Stereochemical Geometries and Photoluminescence in Pseudo-Halido-Zinc(II) Complexes. Structural Comparison between the Corresponding Cadmium(II) Analogs
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Physical Measurements
Caution! Salts of perchlorate and azide as well as their metal complexes are potentially explosive and should be handled with great care and in small quantities.
2.2. Preparation of the Compounds
2.2.1. [Zn(Meepmqa)(N3)2] (1)
2.2.2. [Zn(Meepmqa)(dca)]ClO4·½H2O (2)
2.2.3. [Zn(NTB)(N3)]ClO4·½H2O (3)
2.2.4. [Zn(TPA)(NCS)]ClO4 (4)
2.2.5. [Zn(1,8-damnph)2(dca)2] (5)
2.2.6. [Zn(8-amq)2(dca)]ClO4 (6)
2.2.7. Catena-[Zn(isq)2(μ1,5-dca)2] (7)
2.2.8. Catena-[Zn(N,N-Me2en)2(μ1,5-dca)]dca (8)
2.3. X-ray Crystal Structure Analysis
3. Results and Discussion
3.1. Synthetic Aspects
3.2. IR Spectra of the Complexes
3.3. Description of the Structures
3.3.1. [Zn(NTB)(N3)]ClO4·½H2O (3) and [Zn(TPA)(NCS)]ClO4 (4)
3.3.2. [Zn(1,8-damnph)2(dca)2] (5) and [Zn(8-amq)2(dca)2] (6a)
3.3.3. Catena-[Zn(isq)2(μ1,5-dca)2] (7) and Catena-[Zn(N,N-Me2en)(μ1,5-dca)]dca (8)
3.4. Structural Comparison between Zinc(II) Complexes and Cadmium(II) Analogs
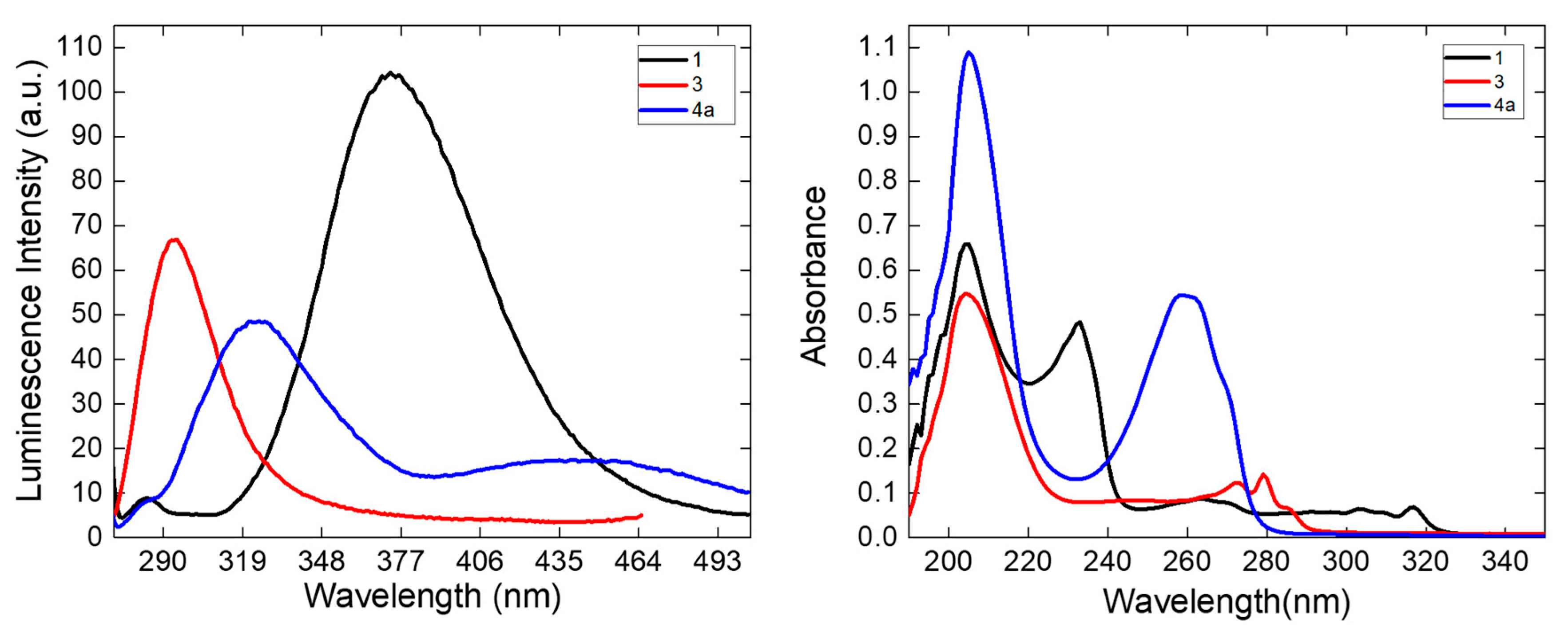
3.5. Luminescence Emission
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maret, W. Zinc in cellular regulation: The Nature and significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinc (In Biological Systems). In Van Nostrand’s Scientific Encyclopedia; Wiley Online: Hoboken, NJ, USA, 2006. [CrossRef]
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clinic Toxicol. 2011, 3. [Google Scholar] [CrossRef] [Green Version]
- Sigel, H.; Bruce Martin, R. The colorless ‘chameleon’ or the peculiar properties of Zn2+ in complexes in solution. Quantification of equilibria involving a change of the coordination number of the metal ion. Chem. Soc. Rev. 1994, 23, 83–91. [Google Scholar] [CrossRef]
- Maret, W.; Moulis, J.-M. The bioinorganic chemistry of cadmium in the context of its toxicity. In Cadmium: From Toxicity to Essentiality; (Book Series); Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA; London, UK, 2011; Volume 11. [Google Scholar] [CrossRef]
- Burgess, J.; Prince, R.H. Inorganic & Coordination Chemistry: Zinc: Inorganic & Coordination Chemistry. In The Encyclopedia of Inorganic and Bioinorganic Chemistry, 1st ed.; John Wiley & Sons, Ltd. (Wiley Online Library): Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Tetrahedral vs octahedral zinc complexes with ligands of biological interest: A DFT/CDM study. J. Am. Chem. Soc. 2000, 122, 11146–11153. [Google Scholar] [CrossRef]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Massoud, S.S. Synthesis, characterization, and luminescence properties of zinc(II) complexes of pseudohalides and nitrite derived from 4-azido-pyridine. Inorg. Chim. Acta 2015, 425, 46–51. [Google Scholar] [CrossRef]
- Mautner, F.A.; Berger, C.; Dartez, M.J.; Nguyen, Q.L.; Favreau, J.; Massoud, S.S. Cadmium(II) and zinc(II) azido complexes with different nuclearity and dimensionality. Polyhedron 2014, 69, 48–54. [Google Scholar] [CrossRef]
- Nath, J.; Tarai, A.; Baruah, J.B. Copper(II), zinc(II), and cadmium(II) formylbenzoate complexes: Reactivity and emission properties. ACS Omega 2019, 4, 18444–18455. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Obaid, Y.K.; Vicente, R.; Ribas, J.; Fischer, R.C.; Mautner, F.A. Metal ions directing the geometry and nuclearity of azido-metal(II) complexes derived from bis(2-(3,5-dimethyl-1H-pyrazol-1-yl)ethyl)amine. Dalton Trans. 2013, 42, 3968–3978. