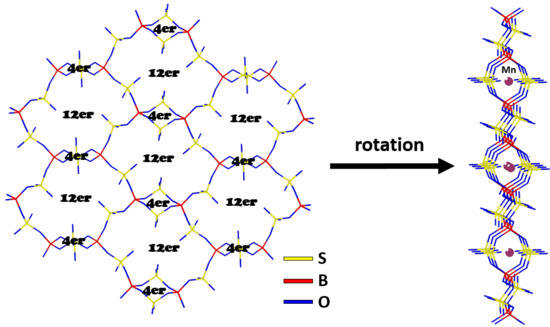
M[B2(SO4)4] (M = Mn, Zn)—Syntheses and Crystal Structures of Two New Phyllosilicate Analogue Borosulfates
Abstract
1. Introduction
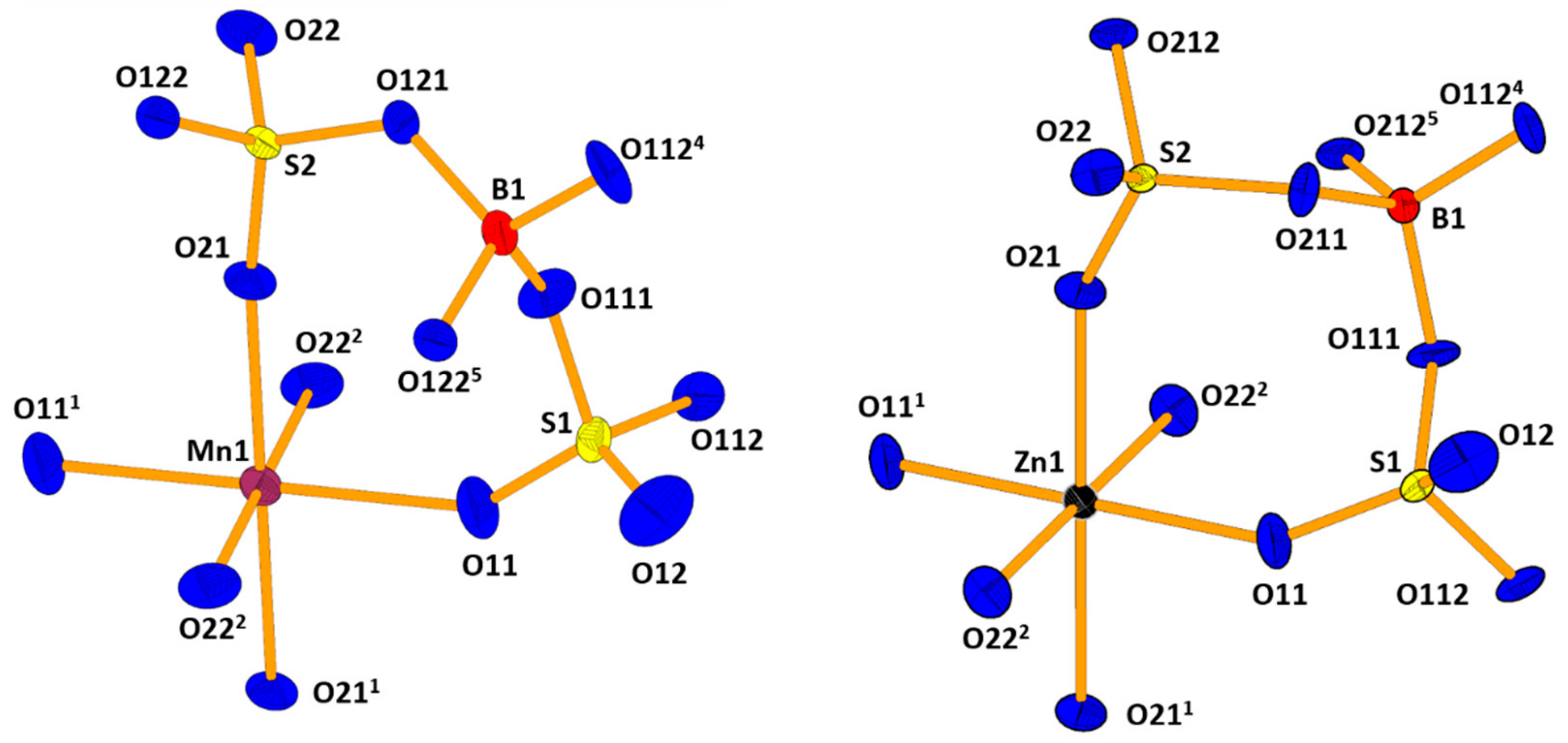
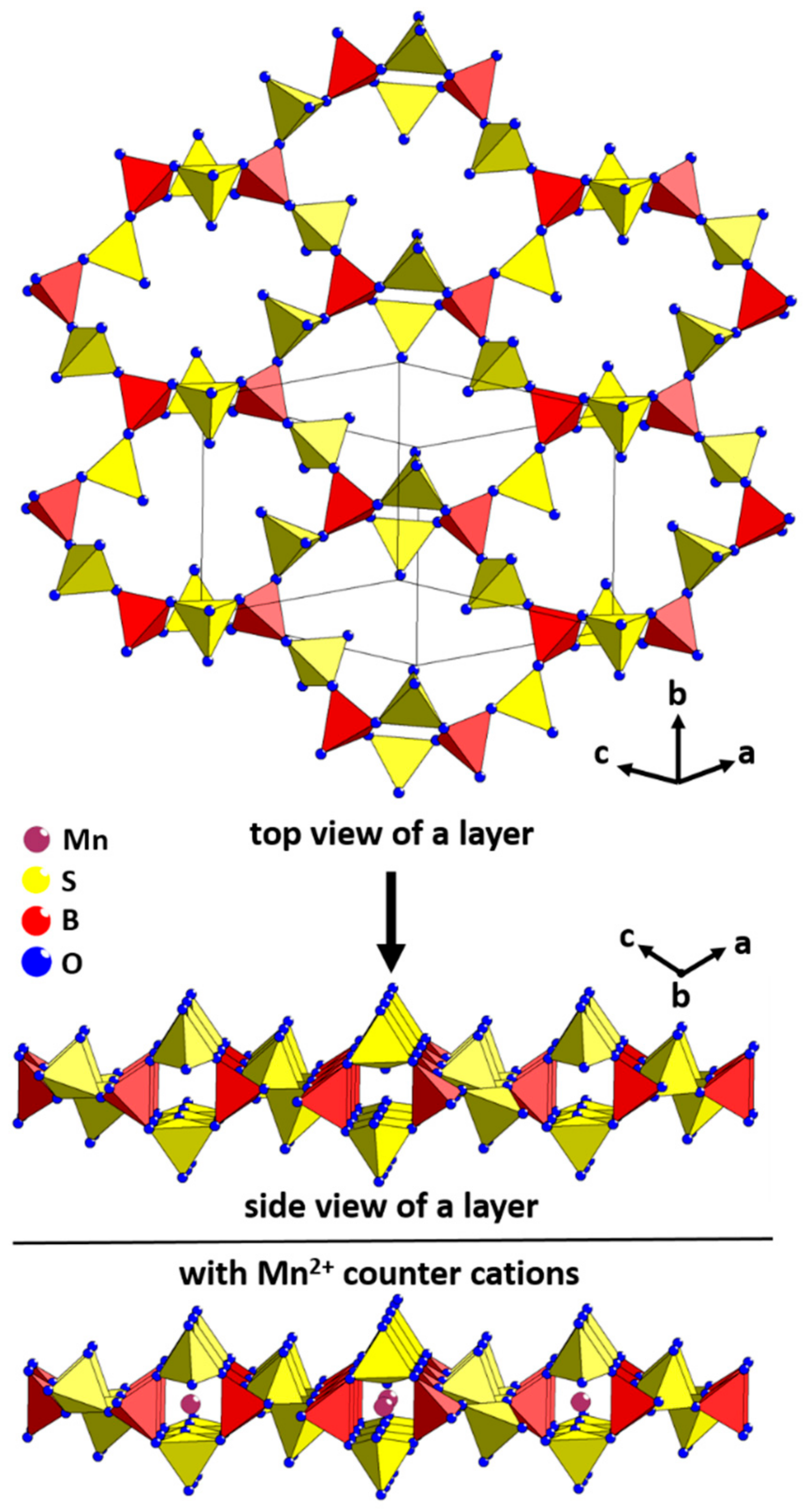
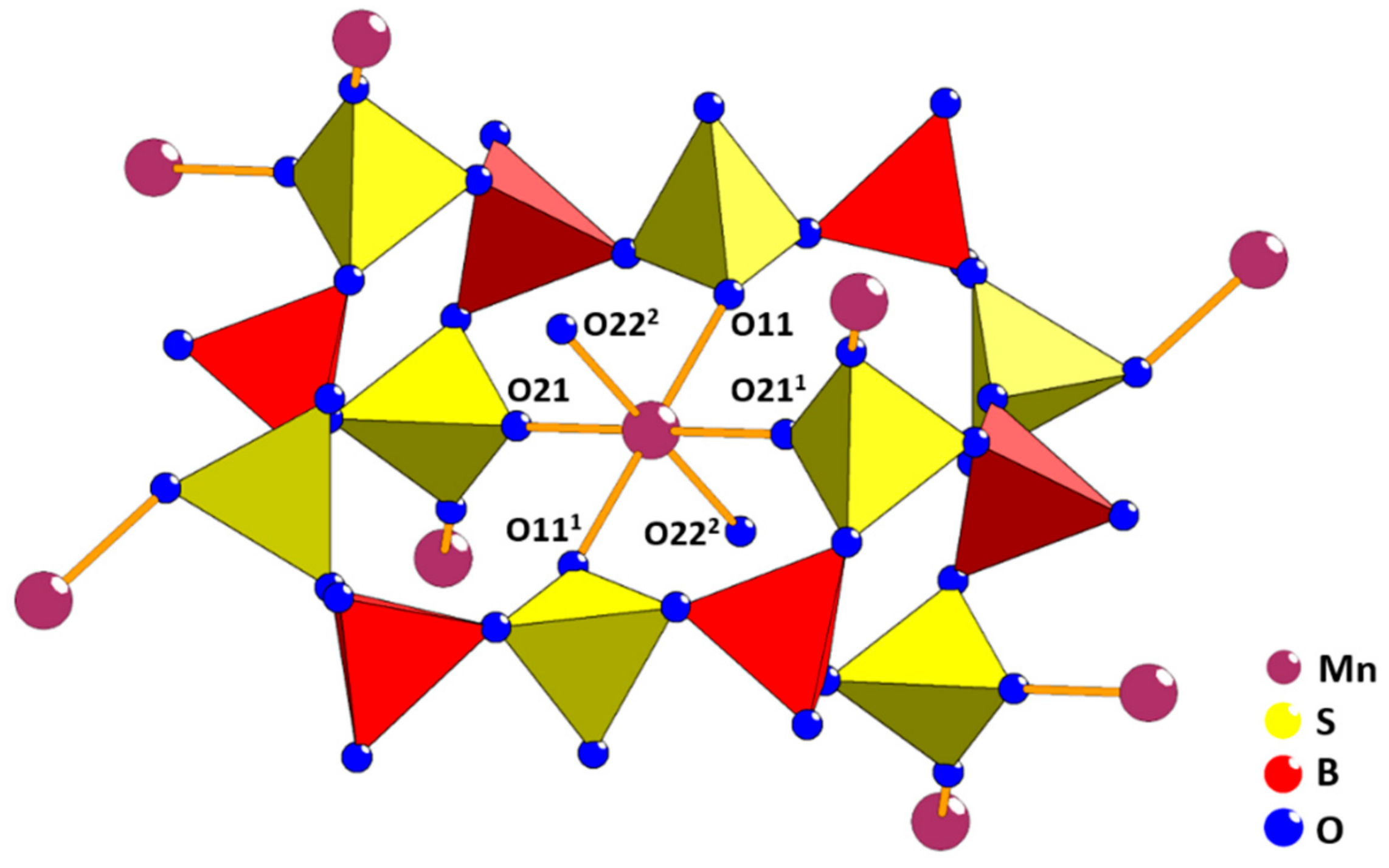
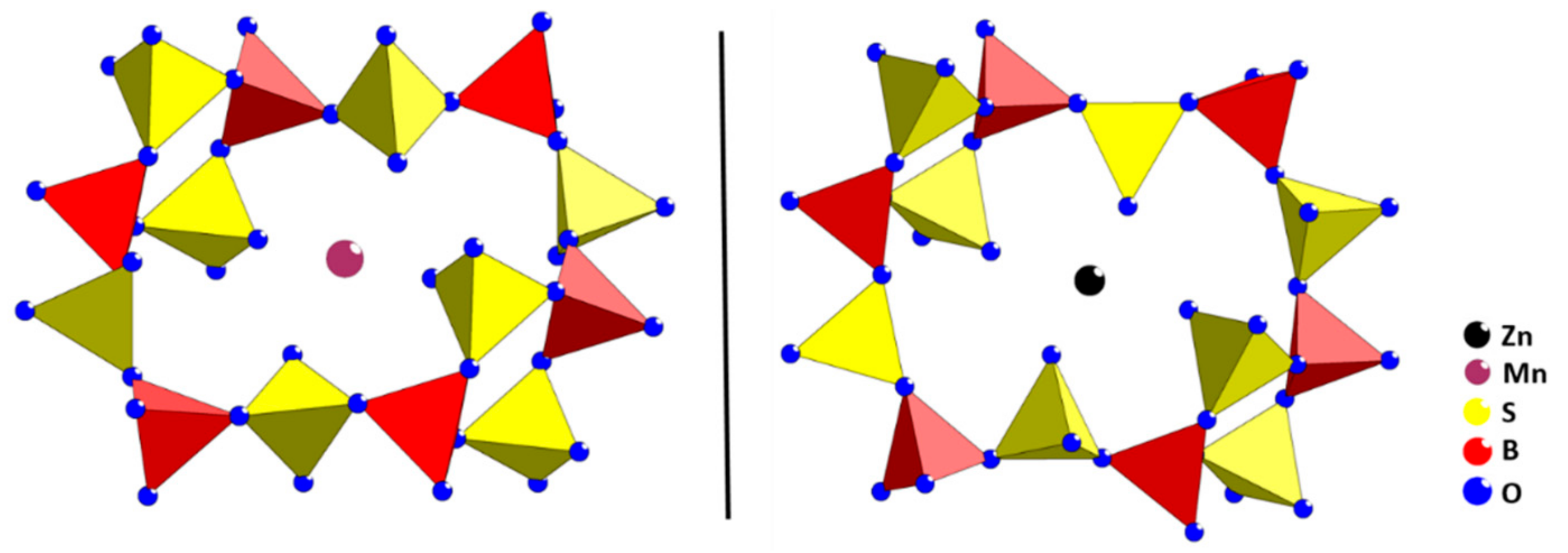
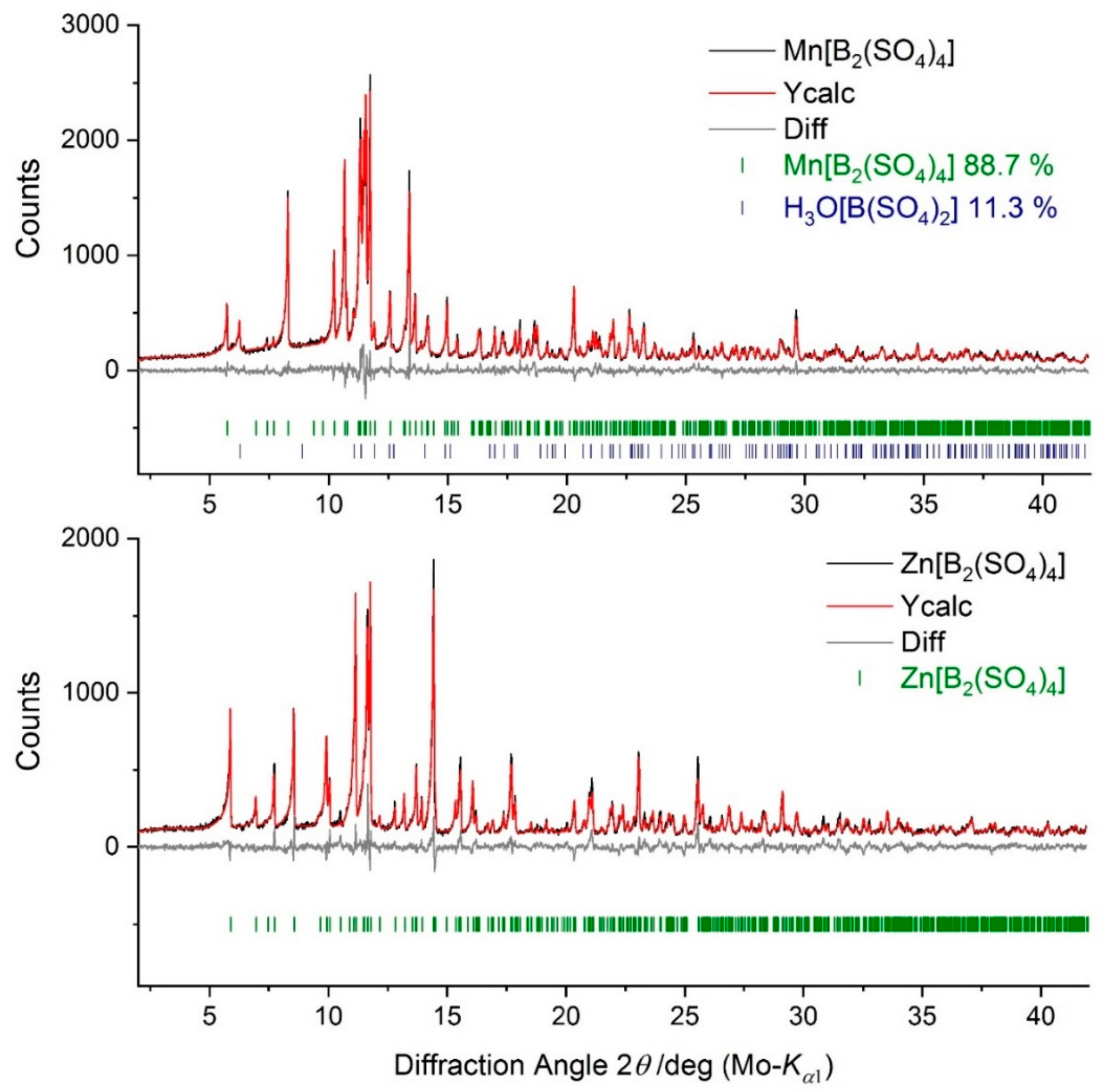
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgment
Conflicts of Interest
References
- Liebau, F. Structural Chemistry of Silicates; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussmann, J. An Introduction to Rock-Forming Minerals; Langmanns: London, UK, 1966. [Google Scholar]
- Krivovichev, S.V. Minerals as Advanced Materials II; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Chen, J.; Zhou, K.; Li, Y. Thermally Stable Silicate Nanotubes. Angew. Chem. Int. Ed. 2004, 43, 2017–2020. