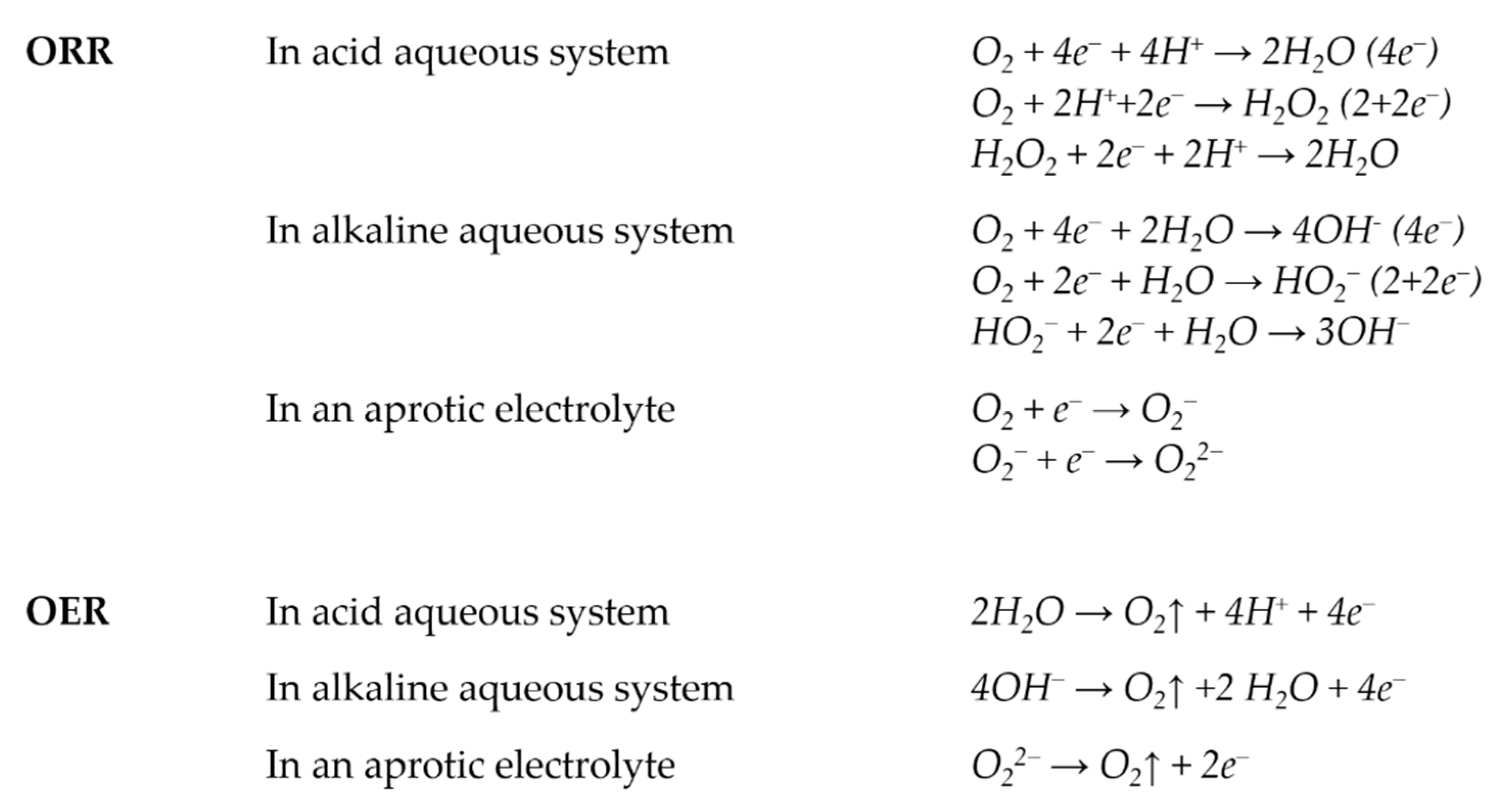
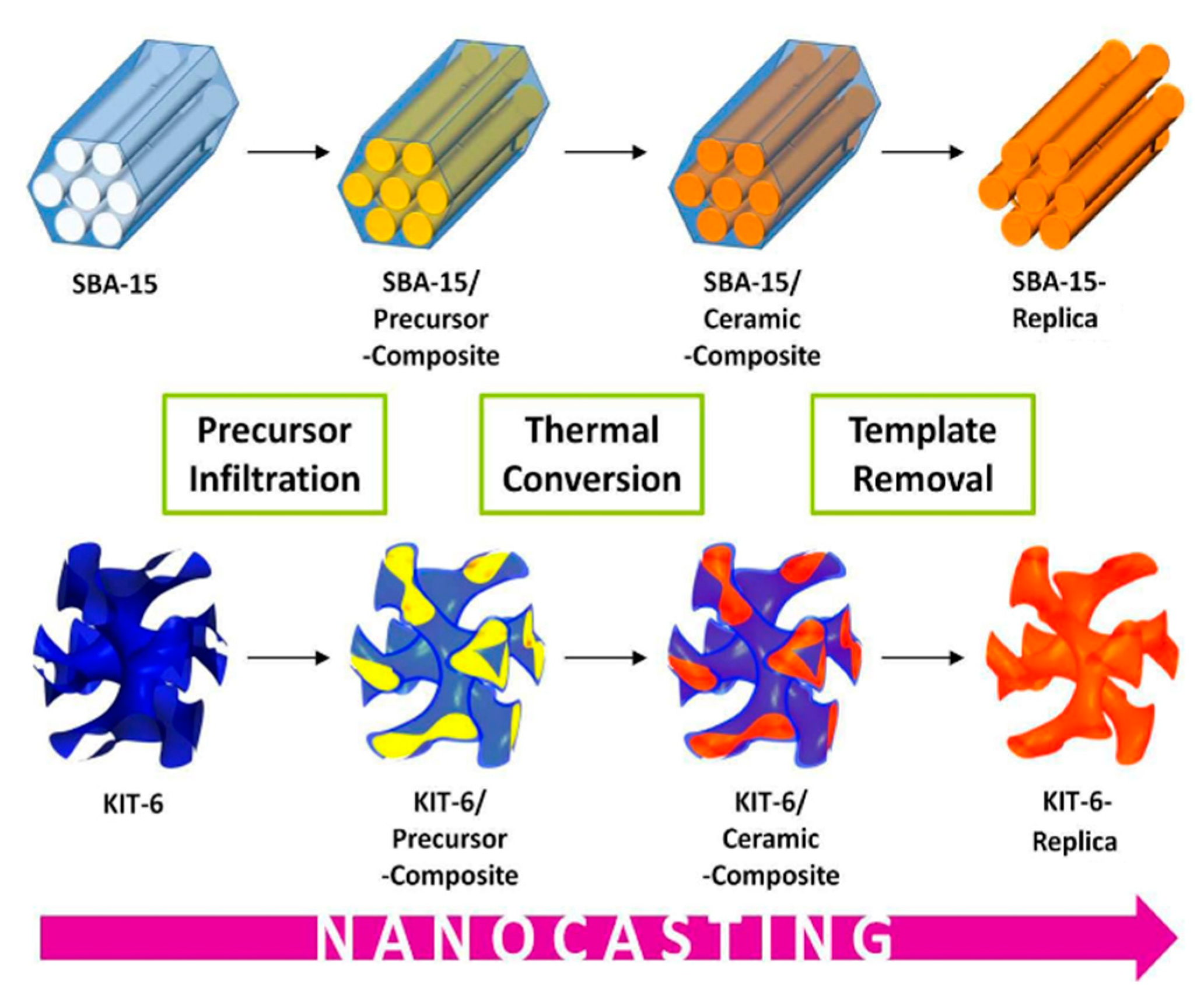
2.2. Application to Water Splitting (OER)
In an early study, Tüysüz et al. [
40] synthesized ordered mesoporous (m)Co
3O
4 from KIT-6 templates aged at different temperatures (35, 100, and 130 °C) via wetness impregnation nanocasting and tested their electrocatalytic activity towards OER at the different pH. The obtained oxides exhibited different specific surface areas (SSAs) (156, 113, and 72 m
2/g for Co
3O
4-35, -100, -130 respectively) and were compared to a bulk Co
3O
4 with a SSA of 2 m
2/g. The catalysts were tested using a three electrode standard cell with rotating disk electrode (RDE). The working electrode (WE) was a gold disk electrode (GE) coated with the catalytic ink (loading of 0.13 mg/cm
2). Pt gauze was used as a counter electrode (CE) and Ag/AgCl was the reference electrode (RE). Catalytic current measurements were performed in aqueous KOH electrolytes. The current density for ordered mCo
3O
4 samples was found to increase with increasing specific surface area (27.2 mA/cm
2 was achieved at 1 V (vs Ag/AgCl) for Co
3O
4-35). Similar results were obtained regarding the oxidative catalytic activity of the mCo
3O
4 as a function of the pH of the electrolyte. Of particular interest, chronoamperometric measurement biased at 0.8 V vs Ag/AgCl at pH = 13 to evaluate the stability of the catalysts showed that the Co
3O
4-35 sample reached a current density of 8 mA/cm
2 and did not exhibit any deactivation over 100 min. Following this, doping of cobalt oxides by various transition metals has been intensively investigated. For instance, Lu et al. [
41] embedded gold nanoparticles (Au NPs, nanoparticles) within mCo
3O
4. The use of Au NPs was motivated by the electronegativity of the metal that generates a stronger electronic field for the adsorption of oxygen, making the generation of Co(IV) species easier and ultimately increasing the overall activity of the Au/Co catalyst [
42,
43]. Among all materials, 2.5 wt % Au/mCo
3O
4 revealed a highly ordered structure with a narrow pore size distribution, high SSA (109 m
2/g) and uniformly distributed Au NPs within the mCo
3O
4. The catalytic performance of this material was then tested with the 3-electrode standard system with Ag/AgCl in 3M KCl as a reference electrode, and modified glassy-carbon RDE as a working electrode. The polarization curves confirmed the improved catalytic activity of the Au-doped cobalt oxide as compared to pure mCo