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Rashmawi, L.G.; Louka, F.R.; Massoud, S.S. Structural characterization of metal(II) thiocyanato complexes derived from bis(2-(1-H-pyrazol-1-yl)ethyl)amine. Polyhedron 2017, 124, 237–242. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Williams, B.R.; Massoud, S.S.; Salem, N.M.H. Hexanuclear cadmium(II) cluster constructed from tris(2-methylpyridyl)amine (TPA) and azides. Crystals 2020, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Mautner, F.A.; Jantscher, P.V.; Fischer, R.C.; Torvisco, A.; Reichmann, K.; Salem, N.M.H.; Gordon, K.J.; Louka, F.R.; Massoud, S.S. Coordination polymers in dicyanamido-cadmium(II) with diverse network dimensionalities. Crystals 2021, 11, 181. [Google Scholar] [CrossRef]
- Brahma, R.; Baruah, J.B. Self-assemblies of zinc complexes for aggregation-induced emission luminogen precursors. ACS Omega 2020, 5, 3774–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xhaferaj, N.; Tăbăcaru, A.; Moroni, M.; Senchyk, G.A.; Domasevitch, K.V.; Pettinari, C.; Galli, S. New coordination polymers of zinc(II), copper(II) and cadmium(II) with 1,3-bis(1,2,4-triazol-4-yl)adamantane. Inorganics 2020, 8, 60. [Google Scholar] [CrossRef]
- Gusev, A.; Shul’gin, V.; Braga, E.; Zamnius, E.; Kryukova, M.; Linert, W. Luminescent properties of Zn complexes based on tetradentate N2O2-donor pyrazolone Schiff bases. Dyes Pigments 2020, 183, 108626. [Google Scholar] [CrossRef]
- Endo, K.; Liu, Y.; Ube, H.; Nagata, K.; Shionoya, M. Asymmetric construction of tetrahedral chiral zinc with high configurational stability and catalytic activity. Nat. Commun. 2020, 11, 6263. [Google Scholar] [CrossRef]
- Gusev, A.; Braga, E.; Baluda, Y.; Kiskin, M.; Kryukova, M.; Karaush-Karmazin, N.; Baryshnikov, G.; Kuklin, A.; Minaev, B.; Ågren, H.; et al. Structure and tunable luminescence in polymeric zinc compounds based on 3-(3-pyridyl)-5-(4-pyridyl)-1,2,4-triazole. Polyhedron 2020, 191, 114768. [Google Scholar] [CrossRef]
- Artesani, A.; Gherardi, F.; Nevin, A.; Gianluca Valentini, G.; Comelli, D. A Photoluminescence study of the changes induced in the zinc white pigment by formation of zinc complexes. Materials 2017, 10, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; So, G.K.-M.; To, W.-P.; Chen, Y.; Kwok, C.-C.; Ma, C.; Guan, X.; Chang, X.; Kwoke, W.-M.; Che, C.-M. Luminescent zinc(ii) and copper(i) complexes for high-performance solution-processed monochromic and white organic light-emitting devices. Chem. Sci. 2015, 6, 4623–4635. [Google Scholar] [CrossRef] [Green Version]
- Dumur, F.; Contal, E.; Wantz, G.; Gigmes, D. Photoluminescence of zinc complexes: Easily tunable optical properties by variation of the bridge between the imido groups of Schiff Base ligands. Eur. J. Inorg. Chem. 2014, 2014, 4186–4198. [Google Scholar] [CrossRef]
- Gusev, A.; Shul’gin, V.; Brag, E.; Zamnius, E.; GalinaStarov, G.; Lyssenko, K.; Eremenko, I.; Linert, W. Luminescent properties of zinc complexes of 4-formylpyrazolone based azomethine ligands: Excitation-dependent emission in solution. J. Lumin. 2018, 202, 370–376. [Google Scholar] [CrossRef]
- Haas, R.M.; Arshad, M.; Anthony, J.; Altmann, P.J.; Pöthig, A.; Köhler, F.H.; Hess, C.R. Six- and seven-coordinate Fe(II) and Zn(II) compounds ligated by unsymmetric xanthene-based ligands: Characterization and magnetic properties. Inorg. Chem. Front. 2016, 3, 616–629. [Google Scholar] [CrossRef]
- Sarkar, B.N.; Bhar, K.; Chattopadhyay, S.; Das, S.; Mitra, P.; Ghosh, B.K. Synthesis, structure and luminescence behavior of heptacoordinated one-dimensional coordination polymers of the type [Cd(L)(dca)]n(X)n (L = a pentadentate Schiff base; dca = dicyanamide; X = ClO4−, PF6−). J. Mol. Struct. 2010, 963, 35–40. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Gallucci, J.C.; Zhen, H.; Huffman, J.C. Three-coordinate zinc amide and phenoxide complexes supported by a bulky Schiff Base ligand. Inorg. Chem. 2001, 40, 5051–5054. [Google Scholar] [CrossRef]
