High-Pressure Synthesis, Crystal Structure, and Photoluminescence Properties of β-Y2B4O9:Eu3+
Abstract
:1. Introduction
2. Results and Discussion
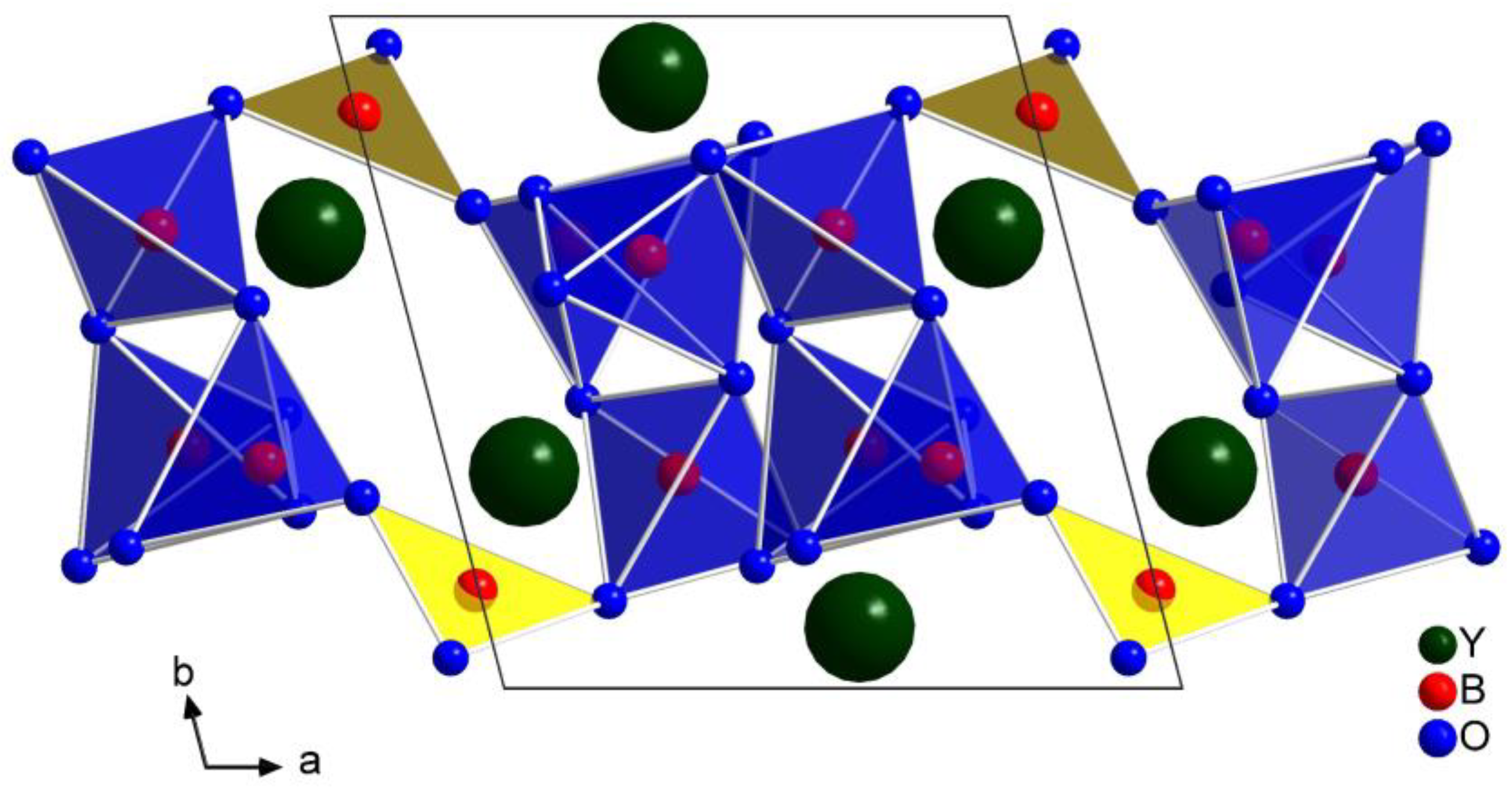
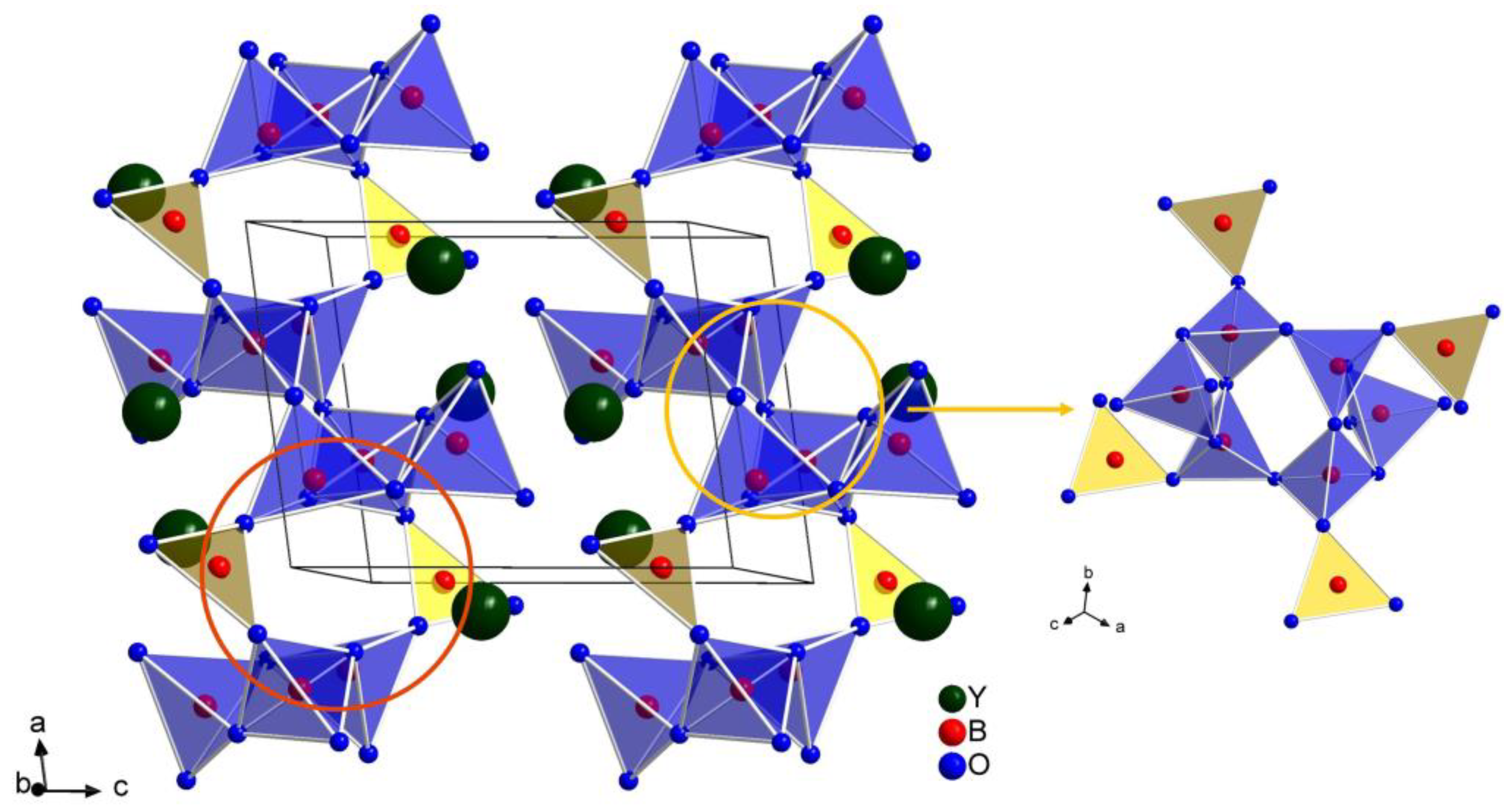
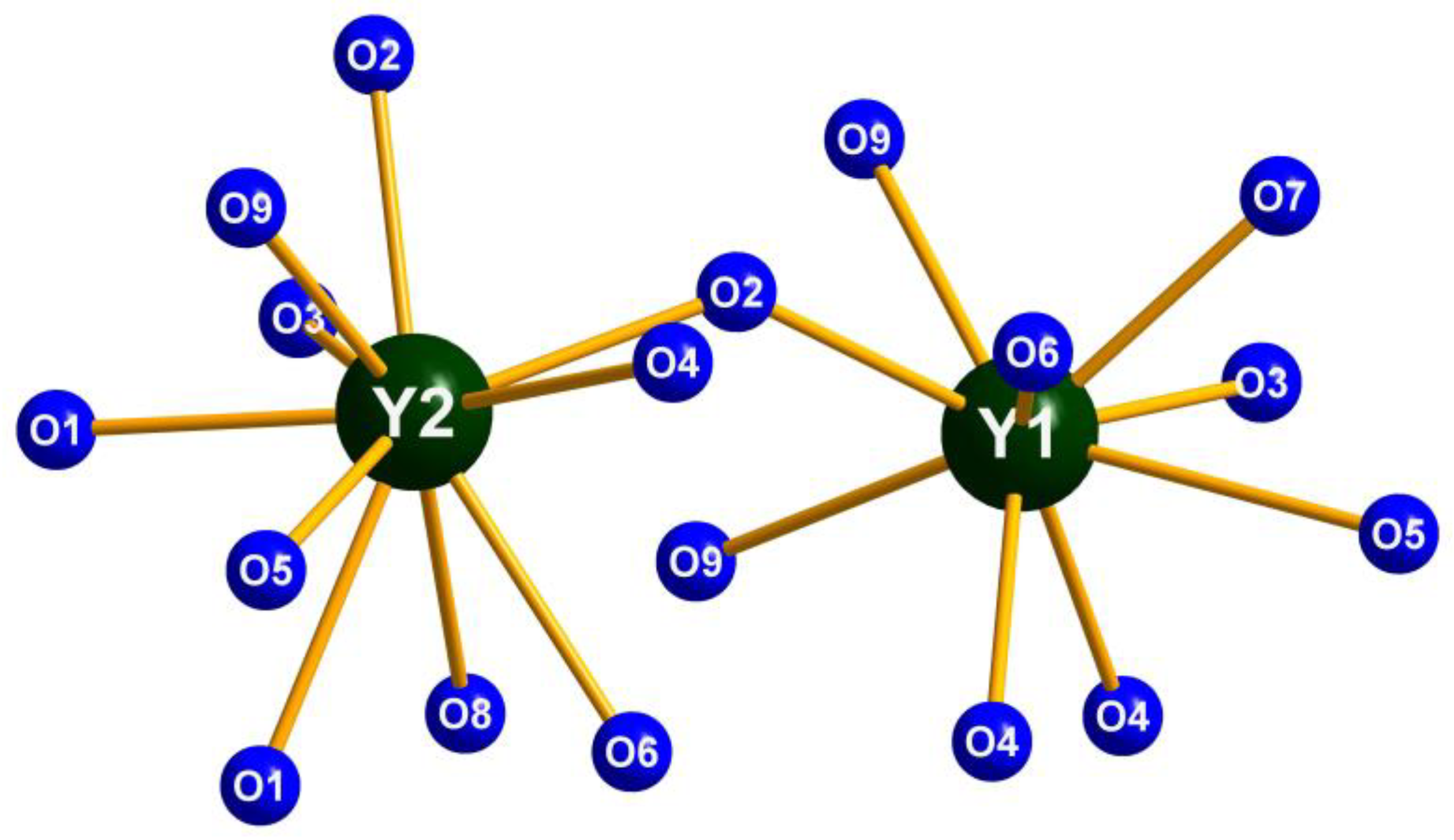
2.1. Crystal Structure
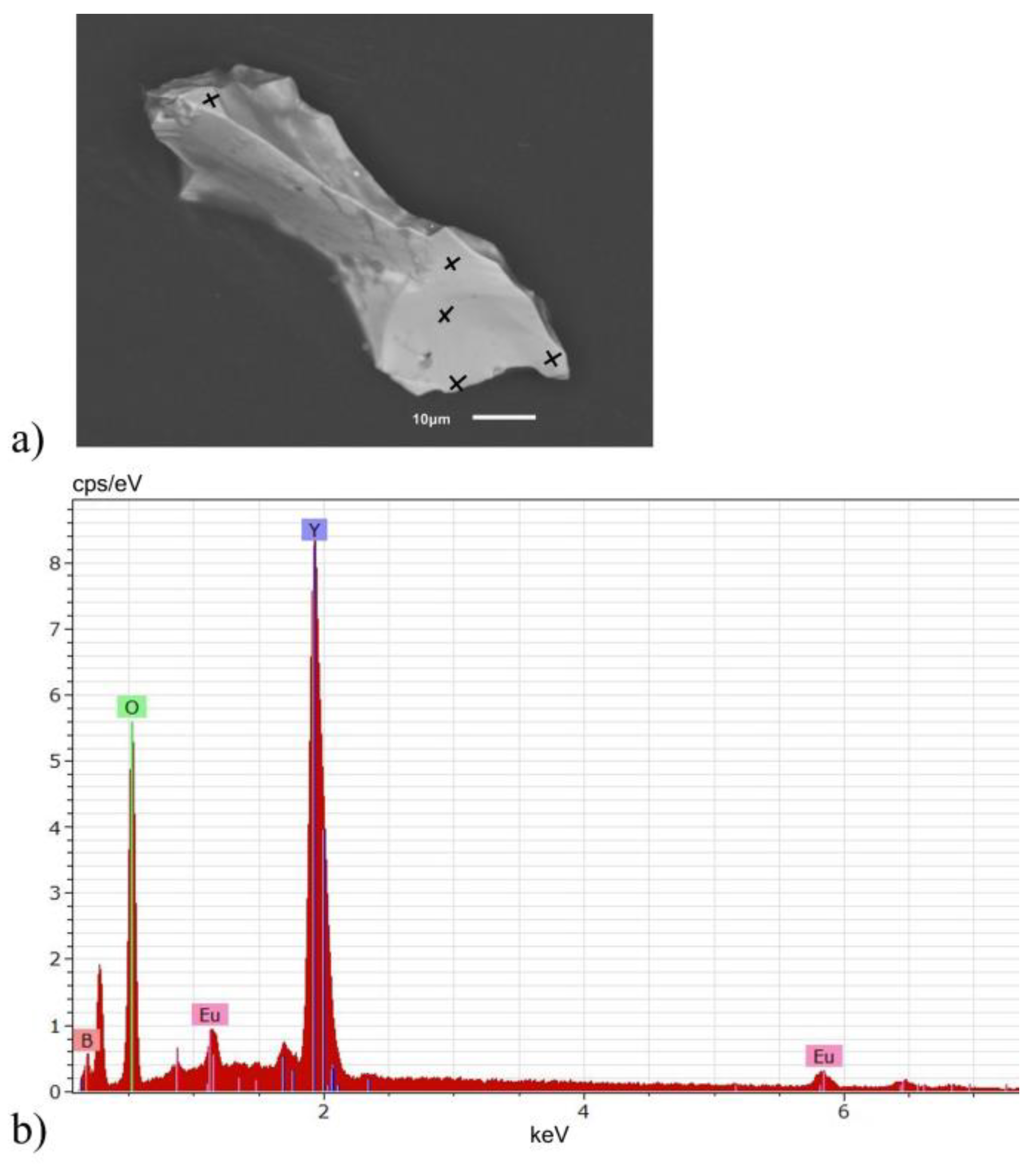
2.2. Elemental Analysis
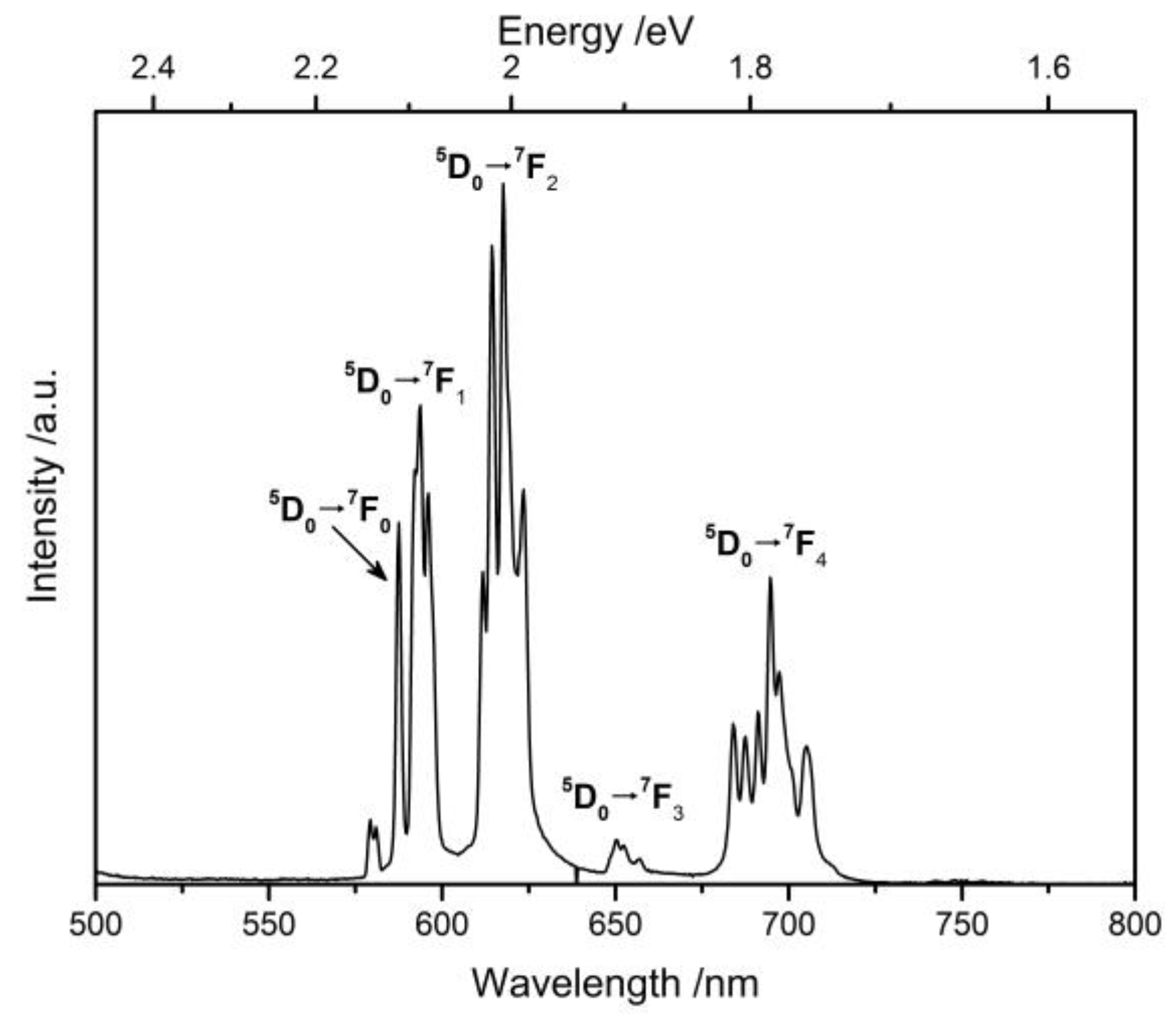
2.3. Photoluminescence Properties
3. Experimental Section
3.1. Synthesis
3.2. Single-Crystal Structure Analysis
3.3. Energy-Dispersive X-ray Spectroscopy (EDX)
3.4. Luminescence Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levin, E.M.; Roth, R.S.; Martin, J.B. Polymorphism of ABO3 type Rare Earth Borates. Am. Mineral. J. Earth Planet. Mater. 1961, 46, 1030–1055. [Google Scholar]
- Lin, J.H.; Zhou, S.; Yang, L.Q.; Yao, G.Q.; Su, M.Z.; You, L.P. Structure and Luminescent Properties of Y17.33(BO3)4(B2O5)2O16. J. Solid State Chem. 1997, 134, 158–163. [Google Scholar] [CrossRef]
- Schmitt, M.K.; Huppertz, H. β-Y(BO2)3—A new member of the β-Ln(BO2)3 (Ln = Nd, Sm, Gd–Lu) structure family. Z. Naturforsch. B 2017, 72, 983–988. [Google Scholar] [CrossRef]
- Schmitt, M.K.; Huppertz, H. High-pressure synthesis and crystal structure of α-Y2B4O9. Z. Naturforsch. B 2017, 72, 977–982. [Google Scholar] [CrossRef]