3O
4. Moreover, the composite demonstrated an excellent stability during 2000 cycles. Remarkably, the calculated turnover frequency of Au/mCo
3O
4 (0.048 s
−1) was much higher than that found for mCo
3O
4 (0.026 s
−1) and other data reported so far.
Increasing the amount of Au NPs was not beneficial since it led to a lower SSA resulting in lower number of reactive catalytic sites and therefore, a decrease in the activity towards OER. Thus, it was rapidly identified that high SSA in the mesoporous replicas was highly desirable for electrocatalytic activity. Following similar reasoning, a complementary work used palladium nanoparticles (Pd NPs) which were dispersed onto mCo
3O
4 [
44]. Pd has a much higher conductivity and has shown a substantially higher catalytic activity for OER as compared to gold. High amount of Co(IV) species were obtained by dispersion of Pd NPs onto a 3D nanostructured Co
3O
4 material obtained via nanocasting of KIT-6 silica. The resulting Pd/Co
3O
4 sample had a SSA of 81 m
2/g and a relatively small mode pore size of 1.1 nm. However, this value was obtained using the Barrett–Joyner–Halenda (BJH) method, which is known to severely underestimate the pore size of materials, especially below 10 nm [
45]. In the same way, the reported pore volume (0.03 cm
3/g) does not seem to be in agreement with the presented physisorption data. Nevertheless, the Pd-modified sample (1:1 wt ratio) demonstrated an enhanced activity towards oxygen evolution by 1.6 times compared to mCo
3O
4, thanks to synergistic effects between the two metals [
46]. Interestingly, the lowest onset potential and the highest current density were obtained for a sample containing half less Pd (50 wt % of Pd), highlighting the importance of such bimetallic systems. One may aim for a high surface over volume ratio in order to maximize the activity of the dopant. Furthermore, long-term chronoamperometry measurements showed an excellent durability in comparison to the pristine mCo
3O
4. All electrochemical measurements discussed above were carried out with a standard three-electrode cell using 0.1 M KOH solution as an electrolyte, a graphite rod as a working electrode, a platinum foil (3.0 cm
2) as a counter electrode, and a saturated calomel electrode (SCE) with a salt bridge as a reference electrode.
