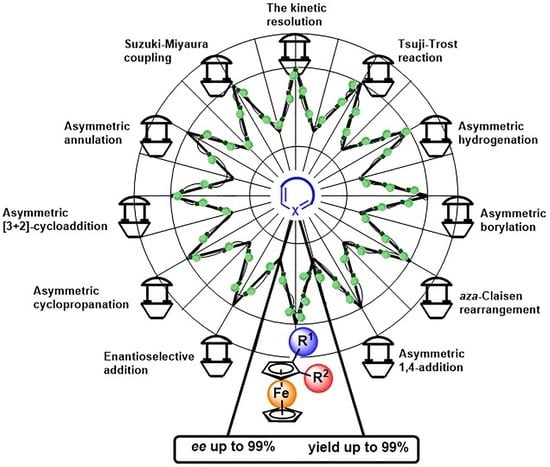
Advanced Application of Planar Chiral Heterocyclic Ferrocenes
Abstract
:1. Introduction
2. Application of Planar Chiral Heterocyclic Ferrocenes
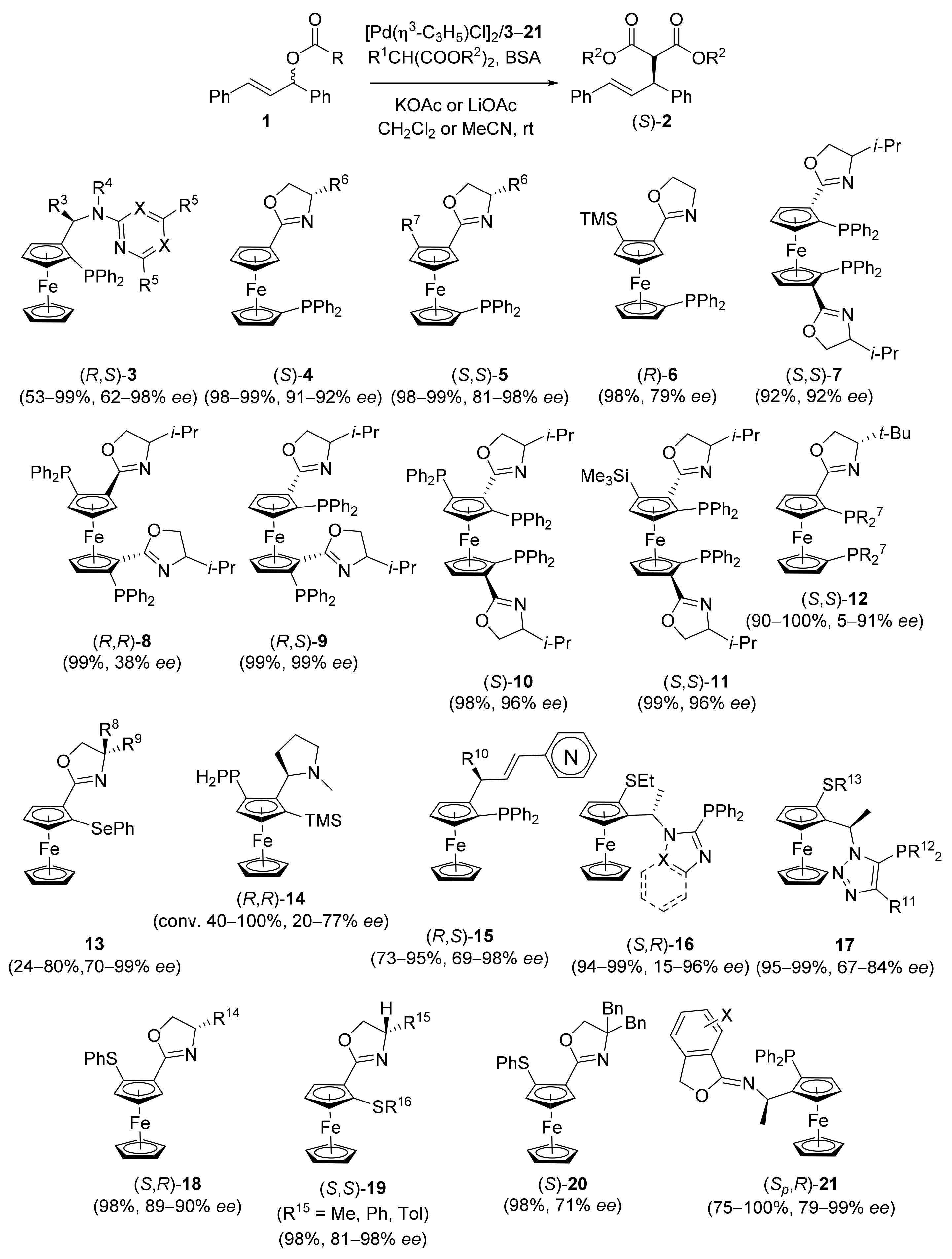
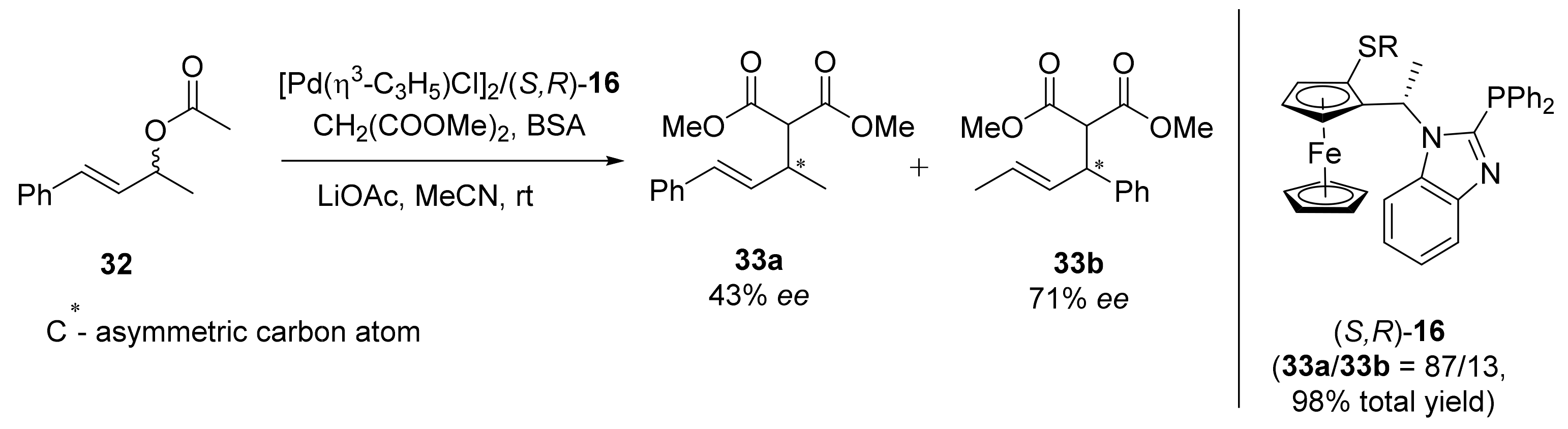
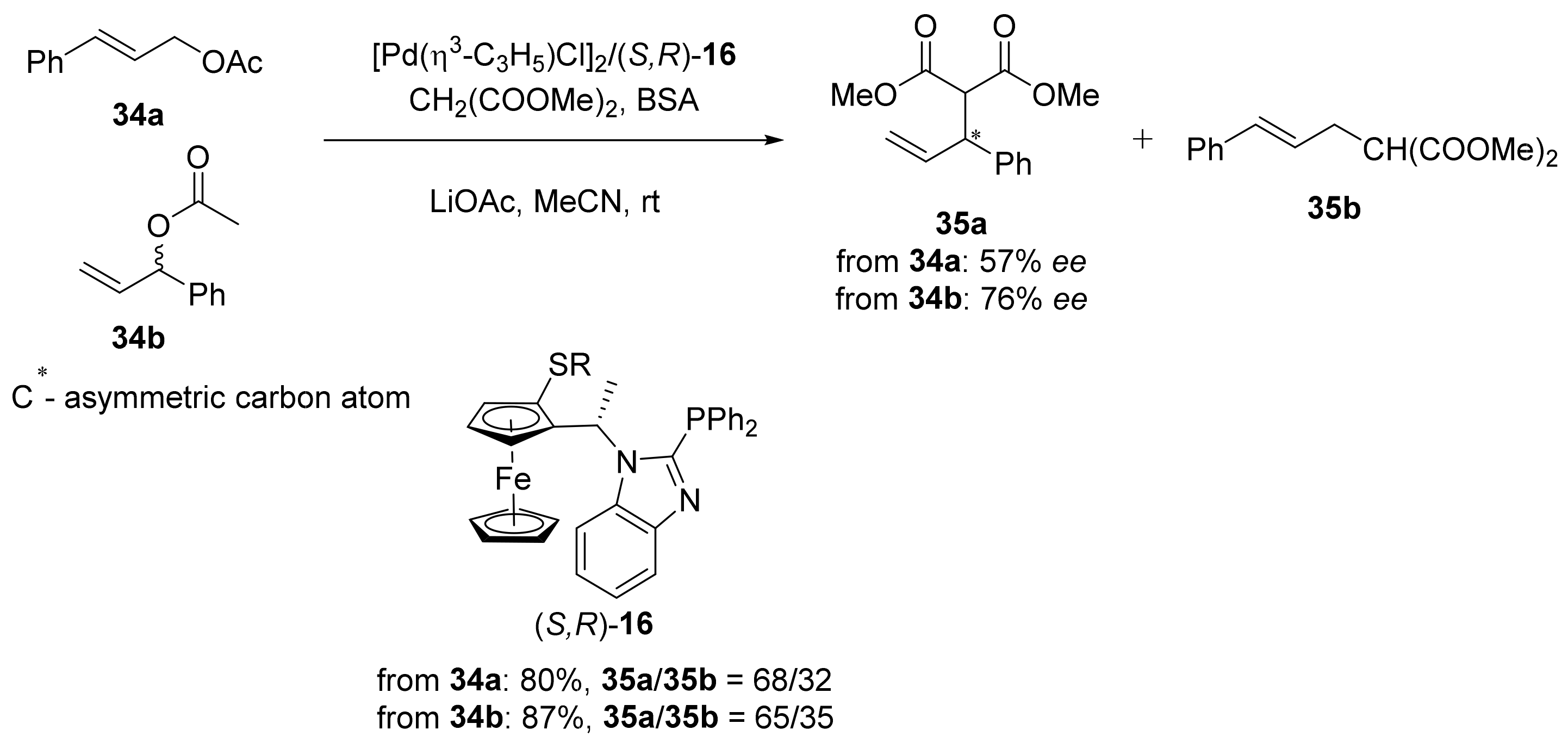
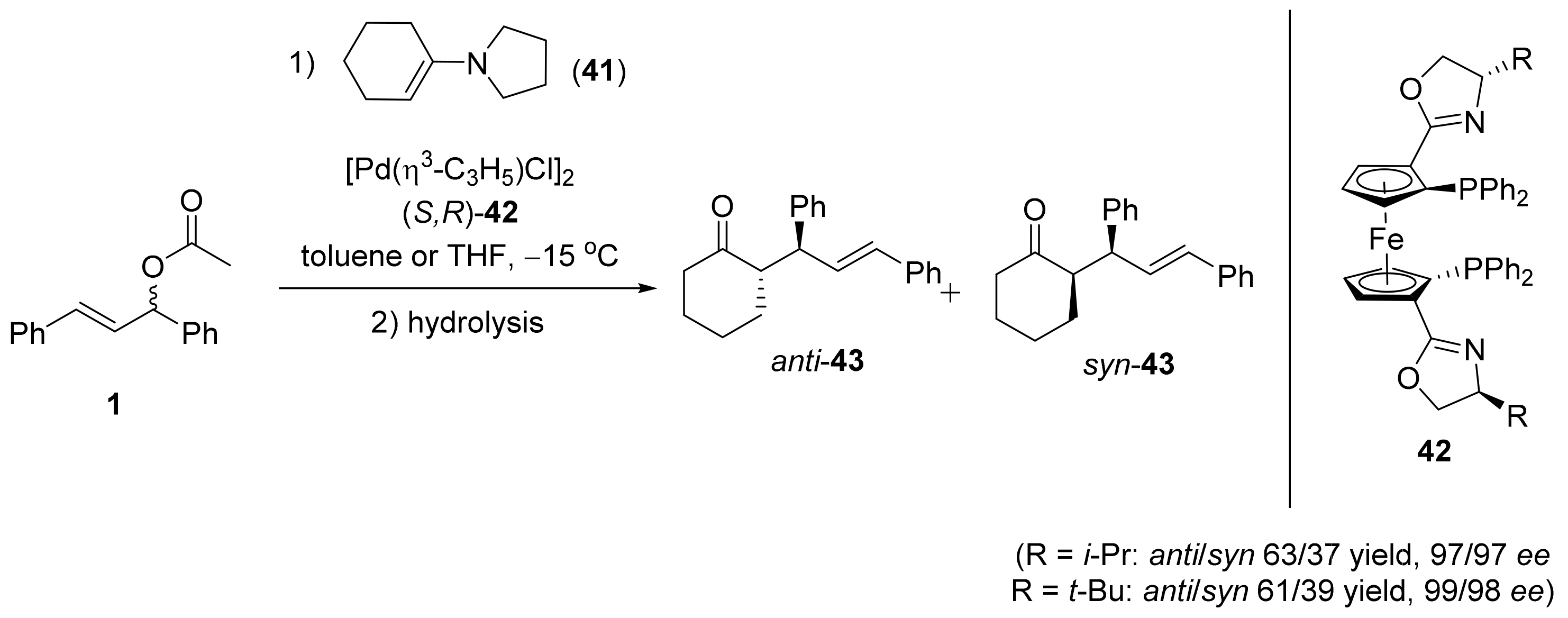
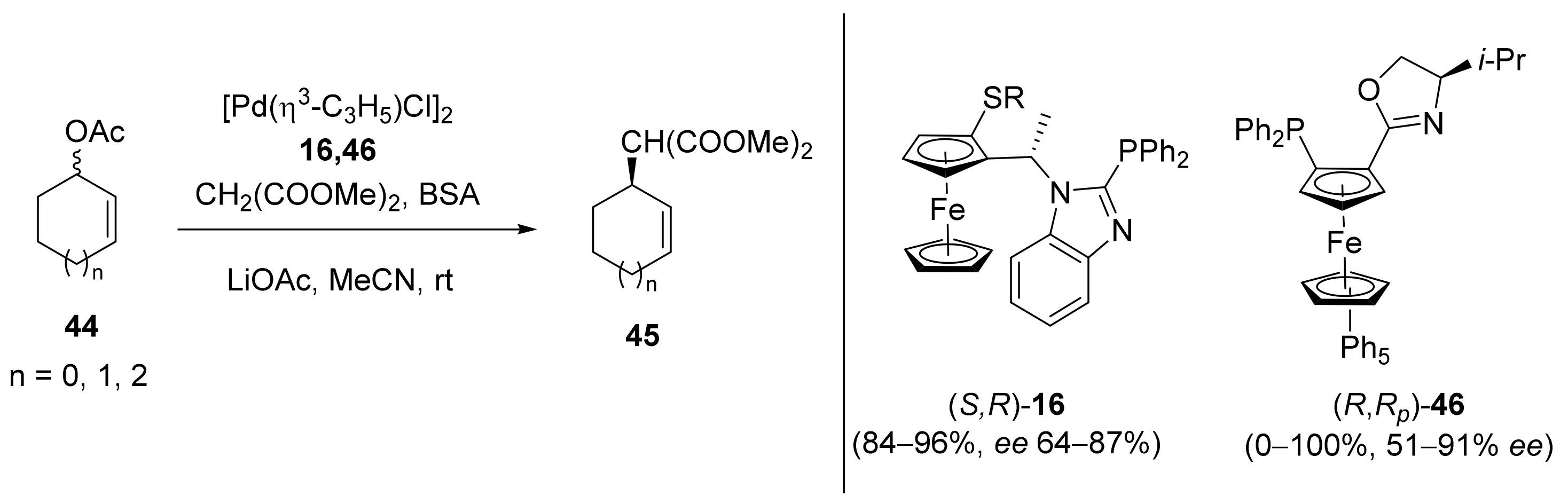
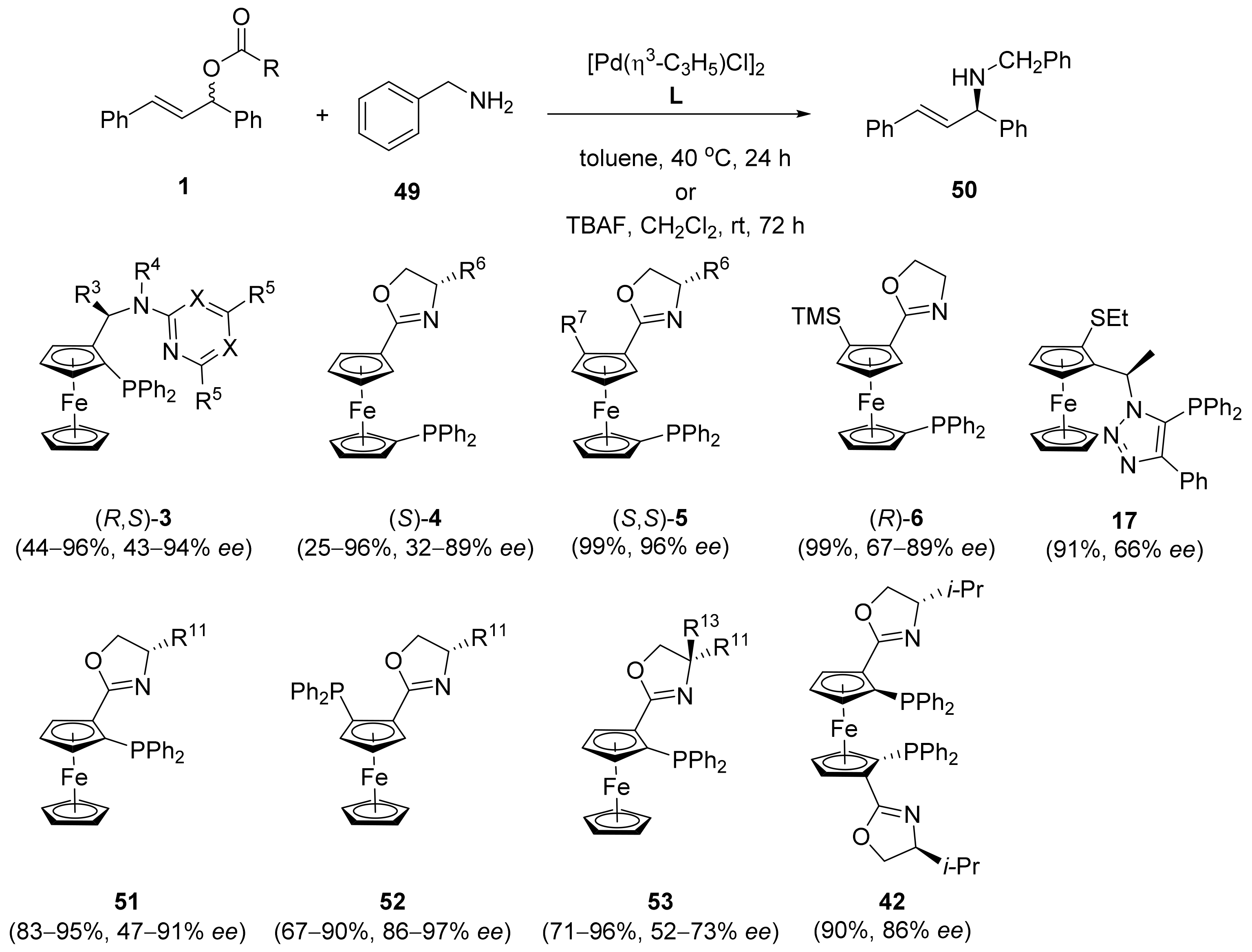
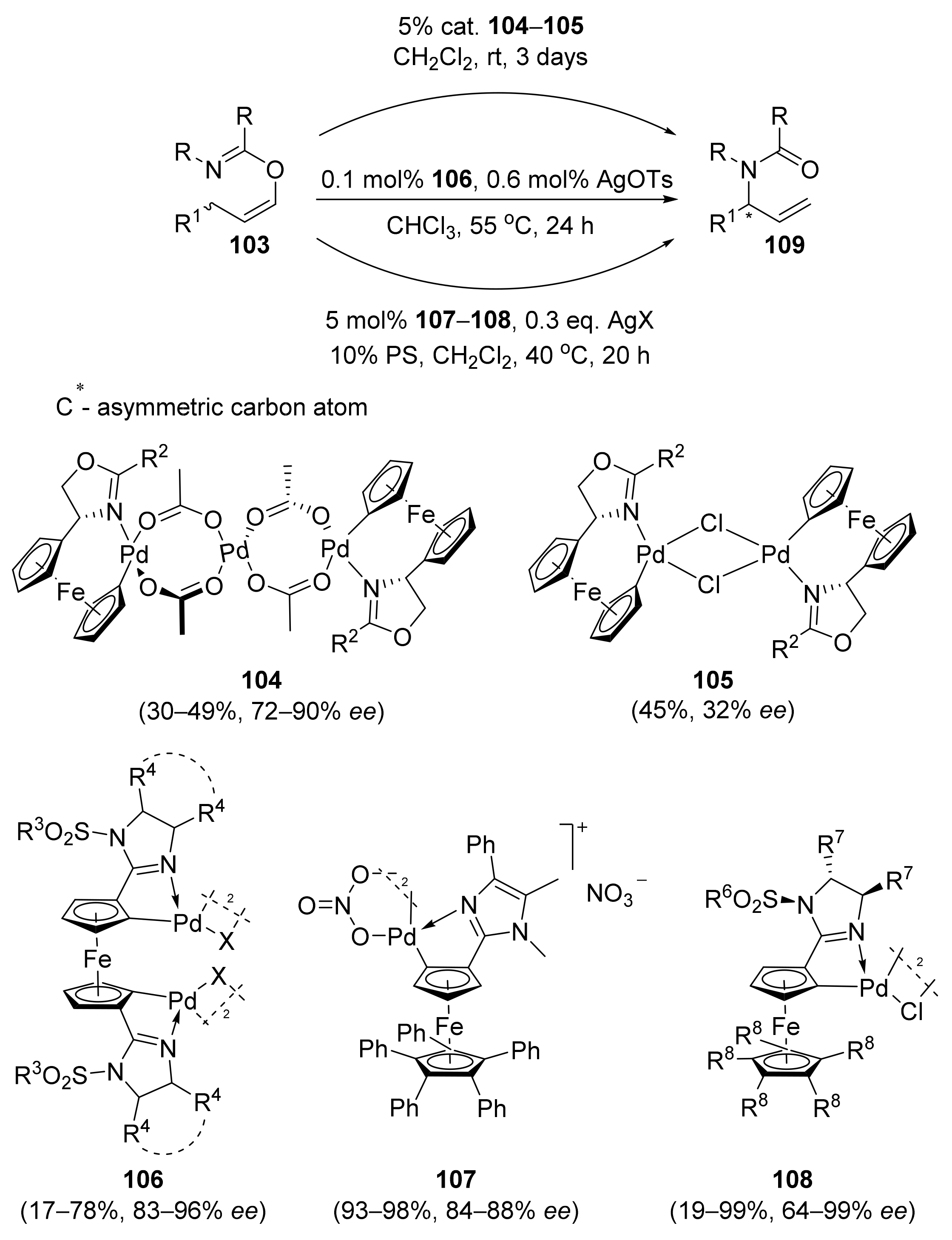
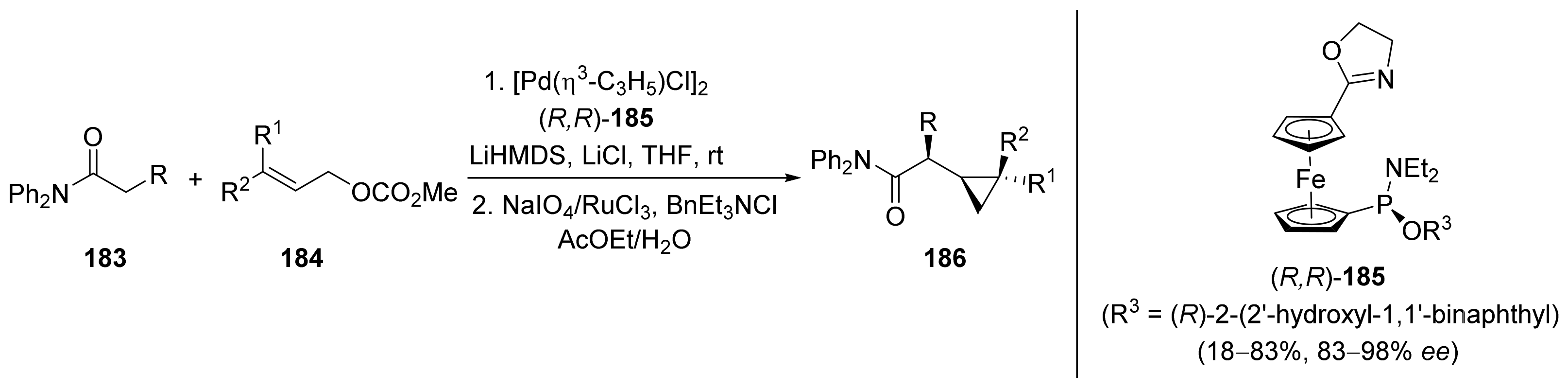
2.1. Tsuji–Trost Reaction
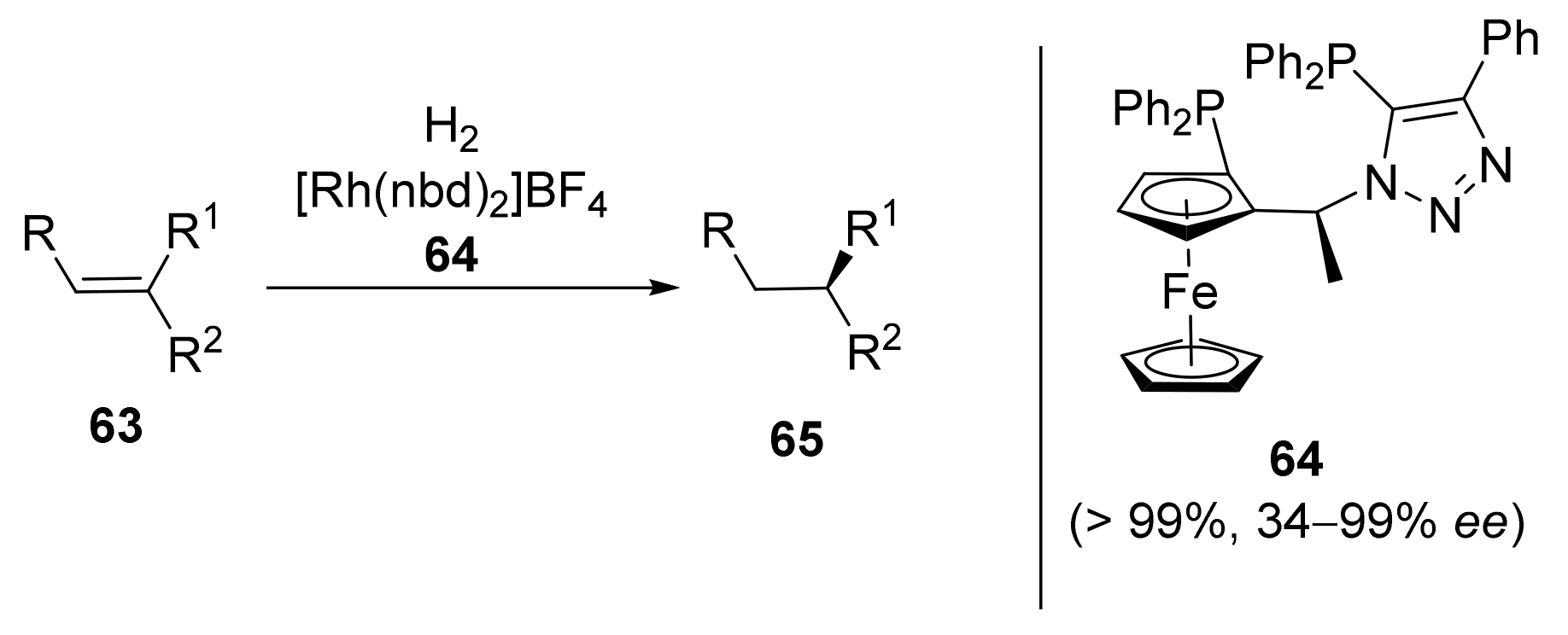
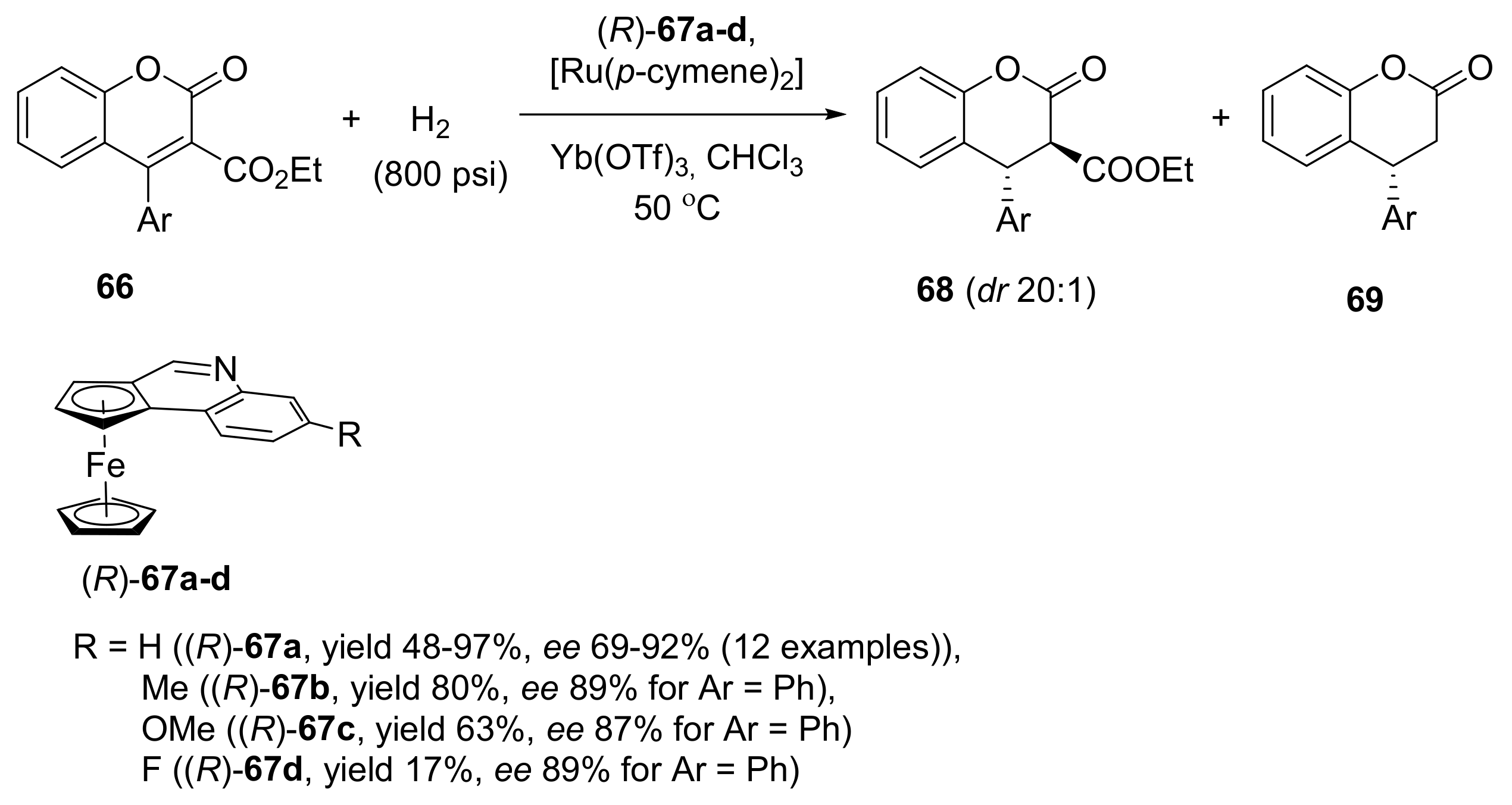
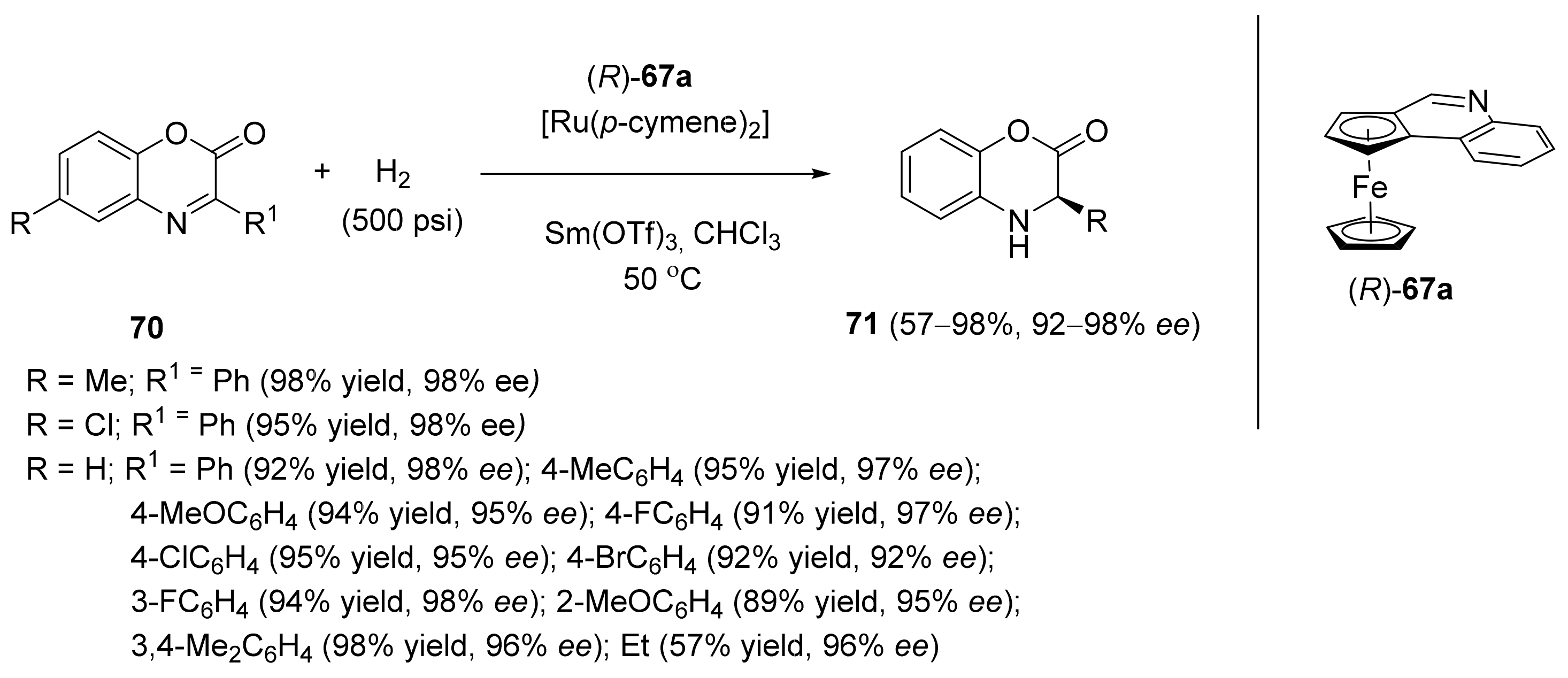
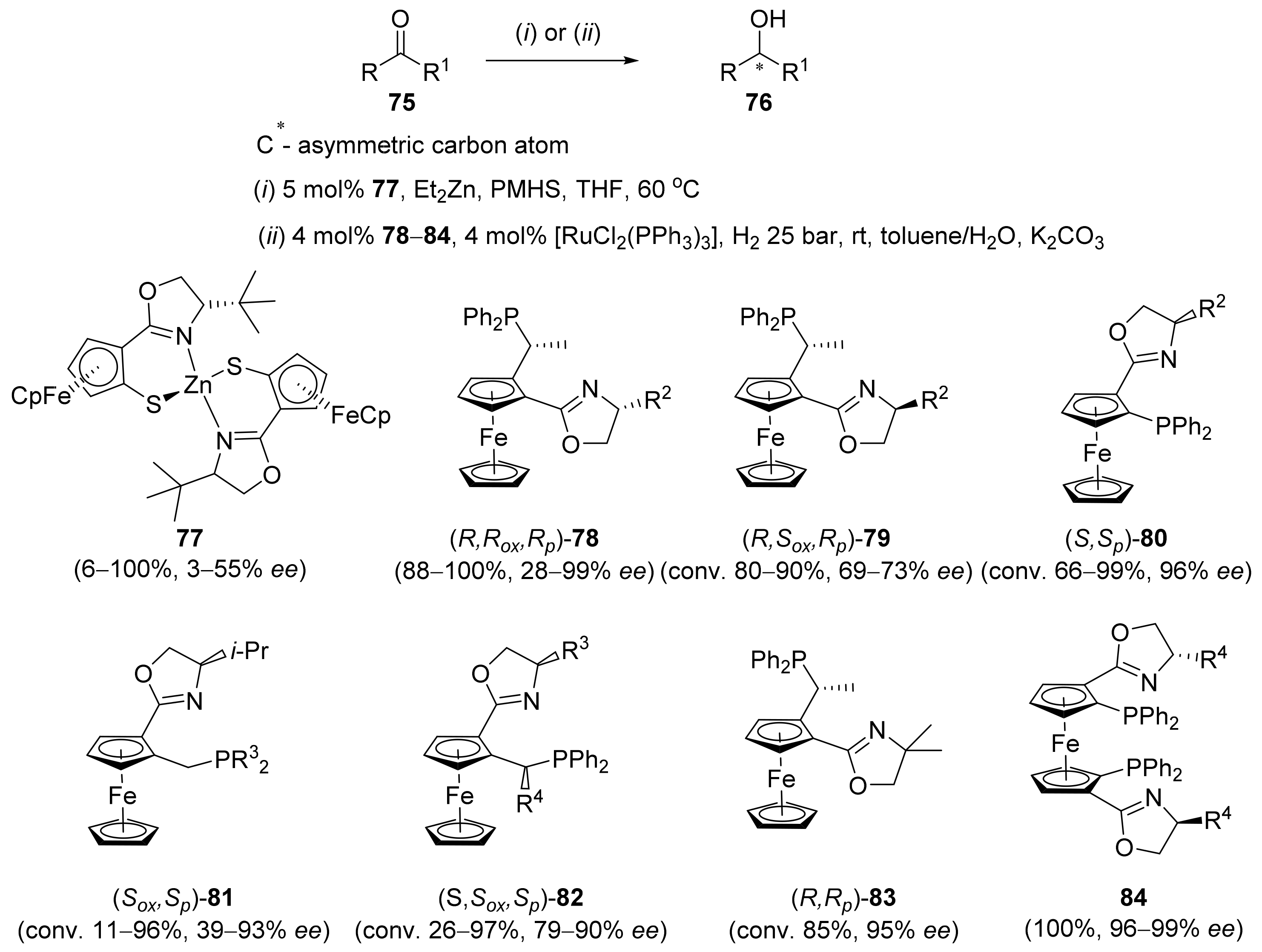
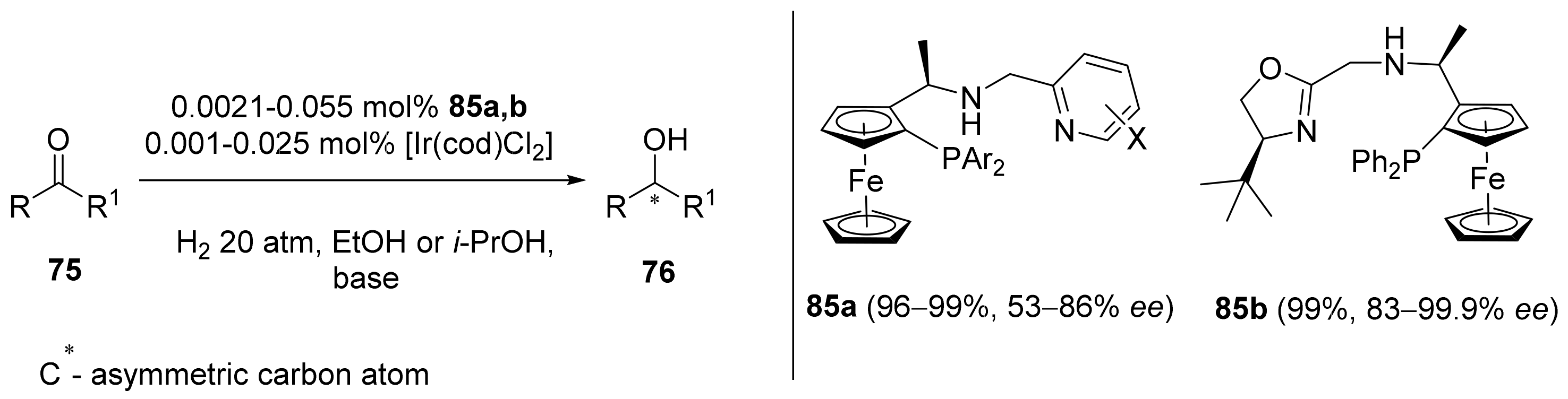
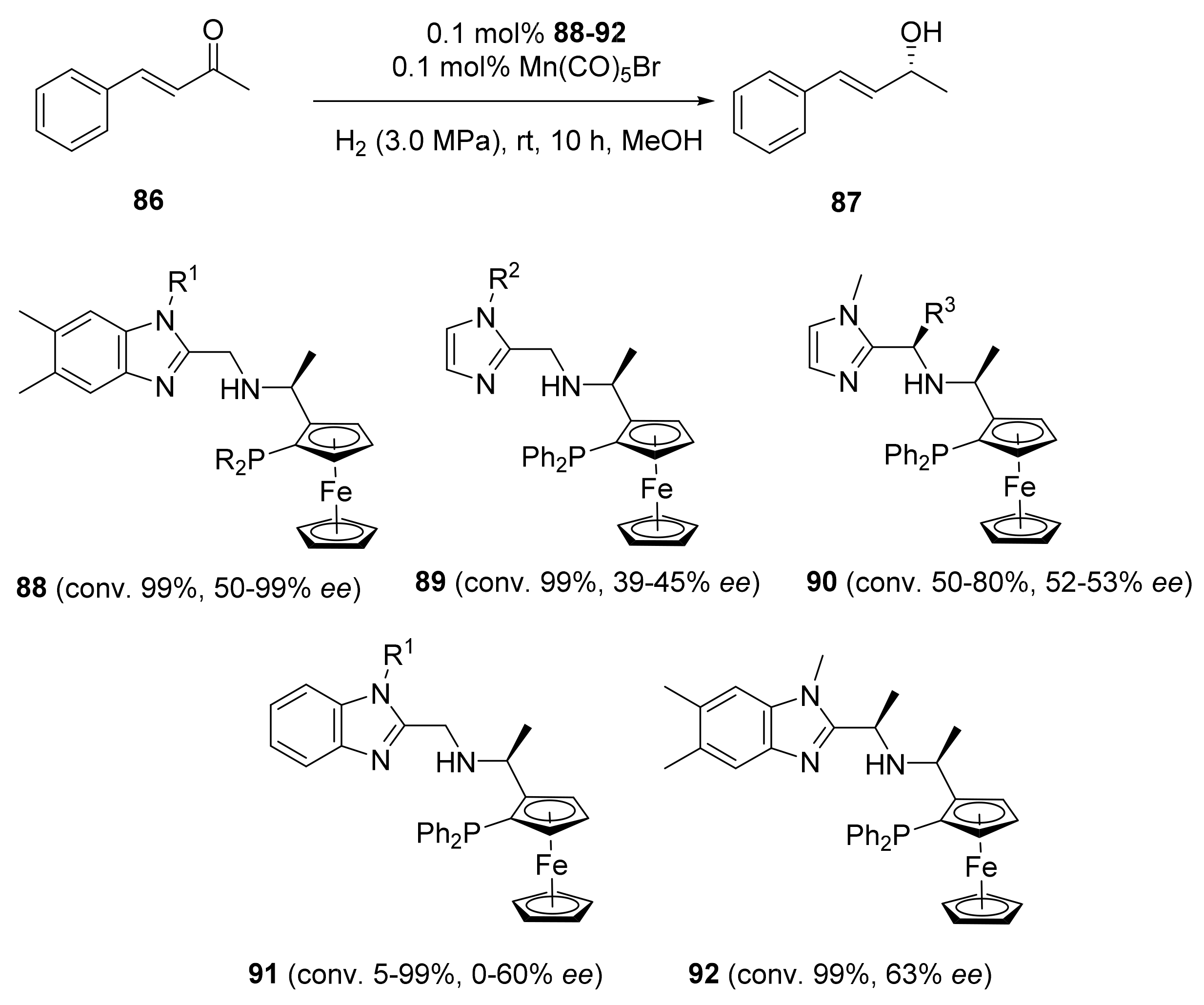
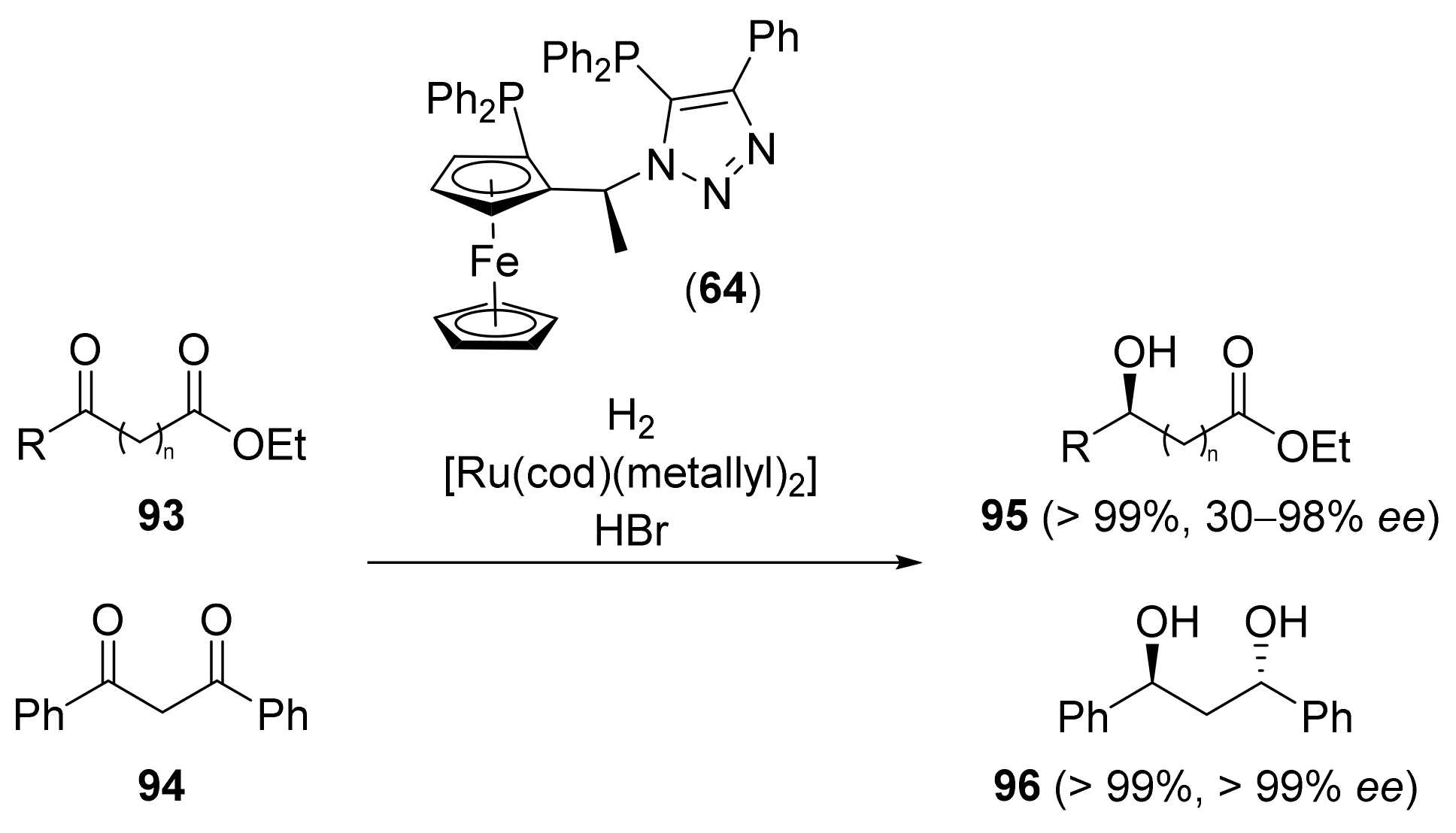
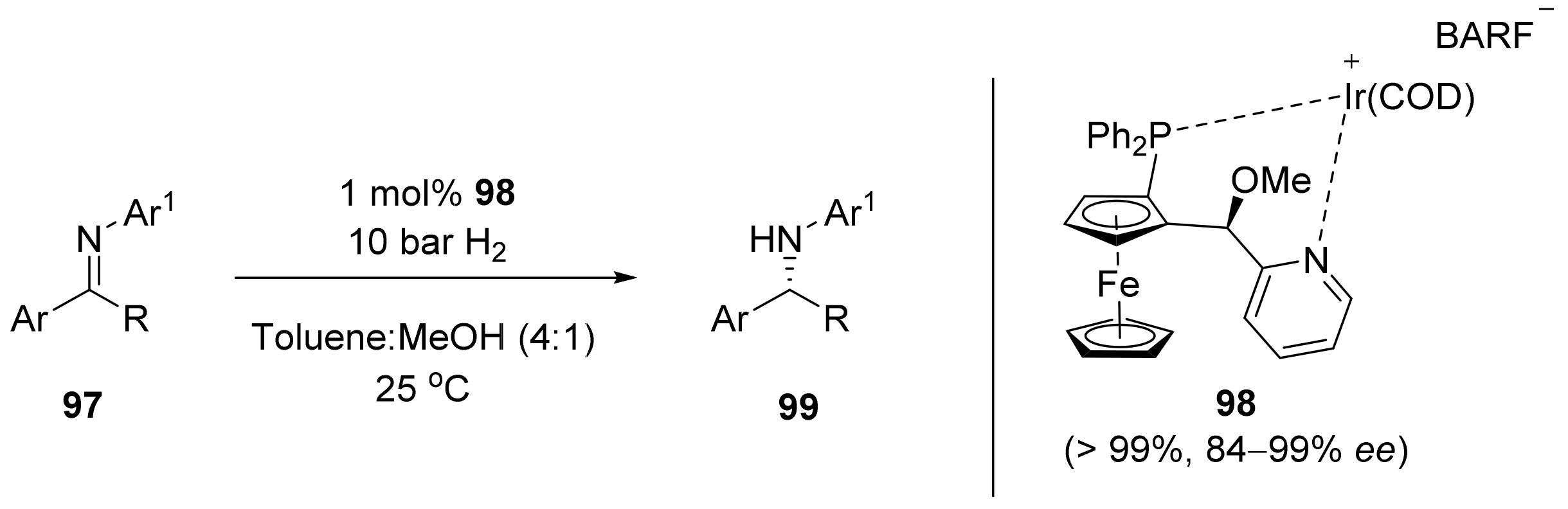
2.2. Asymmetric Hydrogenation
2.3. The Rearrangement
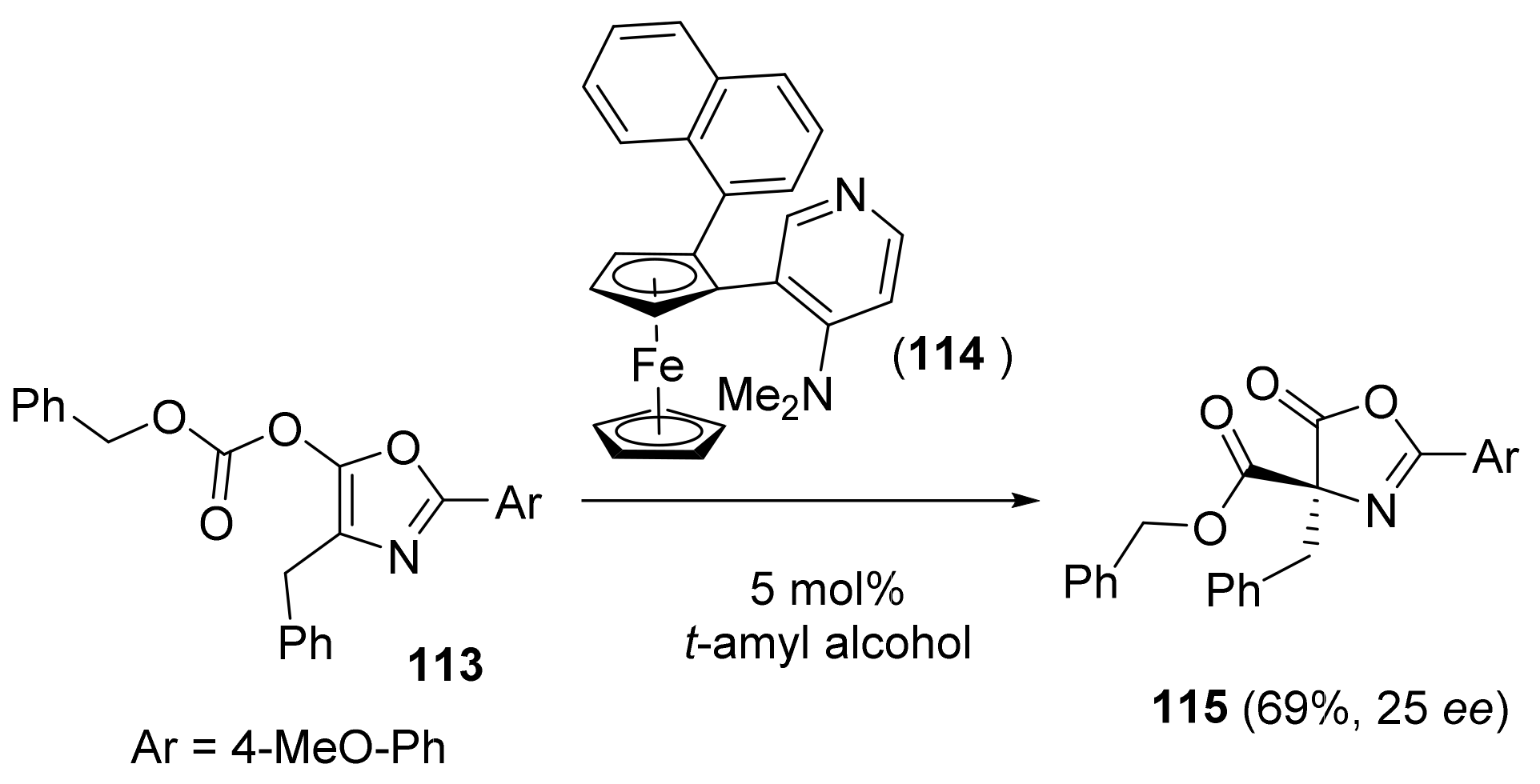
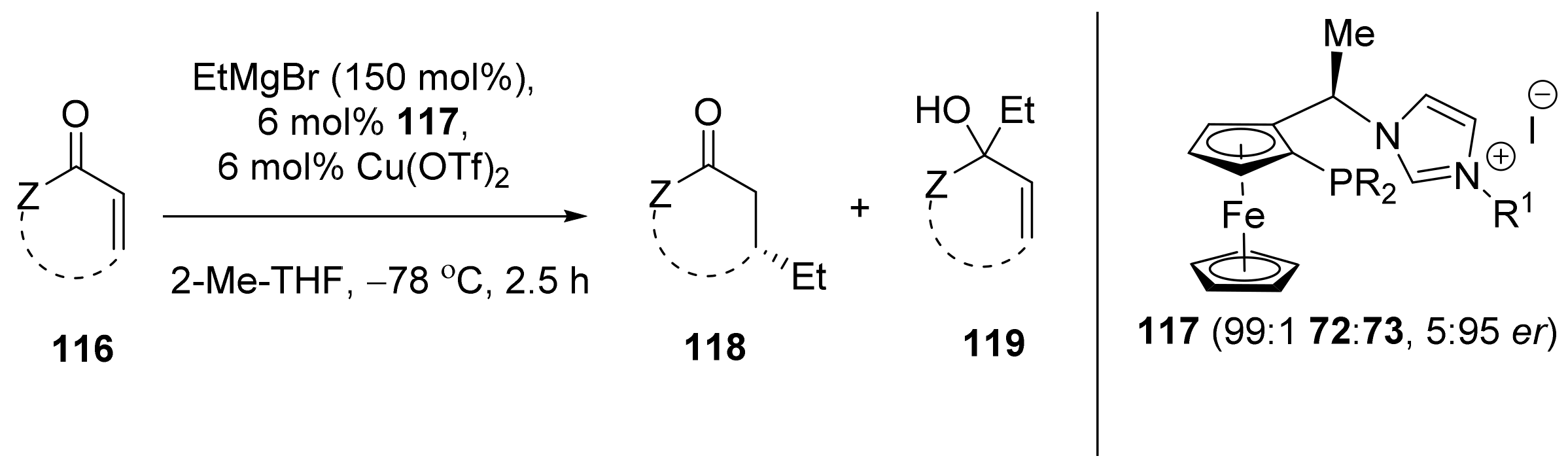
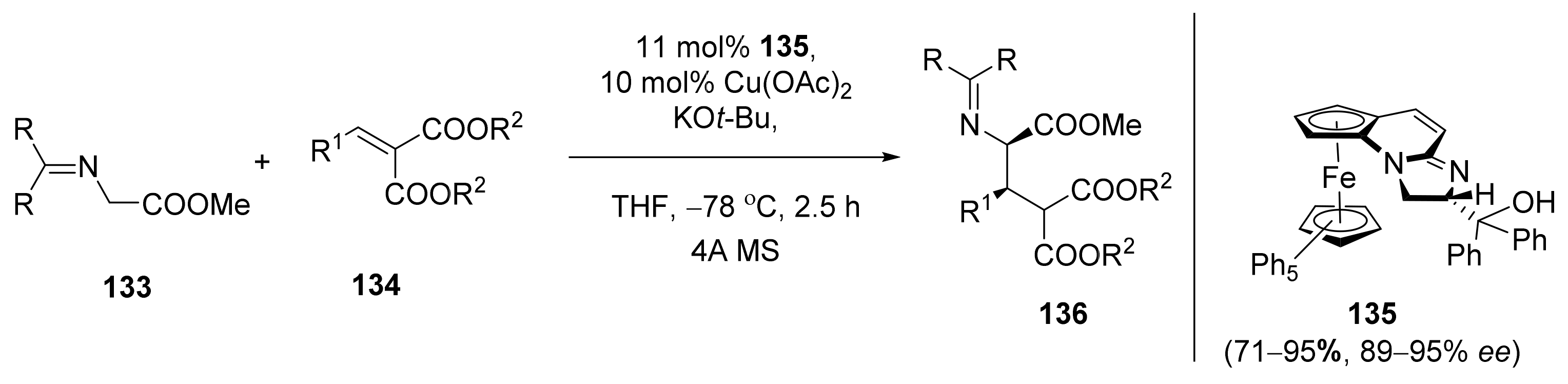
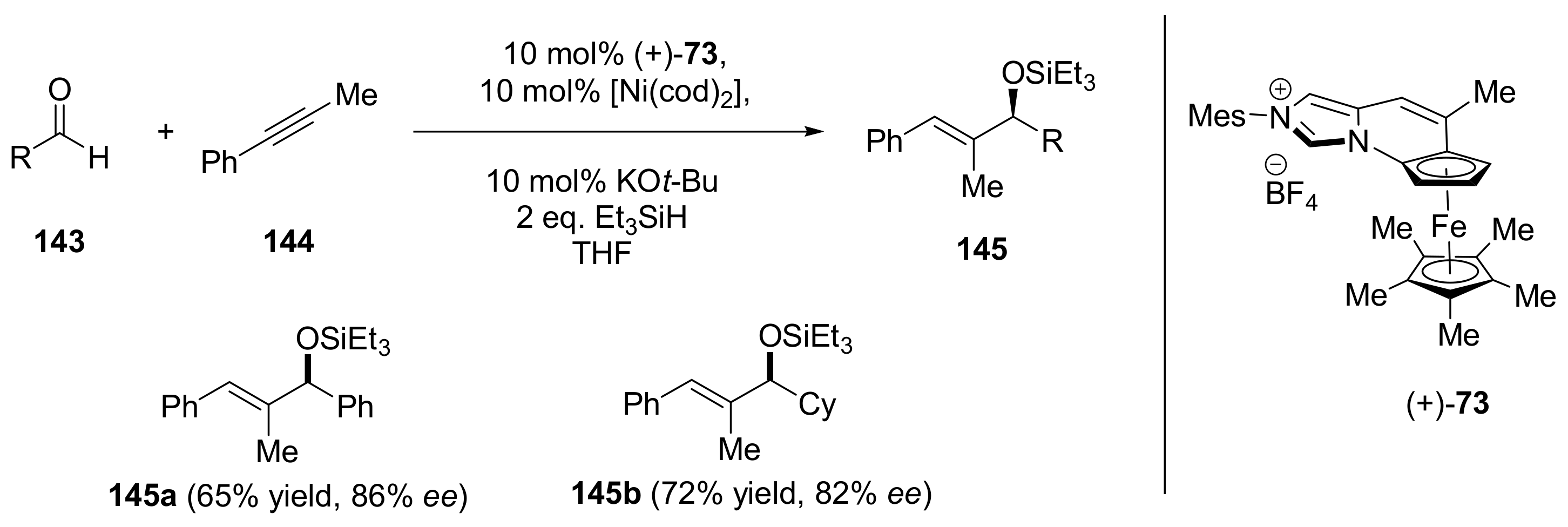
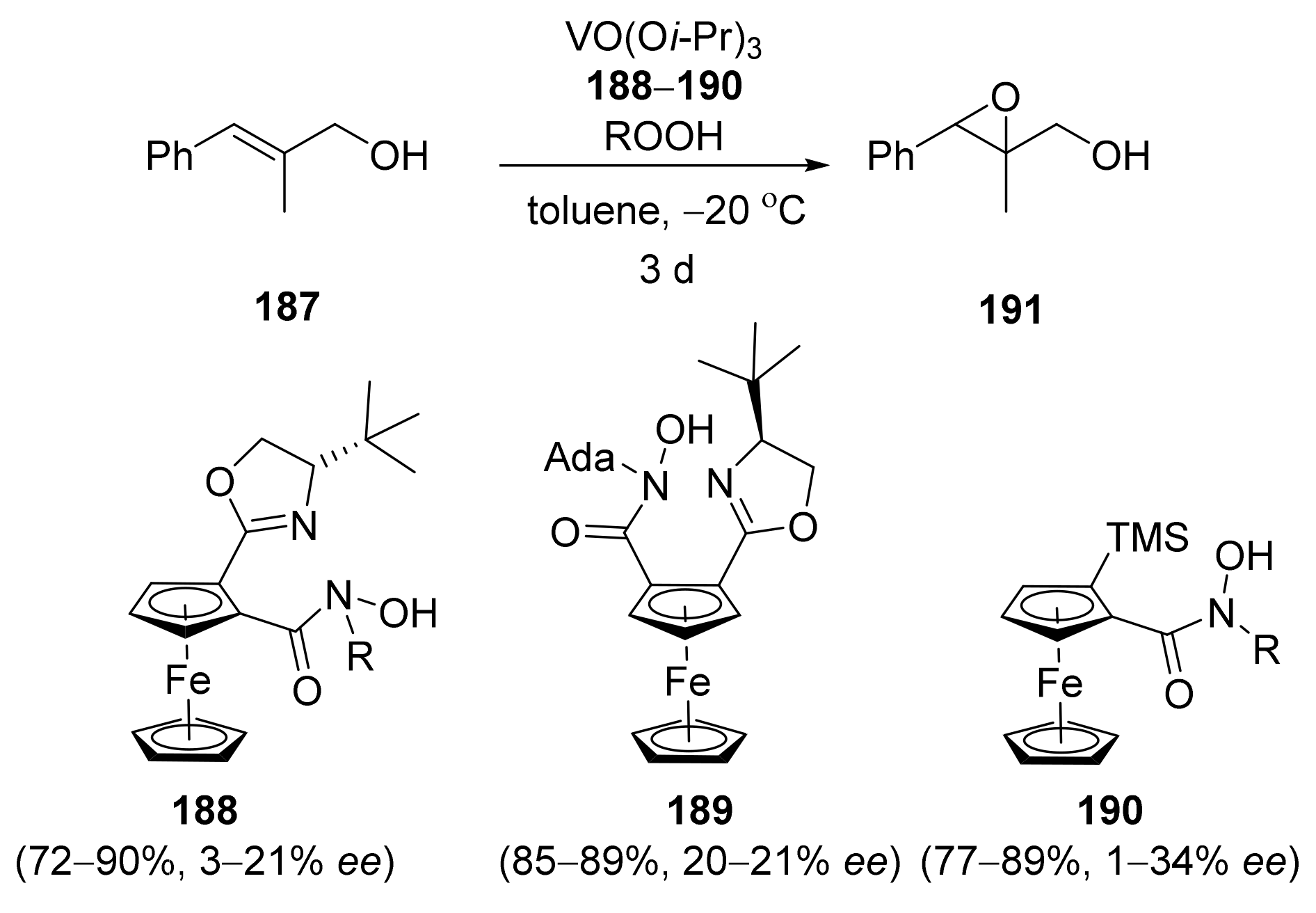
2.4. Asymmetric Addition
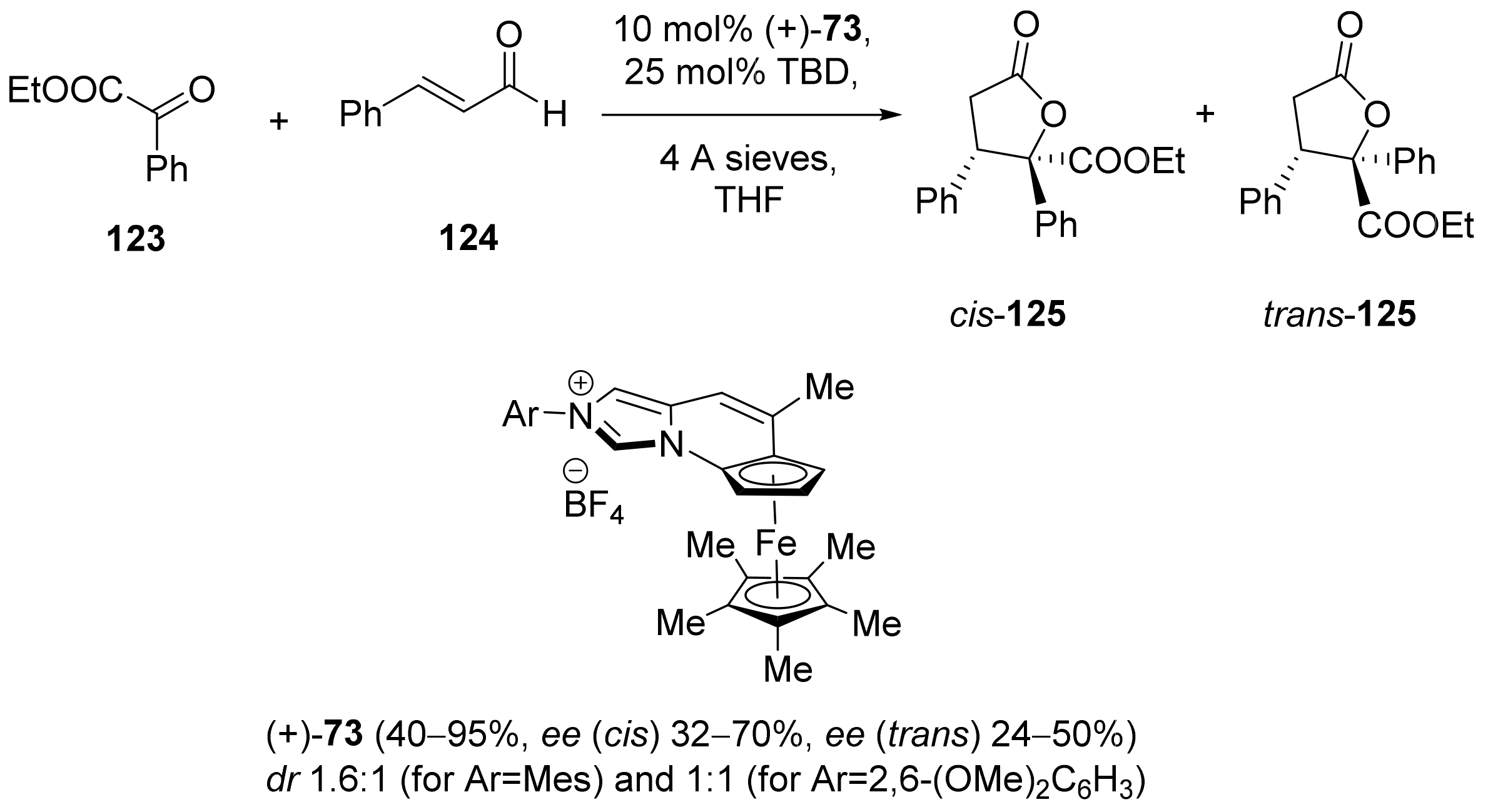
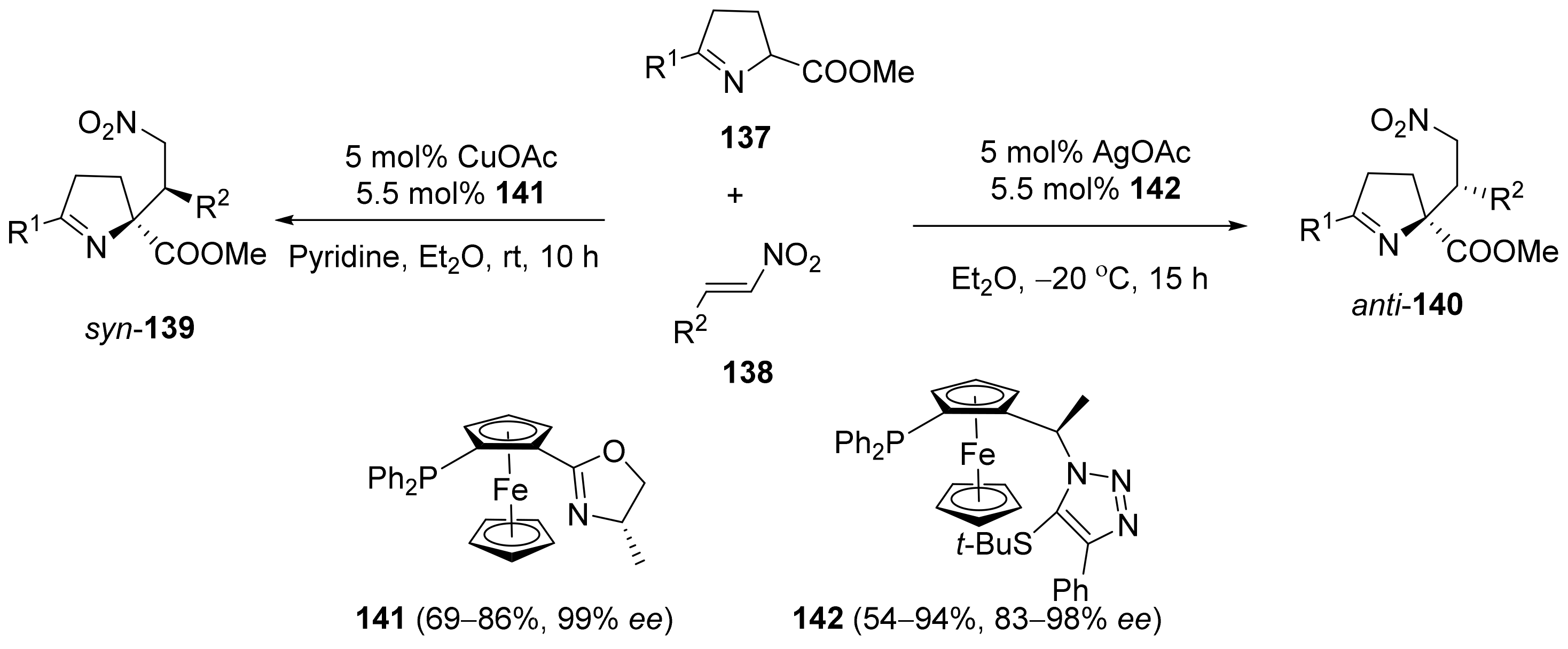
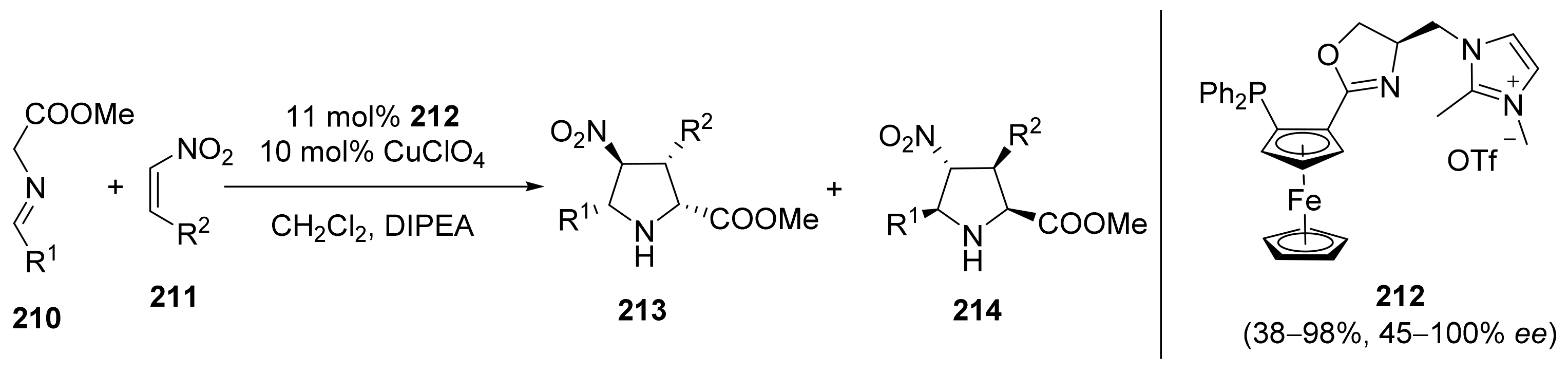
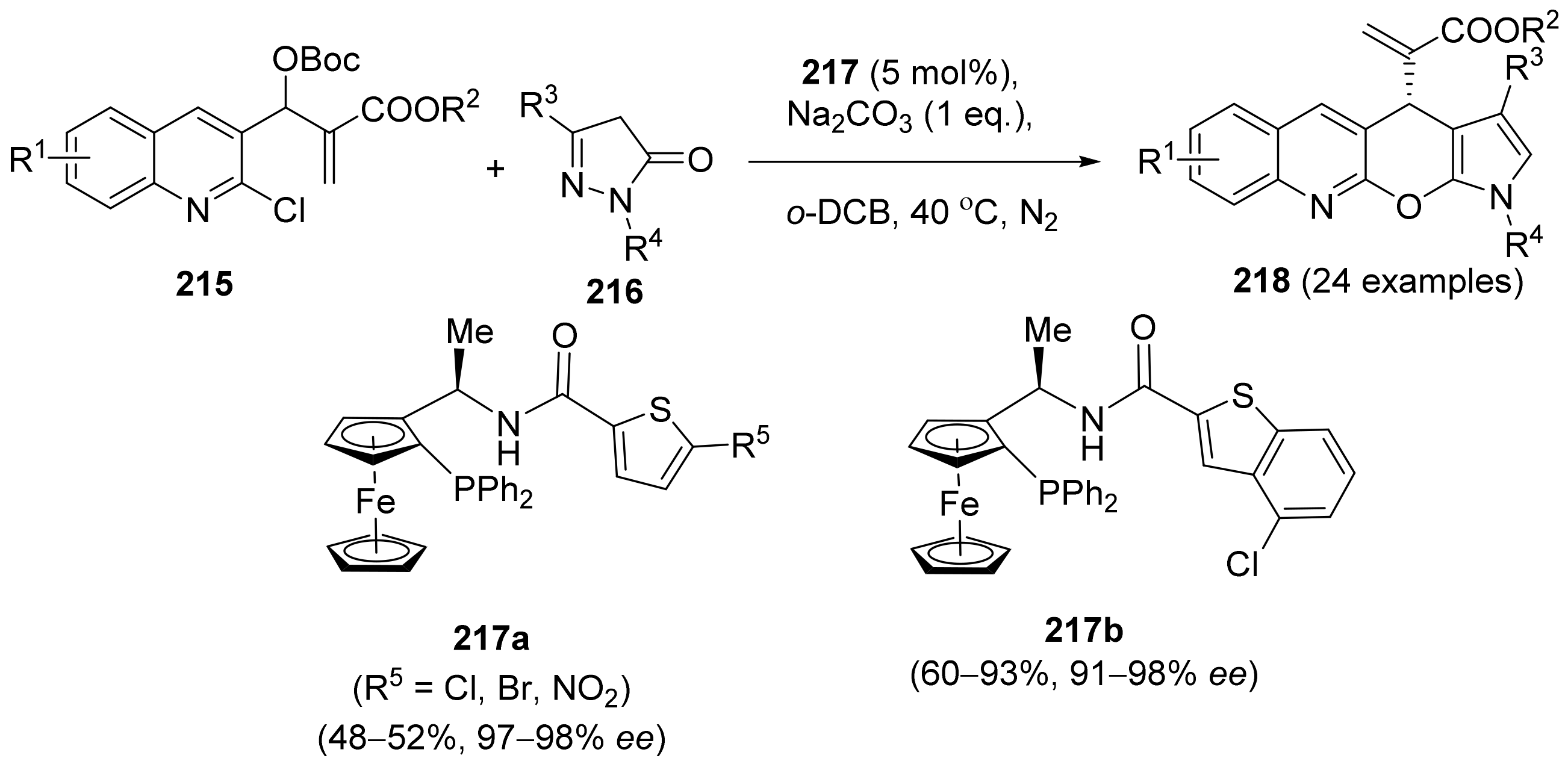
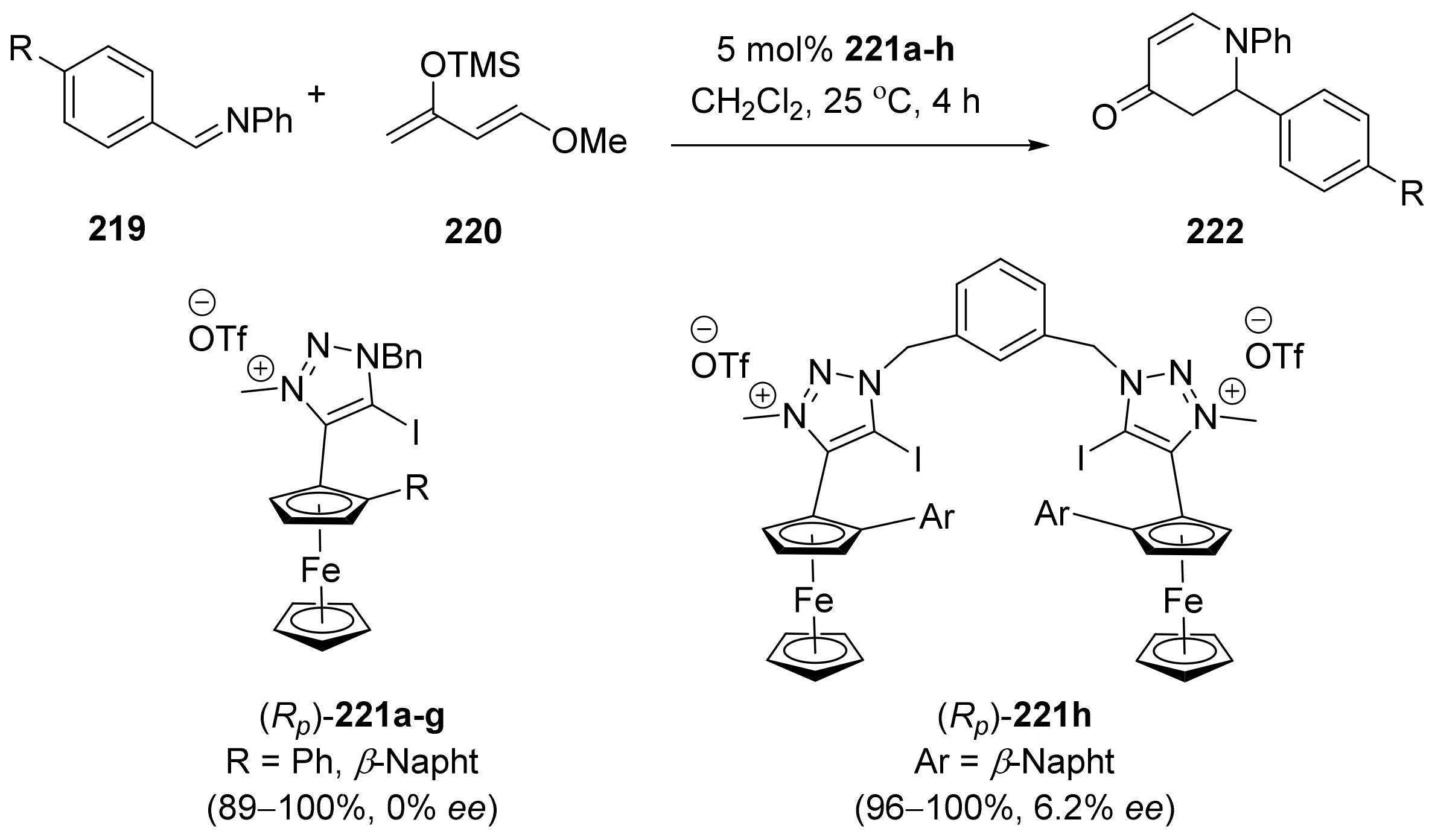
2.5. Annulation and Cycloaddition
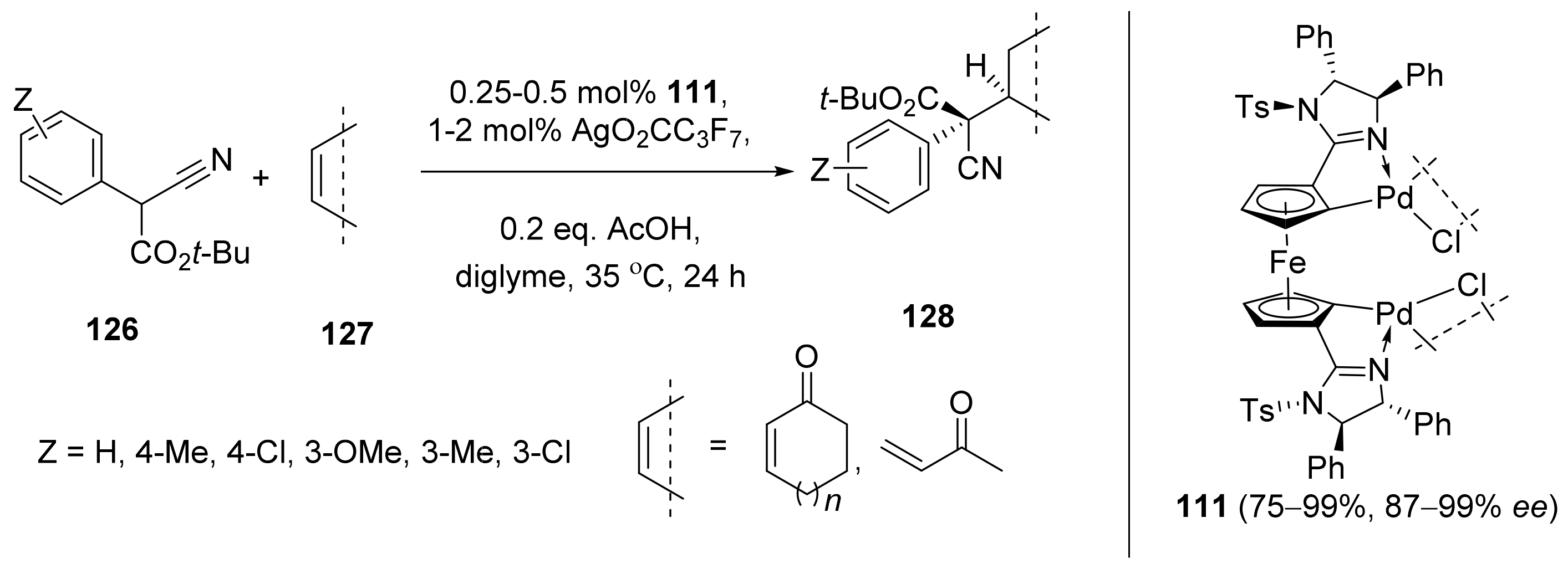
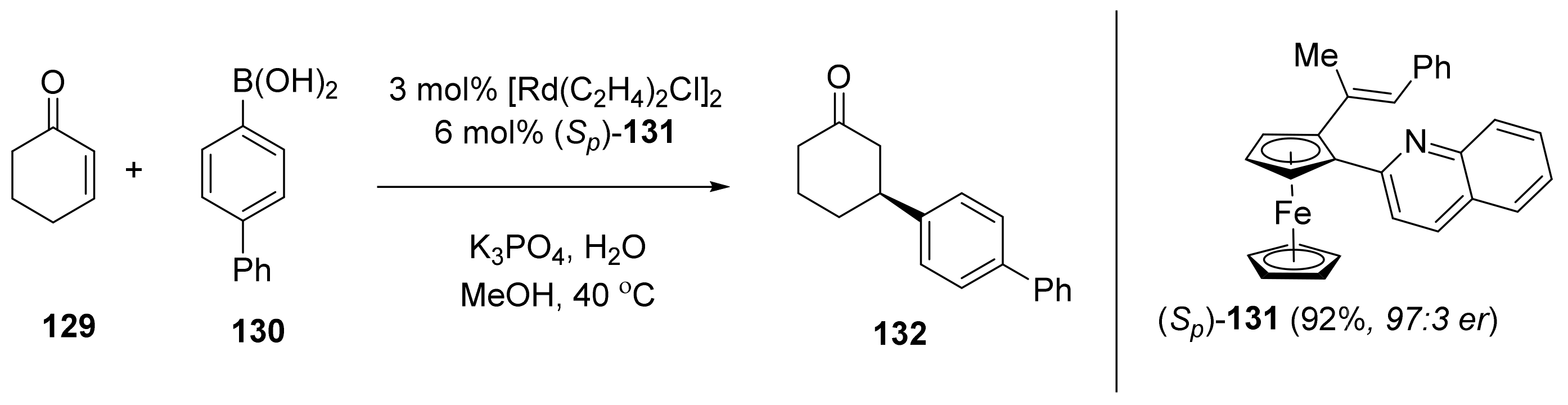
2.6. Cross-Coupling Reactions Catalyzed by Metals
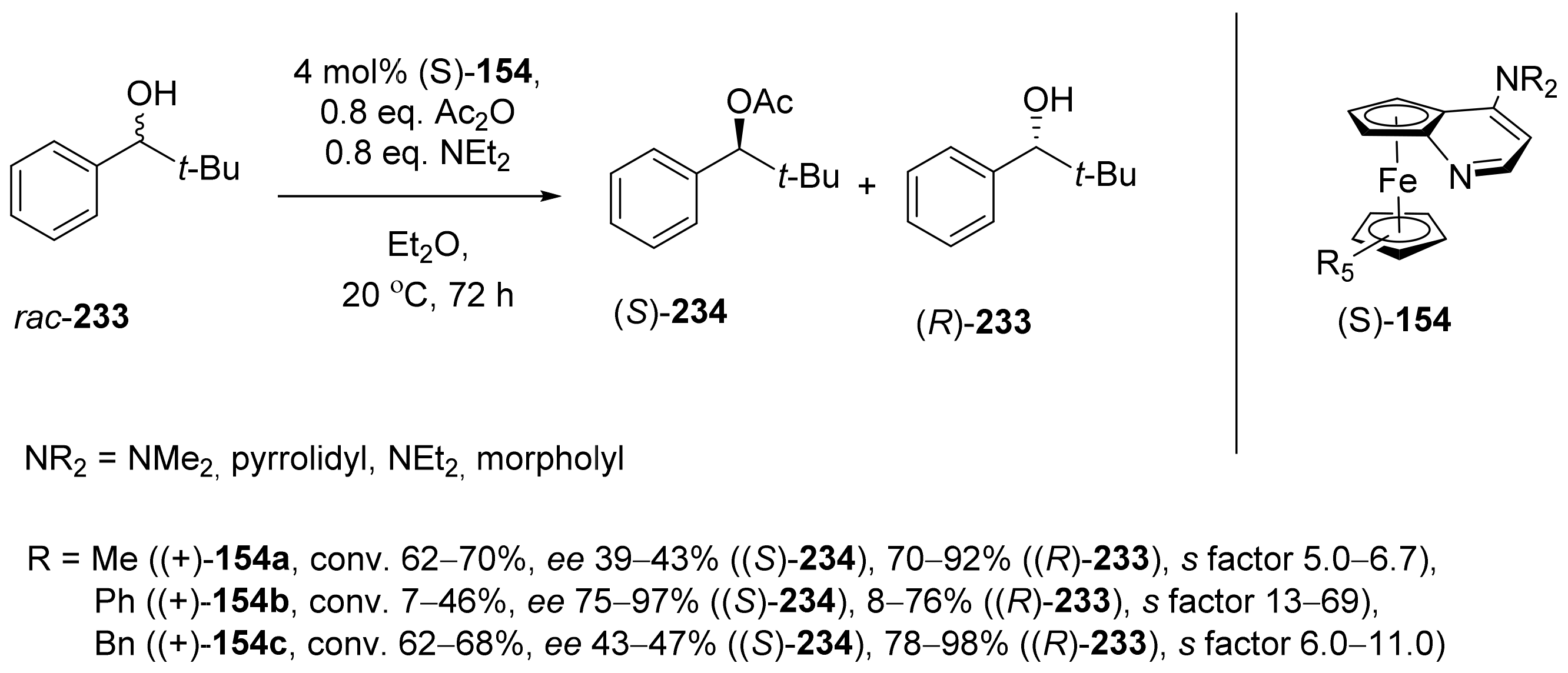
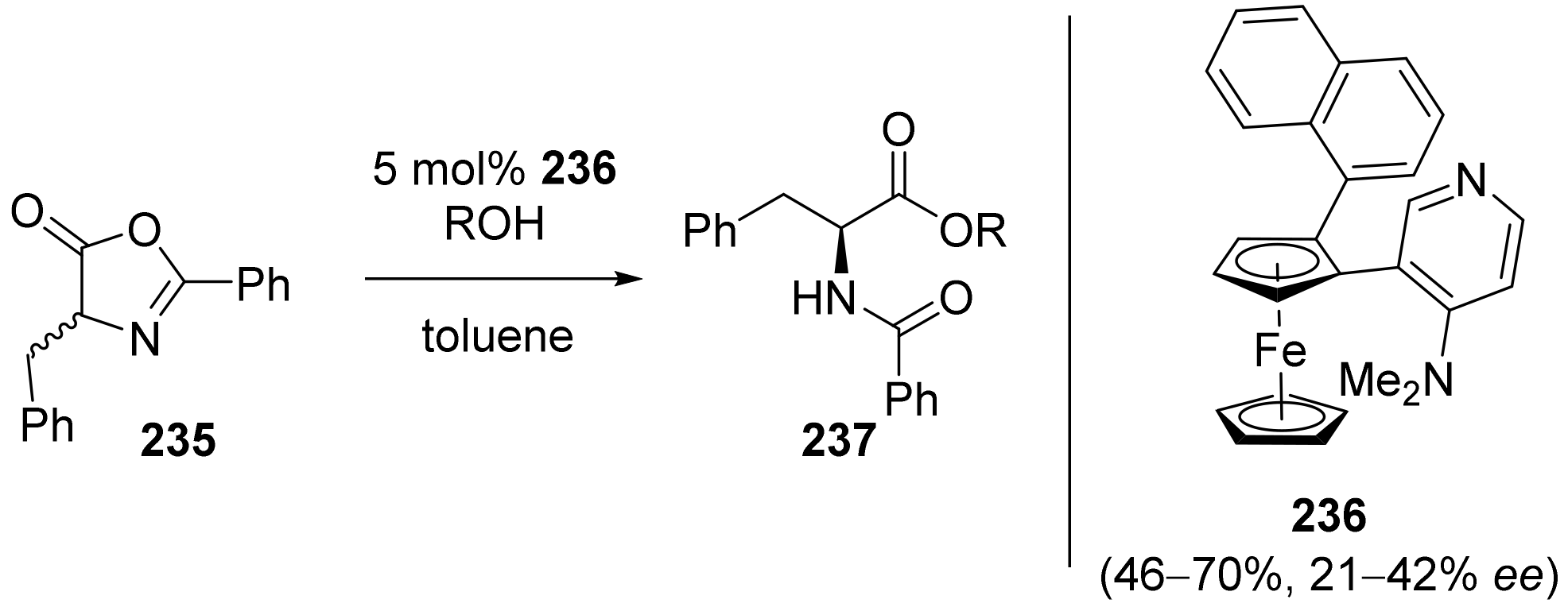
2.7. Resolution of Enantiomers
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ada | adamantly; |
| Ar | aryl; |
| Bn | benzoyl; |
| Boc | tert-butyloxycarbonyl; |
| (Bpin)2 | bis(pinacolato)diborane; |
| BSA | bis(trimethylsilyl)acetamide; |
| t-Bu | t-butyl; |
| CHP | cumene hydroperoxide; |
| o-DCB | 1,2-dichlorobenzene; |
| DIPEA | N,N-diisopropylethylamine; |
| DME | dimethoxyethane; |
| DMF | dimethylformamide; |
| ee | enantiomeric enriched; |
| eq. | equivalent; |
| er | enantiomeric ratio; |
| Et | ethyl; |
| HCA | L-homocysteic acid; |
| [Ir(cod)Cl2] | bis(1,5-cyclooctadiene)diiridium(I) dichloride; |
| LiHMDS | lithium bis(trimethylsilyl)amide; |
| Me | methyl; |
| Mes | mesityl; |
| MPEG | methoxy polyethylene glycol; |
| NHCs | N-heterocyclic carbenes; |
| [Ni(cod)2] | bis(cyclooctadiene)nickel(0); |
| Pd2(dba)3 | tris(dibenzylideneacetone)dipalladium(0); |
| Ph | phenyl; |
| PMHS | polymethylhydrosiloxane; |
| i-Pr | i-propyl; |
| PS | proton sponge, N,N,N′,N′-tetramethyl-1,8-diaminonaphthalene; |
| [Rh(cod)2]BF4 | bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; |
| [Rh(nbd)2]BF4 | bis(norbornadiene)rhodium(I) tetrafluoroborate; |
| [Ru(p-cymene)2] | dichloro(p-cymene)ruthenium(II) dimer; |
| TBAF | tetra-n-butylammonium fluoride; |
| TBD | triazabicyclodecene; |
| TBHP | tert-butyl hydroperoxide; |
| THF | tetrahydrofuran; |
| TMS | trimethylsilyl; |
| Tol | tolyl. |
References
- Liu, Z.-Q. Enhancing Antioxidant Effect against Peroxyl Radical-Induced Oxidation of DNA: Linking with Ferrocene Moiety! Chem. Rec. 2019, 19, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Nataro, C. 5.09—Ferrocene: To Infinity and Back Again. In Comprehensive Coordination Chemistry III; Constable, E.C., Parkin, G., Que, L., Jr., Eds.; Elsevier: Oxford, UK, 2021; pp. 572–656. ISBN 978-0-08-102689-2. [Google Scholar]
- Ludwig, B.S.; Correia, J.D.G.; Kühn, F.E. Ferrocene Derivatives as Anti-Infective Agents. Coord. Chem. Rev. 2019, 396, 22–48. [Google Scholar] [CrossRef]