- Fuchs, B.; Schmitt, M.K.; Wurst, K.; Huppertz, H. High-Pressure Synthesis and Crystal Structure of the Highly Condensed Yttrium Borate YB7O12. Eur. J. Inorg. Chem. 2019, 2019, 271–276. [Google Scholar] [CrossRef]
- Huppertz, H.; Altmannshofer, S.; Heymann, G. High-pressure preparation, crystal structure, and properties of the new rare-earth oxoborate β-Dy2B4O9. J. Solid State Chem. 2003, 170, 320–329. [Google Scholar] [CrossRef]
- Emme, H.; Huppertz, H. High-pressure synthesis of the new rare-earth oxoborate β-Gd2B4O9. Acta Crystallogr. 2005, 61, i23–i24. [Google Scholar]
- Lin, J.; Sheptyakov, D.; Wang, Y.; Allenspach, P. Structures and Phase Transition of Vaterite-Type Rare Earth Orthoborates: A Neutron Diffraction Study. Chem. Mater. 2004, 16, 2418–2424. [Google Scholar] [CrossRef]
- Pitscheider, A.; Kaindl, R.; Oeckler, O.; Huppertz, H. The crystal structure of pi-ErBO3: New single-crystal data for an old problem. J. Solid State Chem. 2011, 184, 149–153. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Zhang, X.; Marathe, A.; Cordes, D.B.; Weeks, B.; Chaudhuri, J. Tunable photoluminescence and energy transfer of YBO3:Tb3+, Eu3+ for white light emitting diodes. J. Mater. Chem. 2013, 1, 7202–7207. [Google Scholar] [CrossRef]