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Kupermann, A.; Ozin, G.A. A New Model for Aluminophosphate Formation: Transformation of a Linear Chain Aluminophosphate to Chain, Layer, and Framework Structures. Angew. Chem. Int. Ed. 1998, 37, 46–62. [Google Scholar] [CrossRef]
- Santilli, D.S.; Zones, S.I. Synthesis of Microporous Materials: Vol. 1: Molecular Sieves; Van Nostrand Reinhold: New York, NY, USA, 1992. [Google Scholar]
- Kniep, R.; Gözel, G.; Eisenmann, B.; Röhr, C.; Asbrand, M.; Kizilyalli, M. Borophosphates—A Neglected Class of Compounds: Crystal Structures of MII[BPO5] (MII = Ca, Sr) and Ba3[BP3O12]. Angew. Chem. Int. Ed. 1994, 33, 749–751. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Halgeri, A.B. Metal ion-exchanged Zeolites as highly active Solid acid Catalysts for the Green Synthesis of Glycerol Carbonate from Glycerol. RSC Adv. 2015, 5, 14286–14293. [Google Scholar] [CrossRef]
- Marakatti, V.; Halgeri, A.B.; Shanbhag, G.V. Metal ion-exchanged Zeolites as Solid acid Catalysts for the Green Synthesis of nopol from Prins reaction. Catal. Sci. Technol. 2014, 4, 4065–4074. [Google Scholar] [CrossRef]
- Pauling, L. The Principles determining the structure of complex ionic crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Höppe, H.A.; Kazmierczak, K.; Daub, M.; Förg, K.; Fuchs, F.; Hillebrecht, H. The First Borosulfate K5[B(SO4)4]. Angew. Chem. Int. Ed. 2012, 51, 6255–6257. [Google Scholar] [CrossRef]
- Gross, P.; Kirchhain, A.; Höppe, H.A. The Borosulfates K4[BS4O15(OH)], Ba[B2S3O13], and Gd2[B2S6O24]. Angew. Chem. Int. Ed. 2016, 55, 4353–4355. [Google Scholar] [CrossRef]