- Pang, K.; Rong, Y.; Parkin, G. Molecular structures of three coordinate zinc and cadmium complexes that feature β-diketiminato and anilido-imine ligands. Polyhedron 2010, 29, 1881–1890. [Google Scholar] [CrossRef]
- Mautner, F.A.; Louka, F.R.; Hofer, J.; Spell, M.; Lefèvre, A.; Guilbeau, A.E.; Massoud, S.S. One-dimensional cadmium polymers with alternative di(EO/EE) and di(EO/EO/EO/EE) bridged azide bonding modes. Cryst. Growth Des. 2013, 13, 4518–4525. [Google Scholar] [CrossRef]
- Mautner, F.A.; Berger, C.; Gspan, C.; Yana, B.; Fischer, R.C.; Massoud, S.S. Pyridyl and triazole ligands directing the assembling of zinc(II) into coordination polymers with different dimensionality through azides. Polyhedron 2017, 130, 136–144. [Google Scholar] [CrossRef]
- Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Synthesis and characterization of 1D coordination polymers of metal(II)-dicyanamido complexes. Polyhedron 2019, 166, 36–43. [Google Scholar] [CrossRef]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Vicente, R.; Massoud, S.S. Synthesis and characterization of three new 1-D polymeric [M2(4-azidopyridine)4(µ1,1-N3)2(µ1,3-N3)2]n (M = Ni, Co, Cd) complexes. Polyhedron 2015, 85, 329–336. [Google Scholar] [CrossRef]
- Mautner, F.A.; Traber, M.; Fischer, R.C.; Massoud, S.S.; Vicente, R. Synthesis, crystal structures, spectral and magnetic properties of 1-D polymeric dicyanamido metal(II) complexes. Polyhedron 2017, 138, 13–20. [Google Scholar] [CrossRef]
- Xu, H.; Guo, C. catena-poly[[(8-aminoquinoline-κ2 N,N′)cadmium]-di-μ-thioyanate-κ2 N:S;κ2 S:N-[(8-aminoquinoline-κ2 N,N′)cadmium]-di-μ-chlorido]. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, m3. [Google Scholar] [CrossRef]
- Xu, H.; Huang, L.-F.; Guo, L.-M.; Zhang, Y.-F.; Ren, X.-M.; Song, Y.; Xie, J. Three green luminescent cadmium complexes containing 8-aminoquinoline ligands: Syntheses, crystal structures, emission spectra and DFT calculations. J. Lumin. 2008, 128, 1665–1672. [Google Scholar] [CrossRef]
- Cui, P.; Chen, Z.; Gao, D.; Zhao, B.; Shi, W.; Cheng, P. Syntheses, structures, and photoluminescence of a series of three-dimensional Cd(II) frameworks with a flexible ligand, 1,5-bis(5-tetrazolo)-3-oxapentane. Cryst. Growth Des. 2010, 10, 4370–4378. [Google Scholar] [CrossRef]
- Ding, B.; Li, J.; Yang, E.-C.; Wang, X.-G.; Zhao, X.-J. Synthesis, structure, and characterization of a novel one-dimensional tube-like cadmium coordination polymer. Z. Anorg. Allg. Chem. 2007, 633, 1062–1065. [Google Scholar] [CrossRef]
- Jurgens, B.; Irran, E.; Hoppe, H.A.; Schnick, W. Phase transition of a dicyanamide with rutile-like structure: Syntheses and crystal structures of α- and β-Cd[N(CN)2]2. Z. Anorg. Allg. Chem. 2004, 630, 219–223. [Google Scholar] [CrossRef]
- Adhikary, C.; Koner, S. Structural and magnetic studies on copper(II) azido complexes. Coord. Chem. Rev. 2010, 254, 2933–2958. [Google Scholar] [CrossRef]
- Louka, F.R.; Massoud, S.S.; Haq, T.K.; Koikawa, M.; Mikuriya, M.; Omote, M.; Fischer, R.C.; Mautner, F.A. Synthesis, structural characterization, and magnetic properties of one-dimensional Cu(II)-azido coordination polymers. Polyhedron 2017, 138, 177–184. [Google Scholar] [CrossRef]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Vicente, R.; Massoud, S.S. Synthesis and characterization of five new thiocyanato- and yanate-metal(II) complexes with 4-azidopyridine as co-ligand. Polyhedron 2015, 85, 20–26. [Google Scholar] [CrossRef]