- Brunel, D.; Noirbent, G.; Dumur, F. Ferrocene: An Unrivaled Electroactive Building Block for the Design of Push-Pull Dyes with near-Infrared and Infrared Absorptions. Dye. Pigment. 2019, 170, 107611. [Google Scholar] [CrossRef]
- Pal, A.; Ranjan Bhatta, S.; Thakur, A. Recent Advances in the Development of Ferrocene Based Electroactive Small Molecules for Cation Recognition: A Comprehensive Review of the Years 2010–2020. Coord. Chem. Rev. 2021, 431, 213685. [Google Scholar] [CrossRef]
- Chaudhary, A.; Poonia, K. The Redox Mechanism of Ferrocene and Its Phytochemical and Biochemical Compounds in Anticancer Therapy: A Mini Review. Inorg. Chem. Commun. 2021, 134, 109044. [Google Scholar] [CrossRef]
- Štěpnička, P. (Ed.) Ferrocenes: Ligands, Materials and Biomolecules; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; ISBN 978-0-470-03585-6. [Google Scholar]
- Korb, M.; Lang, H. Rearrangements and Migrations along the Ferrocene Periphery: On the Way to Planar-Chiral and (Multi)Substitution Patterns. Eur. J. Inorg. Chem. 2022, 2022, e202100946. [Google Scholar] [CrossRef]
- Pérez-Sánchez, J.C.; Herrera, R.P.; Gimeno, M.C. Ferrocenyl Gold Complexes as Efficient Catalysts. Eur. J. Inorg. Chem. 2022, 2022, e202101067. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.; Zhang, Z.; Jin, S.; Wen, S.; Ji, J.; Chung, L.W.; Dong, X.-Q.; Zhang, X. Ferrocenyl Chiral Bisphosphorus Ligands for Highly Enantioselective Asymmetric Hydrogenation via Noncovalent Ion Pair Interaction. Chem. Sci. 2016, 7, 6669–6673. [Google Scholar] [CrossRef]
- Yu, J.; Duan, M.; Wu, W.; Qi, X.; Xue, P.; Lan, Y.; Dong, X.-Q.; Zhang, X. Readily Accessible and Highly Efficient Ferrocene-Based Amino-Phosphine-Alcohol (f-Amphol) Ligands for Iridium-Catalyzed Asymmetric Hydrogenation of Simple Ketones. Chem.-Eur. J. 2017, 23, 970–975. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Dai, L. Chiral Oxazolinyl Hydroxyl Ferrocene Catalysts: Synthesis and Applications in Asymmetric Reactions. ChemistrySelect 2020, 5, 9443–9456. [Google Scholar] [CrossRef]
- Drusan, M.; Šebesta, R. Enantioselective C–C and C–Heteroatom Bond Forming Reactions Using Chiral Ferrocene Catalysts. Tetrahedron 2014, 70, 759–786. [Google Scholar] [CrossRef]
- Toma, Š.; Csizmadiová, J.; Mečiarová, M.; Šebesta, R. Ferrocene Phosphane-Heteroatom/Carbon Bidentate Ligands in Asymmetric Catalysis. Dalton Trans. 2014, 43, 16557–16579. [Google Scholar] [CrossRef]
- Erb, W.; Mongin, F. Twofold Ferrocene C–H Lithiations For One-Step Difunctionalizations. Synthesis 2019, 51, 146–160. [Google Scholar] [CrossRef]
- Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Asymmetric Catalysis with Chiral Ferrocene Ligands. Acc. Chem. Res. 2003, 36, 659–667. [Google Scholar] [CrossRef]
- Yu, J.; Long, J.; Yang, Y.; Wu, W.; Xue, P.; Chung, L.W.; Dong, X.-Q.; Zhang, X. Iridium-Catalyzed Asymmetric Hydrogenation of Ketones with Accessible and Modular Ferrocene-Based Amino-Phosphine Acid (f-Ampha) Ligands. Org. Lett. 2017, 19, 690–693. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, T.; Gu, G.; Dang, L.; Zhang, X. Scope and Mechanism on Iridium-f-Amphamide Catalyzed Asymmetric Hydrogenation of Ketones. Chin. J. Chem. 2018, 36, 851–856. [Google Scholar] [CrossRef]
- Ling, F.; Nian, S.; Chen, J.; Luo, W.; Wang, Z.; Lv, Y.; Zhong, W. Development of Ferrocene-Based Diamine-Phosphine-Sulfonamide Ligands for Iridium-Catalyzed Asymmetric Hydrogenation of Ketones. J. Org. Chem. 2018, 83, 10749–10761. [Google Scholar] [CrossRef]