- Bruns, J.; Podewitz, M.; Janka, O.; Pöttgen, R.; Liedl, K.; Huppertz, H. Cu[B2(SO4)4] and Cu[B(SO4)2(HSO4)]—Two silicate analogue borosulfates differing in their dimensionality: A comparative study of stability and acidity. Angew. Chem. Int. Ed. 2018, 130, 9693–9697. [Google Scholar] [CrossRef]
- Netzsch, P.; Hämmer, M.; Gross, P.; Bariss, H.; Block, T.; Heletta, L.; Pöttgen, R.; Bruns, J.; Huppertz, H.; Höppe, H.A. RE2[B2(SO4)6] (RE = Y, La–Nd, Sm, Eu, Tb–Lu): A silicate-analogues host structure with weak coordination behaviour. Dalton Trans. 2019, 48, 4387–4397. [Google Scholar] [CrossRef]
- Bruns, J.; Podewitz, M.; Schauperl, M.; Liedl, K.; Janka, O.; Pöttgen, R.; Huppertz, H. Ag[B(SO4)2]—Synthesis, crystal structure and characterization of the first precious-metal borosulfate. Eur. J. Inorg. Chem. 2017, 34, 3981–3989. [Google Scholar] [CrossRef]
- Schönegger, S.; Bruns, J.; Gartner, B.; Wurst, K.; Liedl, K.; Huppertz, H. Synthesis and characterization of the first lead(II) borosulfate Pb[B2(SO4)4]. Z. Allg. Anorg. Chem. 2018, 644, 1702–1706. [Google Scholar] [CrossRef]
