Arsenates of Divalent Metals Comprising Arsenic Acid—An Update
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structures of the M(H2AsO4)2(H3AsO4)2 Series (M = Mg, Mn, Co, Ni)
2.2. Crystal Structures of M(HAsO4)(H3AsO4)(H2O)0.5 (M = Mn, Cd) and Zn(HAsO4)(H3AsO4)
2.3. Hydrogen Bonding Schemes
2.4. AsO4 Tetrahedra in the Sructures
2.5. Comparison of the Crystal Structures
3. Materials and Methods
3.1. Synthesis and Crystal Growth
3.2. Single Crystal X-ray Diffraction
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Salmon, J.E.; Terrey, H. The systems zinc oxide–phosphoric oxide–water and manganous oxide–phosphoric oxide–water at temperatures between 25° and 100°. J. Chem. Soc. 1950, 2813–2824. [Google Scholar] [CrossRef]
- Herak, R.; Prelesnik, B.; Čurić, M.; Djurić, S. The crystal structure of Co(H2PO4)2·2H3PO4. Z. Kristallogr. 1983, 164, 25–30. [Google Scholar] [CrossRef]
- Averbuch-Pouchot, M.T.; Durif, A. Topics in Phosphate Chemistry; World Scientific: Singapore, 1996; p. 346. [Google Scholar]
- Boudjada, A.; Durif, A.; Guitel, J.C. Structure d’un orthoarsenate acide de cadmium: CdH10(AsO4)4. Acta Crystallogr. Sect. B 1980, B36, 133–135. [Google Scholar] [CrossRef]
- Tran Qui, D.; Chiadmi, M. Structure de Décahydrogénotétraarsenate de Cuivre et Isotypie dans la Série MIIH10(AsO4)4 (MII = Mg, Mn, Co, Ni, Cu et Zn). Acta Crystallogr. Sect. C 1986, C42, 391–393. [Google Scholar] [CrossRef]
- Sure, S.; Guse, W. The crystal structure of zinc-decahydrogen-tetraarsenate ZnH10(AsO4)4. Neues Jahrb. Mineral. Monatsh. 1989, 9, 401–409. [Google Scholar]