- Lokeswara Reddy, G.V.; Rama Moorthy, L.; Chengaiah, T.; Bungala Chinna, J. Multi-color emission tunability and energy transfer studies of YAl3(BO3)4:Eu3+/Tb3+ phosphors. Ceram. Int. 2014, 40, 3399–3410. [Google Scholar] [CrossRef]
- Lokeswara Reddy, G.V.; Rama Moorthy, L.; Packiyaraj, P.; Jamalaiah, B.C. Optical characterization of YAl3(BO3)4:Dy3+–Tm3+ phosphors under near UV excitation. Opt. Mater. 2013, 35, 2138–2145. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, L.; Jiang, P.; Gao, W.; Cong, R.; Yang, T. Photoluminescence of complete solid solutions α-Y1−xEuxB5O9 by sol-gel synthesis and thermal decomposition from Y1−xEux[B6O9(OH)3]. J. Solid State Chem. 2019, 277, 731–737. [Google Scholar] [CrossRef]
- Zobetz, E. Geometrische Größen und einfache Modellrechnungen für BO4-Gruppen. Z. Kristallogr. 1990, 191, 45–57. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Burns, P.C.; Grice, J.D. Boron: Mineralogy, Petrology, and Geochemistry. In Reviews in Mineralogy; Anovitz, L.M., Grew, E.S., Eds.; Mineralogical Society of America: Washington, DC, USA, 1996; Volume 33. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-Valence Parameters for Solids. Acta Crystallogr. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Hoppe, R.; Voigt, S.; Glaum, H.; Kissel, J.; Müller, H.P.; Bernet, K. A new route to charge distribution in ionic solids. J. Less-Common Met. 1989, 156, 105–122. [Google Scholar] [CrossRef]
- Binnemanns, K. Interpretation of europium(III) spectra. Coordin. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Dieke, G.H. Spectra and Energy Levels of Rare Earth Ions in Crystals; InterScience, Johan Wiley and Sons: New York, NY, USA, 1968. [Google Scholar]
- Walker, D.; Carpenter, M.A.; Hitch, C.M. Some simplifications to multianvil devoces for high pressure experiments. Am. Mineral. 1990, 75, 1020–1028. [Google Scholar]
- Walker, D. Lubrication, gasketing, and precision in multianvil exeriments. Am. Mineral. 1991, 76, 1092–1100. [Google Scholar]
- Huppertz, H. Multianvil high-pressure/high-temperature synthesis in solid state chemistry. Z. Kristallogr. 2004, 219, 330–338. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, v2014/5; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELX. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
| Empirical Formula | β-Y2B4O9 |
| Molar mass, g·mol−1 | 365.06 |
| Crystal system | triclinic |
| Space group | P (no. 2) |
| Single-crystal data | |
| T, K | 277(2) |
| Radiation | Mo Kα (λ = 71.07 pm) |
| a, Å | 6.1463(2) |
| b, Å | 6.4053(2) |
| c, Å | 7.4642(2) |
| α, ° | 102.59(2) |
| β, ° | 97.11(2) |
| γ, ° | 102.46(2) |
| V, Å3 | 257.50(2) |
| Z | 2 |
| Calculated density, g·cm−3 | 4.401 |
| Absorption coeff., mm−1 | 20.993 |
| F(000) | 340 |
| Crystal size, mm3 | 0.050 × 0.040 × 0.020 |
| θ range, ° | 2.8–41.3 |
| Index ranges | −11 ≤ h ≤11, −11 ≤ k ≤ 11, −13 ≤l ≤13 |
| Reflections collected | 28267 |
| Independent reflections | 3675 [Rint = 0.0398] |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3675/0/137 |
| Goodness-of-fit on F2 | 1.058 |
| Final R1/wR2 indices [I ≥ 2σ(I)] | 0.0210/0.0414 |
| Final R1/wR2 indices (all data) | 0.0286/0.0431 |
| Largest diff. peak/hole, e Å−3 | 1.42/−0.90 |
| Atom | x | y | z | Ueq |
|---|---|---|---|---|
| Y1 | 0.8881(1) | 0.6775(1) | 0.3598(1) | 0.0022(1) |
| Y2 | 0.5473(1) | 0.0910(1) | 0.2854(1) | 0.0030(1) |
| B1 | 0.7321(3) | 0.3347(3) | 0.9806(2) | 0.0052(2) |
| B2 | 0.6618(3) | 0.6822(3) | 0.9275(2) | 0.0053(2) |
| B3 | 0.6255(3) | 0.3572(3) | 0.6505(2) | 0.0056(2) |
| B4 | 0.0050(3) | 0.8543(3) | 0.8111(2) | 0.0064(2) |
| O1 | 0.5040(2) | 0.7902(2) | 0.0204(2) | 0.0057(2) |