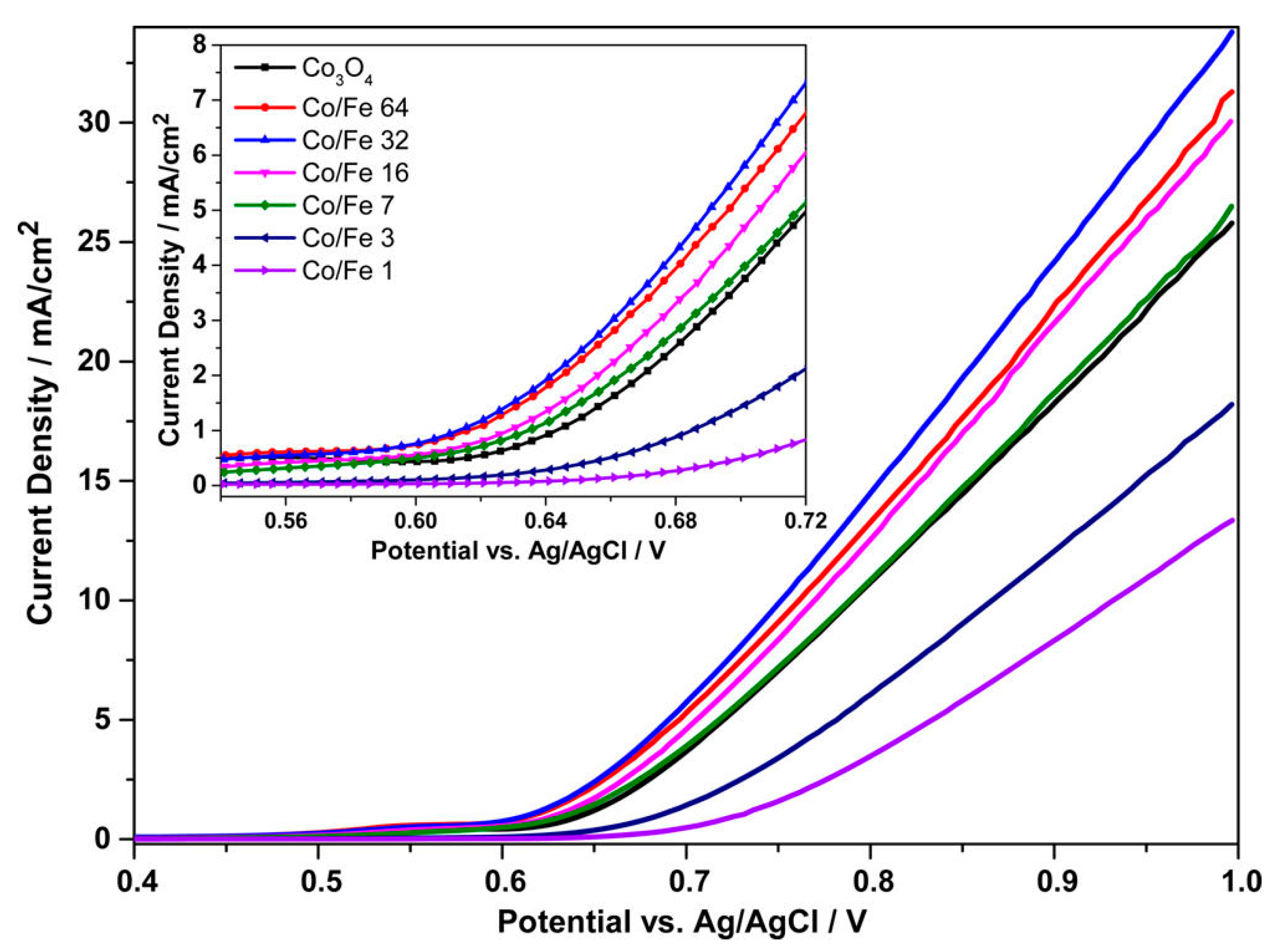
Another example of “doped” cobalt oxides was reported by Grewe et al. [
39]. However, in this study, instead of noble metals, a non-noble transition metal, i.e., Fe, was added. Fe-mCo
3O
4 oxides were prepared via a wetness impregnation protocol using a standard KIT-6 as the template and a solution of cobalt and iron precursors in different ratios. The extent of the pore ordering in the nanocast replicas was found to be inversely correlated with the Fe/Co ratios, i.e., higher ratios led to less ordered structures. All the doped samples had a similar SSA around 120 m
2/g, and a total pore volume in the range of 0.3–0.4 cm
3/g. For the electrocatalytic characterization, a three-electrode cell with Ag/AgCl was used as the reference electrode, Pt wire as the counter electrode, and aqueous KOH solution was chosen as the electrolyte. The working electrodes were fabricated by deposition of the catalytic ink on glassy carbon (GC) electrodes. The catalytic activities towards OER of pure mCo
3O
4 and Fe-doped ones are presented in
Figure 4.
Fe-mCo
3O
4 samples with ratios Co/Fe = 32 and Co/Fe = 64 had the highest catalytic activity and high current density at 1 V (vs Ag/AgCl): 33.8 mA/cm
2 and 31.3 mA/cm
2, respectively. However, the introduction of large amounts of iron (atomic ratio > 0.1) led to the co-formation of CoFe
2O
4 inverse spinel, which resulted in lower catalytic activities. It was thus suggested that fine distribution of Fe atoms within the Co
3O
4 crystal structure was preferential for enhanced catalytic activities, in good agreement with the results obtained for Pd-mCo
3O
4 discussed above. This effect was attributed to a change in electronic structure of the material that might affect the conductivity and charge transfer properties. Accurate control of the dopant metal is thus mandatory for the successful synthesis of a potent electrocatalyst. However, since each metal may present a different behavior and affect the final catalyst pore structure in various ways, it appears difficult to predict the optimal loading and a trial and error approach might unfortunately be applied to find the best composition. In this way, Xiao et al. [
47] also incorporated Fe and/or Ni into the structure of mesoporous Co
3O
4. Similar to Grewe et al. [
39], the catalysts were prepared via wetness impregnation nanocasting using KIT-6 silica as the template. In line with the literature, high-quality replicas of mesoporous cobalt oxide with a cubic pore symmetry were obtained. Further, the effect of the dopant nature onto the pore structure was rationalized. The addition of 10 wt % of Ni into mCo
3O
4 did not significantly change the porous architecture whereas addition of Fe (10 wt %) or Fe-Ni (5 wt %–5 wt %) led to the formation of slit-like pores of broad pore size distribution, as suggested by nitrogen physisorption experiments. Electrocatalytic activity of the resulting oxides was tested using a standard three-electrode cell, a catalyst-loaded glassy carbon working electrode, an Ag/AgCl (3 M KCl) reference electrode and a Pt wire counter electrode. Among all the synthesized catalysts, Fe-mCo
3O
4 demonstrated the highest OER activity, which was also higher than the activity of the Au-mCo
3O
4 system described above [
41]. The enhanced activity was attributed to the synergistic effect between Fe and Co, the changes of the mesoporous structure related with the introduction of Fe and to the increased conductivity as compared to the pristine mCo
3O
4. Unfortunately, no low angle X-ray diffraction (XRD) experiments were performed to further investigate the extent of modifications in the pore structure and ordering, making it hard to conclude on the importance of the pore shape, network structure and pore ordering. The synergistic effect between Fe and Co was indirectly substantiated by the results obtained on Ni-mCo
3O
4 and NiFe-mCo
3O
4. Both materials showed similar lower catalytic activity. Therefore, it was proposed by the authors that the sites occupied by some Ni in the crystal structure suppressed the synergistic effects between Fe and Co. Durability tests demonstrated that the Fe-mCo
3O
4 catalyst retained excellent stability and activity over 450 cycles. Mesoporous oxides nanocasted from KIT-6 silica with variable Co/Ni ratios were further reported by Deng et al. [
48]. The materials had Brunauer–Emmett–Teller (BET) SSAs in the range of 90–110 m
2/g and pore volumes around 0.35 cm
3/g, which is rather typical for the replicas templated from KIT-6 aged at 100 °C [
40,
47]. Moreover, all samples had a narrow pore size distribution centered at around 3 nm. The electrochemical tests were performed in standard three-electrode system using a modified glassy carbon RDE as a working electrode, reversible hydrogen electrode (RHE) as a reference, and Pt wire as a counter electrode. The importance of this work lies in the study of the electrochemical “activation” of Ni-containing oxides, and how the purity of the KOH electrolyte influences the catalytic activity. Indeed, several groups [
49,
50] previously reported that nickel-based oxides/hydroxides could be converted to oxyhydroxide species resulting in a more efficient water oxidation reaction. Higher activity can thus be achieved through an activation procedure. Results published by Deng and co-workers validated this observation [
48]. Linear scanning voltammetry (LSV) polarization curves were collected before each cycle of 50 cyclic voltammetry (CV) runs, ultimately aiming to obtain an invariant LSV curve. The total number of CV runs performed was dependent on the catalyst composition. Still, the activation process was found to shift the overpotential to lower values and increased the catalytic activity for all Ni-containing oxides. The effect was even more pronounced for doped spinel-form oxides while deactivation after the long-term tests was observed for pure mCo