- Batten, R.S.; Murray, R.K. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Wang, S. Luminescence, and electroluminescence of Al(III), B(III), Be(II) and Zn(II) complexes with nitrogen donors. Coord. Chem. Rev. 2001, 215, 79–98. [Google Scholar] [CrossRef]
- Li, Y.; Shi, L.; Qin, L.-X.; Qu, L.-L.; Jing, C.; Lan, M.; James, T.D.; Long, Y.-T. An OFF–ON fluorescent probe for Zn2+ based on a GFP-inspired imidazolone derivative attached to a 1,10-phenanthroline moiety. Chem. Commun. 2011, 47, 4361–4363. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Q.; Luo, Z.-D.; Pan, Y.; Singh, A.K.; di Trive, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Liang, X.-X.; Cai, Y.; Wu, J.; Shi, Z.-F.; Kirillov, A.M. Hydrothermal assembly, structures, topologies, luminescence, and magnetism of a novel series of coordination polymers driven by a trifunctional nicotinic acid building block. Dalton Trans. 2017, 46, 10908–10925. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]
- De Silva, A.P.; Fox, D.P.; Huxley, A.J.M.; Moody, T.S. Combining luminescence, coordination, and electron transfer for signaling purposes. Coord. Chem. Rev. 2000, 205, 41–57. [Google Scholar] [CrossRef]
- Alam, R.; Bhowmick, R.; Islam, A.S.M.; Chaudhuri, K.; Ali, M. A rhodamine based fluorescent trivalent sensor (Fe3+, Al3+, Cr3+) with potential applications for live cell imaging and combinational logic circuits and memory devices. New J. Chem. 2017, 41, 8359–8369. [Google Scholar] [CrossRef]
- Aragoni, M.C.; Arca, M.; Bencini, A.; Caltagirone, C.; Garau, A.; Isaia, F.; Light, M.E.; Lippolis, V.; Lodeiro, C.; Mameli, M. Zn2+/Cd2+ optical discrimination by fluorescent chemosensors based on 8-hydroxyquinoline derivatives and sulfur-containing macrocyclic units. Dalton Trans. 2013, 42, 14516–14530. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.S.; Reibenspies, J.H.; Hancock, R.D. Mechanism of “turn-on” fluorescent sensors for mercury(II) in solution and its implications for ligand design. Inorg. Chem. 2012, 51, 10904–10915. [Google Scholar] [CrossRef]
- Deems, J.C.; Reibenspies, J.H.; Lee, H.-S.; Hancock, R.D. Strategies for a fluorescent sensor with receptor and fluorophore designed for the recognition of heavy metal ions. Inorg. Chim. Acta 2020, 499, 119181. [Google Scholar] [CrossRef]
- Lee, H.; Hancock, R.D.; Lee, H.S. Role of fluorophore-metal interaction in photoinduced electron transfer (PET) sensors: Time-dependent density functional theory (TDDFT) study. J. Phys. Chem. A 2013, 117, 13345–13355. [Google Scholar] [CrossRef]
- Rosita, D.; Panunzi, B. The role of zinc(II) ion in fluorescence tuning of tridentate pincers: A Review. Molecules 2020, 25, 4984. [Google Scholar] [CrossRef]
- Oki, A.R.; Bommarreddy, P.R.; Zhang, H.; Nosmane, N. Manganese(II) complex of the ‘tripod’ ligand tris(2-benzimidazolyl-methyl)amine. Five-coordinate and six-coordinate Mn(II) in the crystal structure. Inorg. Chim. Acta 1995, 231, 109–114. [Google Scholar] [CrossRef]
- Anderegg, G.; Wenk, F. Pyridine derivatives as complexing agents. VIII. Preparation of a new quadridentate and a new hexadentate ligand. Helv. Chim. Acta 1967, 50, 2330. [Google Scholar] [CrossRef]
- Tyekla´r, Z.; Jacobson, R.R.; Wei, N.; Murthy, N.N.; Zubieta, J.; Karlin, K.D. Reversible reaction of dioxygen (and carbon monoxide) with a copper(I) complex. X-ray structures of relevant mononuclear Cu(I) precursor adducts and the trans-(µ1,2-peroxo)dicopper(II) product. J. Am. Chem. Soc. 1993, 115, 2677–2689. [Google Scholar] [CrossRef]
- Bruker (2005); SAINT v. 7.23; Bruker AXS Inc.: Madison, WI, USA, 2005.