- Yi, X.; Chen, Y.; Huang, A.; Song, D.; He, J.; Ling, F.; Zhong, W. Design of Chiral Ferrocenylphosphine-Spiro Phosphonamidite Ligands for Ruthenium-Catalyzed Highly Enantioselective Coupling of 1,2-Diols with Amines. Org. Chem. Front. 2021, 8, 6830–6836. [Google Scholar] [CrossRef]
- Gómez Arrayás, R.; Adrio, J.; Carretero, J.C. Recent Applications of Chiral Ferrocene Ligands in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7674–7715. [Google Scholar] [CrossRef]
- Peters, R. Chiral Ferrocenes in Asymmetric Catalysis. Synthesis and Applications. Edited by Li-Xin Dai and Xue-Long Hou. Angew. Chem. Int. Ed. 2010, 49, 4163–4164. [Google Scholar] [CrossRef]
- Hu, X.-P.; Chen, H.-L.; Zheng, Z. Ferrocene-Based Chiral Phosphine-Triazines: A New Family of Highly Efficient P,N Ligands for Asymmetric Catalysis. Adv. Synth. Catal. 2005, 347, 541–548. [Google Scholar] [CrossRef]
- Deng, W.-P.; Hou, X.-L.; Dai, L.-X.; Yu, Y.-H.; Xia, W. On the Role of Planar Chirality: A Tunable Enantioselectivity in Palladium-Catalyzed Allylic Alkylation with Planar Chiral 1,1′-P,N-Ferrocene Ligands. Chem. Commun. 2000, 4, 285–286. [Google Scholar] [CrossRef]
- Deng, W.-P.; You, S.-L.; Hou, X.-L.; Dai, L.-X.; Yu, Y.-H.; Xia, W.; Sun, J. Importance of Planar Chirality in Chiral Catalysts with Three Chiral Elements: The Role of Planar Chirality in 2′-Substituted 1,1′-P,N-Ferrocene Ligands on the Enantioselectivity in Pd-Catalyzed Allylic Substitution. J. Am. Chem. Soc. 2001, 123, 6508–6519. [Google Scholar] [CrossRef]
- Lee, S.; Koh, J.H.; Park, J. Ortho-Silylation of 2,2′-Bis(Oxazolinyl)-1,1′-Bis(Diphenylphosphino)Ferrocenes and Remarkable Effect of the Silyl Groups on the Enantioselectivity in Pd-Catalyzed Asymmetric Allylic Alkylation. J. Organomet. Chem. 2001, 637–639, 99–106. [Google Scholar] [CrossRef]
- Tu, T.; Hou, X.-L.; Dai, L.-X. Ligand Electronic Effects in the Palladium Catalyzed Asymmetric Allylic Alkylation Reaction with Planar Chiral Diphosphine-Oxazoline Ferrocenyl Ligands. J. Organomet. Chem. 2004, 689, 3847–3852. [Google Scholar] [CrossRef]
- You, S.-L.; Hou, X.-L.; Dai, L.-X. Synthesis of Planar Chiral Selenide Derivatives of Ferrocenyl-Oxazoline and Their Application in Enantioselective Palladium Catalyzed Allylic Substitution Reaction. Tetrahedron Asymmetry 2000, 11, 1495–1500. [Google Scholar] [CrossRef]
- Meaney, K.; Goddard, R.; Bronger, R.P.J.; Guiry, P.J. The Preparation of Ferrocene-Containing Phosphinamine Ligands Possessing Central and Planar Chirality and Their Application in Palladium-Catalysed Allylic Substitution. Tetrahedron 2021, 90, 132088. [Google Scholar] [CrossRef]
- Hu, X.; Dai, H.; Bai, C.; Chen, H.; Zheng, Z. Novel Ferrocenylphosphine-Imines Containing a Pyridine Unit as a New Family of Chiral Ligands: The Important Influence of the Position of the Pyridine N-Atom on the Reactivity and Enantioselectivity in Palladium-Catalyzed Asymmetric Allylic Alkylations. Tetrahedron Asymmetry 2004, 15, 1065–1068. [Google Scholar] [CrossRef]
- Cheung, H.Y.; Yu, W.-Y.; Au-Yeung, T.T.L.; Zhou, Z.; Chan, A.S.C. Effective Chiral Ferrocenyl Phosphine-Thioether Ligands in Enantioselective Palladium-Catalyzed Allylic Alkylations. Adv. Synth. Catal. 2009, 351, 1412–1422. [Google Scholar] [CrossRef]
- Kato, M.; Nakamura, T.; Ogata, K.; Fukuzawa, S. Synthesis of Novel Ferrocenyl-Based P,S Ligands (ThioClickFerrophos) and Their Use in Pd-Catalyzed Asymmetric Allylic Substitutions. Eur. J. Org. Chem. 2009, 2009, 5232–5238. [Google Scholar] [CrossRef]
- You, S.-L.; Hou, X.-L.; Dai, L.-X.; Yu, Y.-H.; Xia, W. Role of Planar Chirality of S,N- and P,N-Ferrocene Ligands in Palladium-Catalyzed Allylic Substitutions. J. Org. Chem. 2002, 67, 4684–4695. [Google Scholar] [CrossRef] [PubMed]