3O
4. The most active sample had a Co/Ni ratio of 4 and exhibited an overpotential η ≈ 336 mV after the activation step, which is among the lowest values reported, to the best of our knowledge, for Ni-Co based oxides tested for OER. Of particular importance, this activation led to a far superior mass activity (≈ 8 times higher as compared to a non-activated sample) and current densities, being stable for 14 h. No changes in the mesoporous ordering and crystallinity were noted after the electrochemical measurements, confirming the appreciable stability of the material. Furthermore, impedance spectroscopy demonstrated that the transfer resistance of this electrocatalyst was almost divided by 5 times after activation, which indicates a significant enhancement of oxygen evolution kinetics. For comparison purposes, a sample with the same composition (Co/Ni = 4) was also synthesized using a silica gel as a template. The activation process had a similar effect on the disordered mesoporous oxide, confirming that the activation process is related to the crystal structure and not dependent on the mesoporous ordering of the material. However, nickel cobalt oxide with an ordered mesoporous structure still exhibited higher activity as compared to the disordered one, highlighting again the impact and the importance of an ordered/easily accessible pore structure, e.g., 3D pore network. The synthesis of mesoporous mixed transition metal oxides based on Ni and Fe (NiFe
2O
4, NFO) was also reported by the group of Du [
51]. The authors used a defect engineering strategy to increase the electrocatalytic activity towards OER. All electrochemical measurements were performed in a standard three-electrode system where saturated Ag/AgCl and platinum wires were used as a reference and counter electrodes, respectively. A modified glassy carbon RDE was used as the working electrode. Catalysts were prepared by a one-step impregnation nanocasting method using SBA-15 as a template combined with a reduction under gas at different temperatures [
51]. As expected, higher reduction temperatures led to mesoporous oxides with lower SSA (from 261 m
2/g to 165 m
2/g). One of the most interesting point of this study was the in-depth investigation of the mesoporous oxide structures with extended X-ray absorption fine structure (EXAFS) and X-ray absorption near-edge structure (XANES) analyses that clearly revealed the existence of oxygen vacancies in the samples reduced at high temperatures, i.e., 280, 300, and 320 °C (NFO-280, -300, -320). In addition, using X-ray photoelectron spectroscopy (XPS), oxygen species were shown to preferentially chemisorb at the surface of the NFO defect centers. These results were directly correlated with the electrocatalysis behavior of the materials since all the defective NFO catalysts exhibited higher activity towards OER, i.e., smaller overpotentials and onset potentials, as compared to the defect-free sample (NFO-0). The mesoporous oxide NFO-320 exhibited the highest specific mass and area activities at an overpotential of 0.35 V, i.e., 17.5 A/g and 0.106 A/m
2, being ≈ 23 times and ≈ 36 times higher than the values obtained for NFO-0, respectively. Defective NFO-320 showed an excellent durability for 40 h combined with a conservation of its lattice structure and a good stability over time. Thus, it can be concluded that defect engineering is desirable as it leads to increased amount of the active sites and higher conductivities, ultimately enhancing the catalytic activity towards OER. Moreover, considering that these NFO samples were replicated from a 2D silica template, i.e., SBA-15, and the results described earlier in this paragraph, it would be interesting to apply this approach for NFO materials made from a 3D mold structure.
In addition to transition (bimetallic) oxides, transition metal phosphides have also been investigated as highly affective catalysts towards OER [
52]. The first synthesis of a family of bimetallic Co–Ni phosphides (CoNiP) was done in 2016 by Fu et al. [
53]. Samples were nanocasted via a wetness impregnation procedure from KIT-6 silica and all exhibited ordered mesoporous structures with a pore size around 8 nm regardless of the stoichiometry (Co
3NiP, CoNi
3P, CoNiP), demonstrating the versatility of the nanocasting process. All electrochemical measurements were performed in a standard three-electrode system using a Pt foil and a saturated calomel electrode (SCE) as the counter electrode and the reference electrode, respectively and a coated glassy carbon RDE as a working electrode. Studies of single metal phosphides had shown that CoP exhibited even better activity than commercial RuO
2, which is considered to be the reference catalyst for OER [
54]. To probe the effect of the chemical composition, bimetallic samples with different Co/Ni ratios were investigated in the same way as single metal phosphides. The sample prepared using precursors with a molar ratio of Co/Ni = 3 had the lowest onset potential among all the obtained samples and RuO
2, as well as the highest activity for OER (225.5 mA/cm
2 at 1.65 V). The kinetics, evaluated from the Tafel slope, were also found to be better for the Co
3NiP catalyst as compared to the commercial RuO
2. Moreover, the sample showed excellent stability during 12 h. Overall, the use of bimetallic phosphides catalysts allowed a 60% current enhancement while conventional RuO
2 showed a 37% loss on the current density. The authors proposed that the superior catalytic activity of Co
3NiP was related to the lower reaction-free energy caused by the introduction of bimetallic phosphides and a properly tuned composition.