- Bruker (2006); APEX 2, v. 2.0-2; Bruker AXS Inc.: Madison, WI, USA, 2005.
- Sheldrick, G.M. SADABS v. 2; University of Goettingen: Goettingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure with SHELX. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Speck, A.L. PLATON, a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2001. [Google Scholar]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Mautner, F.A.; Albering, J.H.; Harrelson, E.V.; Gallo, A.A.; Massoud, S.S. N-Bonding vs. S-bonding in thiocyanato-copper(II) complexes. J. Mol. Struct. 2011, 1006, 570–575. [Google Scholar] [CrossRef]
- Köhler, H.; Kolbe, A.; Lux, G. Metall-pseudohalogenide. 27. Zur Struktur der Dicyanamide zweiwertiger 3d-metalle M(N(CN)2)2. Z. Anorg. Allg. Chem. 1977, 428, 103–112. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijin, J.V.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-20 -yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Dahl, E.W.; Kiernicki, J.J.; Zeller, M.; Szymczak, N.K. Hydrogen bonds dictate O2 Capture and release within a zinc tripod. J. Am. Chem. Soc. 2018, 140, 10075–10079. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, E.-X.; Huan, C.-A. Syntheses and characterizations of three new coordination compounds: [M(C9NH7)4(SCN)2] (M = Zn, Cd) and [Zn(C9NH7)2(SCN)2] with a pseudo-merohedral twinning structure. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2014, 44, 1390–1397. [Google Scholar] [CrossRef]
- Xu, H.; Liu, G.-X.; Huang, R.-Y.; Zhao, S.-P.; Kong, X.-J. Synthesis, Crystal Structure, Luminescence Property and DFT Calculations of a New Coordination Compound Containing 8-Aminoquinoline Ligand. Jiegou Huaxue (Chin.) (Chin. J. Struct. Chem.) 2013, 32, 545. [Google Scholar]
- Xu, H.; Huang, L.-F.; Ren, X.-M. Synthesis, crystal structure and fluorescence properties of the green fluorescent complex 8-aminoquinoline cadmium thiocyanate. Wuji Huaxue Xuebao (Chin.) (Chin. J. Inorg. Chem.) 2010, 26, 171. [Google Scholar]
- Li, H.; Zhao, H.Y.; Zhang, S.G. (Dicyanamido)[tris-(2-pyridylmeth-yl)amine]zinc(II) perchlorate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, m495. [Google Scholar] [CrossRef] [Green Version]