| O2 | 0.4200(2) | 0.1827(2) | 0.5554(2) | 0.0064(2) |
| O3 | 0.2360(2) | 0.7364(2) | 0.2113(2) | 0.0060(2) |
| O4 | 0.7795(2) | 0.4063(2) | 0.5228(2) | 0.0064(2) |
| O5 | 0.1375(2) | 0.7163(2) | 0.8570(2) | 0.0069(2) |
| O6 | 0.5394(2) | 0.5388(2) | 0.7479(2) | 0.0065(2) |
| O7 | 0.7741(2) | 0.5720(2) | 0.0412(2) | 0.0058(2) |
| O8 | 0.8122(2) | 0.8700(2) | 0.8812(2) | 0.0072(2) |
| O9 | 0.0677(2) | 0.9547(2) | 0.6761(2) | 0.0077(2) |
| Bond | Distance | Bond | Distance | ||
|---|---|---|---|---|---|
| B1 | –O7 | 1.444(2) | B2 | –O7 | 1.421(2) |
| –O3 | 1.457(2) | –O6 | 1.457(2) | ||
| –O1 | 1.497(2) | –O1 | 1.466(2) | ||
| –O5 | 1.505(2) | –O8 | 1.483(2) | ||
| Ø | 1.476 | Ø | 1.457 | ||
| B3 | –O4 | 1.459(2) | B4 | –O9 | 1.360(2) |
| –O6 | 1.468(2) | –O8 | 1.367(2) | ||
| –O2 | 1.474(2) | –O5 | 1.391(2) | ||
| –O3 | 1.555(2) | ||||
| Ø | 1.489 | Ø | 1.373 | ||
| Bond | Angle | Bond | Angle |
|---|---|---|---|
| O1–B1–O5 | 101.4(2) | O1–B2–O8 | 101.7(2) |
| O7–B1–O5 | 101.7(2) | O6–B2–O8 | 104.9(2) |
| O3–B1–O1 | 104.0(2) | O6–B2–O1 | 108.3(2) |
| O7–B1–O3 | 111.6(2) | O7–B2–O1 | 112.6(2) |
| O7–B1–O1 | 114.9(2) | O7–B2–O6 | 113.6(2) 119.1(5) |
| O3–B1–O5 | 123.4(2) | O7–B2–O8 | 114.8(2) |