As discussed above, the hard-templating approach appears to be an efficient and flexible method to synthesize functional mesoporous electrocatalysts based on transition metals, with narrow pore size distribution and high SSA. However, one of the main drawbacks of this method is the low amount of produced materials. Therefore, it is mandatory to scale up the process to truly evaluate its potential and limitations. In this way, Deng et al. [
55] presented a method to use spent tea leaves (STL) for the preparation of cobalt-based mixed oxide nanocrystals as catalysts for OER. Doped (Cu, Ni, Fe, and Mn, M/Co = 1/8) nanocast mCo
3O
4 were synthesized in a larger amount, i.e., 8 g, using a wet impregnation technique. XRD analyses showed that the obtained materials had spinel structures with different lattice parameters depending on the doping metal used. The calculated BET SSAs were all in the range of 34–63 m
2/g, in good correlation with the crystal size calculated from powder XRD. It is important to note that the materials obtained from the scaled-up synthesis protocol exhibited similar textural and structural characteristics as compared to the samples obtained using the “usual small scale” process. This approach was also shown to be versatile as the authors were able to synthesize relatively high amounts of mCo-based oxides starting from five different types of tea leaves. Electrocatalytic activity was tested in a standard three-electrode system with coated glassy carbon (GC) RDE, RHE and Pt wire as a working, reference, and counter electrodes, respectively. At the exception of the Mn doped sample, Ni-, Cu-, and Fe-mCo oxides demonstrated enhanced catalytic activity, i.e., lower overpotential of 10 mA/cm
2 and improved kinetics according to the Tafel slope, in comparison to pristine mesoporous and bulk Co
3O
4 oxides. mCo oxides synthesized from different tea leaves exhibited an enhanced activity as well, and samples with higher BET SSA showed increased activity, in good agreement with previously reported results [
40,
56]. Although the presented electrocatalytic results were promising, the most appealing aspect of this work still remains the possibility to use the nanocasting method to synthesize catalytic materials (for fuel cells) in larger amounts. Yu et al. later extended their work to Ni/Fe-based oxides for OER [
57]. Variable Ni/Fe ratios were investigated in order to find an optimal composition regarding the electrocatalytic activity. The optimal catalyst (Ni/Fe = 32/1) performed with a high activity (current density after activation is 32.4 mA/cm
2 at 1.7 V vs RHE), a small reaction resistance, large exposed electrochemical surface area (ECSA) and a superior intrinsic activity due to the introduction of Fe. Aiming to demonstrate the industrial applicability of their process, the authors fabricated a catalyst-coated nickel foam electrode, which exhibited large current density, and an activity maintained over 2 days. Finally, these two additional reports confirmed the essential role of the stoichiometry in the electrocatalytic activity of the mixed metal oxides. Here also, the trial and error/screening approach appeared as mandatory to investigate the different metals and isolate the best composition. One may keep in mind that such experiment design is unfortunately quite time and resource consuming. Also, since it may be metal-dependent, it is difficult to draw clear conclusions regarding the role of the pore structure ordering as well as the optimal pore size or pore size range for the application in OER.
2.3. Prospects for Applications in Metal–Air/Ion Batteries (Bifunctional; Only ORR)
Bifunctional catalysts are interesting for metal–air/oxygen batteries since they might reduce the production costs and simplify the manufacturing process. During the last years, various studies were reported regarding the use of single or mixed metal oxides for both OER and ORR. For instance, Sa et al. [
56] prepared two mCo
3O
4 using wetness impregnation nanocasting and KIT-6 silica aged at different temperatures, i.e., 35 and 100 °C, as templates. The BET SSAs for the mCo
3O
4-based samples were 114 m
2/g and 135 m
2/g, for materials aged at 100 and 35 °C, respectively. The resulting electrocatalytic activity of the nanocast mCo
3O
4 was then compared to the one of Co