- Lamperti, M.; Giani, A.M.; Maspero, A.; Vesco, G.; Cimino, A.; Negri, R.; Giovenzana, G.B.; Palmisano, G.; Mella, M.; Nardo, L. Synthesis and spectroscopic characterization of 2-(het)Aryl perimidine derivatives with enhanced fluorescence quantum yields. J. Fluoresc. 2019, 29, 495–504. [Google Scholar] [CrossRef]
- Schulman, S.G.; Sanders, L.B. Fluorescence, and phosphorescence of 5- and 8-aminoquinoline. Anal. Chim. Acta 1971, 56, 83–89. [Google Scholar] [CrossRef]
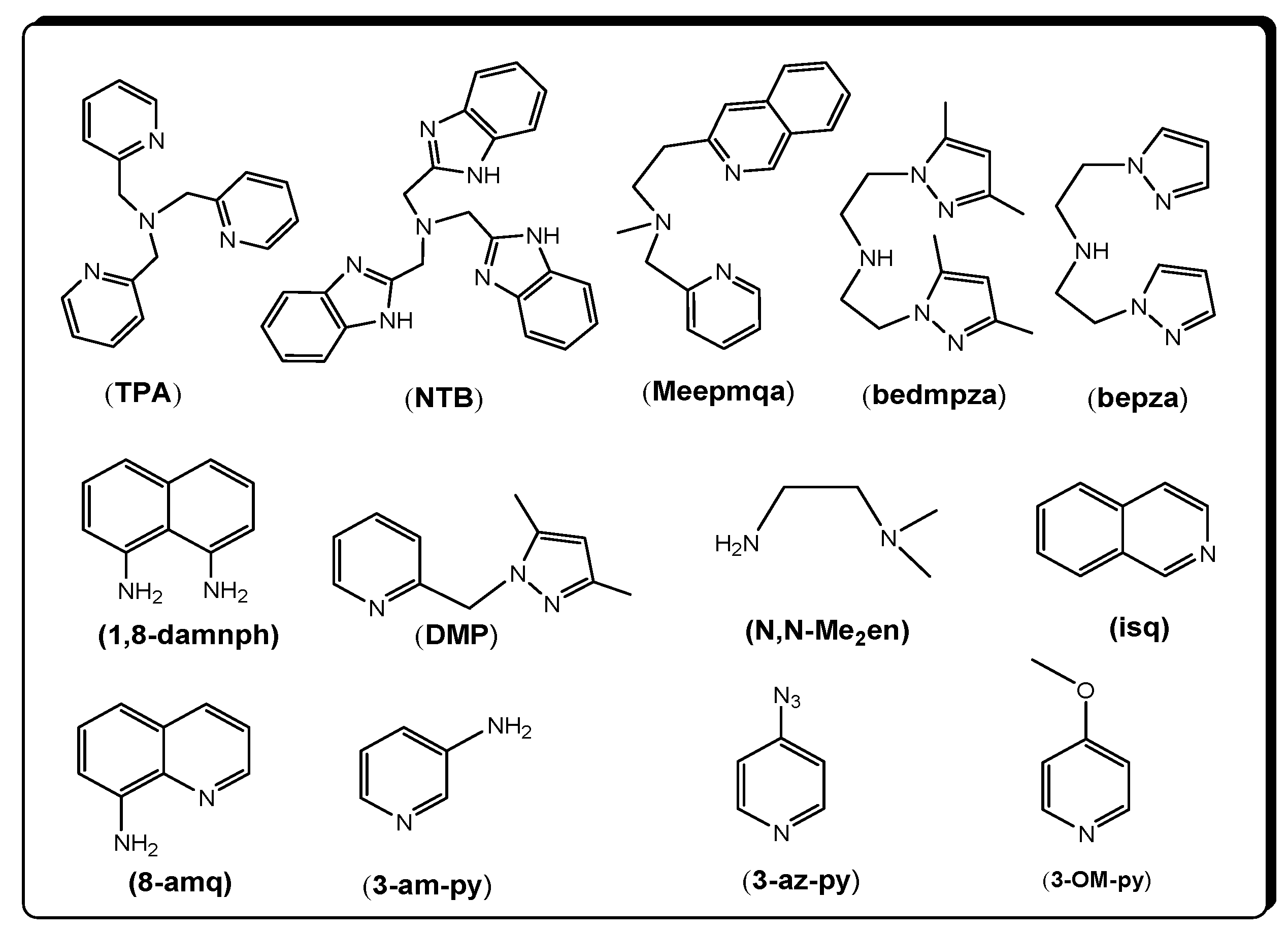
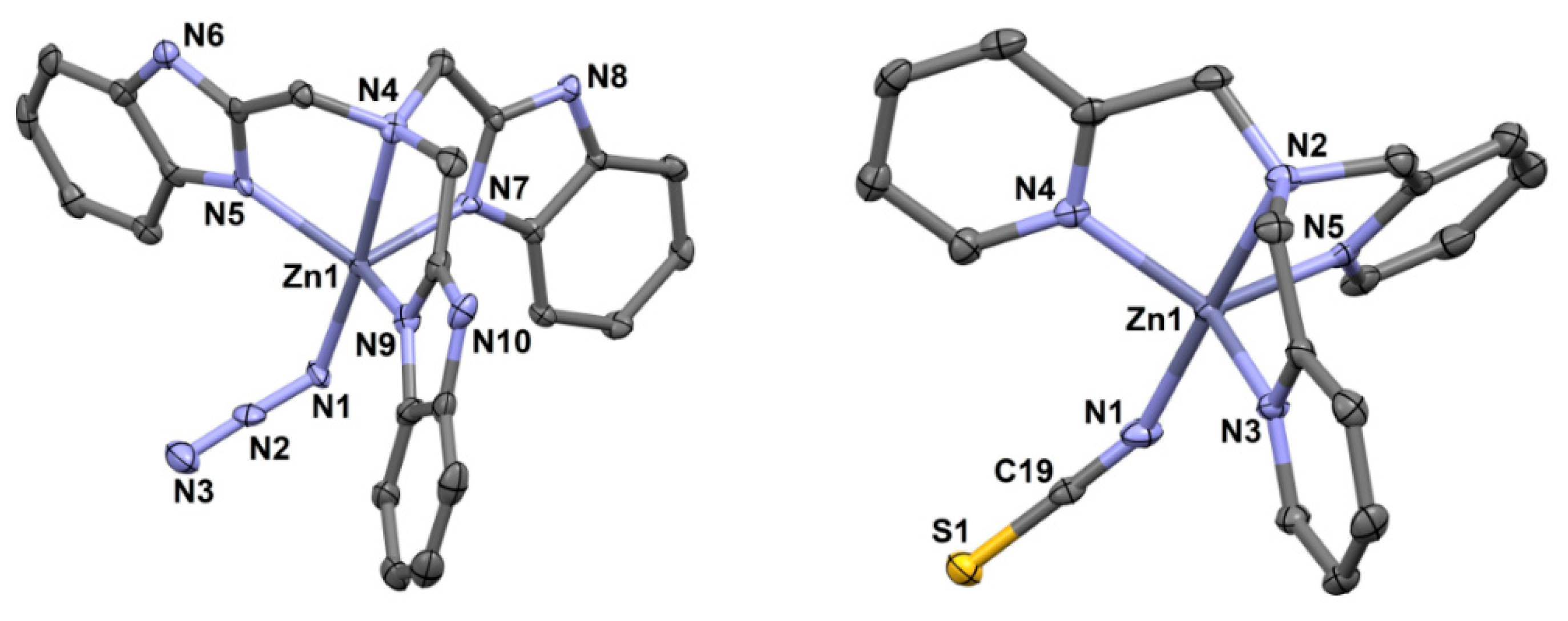




| Compound | 3 | 4 | 5 |
|---|---|---|---|
| Empirical formula | C48H44Cl2N20O9Zn2 | C19H18ClN5O4SZn | C24H20N10Zn |
| Formula mass | 1246.71 | 513.28 | 513.87 |
| System | Triclinic | Monoclinic | Monoclinic |
| Space group | P-1 | P21/c | P21/n |
| a (Å) | 13.7520(9) | 15.2869(9) | 7.4176(4) |
| b (Å) | 13.8644(8) | 9.6319(5) | 11.2397(6) |
| c (Å) | 17.3919(10) | 14.7015(9) | 13.3287(6) |
| α (°) | 99.093(3) | 90 | 90 |
| β (°) | 102.308(3) | 91.591(4) | 98.738(3) |
| γ (°) | 119.270(2) | 90 | 90 |
| V (Å3) | 2689.0(3) | 2163.8(2) | 1098.34(10) |
| Z | 2 | 4 | 2 |
| Dcalc (Mg/m3) | 1.540 | 1.576 | 1.554 |
| θ max (°) | 27.544 | 28.782 | 33.204 |
| Data collected | 24602 | 36586 | 49264 |
| Unique refl./Rint | 12326/0.0642 | 5570/0.0853 | 4201/0.1122 |
| Parameters/Restraints | 725/140 | 280/0 | 176/0 |
| Goodness-of-Fit on F2 | 1.081 | 1.051 | 1.023 |
| R1/wR2 (all data) | 0.0897/0.2605 | 0.0520/0.1274 | 0.0392/0.0826 |
| Residual extrema (e/Å3) | 3.202/−2.305 | 1.97/−0.73 | 0.59/−0.55 |
| Compound | 6a | 7 | 8 |
| Empirical formula | C22H16N10Zn | C22H14N8Zn | C36H72N30Zn3 |
| Formula mass | 485.84 | 455.78 | 1121.34 |
| System | Monoclinic | Triclinic | Monoclinic |
| Space group | P21/n | P-1 | C m |
| a (Å) | 8.8263(3) | 7.406(3) | 13.4600(13) |
| b (Å) | 7.2601(3) | 10.549(4) | 26.939(3) |
| c (Å) | 16.0965(6) | 13.314(5) | 7.4731(7) |
| α (°) | 90 | 102.690(14) | 90 |
| β (°) | 94.614(2) | 90.274(17) | 109.599(6) |
| γ (°) | 90 | 110.278(16) | 90 |
| V (Å3) | 1028.12(7) | 948.1(6) | 2522.8(5) |
| Z | 2 | 2 | 2 |
| Dcalc (Mg/m3) | 1.569 | 1.596 | 1.459 |
| θ max (°) | 33.160 | 33.282 | 30.104 |
| Data collected | 101990 | 76319 | 38697 |
| Unique refl./Rint | 3932/0.0740 | 7224/0.0762 | 7333/0.0436 |
| Parameters/Restraints | 159/0 | 283/0 | 364/50 |