| Ø | 109.5 | Ø | 109.3 |
| O4–B3–O3 | 103.2(2) | O9–B4–O5 | 114.9(2) |
| O6–B3–O2 | 104.3(2) | O9–B4–O8 | 122.1(2) |
| O2–B3–O3 | 107.8(2) | O8–B4–O5 | 122.7(2) |
| O4–B3–O2 | 112.0(2) | ||
| O6–B3–O3 | 112.0(2) | ||
| O4–B3–O6 | 117.4(2) | ||
| Ø | 109.5 | Ø | 119.9 |
| Bond | Distance | Bond | Distance | ||
|---|---|---|---|---|---|
| Y1 | –O7 | 2.299(2) | Y2 | –O2 | 2.256(2) |
| –O4 | 2.338(2) | –O2 | 2.351(2) | ||
| –O4 | 2.359(2) | –O1 | 2.384(2) | ||
| –O2 | 2.359(2) | –O4 | 2.414(2) | ||
| –O9 | 2.390(2) | –O9 | 2.436(2) | ||
| –O3 | 2.523(2) | –O8 | 2.487(2) | ||
| –O9 | 2.573(2) | –O3 | 2.538(2) | ||
| –O6 | 2.625(2) | –O5 | 2.541(2) | ||
| –O5 | 2.645(2) | –O1 | 2.563(2) | ||
| –O6 | 2.596(2) | ||||
| Ø | 2.457 | Ø | 2.457 | ||
| Method | Y1 | Y2 | B1 | B2 | B3 | B4 | |||
| ∑V | +2.92 | +3.19 | +3.02 | +3.18 | +2.92 | +2.99 | |||
| ∑Q | +2.97 | +3.01 | +3.00 | +2.97 | +3.03 | +3.03 | |||
| O1 | O2 | O3 | O4 | O5 | O6 | O7 | O8 | O9 | |
| ∑V | −2.09 | −2.09 | −1.90 | −1.95 | −2.07 | −1.97 | −2.16 | −2.03 | −1.94 |
| ∑Q | −1.98 | −2.05 | −1.86 | −2.03 | −1.99 | −1.95 | −2.18 | −1.97 | −1.99 |
| Element | Y | B | O | Eu | Si |
|---|---|---|---|---|---|
| M1 | 45.0 | 15.5 | 36.1 | 3.0 | 0.4 |
| M2 | 41.2 | 13.0 | 33.2 | 12.5 | 0.1 |