3O
4 nanoparticles synthesized via a hydrothermal method and with Ir loaded (20 wt %, incipient wetness) Ketjen black carbon. A three-electrode cell with glassy-carbon RDE coated with catalytic ink was used as a working electrode, Hg/HgO as a reference electrode, and Pt-wire as a counter electrode. The catalyst loading on the WE was set at 0.1 mg/cm
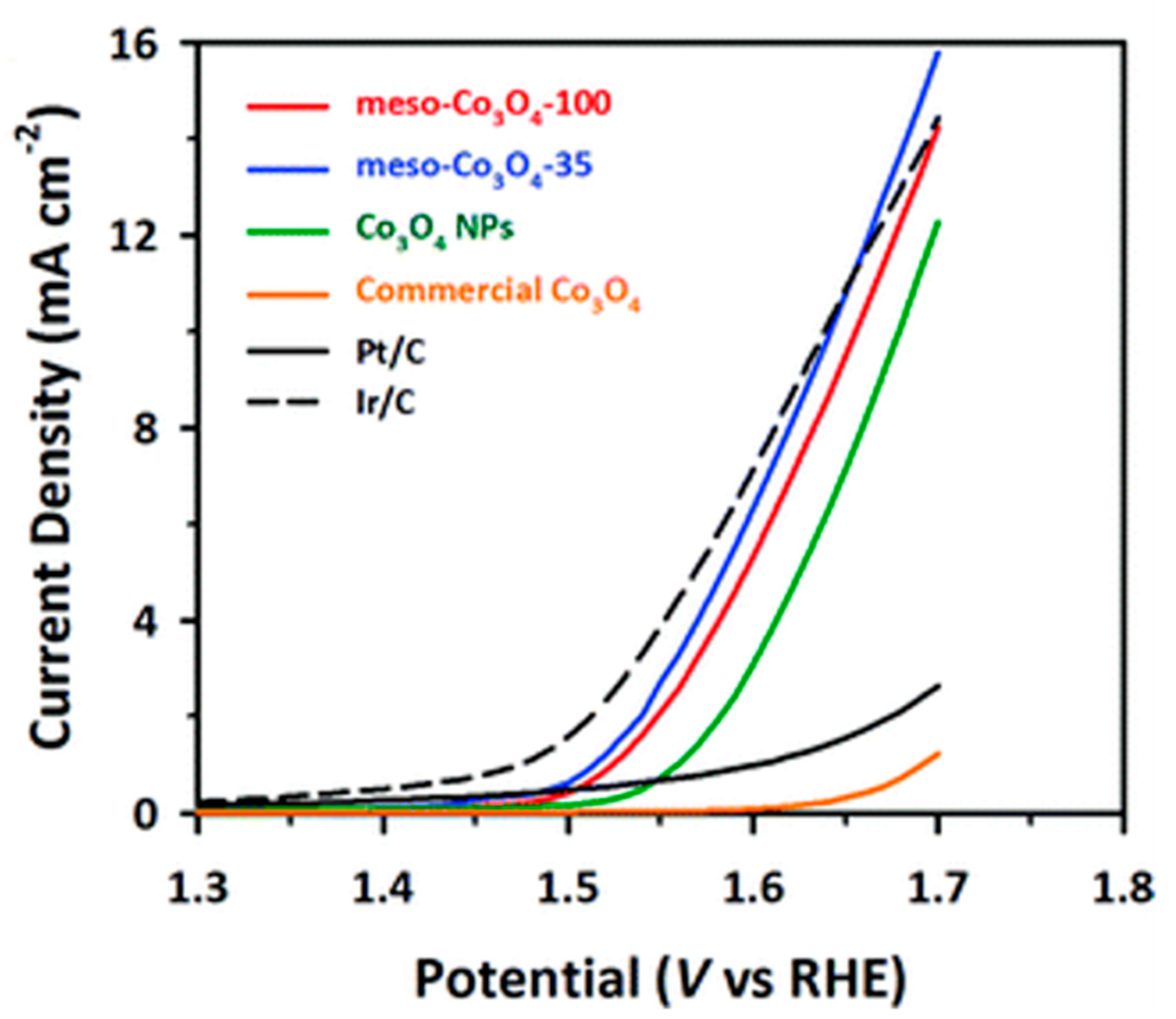
2. The OER activities of the catalysts were measured using a RDE in a 0.1 M KOH electrolyte solution with LSV from 1.0 to 1.7 V (vs RHE) at a scan rate of 5 mV/s. As shown in
Figure 5, mCo
3O
4 was more active towards OER as compared to bulk and Co
3O
4 NPs as well as Pt/C. It also showed current densities close to those obtained with Ir/C. Moreover, the activity was enhanced with the mesoporous samples exhibiting higher specific surface areas, which correlates well with the results of Tüysüz and coworkers [
40]. Interestingly, the two mCo
3O
4 catalysts showed nearly identical polarization curves for ORR, i.e., the onset and half-wave potentials values were around 0.75 V and 0.62 V, respectively, and curves had well-defined plateaus corresponding to diffusion-limiting current at around 5.5 mA/cm
2. These results clearly indicate that the mesoporous cobalt oxides had good catalytic activities towards both ORR and OER, ultimately improving the kinetics of these reactions. Regarding the catalyst stability, the OER activity (at 1.65 V) of mCo
3O
4-35, i.e., the sample nanocasted from a KIT-6 aged at 35 °C, showed a 29% loss of activity only after 1500 CV cycles while Co
3O
4 NPs showed a much more severe degradation, i.e., an activity loss of 41%. This result was postulated to be related with the 3D interconnected pore structure of mCo
3O
4-35 that may improve the durability via a better retention of the original structure after the cycling. Unfortunately, it was not really possible to conclude on the effect of the pore size of the starting template since the mCo
3O
4-100 material was not fully compared to its analogue synthesized at 35 °C. However, since mCo
3O
4-35 exhibited a higher SSA, it may explain its superior electrocatalytic and durability behavior. In a similar study, using MnO
2 this time, Selvakumar et al. [
58] synthesized 2D and 3D mesoporous oxides from a variety of different templates, i.e., MCM-41, SBA-15, MCM-48 and KIT-6. The resulting ordered mesoporous samples had moderate SSAs (28–50 m
2/g) and pore volumes in the range of 0.1–0.8 cm
3/g, with the highest values obtained for the replica of the KIT-6 silica. Moreover, it was demonstrated that the replication process was more successful for materials exhibiting larger mesopores, i.e., SBA-15 and KIT-6. All electrochemical tests were performed in a standard three-electrode system with R(R)DE as a working electrode, Ag/AgCl as a reference electrode, and Pt wire as a counter electrode. Among all the samples, 3D mMnO
2 showed enhanced catalytic activity towards both OER and ORR in comparison to bulk and nanocast materials based on a 2D template. These results, being in line with the observations discussed above, may be explained by the enhanced electronic mobility and favored mass transport of charges in 3D materials compared to their 2D analogues. The stability tests performed on 3D materials also revealed a better durability (8000 s) for MnO
2-KIT-6 as compared to MnO
2-MCM-48 (less than 5000 s). The durability of MnO
2-KIT-6 was comparable to the one of commercial IrO
2. Overall, 3D ordered mesoporous manganese oxide, nanocasted from “large pores” silica showed excellent and promising bifunctional performances towards ORR and OER. However, it is difficult to conclude on the effect of the pore size from this study, except for the ease of replication, since the mesoporous oxides nanocasted from MCM-48, MCM-41 and KIT-6 silicas all exhibited a similar pore size of 6.2 nm, whereas the one made from SBA-15 had bigger pores, i.e., about 9 nm. It remains that replicas made from large pore silica, SBA-15 and KIT-6, resulted in materials with larger pore volumes. Therefore, according to the results presented by the authors, the optimal combination for efficient nanocasting seems to be the use of a 3D silica template having sufficiently large mesopores and pore volume, typically, KIT-6 and related materials.