| Goodness-of-Fit on F2 | 1.074 | 1.104 | 1.038 |
| R1/wR2 (all data) | 0.0282/0.0745 | 0.0379/0.0899 | 0.0253/0.0637 |
| Residual extrema (e/Å3) | 0.49/−0.44 | 0.60/−0.70 | 0.72/−0.35 |
| # | Zn(II) or Cd(II) Complex | Pseudoh. Bonding | Geom. | Dim./Nuc. | Ref. |
|---|---|---|---|---|---|
| 1 | [Zn(TPA)(N3)]ClO4 (4a) | monodentate N3− | TBP | mononuclear | [68] |
| 2 | [Cd6(TPA)4(µ1,1,3-N3)4(µ1,1N3)6](ClO4)2·2H2O | µ1,1,3- N3−, µ1,1- N3− | dist. Oh, and PBP | hexanuclear | [13] |
| 3 | [Zn(TPA)(N3)2] | monodentate N3− | dist. Oh, | mononuclear | [68] |
| 4 | catena-[Zn(N,N-Me2en)2(μ1,5-dca)]dca (8) | μ1,5-dca | dist. Oh | CPs,1D | This work |
| 5 | catena-[Cd(N,N-Me2en)(µ1,5-dca)2] | μ1,5-dca | dist. Oh | CPs, 1D double chains | [14] |
| 6 | [Zn(isq)2(NCS)2] | monodentate NCS− | dist. Td | mononuclear | [69] |
| 7 | trans-[Zn(isq)4(NCS)2] | monodentate NCS− | trans-Oh | mononuclear | [69] |
| 8 | trans-[Cd(isq)4(NCS)2] | monodentate NCS− | trans-Oh | mononuclear | [69] |
| 9 | cis-[Zn(8-amq)2(NCS)2] | monodentate NCS− | cis-Oh | mononuclear | [70] |
| 10 | cis-[Cd(8-amq)2(NCS)2] | monodentate NCS− | cis-Oh | mononuclear | [34] |
| 11 | catena-[Cd(8-amq)(μ1,3-NCS)2] | μ1,3-NCS− | dis. Oh | CPs, 1D | [71] |
| 12 | trans-[Zn(1,8-damnph)2(dca)2] (5) | monodentate dca | trans-Oh | mononuclear | This work |
| 13 | trans-[Cd(1,8-damnph)2(dca)2] | monodentate dca | trans-Oh | mononuclear | [14] |
| 14 | [Zn(bedmpza)(N3)]ClO4 | monodentate N3− | dist. TBP | mononuclear | [11] |
| 15 | [Cd(bedmpza)(μ1,1-N3)(N3)]2·1.5H2O | μ1,1-N3−, monodentate N3− | dist. Oh | dinuclear | [11] |
| 16 | [Zn(bepza)(NCS)2] | monodentate NCS− | dist. TBP | monomer | [12] |
| 17 | [Cd2(bepza)2(μ1,3-NCS)2(NCS)2] | μ1,3-NCS−, mono-dentate NCS− | dist. Oh | dinuclear | [12] |
| 18 | [Zn(DMP)(μ1,1-N3)(N3)]2 | μ1,1- N3− | dist. TBP | dinuclear | [28] |
| 19 | catena-[Cd(DMP)(μ1,1-N3)2] | μ1,1- N3− | dist. Oh | CPs, 1D | [28] |
| 20 | catena-[Zn(3-ampy)2(μ1,5-dca)2] | μ1,5-dca | dist. Oh | CPs, 3D | [30] |
| 21 | catena-[Cd(3-ampy)(µ1,3-dca)(µ1,5-dca)] | μ1,5-dca, µ1,3-dca | dist. Oh | CPs, 3D | [30] |
| 22 | catena-[Zn(4-OMP)2(μ1,5-dca)2] | μ1,5-dca | dist. Oh | CPs, 1D | [32] |
| 23 | catena-[Cd(4-OMP)2(μ1,5-dca)2] | μ1,5-dca | dist. Oh | CPs, 1D | [32] |