| M3 | 43.4 | 14.0 | 34.1 | 8.6 | 0.0 |
| M4 | 42.1 | 15.3 | 34.9 | 7.7 | 0.1 |
| M5 | 42.5 | 13.6 | 34.1 | 9.8 | 0.0 |
| average | 42.8(3) | 14.3(2) | 34.5(2) | 8.3(7) | 0.1 |
| expected | 46.1 | 11.7 | 38.9 | 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, B.; Schröder, F.; Heymann, G.; Jüstel, T.; Huppertz, H. High-Pressure Synthesis, Crystal Structure, and Photoluminescence Properties of β-Y2B4O9:Eu3+. Inorganics 2019, 7, 136. https://doi.org/10.3390/inorganics7110136
Fuchs B, Schröder F, Heymann G, Jüstel T, Huppertz H. High-Pressure Synthesis, Crystal Structure, and Photoluminescence Properties of β-Y2B4O9:Eu3+. Inorganics. 2019; 7(11):136. https://doi.org/10.3390/inorganics7110136
Chicago/Turabian StyleFuchs, Birgit, Franziska Schröder, Gunter Heymann, Thomas Jüstel, and Hubert Huppertz. 2019. "High-Pressure Synthesis, Crystal Structure, and Photoluminescence Properties of β-Y2B4O9:Eu3+" Inorganics 7, no. 11: 136. https://doi.org/10.3390/inorganics7110136
APA StyleFuchs, B., Schröder, F., Heymann, G., Jüstel, T., & Huppertz, H. (2019). High-Pressure Synthesis, Crystal Structure, and Photoluminescence Properties of β-Y2B4O9:Eu3+. Inorganics, 7(11), 136. https://doi.org/10.3390/inorganics7110136