In comparison to other metal oxides, cobalt oxides have still drawn the most promises because of their reasonably high catalytic activity, ease of preparation and good chemical stability. However, cobalt is potentially toxic and is relatively costly. Combined with the fact that cobalt is now included in the list of critical raw materials [
59], there is a strong interest to at least partially replace some Co in Co
3O
4-based materials by cheaper and more ecofriendly metals. In this direction, Li et al. [
60] reported the synthesis of 3D ordered mZnCo
2O
4 (3DOM (three-dimensional ordered mesoporous) ZnCo
2O
4) via a wetness impregnation approach, using KIT-6 silica as a template and Zn and Co nitrates as precursors. The obtained material had a relatively high BET SSA of 127 m
2/g with a narrow pore size distribution, centered at 3.4 nm. In contrast to most studies reported in the literature, the resulting catalysts were incorporated in Li-O
2 coin batteries (2025 type) in order to perform advanced and more realistic electrochemical tests. The oxygen cathodes were prepared by coating a current collector with the homogenous catalytic ink (1.1 mg/cm
2 loading). An aprotic electrolyte, i.e., 1 M LiCF
3SO
3 in tetraethyleneglycoldimethylether (TEGDME), was used and the discharge/charge tests were carried out under an oxygen atmosphere. Specific capacities of 6024, 3710, 3119, and 1674 mA·h/g were obtained for Li-oxygen batteries containing 3DOM ZnCo
2O
4 at 100, 200, 500, and 1000 mA/g, respectively. These values are much higher than the ones reached with pure carbon at all investigated current densities. In addition, it was observed that the ZnCo
2O
4 cathode could be discharged and charged stably for at least 27 cycles above 2.0 V, whereas the carbon electrode was stable only during 7 cycles (at 100 mA/g under a specific capacity limit of 500 mAh/g). Here also, these encouraging results were thought to be related with the enhanced catalytic activity of spinel-type ZnCo
2O
4 and its 3DOM large pore structure. Furthermore, XPS analyses showed the apparent reversible formation and decomposition of Li
2O
2 during charge and discharge cycles onto the carbon electrode. SEM observations further confirmed some differences between the electrode surface before and after the discharge process. From these data, the authors concluded that the process of formation and decomposition of Li
2O
2 was reversible. Following this, a more promising composition, i.e., spinel CuCo
2O
4, was proposed by the same group [
61]. The electrocatalytic tests were conducted on a battery constituted of an oxygen cathode (a current collector coated with the catalytic ink), a glass filter separator, a non-carbonate electrolyte containing 1M LiCF
3SO
3 in TEGDME and a metallic lithium foil anode. The CuCo
2O
4/carbon electrode exhibited a higher cathodic peak voltage, a lower anodic onset potential, and much larger cathodic and anodic currents in comparison to the pure carbon electrode. More importantly, the specific capacity for the Li–O
2 battery with CuCo
2O
4 electrode was again much higher than the one observed for the pure carbon electrode, i.e., 7456, 3606, and 2692 mA·h/g at 100, 200, and 500 mA/g, respectively. An energy density of 1350 W·h/kg (500 mA·h/g × 2.7 V) could be achieved, which is about twice the energy density of commercial cathodes in Li-ion batteries (640 W·h/kg, 160 mA·h/g × 4 V).
Oxides based on both cobalt and nickel were applied as highly active catalysts towards OER. In 2014, Li et al. [
62] synthesized a 3DOM NiCo
2O
4 from a KIT-6 template using the wetness impregnation technique. The obtained material, exhibiting a specific surface area of 95 m
2/g and bimodal mesopores of about 3.5–5.0 and 7.5–8.0 nm, was then tested as a bifunctional catalyst towards both ORR and OER (with inks containing variable oxide wt %). A swagelok cell with an air hole of 10 mm diameter was used. The electrolyte was made out of 1 M LiCF
3SO
3 in TEGDME. A metallic Li foil with a thickness of 0.50 mm was used as the anode, and a carbon paper coated with the catalytic ink was used as the cathode. Higher current densities, higher ORR and lower OER onset potentials were obtained for most 3DOM NiCo
2O
4/carbon electrodes as compared to the classical carbon one. The electrodes with 0 wt% (pure carbon), 20 wt %, 45 wt % and 70 wt % mNiCo
2O
4 showed specific capacity of 3993, 4357, 4120 and 1881 mA·h/g, respectively. Moreover, the addition of mNiCo
2O
4 increased the round-trip capacity efficiency of Li-O
2 batteries from 23.2% (for the pure carbon) to 78% (for the mixture). The optimal ink, containing 20% mNiCo
2O
4, not only provided the best specific capacity, but also a promising capacity retention rate after 5 cycles, i.e., 41.9%. Ren et al. reported similar research on ordered mNiCoMnO
4 [
63] synthesized via the wetness impregnation method and NiMn
2O
x [
64] synthesized via the two-solvent method, using KIT-6 as a hard template in both cases. The authors expanded on the synthesis conditions and explored the effect of the annealing temperature (200, 550, 700, and 800 °C for NiCoMnO
4 and 600 and 800 °C for NiMn
2O
x) on the properties of the metal oxides. Most of the resulting catalysts exhibited SSAs in the range of 100–150 m
2/g and pore volumes between 0.21 and 0.26 cm
3. One particular material, NiMn
2O
x-600, showed a rather large pore volume of 0.48 cm
3/g (with a BET SSA of 143 m
2/g). When compared to its analogue annealed at 800 °C in CV, NiMn
2O
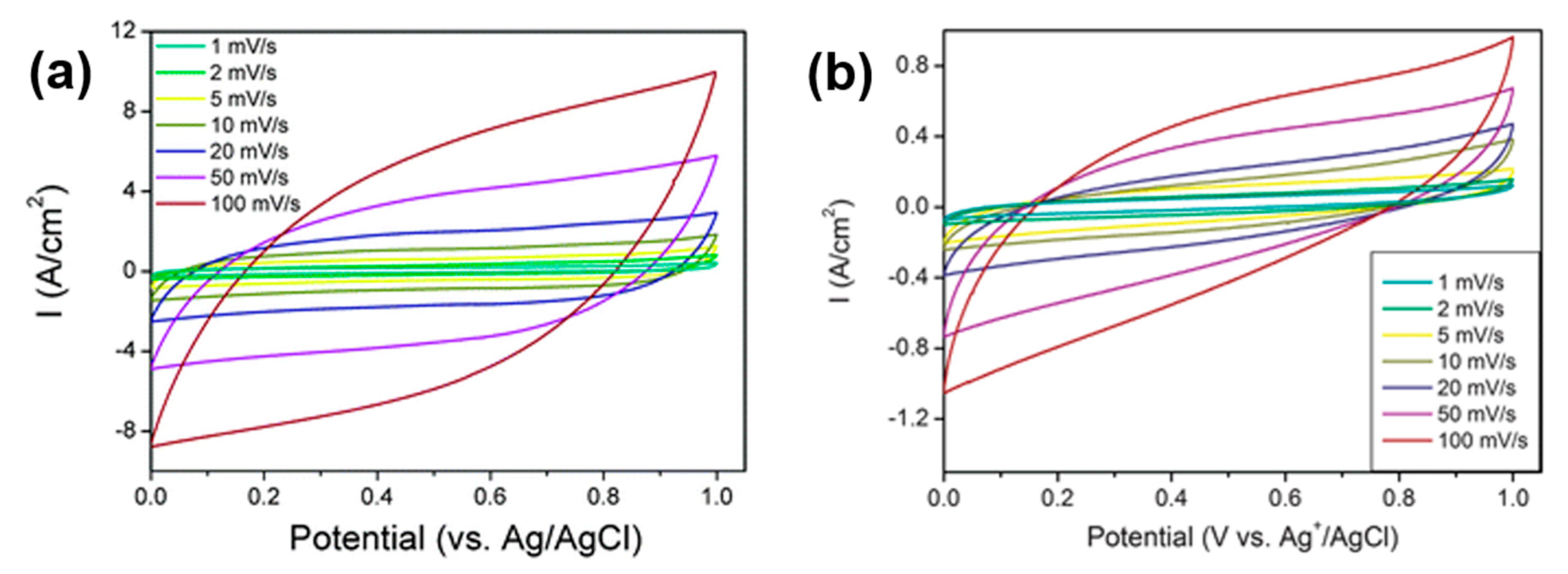
x-600 reached much higher current densities (
Figure 6a,b). Moreover, the capacitance of NiCoMnO
4-800 at 1 mV/s was about 2/3 of the value of NiMn
2O
