https://doi.org/10.3390/inorganics9070058
Escudero A, Carrillo-Carrión C, Romero-Ben E, Franco A, Rosales-Barrios C, Castillejos MC, Khiar N. Molecular Bottom-Up Approaches for the Synthesis of Inorganic and Hybrid Nanostructures. Inorganics. 2021; 9(7):58. https://doi.org/10.3390/inorganics9070058
Chicago/Turabian StyleEscudero, Alberto, Carolina Carrillo-Carrión, Elena Romero-Ben, Ana Franco, Christian Rosales-Barrios, Mª Carmen Castillejos, and Noureddine Khiar. 2021. "Molecular Bottom-Up Approaches for the Synthesis of Inorganic and Hybrid Nanostructures" Inorganics 9, no. 7: 58. https://doi.org/10.3390/inorganics9070058
APA StyleEscudero, A., Carrillo-Carrión, C., Romero-Ben, E., Franco, A., Rosales-Barrios, C., Castillejos, M. C., & Khiar, N. (2021). Molecular Bottom-Up Approaches for the Synthesis of Inorganic and Hybrid Nanostructures. Inorganics, 9(7), 58. https://doi.org/10.3390/inorganics9070058