- Noël, T.; Bert, K.; Van der Eycken, E.; Van der Eycken, J. Imidate–Phosphanes as Highly Versatile N,P Ligands and Their Application in Palladium-Catalyzed Asymmetric Allylic Alkylation Reactions. Eur. J. Org. Chem. 2010, 2010, 4056–4061. [Google Scholar] [CrossRef]
- Utepova, I.A.; Chupakhin, O.N.; Serebrennikova, P.O.; Musikhina, A.A.; Charushin, V.N. Two Approaches in the Synthesis of Planar Chiral Azinylferrocenes. J. Org. Chem. 2014, 79, 8659–8667. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-H.; Zheng, B.-H.; Zhang, Y.; Hou, X.-L. Highly Regio-, Diastereo-, and Enantioselective Pd-Catalyzed Allylic Alkylation of Acyclic Ketone Enolates with Monosubstituted Allyl Substrates. J. Am. Chem. Soc. 2007, 129, 7718–7719. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, F.; Zhang, W. Palladium-Catalyzed Asymmetric Allylic Alkylation with an Enamine as the Nucleophilic Reagent. Tetrahedron Lett. 2007, 48, 7591–7594. [Google Scholar] [CrossRef]
- Ayerbe Garcia, M.; Frey, W.; Peters, R. Sterically Demanding Planar Chiral P,N Ligands by Diastereoselective Ortho Lithiation of Pentaphenylferrocenyloxazolines and Their Application to Palladium-Catalyzed Substitutions with Cyclic Allylic Acetates. Organometallics 2014, 33, 1068–1078. [Google Scholar] [CrossRef]
- Wu, H.; Xie, F.; Wang, Y.; Zhao, X.; Liu, D.; Zhang, W. Pd-Catalyzed Asymmetric Allylic Amination Using Easily Accessible Metallocenyl P,N-Ligands. Org. Biomol. Chem. 2015, 13, 4248–4254. [Google Scholar] [CrossRef]
- Lai, Z.-W.; Yang, R.-F.; Ye, K.-Y.; Sun, H.; You, S.-L. Synthesis of 1-[Bis(Trifluoromethyl)Phosphine]-1′-Oxazolinylferrocene Ligands and Their Application in Regio- and Enantioselective Pd-Catalyzed Allylic Alkylation of Monosubstituted Allyl Substrates. Beilstein J. Org. Chem. 2014, 10, 1261–1266. [Google Scholar] [CrossRef]
- Abbas, Z.; Hu, X.-H.; Ali, A.; Xu, Y.-W.; Hu, X.-P. New Chiral Ferrocene/Indole-Based Diphosphine Ligands for Rh-Catalyzed Asymmetric Hydrogenation of Functionalized Olefins. Tetrahedron Lett. 2020, 61, 151860. [Google Scholar] [CrossRef]
- Fukuzawa, S.; Oki, H.; Hosaka, M.; Sugasawa, J.; Kikuchi, S. ClickFerrophos: New Chiral Ferrocenyl Phosphine Ligands Synthesized by Click Chemistry and the Use of Their Metal Complexes as Catalysts for Asymmetric Hydrogenation and Allylic Substitution. Org. Lett. 2007, 9, 5557–5560. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Z.-H.; Chen, M.-W.; Chen, Q.-A.; Zhou, Y.-G. Catalytic Biomimetic Asymmetric Reduction of Alkenes and Imines Enabled by Chiral and Regenerable NAD(P)H Models. Angew. Chem. Int. Ed. 2019, 58, 1813–1817. [Google Scholar] [CrossRef]
- Check, C.T.; Jang, K.P.; Schwamb, C.B.; Wong, A.S.; Wang, M.H.; Scheidt, K.A. Ferrocene-Based Planar Chiral Imidazopyridinium Salts for Catalysis. Angew. Chem. Int. Ed. 2015, 54, 4264–4268. [Google Scholar] [CrossRef]
- Fitzpatrick, K.P.; Schwamb, C.B.; Check, C.T.; Jang, K.-P.; Barsoum, D.N.; Scheidt, K.A. Development of Ferrocene-Based Planar Chiral Imidazopyridinium Salts for Catalysis. Organometallics 2020, 39, 2705–2712. [Google Scholar] [CrossRef]
- Gérard, S.; Pressel, Y.; Riant, O. Application of N,S-Chelating Chiral Ligands and Zinc Complexes in Catalytic Asymmetric Hydrosilylation Using Polymethylhydrosiloxane. Tetrahedron Asymmetry 2005, 16, 1889–1891. [Google Scholar] [CrossRef]
- Schuecker, R.; Zirakzadeh, A.; Mereiter, K.; Spindler, F.; Weissensteiner, W. Synthesis, Coordination Behavior, and Structural Features of Chiral Amino-, Pyrazolyl-, and Phosphino-Substituted Ferrocenyloxazolines and Their Application in Asymmetric Hydrogenations. Organometallics 2011, 30, 4711–4719. [Google Scholar] [CrossRef]