x-600, i.e., 90 F/g. These data were recorded using a three-electrode cell at room temperature. The catalytic inks were pressed onto nickel foam current-collectors to make the electrodes (mass loading 1.0 mg/cm
2). A mixture of 0.1 mol/L Na
2SO
4 was used as the electrolyte. Platinum foil and an Ag/AgCl electrode were used as the counter and reference electrodes, respectively. Here, it is interesting to discuss the role of specific surface area. Indeed, when the capacitance values are normalized vs. the specific surface area, the two materials have almost the same capacitance, i.e., 0.67 F/m
2 for NiCoMnO
4-800 and 0.63 F/m
2 for NiMn
2O
x-600. Therefore, being able to produce stable and durable ordered mesoporous catalysts with high SSA and 3D porosity appears as a key objective to reach optimal efficiency in both OER and ORR. To further investigate the effect of porosity, Li et al. [
65] synthesized four KIT-6-like templates with different acid concentrations, i.e., 0.5–1.5 M HCl, and different aging temperatures, i.e., 40–130 °C. The NiFe
2O
4 nanocasts obtained from these templates were mixed in a catalytic ink and then coated on a carbon paper (loading of 1.0−1.2 mg/cm
2). 1M LiCF
3SO
3−TEGDME was used as an electrolyte. LSV was conducted in a three-electrode electrochemical cell with the previously mentioned oxygen electrode as the working electrode and lithium metal foils as the counter and reference electrodes. Again, all 3DOM NiFe
2O
4/carbon electrodes exhibited higher activity towards both ORR and OER as compared to the standard carbon electrode. Interestingly, the activity of 3D ordered mNiFe
2O
4 gradually increased as a function of the pore size and pore volume. The specific capacities of Li−O
2 batteries with 3DOM NiFe
2O
4 catalysts were all above 10,000 mA·h/g
C, which is much higher than the value obtained for the standard carbon electrode, i.e., 4454 mA·h/g
C. The best catalyst was found to be that with the largest pore size and pore volume but exhibiting the least ordered structure. All BET SSAs were in the range of 90–123 m
2/g. From this work, it thus appears that a high SSA only is not sufficient for a catalyst to be efficient. Clearly, the role of the pore structure, i.e., 3D vs. 2D, and its mode pore size are also important parameters that cannot be overlooked. The impact of mesostructured ordering is however still unclear. Yet, from the current state-of-the-art, it seems that the most promising catalysts are again based on a 3D structure, with a BET SSA around 100–150 m
2/g and a relatively large pore volume.
Nanoporous carbons are intensively investigated as catalysts that are expected to be highly active in ORR. They are usually highly porous with large accessible surface area, high conductivity and stability, and offer large interfacial area for the reactions, faster charge transport and short diffusion path for the oxygen gas. All these appealing properties make ordered mesoporous carbons (OMC) prospective catalysts for ORR. Doping carbon with N, P, S, or metal atoms is a common way to improve their catalytic activity. Unfortunately, the synthesis of a transition metal- and nitrogen co-doped mesoporous carbon (M-N-MC) is challenging using a classical nanocasting approach due to the limitations associated with the precursors, e.g., poor solubility of metal-macrocyclic complexes in most solvents and poor wettability of the precursor solution on the pore surface of the silica template [
66]. Nevertheless, Tang et al. [
66] reported a novel pathway to do this, i.e., the sublimation and capillary-assisted nanocasting method (SCANC). In this work, SBA-15 silica was used as a template and metallophthalocyanines (MPc, wherein M = Fe, Co, Ni or Cu) were used as carbon precursors. The authors compared the catalytic activity of Fe-N-MC pyrolized at different temperatures and once the optimal one was found, i.e., 700 °C, they synthesized M-N-MC with different metals and compared their catalytic activities. Elemental analysis, XPS and XRD showed that all elements were uniformly distributed throughout the porous carbon framework for Fe-N-MC obtained at 700 °C. This particular OMC exhibited a highly ordered porous structure with high SSA (988 m
2/g), narrow pore size distribution (3.5 nm) and large pore volume (0.87 cm
3/g). Electrochemical tests were carried out in a standard three-electrode cell with coated RDE as a working electrode, Ag/AgCl or Hg/HgO and nickel mesh as a reference and counter electrodes, respectively. Finally, a cell with a serpentine flow field with gas diffusion electrode was used as a working electrode. All M-N-MC demonstrated an excellent activity towards ORR. It is to be noted that for the first time, to the best of our knowledge, a nanocast catalyst based on non-noble metals, i.e., Fe-N-MC-700, exhibited substantially better ORR activity compared to commercial Pt/C. More importantly, the great potential of these catalysts in rechargeable zinc-air battery applications was established. Song et al. [
67] performed a one-step synthesis of sulfur and nitrogen co-doped OMC using SBA-15 silica as a template. The resulting SN-OMC material pyrolyzed at 900 °C reached high catalytic activity towards ORR, being comparable to commercial Pt/C catalysts, with a preferable 4-electron reaction process. Moreover, the samples showed excellent stability during 40,000 s (with an excellent remaining current density of 93%), which was far better than the commercial Pt/C catalyst (77% remained). However, so far, all reported studies used SBA-15 silica, a mainly 2D pore structure, as the starting template. Considering the results discussed above, it would appear beneficial to use a material with a 3D pore network and relatively large mesopore size and pore volume as a template, such as KIT-6 silica.