- Bruns, J.; Podewitz, M.; Schauperl, M.; Joachim, B.; Liedl, K.; Huppertz, H. CaB2S4O16: A borosulfate exhibiting a new structure type with phyllosilicate topology. Chem. Eur. J. 2017, 23, 16773–16781. [Google Scholar] [CrossRef]
- Daub, M.; Hillebrecht, H. Borosulfates Cs2B2S3O13, Rb4B2S4O17, and A3HB4S2O14 (A= Rb, Cs)—Crystalline Approximants for Vitreous B2O3? Eur. J. Inorg. Chem. 2015, 2015, 4176–4181. [Google Scholar] [CrossRef]
- Daub, M.; Höppe, H.A.; Hillebrecht, H. Further New Borosulfates: Synthesis, crystal Structure, and vibrational spectra of A[B(SO4)2] (A = Na, K, NH4) and the crystal structures of Li5[B(SO4)4] and NH4[B(S2O7)2]. Z. Anorg. Allg. Chem. 2014, 640, 2914–2921. [Google Scholar] [CrossRef]
- Daub, M.; Kazmierczak, K.; Höppe, H.A.; Hillebrecht, H. The borosulfate story goes on-From alkali and oxonium salts to polyacids. Chem. Eur. J. 2013, 19, 16954–16962. [Google Scholar] [CrossRef] [PubMed]
- Logemann, C.; Wickleder, M.S. B2S2O9—A boron sulfate with phyllosilicate topology. Angew. Chem. Int. Ed. 2013, 52, 14229–14232. [Google Scholar] [CrossRef]
- Netzsch, P.; Gross, P.; Takahashi, H.; Höppe, H.A. Synthesis and characterization of the first borosulfates of magnesium, manganese, cobat nickel, and zinc. Inorg. Chem. 2018, 57, 8530–8539. [Google Scholar] [CrossRef]
- Daub, M.; Kazmierczak, K.; Gross, P.; Höppe, H.A.; Hillebrecht, H. Exploring a New Structure Family: Alkali Borosulfates Na5[B(SO4)4], A3[B(SO4)3] (A = K, Rb), Li[B(SO4)2], and Li[B(S2O7)2]. Inorg. Chem. 2013, 52, 6011–6020. [Google Scholar] [CrossRef]
- CSD-434487. Available online: www.ccdc.cam.ac.uk/structures (accessed on 11 December 2019).