- Zirakzadeh, A.; Schuecker, R.; Gorgas, N.; Mereiter, K.; Spindler, F.; Weissensteiner, W. Ruthenium Complexes of Phosphino-Substituted Ferrocenyloxazolines in the Asymmetric Hydrogenation and Transfer Hydrogenation of Ketones: A Comparison. Organometallics 2012, 31, 4241–4250. [Google Scholar] [CrossRef]
- Guo, H.; Liu, D.; Butt, N.A.; Liu, Y.; Zhang, W. Efficient Ru(II)-Catalyzed Asymmetric Hydrogenation of Simple Ketones with C2-Symmetric Planar Chiral Metallocenyl Phosphinooxazoline Ligands. Tetrahedron 2012, 68, 3295–3299. [Google Scholar] [CrossRef]
- Nie, H.; Zhou, G.; Wang, Q.; Chen, W.; Zhang, S. Asymmetric Hydrogenation of Aromatic Ketones Using an Iridium(I) Catalyst Containing Ferrocene-Based P–N–N Tridentate Ligands. Tetrahedron Asymmetry 2013, 24, 1567–1571. [Google Scholar] [CrossRef]
- Wu, W.; Liu, S.; Duan, M.; Tan, X.; Chen, C.; Xie, Y.; Lan, Y.; Dong, X.-Q.; Zhang, X. Iridium Catalysts with F-Amphox Ligands: Asymmetric Hydrogenation of Simple Ketones. Org. Lett. 2016, 18, 2938–2941. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Huang, A.; Yang, Z.; Cheng, Y.; Chen, J.; Ling, F.; Zhong, W. Manganese Catalyzed Enantio- and Regioselective Hydrogenation of α,β-Unsaturated Ketones Using an Imidazole-Based Chiral PNN Tridentate Ligand. Tetrahedron Lett. 2021, 82, 153389. [Google Scholar] [CrossRef]
- Cheemala, M.N.; Knochel, P. New P,N-Ferrocenyl Ligands for the Asymmetric Ir-Catalyzed Hydrogenation of Imines. Org. Lett. 2007, 9, 3089–3092. [Google Scholar] [CrossRef]
- Jiang, R.; Sun, X.; He, W.; Chen, H.; Kuang, Y. Asymmetric Transfer Hydrogenation Catalyzed by a Novel Planar Chiral N-Heterocyclic Carbene–Rhodium(I) Complex. Appl. Organomet. Chem. 2009, 23, 179–182. [Google Scholar] [CrossRef]
- Kuang, Y.; Sun, X.; Chen, H.; Liu, P.; Jiang, R. A Novel Planar Chiral N-Heterocyclic Carbene–Oxazoline Ligand for the Asymmetric Hydrosilylation of Ketones. Catal. Commun. 2009, 10, 1493–1496. [Google Scholar] [CrossRef]
- Yuan, Y.; Raabe, G.; Bolm, C. Novel Rhodium Complexes with Ferrocene-Based N-Heterocylic Carbenes: Synthesis, Structure and Catalysis. J. Organomet. Chem. 2005, 690, 5747–5752. [Google Scholar] [CrossRef]
- Moyano, A.; Rosol, M.; Moreno, R.M.; López, C.; Maestro, M.A. Oxazoline-Mediated Interannular Cyclopalladation of Ferrocene: Chiral Palladium(II) Catalysts for the Enantioselective Aza-Claisen Rearrangement. Angew. Chem. Int. Ed. 2005, 44, 1865–1869. [Google Scholar] [CrossRef]
- Jautze, S.; Seiler, P.; Peters, R. Synthesis of Nearly Enantiopure Allylic Amines by Aza-Claisen Rearrangement of Z-Configured Allylic Trifluoroacetimidates Catalyzed by Highly Active Ferrocenylbispalladacycles. Chem.-Eur. J. 2008, 14, 1430–1444. [Google Scholar] [CrossRef]
- Fischer, D.F.; Barakat, A.; Xin, Z.; Weiss, M.E.; Peters, R. The Asymmetric Aza-Claisen Rearrangement: Development of Widely Applicable Pentaphenylferrocenyl Palladacycle Catalysts. Chem.-Eur. J. 2009, 15, 8722–8741. [Google Scholar] [CrossRef]
- Weiss, M.; Frey, W.; Peters, R. Asymmetric Synthesis of Heterobimetallic Planar Chiral Ferrocene Pallada-/Platinacycles and Their Application to Enantioselective Aza-Claisen Rearrangements. Organometallics 2012, 31, 6365–6372. [Google Scholar] [CrossRef]
- Hellmuth, T.; Rieckhoff, S.; Weiss, M.; Dorst, K.; Frey, W.; Peters, R. Cooperative Bimetallic Asymmetric Catalysis: Comparison of a Planar Chiral Ruthenocene Bis-Palladacycle to the Corresponding Ferrocene. ACS Catal. 2014, 4, 1850–1858. [Google Scholar] [CrossRef]