| 24 | catena-[Zn(4-azpy)2(μ1,1-N3)(μ1,3-N3)] | μ1,1- N3−, μ1,3- N3− | dist. Oh | CPs, 1D | [31] |
| 25 | catena-[Cd2(4-azpy)4(μ1,1-N3)2(μ1,3-N3)2] | μ1,1- N3−, μ1,3-N3− | dist. Oh | CPs, 1D | [31] |
| Complex | λmax Absorption or λex (nm) | λem Emission (nm) | FL QY |
|---|---|---|---|
| [Zn(Meepmqa)(N3)2] (1) | 260 | 373 | 2.62% |
| [Zn(Meepmqa)(dca)]ClO4·½H2O (2) | 260 | 373 | 1.15% |
| [Zn(NTB)(N3)]ClO4·½H2O (3) | 240 | 294 | 0.98% |
| [Zn(TPA)(NCS)]ClO4 (4) | 260 | 450 | 0.67% |
| [Zn(TPA)(N3)]ClO4 (4a) | 260 | 324, 450 | 1.50% |
| [Zn(TPA)(NCS)]NO3·½H2O (4b) | 260 | 450 | 0.50% |
| [Zn(1,8-damnph)2(dca)2] (5) | 330 | 421 | 9.00% |
| [Zn(8-amq)2(dca)]ClO4 (6) | 260 | 323 | 0.25% |
| [Zn(isq)2(μ1,5-dca)2] (7) | 260 | 335 | 0.56% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Fischer, R.C.; Torvisco, A.; Salem, N.M.H.; Dugas, A.R.; Aaron, S.F.; Sahu, S.P.; Louka, F.R.; Massoud, S.S. Stereochemical Geometries and Photoluminescence in Pseudo-Halido-Zinc(II) Complexes. Structural Comparison between the Corresponding Cadmium(II) Analogs. Inorganics 2021, 9, 53. https://doi.org/10.3390/inorganics9070053
Mautner FA, Fischer RC, Torvisco A, Salem NMH, Dugas AR, Aaron SF, Sahu SP, Louka FR, Massoud SS. Stereochemical Geometries and Photoluminescence in Pseudo-Halido-Zinc(II) Complexes. Structural Comparison between the Corresponding Cadmium(II) Analogs. Inorganics. 2021; 9(7):53. https://doi.org/10.3390/inorganics9070053
Chicago/Turabian StyleMautner, Franz A., Roland C. Fischer, Ana Torvisco, Nahed M. H. Salem, Amber R. Dugas, Shelby F. Aaron, Sushant P. Sahu, Febee R. Louka, and Salah S. Massoud. 2021. "Stereochemical Geometries and Photoluminescence in Pseudo-Halido-Zinc(II) Complexes. Structural Comparison between the Corresponding Cadmium(II) Analogs" Inorganics 9, no. 7: 53. https://doi.org/10.3390/inorganics9070053
APA StyleMautner, F. A., Fischer, R. C., Torvisco, A., Salem, N. M. H., Dugas, A. R., Aaron, S. F., Sahu, S. P., Louka, F. R., & Massoud, S. S. (2021). Stereochemical Geometries and Photoluminescence in Pseudo-Halido-Zinc(II) Complexes. Structural Comparison between the Corresponding Cadmium(II) Analogs. Inorganics, 9(7), 53. https://doi.org/10.3390/inorganics9070053