- Mairesse, G.; Drache, M. The Crystal Structure of Potassium Tetraehlorosulfatoborate, K[B(SO3Cl)4]. Acta Cryst. 1978, 34, 1771–1776. [Google Scholar] [CrossRef]
- Marsch, R.E. Potassium tetrachlorosulfatoborate: Change in space group. Acta Cryst. 1980, 36, 219–220. [Google Scholar] [CrossRef]
- Mairesse, G.; Drache, M. Lithium Tetrakis(chlorosulfato)borate. Acta Cryst. 1980, 36, 2767–2768. [Google Scholar] [CrossRef]
- Ruchkina, E.A.; Belokoneva, E.L. Structural Features of Pb, Fe, and Alkali Metal Borophosphates as analysed in terms of topologically similar structural blocks. Russ. J. Inorg. Chem. 2003, 48, 1812–1821. [Google Scholar]
- Kniep, R.; Schäfer, G.; Engelhardt, G.; Boy, I. K[ZnBP2O8] and A[ZnBP2O8] (A = NH4+, Rb+, Cs+): Zincoborophosphates as a New Class of Compounds with Tetrahedral Framework Structures. Angew. Chem. Int. Ed. 1999, 38, 3642–3644. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- TOPAS4.2; Bruker: Karlsruhe, Germany, 2009.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS 2014/5; University of Göttingen: Gottingen, Germany, 1996. [Google Scholar]
| Sum formula | Mn[B2(SO4)4] | Zn[B2(SO4)4] |
|---|---|---|
| Formula weight | 460.80 | 471.23 |
| Temperature/K | 173(2) | 183(2) |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/n | P21/n |
| a/Å | 8.0435(4) | 7.8338(4) |
| b/Å | 7.9174(4) | 8.0967(4) |
| c/Å | 9.3082(4) | 9.0399(4) |
| β/° | 110.94(1) | 111.26(1) |
| Volume/Å3 | 553.63(5) | 534.36(5) |
| Z | 2 | 2 |
| ρcalc/g/cm3 | 2.764 | 2.929 |
| μ/mm−1 | 2.052 | 3.189 |
| F(000) | 454 | 464 |
| Crystal size/mm3 | 0.07 × 0.055 × 0.03 | 0.5 × 0.3 × 0.11 |
| Radiation | Mo-Kα1 (λ = 0.71073) | Mo-Kα1 (λ = 0.71073) |
| 2Θ range for data collection/° | 5.76 to 82.54 | 5.91 to 78.95 |
| Index ranges | −14 ≤ h ≤ 14, −14 ≤ k ≤ 14, −17 ≤ l ≤ 17 | −14 ≤ h ≤ 13, −14 ≤ k ≤ 14, −16 ≤ l ≤ 16 |
| Reflections collected | 58531 | 26941 |
| Independent reflections | 3710 [Rint = 0.0417, Rsigma = 0.0169] | 3202 [Rint = 0.0278, Rsigma = 0.0151] |
| Data/restraints/parameters | 3710/0/106 | 3202/0/107 |
| Goodness-of-fit on F2 | 1.078 | 1.110 |
| Final R indexes [I ≥ 2σ(Io)] | R1 = 0.0209, wR2 = 0.0534 | R1 = 0.0168, wR2 = 0.0460 |
| Final R indexes [all data] | R1 = 0.0271, wR2 = 0.0553 | R1 = 0.0186, wR2 = 0.0468 |
| Largest diff. peak/hole/eÅ−3 | 0.48/−0.61 | 0.64/−0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqualini, L.C.; Huppertz, H.; Bruns, J. M[B2(SO4)4] (M = Mn, Zn)—Syntheses and Crystal Structures of Two New Phyllosilicate Analogue Borosulfates. Inorganics 2019, 7, 145. https://doi.org/10.3390/inorganics7120145
Pasqualini LC, Huppertz H, Bruns J. M[B2(SO4)4] (M = Mn, Zn)—Syntheses and Crystal Structures of Two New Phyllosilicate Analogue Borosulfates. Inorganics. 2019; 7(12):145. https://doi.org/10.3390/inorganics7120145
Chicago/Turabian StylePasqualini, Leonard C., Hubert Huppertz, and Jörn Bruns. 2019. "M[B2(SO4)4] (M = Mn, Zn)—Syntheses and Crystal Structures of Two New Phyllosilicate Analogue Borosulfates" Inorganics 7, no. 12: 145. https://doi.org/10.3390/inorganics7120145
APA StylePasqualini, L. C., Huppertz, H., & Bruns, J. (2019). M[B2(SO4)4] (M = Mn, Zn)—Syntheses and Crystal Structures of Two New Phyllosilicate Analogue Borosulfates. Inorganics, 7(12), 145. https://doi.org/10.3390/inorganics7120145