- Seitzberg, J.G.; Dissing, C.; Søtofte, I.; Norrby, P.-O.; Johannsen, M. Design and Synthesis of a New Type of Ferrocene-Based Planar Chiral DMAP Analogues. A New Catalyst System for Asymmetric Nucleophilic Catalysis. J. Org. Chem. 2005, 70, 8332–8337. [Google Scholar] [CrossRef]
- Csizmadiová, J.; Mečiarová, M.; Almássy, A.; Horváth, B.; Šebesta, R. Ferrocene Phosphane–Carbene Ligands in Cu-Catalyzed Enantioselective 1,4-Additions of Grignard Reagents to α,β-Unsaturated Carbonyl Compounds. J. Organomet. Chem. 2013, 737, 47–52. [Google Scholar] [CrossRef]
- Sorádová, Z.; Máziková, J.; Mečiarová, M.; Šebesta, R. Stereoselective Domino Conjugate Addition of Grignard Reagents to Lactones Followed by Reaction with Activated Alkenes Catalyzed by Ferrocenyl Carbene Ligands. Tetrahedron Asymmetry 2015, 26, 271–275. [Google Scholar] [CrossRef]
- Lou, S.-J.; Zhuo, Q.; Nishiura, M.; Luo, G.; Hou, Z. Enantioselective C–H Alkenylation of Ferrocenes with Alkynes by Half-Sandwich Scandium Catalyst. J. Am. Chem. Soc. 2021, 143, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, Y.-H.; Luo, J.-F.; Du, W.; Shi, X.-X.; Fossey, J.S.; Deng, W.-P. Novel N,O-Cu(OAc)2 Complex Catalysed Diastereo- and Enantioselective 1,4-Addition of Glycine Derivatives to Alkylidene Malonates. Catal. Sci. Technol. 2011, 1, 100–103. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Wang, Z.; Hu, B.; Wang, M.; Fossey, J.S.; Deng, W.-P. A Ferrocenyl-DHIPOH/Cu(OAc)2 Complex for Diastereo- and Enantioselective Catalysis of the 1,4-Addition of Glycine Derivatives to Alkylidene Malonates. Org. Lett. 2011, 13, 6010–6013. [Google Scholar] [CrossRef]
- Koizumi, A.; Kimura, M.; Arai, Y.; Tokoro, Y.; Fukuzawa, S. Copper- and Silver-Catalyzed Diastereo- and Enantioselective Conjugate Addition Reaction of 1-Pyrroline Esters to Nitroalkenes: Diastereoselectivity Switch by Chiral Metal Complexes. J. Org. Chem. 2015, 80, 10883–10891. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, M.R.; Sormunen, G.J.; Montgomery, J. New N-Heterocyclic Carbene Ligand and Its Application in Asymmetric Nickel-Catalyzed Aldehyde/Alkyne Reductive Couplings. J. Am. Chem. Soc. 2007, 129, 9568–9569. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Montgomery, J.; Houk, K.N. Ligand Steric Contours To Understand the Effects of N-Heterocyclic Carbene Ligands on the Reversal of Regioselectivity in Ni-Catalyzed Reductive Couplings of Alkynes and Aldehydes. J. Am. Chem. Soc. 2011, 133, 6956–6959. [Google Scholar] [CrossRef]
- Heberle, M.; Legendre, S.; Wannenmacher, N.; Weber, M.; Frey, W.; Peters, R. Bispalladacycle Catalyzed Nucleophilic Enantioselective Allylation of Aldehydes by Allylstannanes. ChemCatChem 2022, 14, e202200093. [Google Scholar] [CrossRef]
- Yoshida, K.; Liu, Q.; Yasue, R.; Wada, S.; Kimura, R.; Konishi, T.; Ogasawara, M. Versatile and Enantioselective Preparation of Planar-Chiral Metallocene-Fused 4-Dialkylaminopyridines and Their Application in Asymmetric Organocatalysis. ACS Catal. 2020, 10, 292–301. [Google Scholar] [CrossRef]
- Ogasawara, M.; Wada, S.; Isshiki, E.; Kamimura, T.; Yanagisawa, A.; Takahashi, T.; Yoshida, K. Enantioselective Synthesis of Planar-Chiral Ferrocene-Fused 4-Pyridones and Their Application in Construction of Pyridine-Based Organocatalyst Library. Org. Lett. 2015, 17, 2286–2289. [Google Scholar] [CrossRef]
- Šebesta, R.; Sališová, M. Derivatives of (S)-{[2-(Methoxymethyl)Pyrrolidin-1-Yl]Methyl}ferrocene—New Planar Chiral Ligands. Collect. Czechoslov. Chem. Commun. 2002, 67, 1700–1708. [Google Scholar] [CrossRef]
- Stepnicka, P.; Base, T.; Cisarova, I.; Kubista, J.; Vyskocil, S.; Sticha, M. Synthesis and Catalytic Activity of Spaced Ferrocene Oxazolines. ChemInform 2003, 68, 1206–1232. [Google Scholar] [CrossRef]
- Li, M.; Yuan, K.; Li, Y.-Y.; Cao, B.-X.; Sun, J.; Hou, X.-L. On the Role of Planar Chirality in Asymmetric Catalysis: Improvement of Enantioselectivity in the Addition of Diethylzinc to Aldehydes with Planar Chiral 1,1′-N,O-Ferrocenyl Ligands. Tetrahedron Asymmetry 2003, 14, 3347–3352. [Google Scholar] [CrossRef]
- Garabatos-Perera, J.R.; Butenschön, H. New Chiral Ferrocenyloxazolines: The First Planar Chiral Triferrocenylmethane Derivative and Its Use in Asymmetric Catalysis. J. Organomet. Chem. 2009, 694, 2047–2052. [Google Scholar] [CrossRef]
- Bolm, C.; Hermanns, N.; Claßen, A.; Muñiz, K. Polymer-Supported Ferrocenyl Oxazolines for the Catalyzed Highly Enantioselective Phenyl Transfer to Aldehydes. Bioorganic Med. Chem. Lett. 2002, 12, 1795–1798. [Google Scholar] [CrossRef]
- Özçubukçu, S.; Schmidt, F.; Bolm, C. Organosilanols as Catalysts in Asymmetric Aryl Transfer Reactions. Org. Lett. 2005, 7, 1407–1409. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.-Z.; Yuan, K.; Cao, B.-X.; Hou, X.-L. Highly Enantioselective Phenylacetylene Addition to Aldehydes Catalyzed by a Chiral N,O-Ferrocene Ligand. Tetrahedron Asymmetry 2004, 15, 219–222. [Google Scholar] [CrossRef]
- Haraguchi, R.; Yamazaki, T.; Torita, K.; Ito, T.; Fukuzawa, S. Planar-Chiral Ferrocene-Based Triazolylidene Copper Complexes: Synthesis, Characterization, and Catalysis in Asymmetric Borylation of α,β-Unsaturated Ester. Dalton Trans. 2020, 49, 17578–17583. [Google Scholar] [CrossRef]
- Hegedus, L.S.; Darlington, W.H.; Russell, C.E. Cyclopropanation of Ester Enolates by.Pi.-Allylpalladium Chloride Complexes. J. Org. Chem. 1980, 45, 5193–5196. [Google Scholar] [CrossRef]
- Patti, A.; Pedotti, S. Preparation of a Chiral Ferrocenyl Derivative Containing a Biphenyl Unit via Nickel-Mediated Homocoupling. Chirality 2005, 17, 233–236. [Google Scholar] [CrossRef]
- Liu, W.; Chen, D.; Zhu, X.-Z.; Wan, X.-L.; Hou, X.-L. Highly Diastereo- and Enantioselective Pd-Catalyzed Cyclopropanation of Acyclic Amides with Substituted Allyl Carbonates. J. Am. Chem. Soc. 2009, 131, 8734–8735. [Google Scholar] [CrossRef]
- Bolm, C.; Kühn, T. Planar Chiral Ferrocenes as Ligands in the Vanadium-Catalyzed Asymmetric Epoxidation of Allylic Alcohols. Isr. J. Chem. 2001, 41, 263–270. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Luo, W.; Shao, B.; Li, J.; Xiao, X.; Song, D.; Ling, F.; Zhong, W. Construction of Spirooxindole-Fused Spiropyrazolones Containing Contiguous Three Stereogenic Centres via [3 + 2] Annulation Utilizing a Ferrocene Derived Bifunctional Phosphine Catalyst. Org. Chem. Front. 2020, 7, 1016–1021. [Google Scholar] [CrossRef]
- Han, M.-L.; Wang, D.-Y.; Zeng, P.-W.; Zheng, Z.; Hu, X.-P. New Chiral Ferrocenyl P,S-Ligands for Highly Diastereo- and Enantioselective Ag(I)-Catalyzed Asymmetric [3+2] Cycloaddition of Azomethine Ylides. Tetrahedron Asymmetry 2012, 23, 306–312. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, C.; Wang, Y.-H.; Cao, Z.; Zheng, Z.; Hu, X.-P. Synthesis of New Chiral Ferrocenyl P,N-Ligands with a Benzoxazole Ring and Their Application in Ag-Catalyzed Asymmetric [3+2] Cycloaddition. Tetrahedron Lett. 2013, 54, 3669–3672. [Google Scholar] [CrossRef]
- Utepova, I.A.; Serebrennikova, P.O.; Streltsova, M.S.; Musikhina, A.A.; Fedorchenko, T.G.; Chupakhin, O.N.; Antonchick, A.P. Enantiomerically Enriched 1,2-P,N-Bidentate Ferrocenyl Ligands for 1,3-Dipolar Cycloaddition and Transfer Hydrogenation Reactions. Molecules 2018, 23, 1311. [Google Scholar] [CrossRef]
- Dai, L.; Xu, D.; Dong, X.; Zhou, Z. The Design, Synthesis and Application of Imidazolium-Tagged Ferrocenyl Oxazoline Phosphine Ligands for the Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides with Nitroalkenes: Ion Effect for Enhancing the Reactivity, Stereoselectivity and Recyclability. Tetrahedron Asymmetry 2015, 26, 350–360. [Google Scholar] [CrossRef]
- Xiao, X.; Shao, B.; Li, J.; Yang, Z.; Lu, Y.-J.; Ling, F.; Zhong, W. Enantioselective Synthesis of Functionalized 1,4-Dihydropyrazolo-[4′,3′:5,6]Pyrano [2,3-b]Quinolines through Ferrocenyl-Phosphine-Catalyzed Annulation of Modified MBH Carbonates and Pyrazolones. Chem. Commun. 2021, 57, 4690–4693. [Google Scholar] [CrossRef] [PubMed]
- Aubert, E.; Doudouh, A.; Wenger, E.; Sechi, B.; Peluso, P.; Pale, P.; Mamane, V. Chiral Ferrocenyl−Iodotriazoles and −Iodotriazoliums as Halogen Bond Donors. Synthesis, Solid State Analysis and Catalytic Properties. Eur. J. Inorg. Chem. 2022, 2022, e202100927. [Google Scholar] [CrossRef]
- Sammakia, T.; Latham, H.A.; Schaad, D.R. Highly Diastereoselective Ortho Lithiations of Chiral Oxazoline-Substituted Ferrocenes. J. Org. Chem. 1995, 60, 10–11. [Google Scholar] [CrossRef]
- Richards, C.J.; Mulvaney, A.W. Synthesis of Phosphinoferrocenyloxazolines. New Ligands for Asymmetric Catalysis. Tetrahedron Asymmetry 1996, 7, 1419–1430. [Google Scholar] [CrossRef]
- Nishibayashi, Y.; Segawa, K.; Ohe, K.; Uemura, S. Chiral Oxazolinylferrocene-Phosphine Hybrid Ligand for the Asymmetric Hydrosilylation of Ketones. Organometallics 1995, 14, 5486–5487. [Google Scholar] [CrossRef]
- Corona-Sánchez, R.; Toscano, R.A.; Ortega-Alfaro, M.C.; Sandoval-Chávez, C.; López-Cortés, J.G. 2-Ferrocenyl-2-Thiazoline as a Building Block of Novel Phosphine-Free Ligands. Dalton Trans. 2013, 42, 11992–12004. [Google Scholar] [CrossRef]
- Sakai, S.; Kanemoto, K.; Fukuzawa, S. Synthesis and Evaluation of Novel Planar-Chiral Monophosphine Ligands Bearing Ferrocene-Triazole Backbones. Eur. J. Inorg. Chem. 2022, 2022, e202100967. [Google Scholar] [CrossRef]
- Debono, N.; Labande, A.; Manoury, E.; Daran, J.-C.; Poli, R. Palladium Complexes of Planar Chiral Ferrocenyl Phosphine-NHC Ligands: New Catalysts for the Asymmetric Suzuki−Miyaura Reaction. Organometallics 2010, 29, 1879–1882. [Google Scholar] [CrossRef]









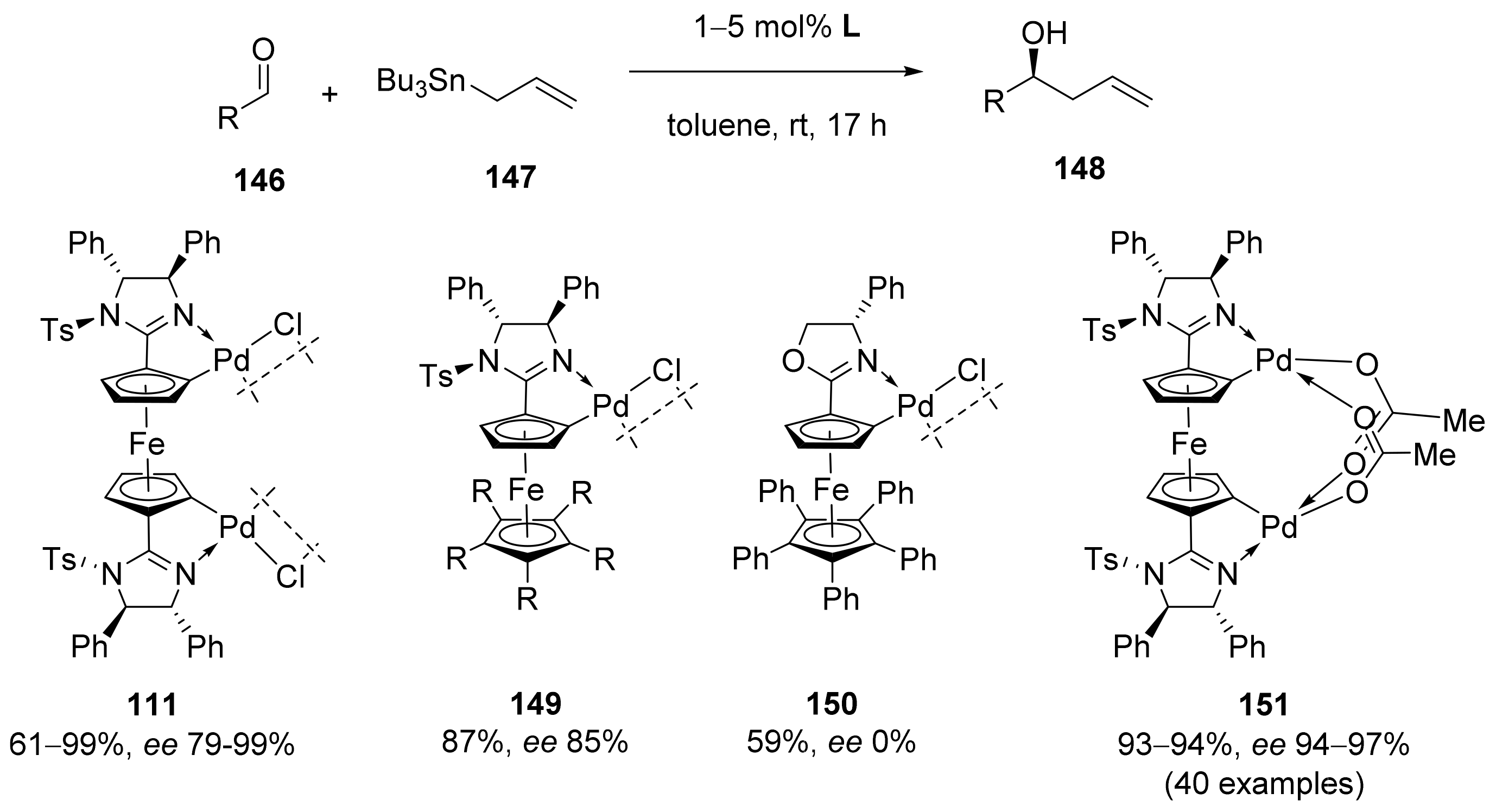


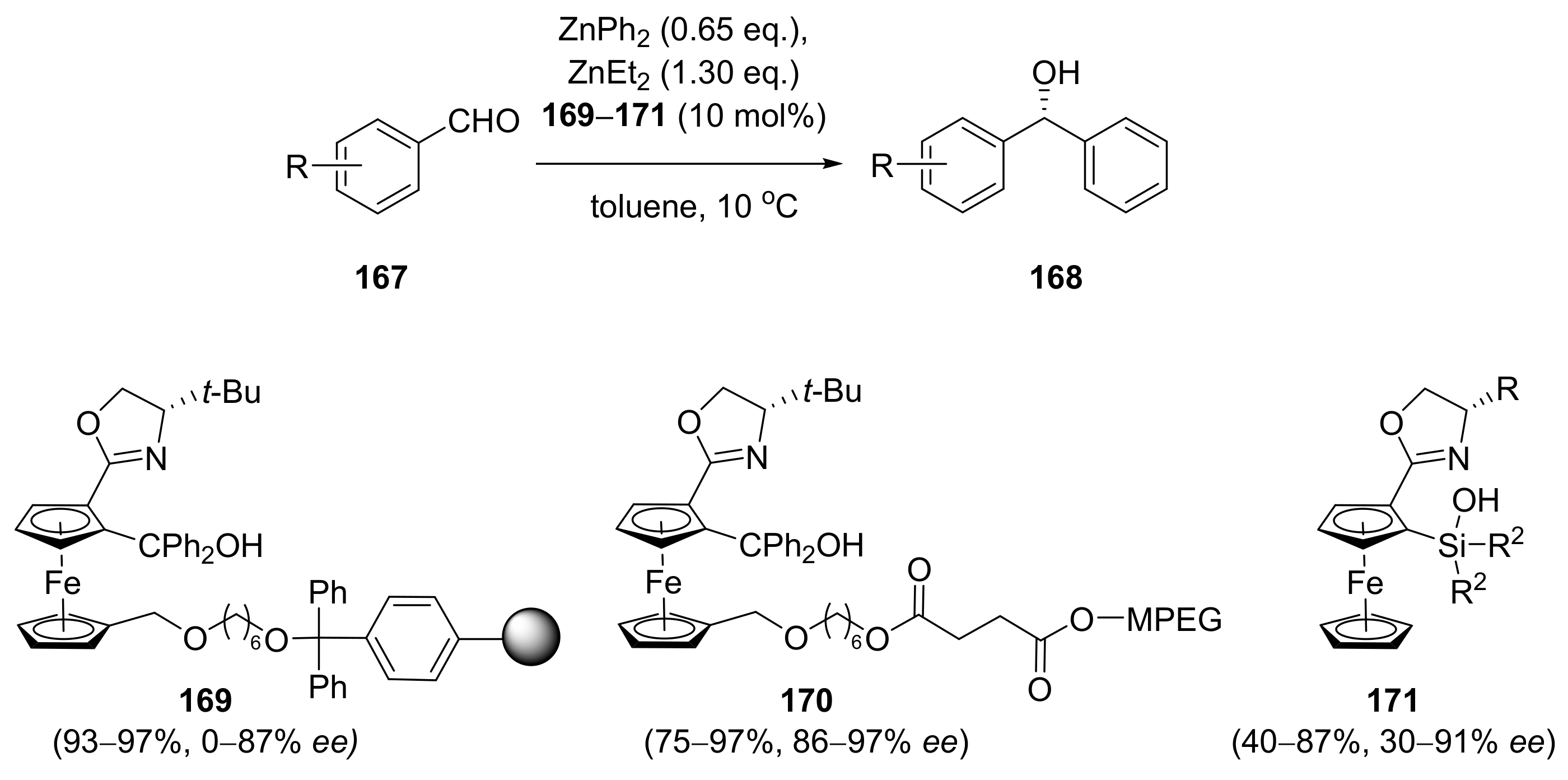

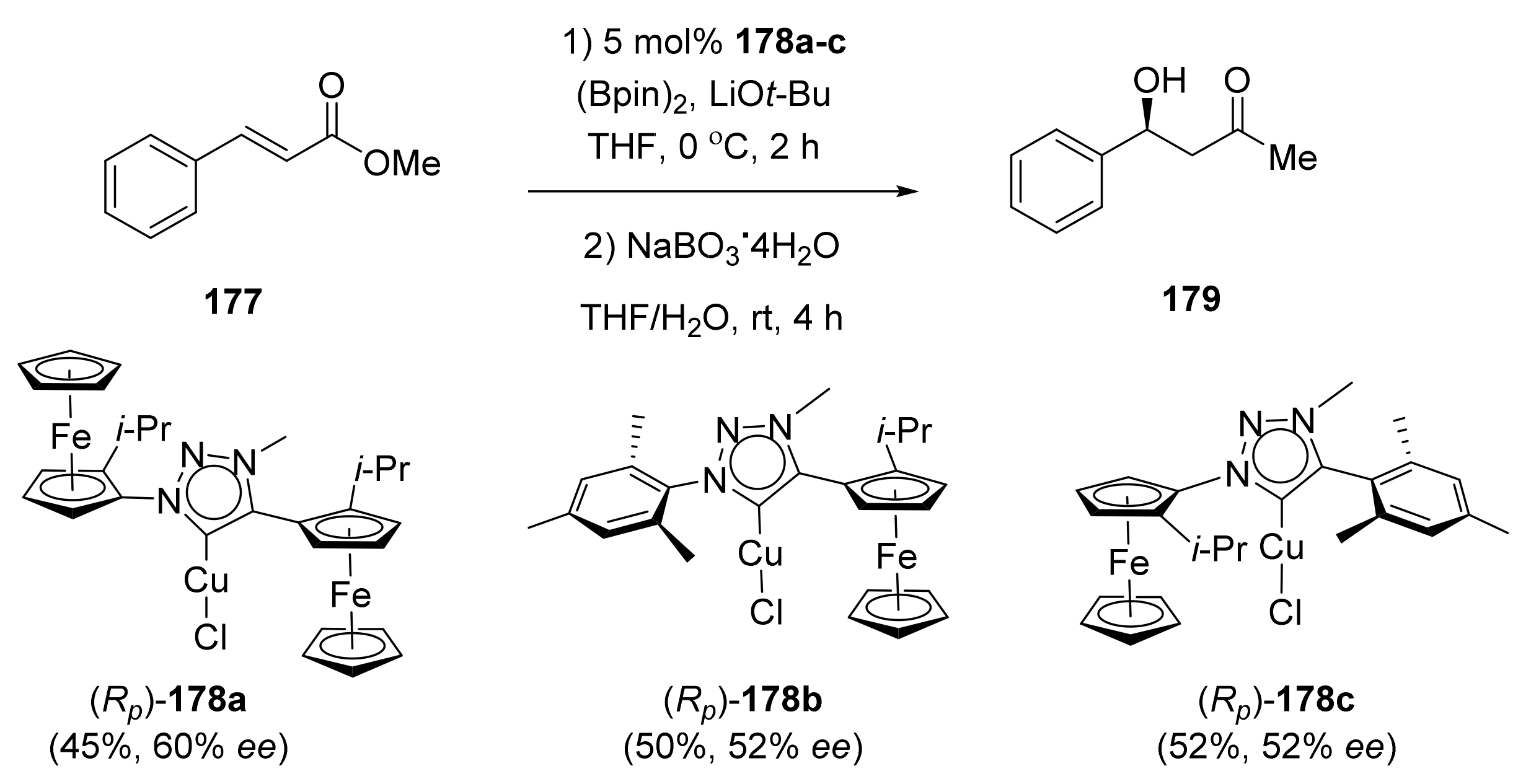
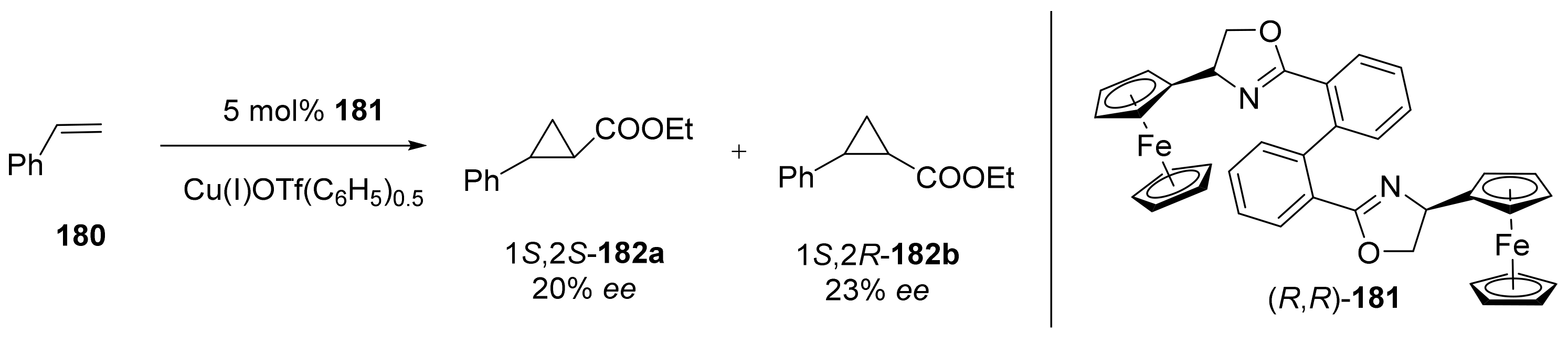




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musikhina, A.A.; Serebrennikova, P.O.; Zabelina, O.N.; Utepova, I.A.; Chupakhin, O.N. Advanced Application of Planar Chiral Heterocyclic Ferrocenes. Inorganics 2022, 10, 152. https://doi.org/10.3390/inorganics10100152
Musikhina AA, Serebrennikova PO, Zabelina ON, Utepova IA, Chupakhin ON. Advanced Application of Planar Chiral Heterocyclic Ferrocenes. Inorganics. 2022; 10(10):152. https://doi.org/10.3390/inorganics10100152
Chicago/Turabian StyleMusikhina, Alexandra A., Polina O. Serebrennikova, Olga N. Zabelina, Irina A. Utepova, and Oleg N. Chupakhin. 2022. "Advanced Application of Planar Chiral Heterocyclic Ferrocenes" Inorganics 10, no. 10: 152. https://doi.org/10.3390/inorganics10100152
APA StyleMusikhina, A. A., Serebrennikova, P. O., Zabelina, O. N., Utepova, I. A., & Chupakhin, O. N. (2022). Advanced Application of Planar Chiral Heterocyclic Ferrocenes. Inorganics, 10(10), 152. https://doi.org/10.3390/inorganics10100152