- De la Flor, G.; Orobengoa, D.; Tasci, E.; Perez-Mato, J.M.; Aroyo, M.I. Comparison of structures applying the tools available at the Bilbao Crystallographic Server. J. Appl. Crystallogr. 2016, 49, 653–664. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. Sect. B 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. Sect. B 2015, B71, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Gagné, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Metalloids and post-transition metals. Acta Crystallogr. Sect. B 2018, B74, 63–78. [Google Scholar] [CrossRef]
- Schwendtner, K.; Kolitsch, U. Three new acid M+ arsenates and phosphates with multiply protonated As/PO4 groups. Acta Crystallogr. Sect. C 2019, C75, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Aroyo, M.I.; Perez-Mato, J.M.; Capillas, C.; Kroumova, E.; Ivantchev, S.; Madariaga, G.; Kirov, A.; Wondratschek, H. Bilbao Crystallographic Server I: Databases and crystallographic computing programs. Z. Kristallogr. 2006, 221, 15–27. [Google Scholar] [CrossRef]
- Lima-de-Faria, J.; Hellner, E.; Liebau, F.; Makovicky, E.; Parthé, E. Nomenclature of inorganic structure types. Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types. Acta Crystallogr. Sect. A 1990, A46, 1–11. [Google Scholar] [CrossRef]
- Gelato, L.M.; Parthé, E. STRUCTURE TIDY—A computer program to standardize crystal structure data. J. Appl. Crystallogr. 1987, 20, 139–143. [Google Scholar] [CrossRef]
- Keller, P.; Hess, H.; Riffel, H. Die Kristallstruktur von Koritnigit, Zn[H2O|HOAsO3]. Neues Jahrb. Mineral. Abh. 1980, 138, 316–332. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. TWINABS; University of Göttingen: Göttingen, Germany, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar]
- Dowty, E. Atoms for Windows; Version 6.3; Shape Software: Kingsport, TN, USA, 2006. [Google Scholar]
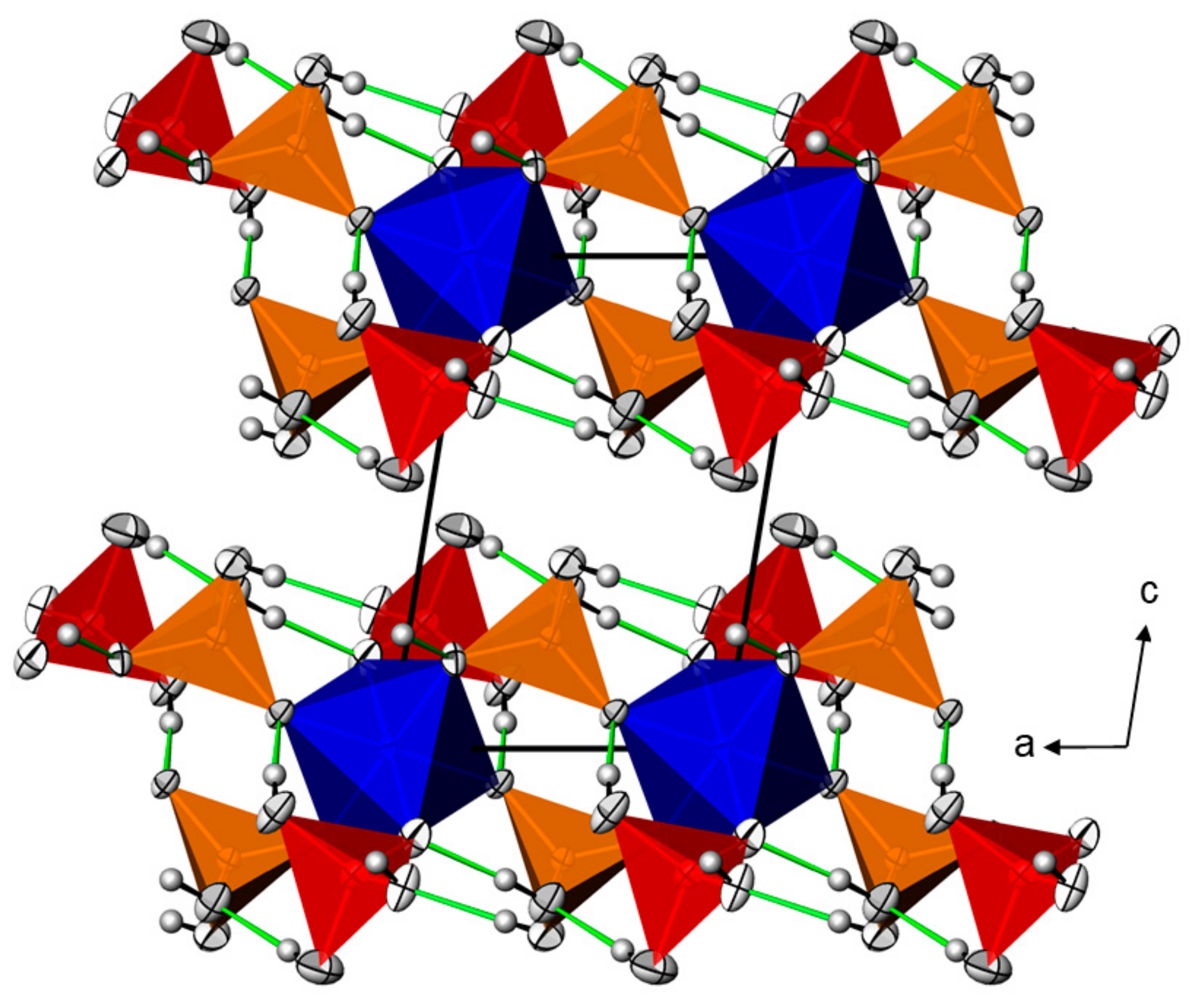
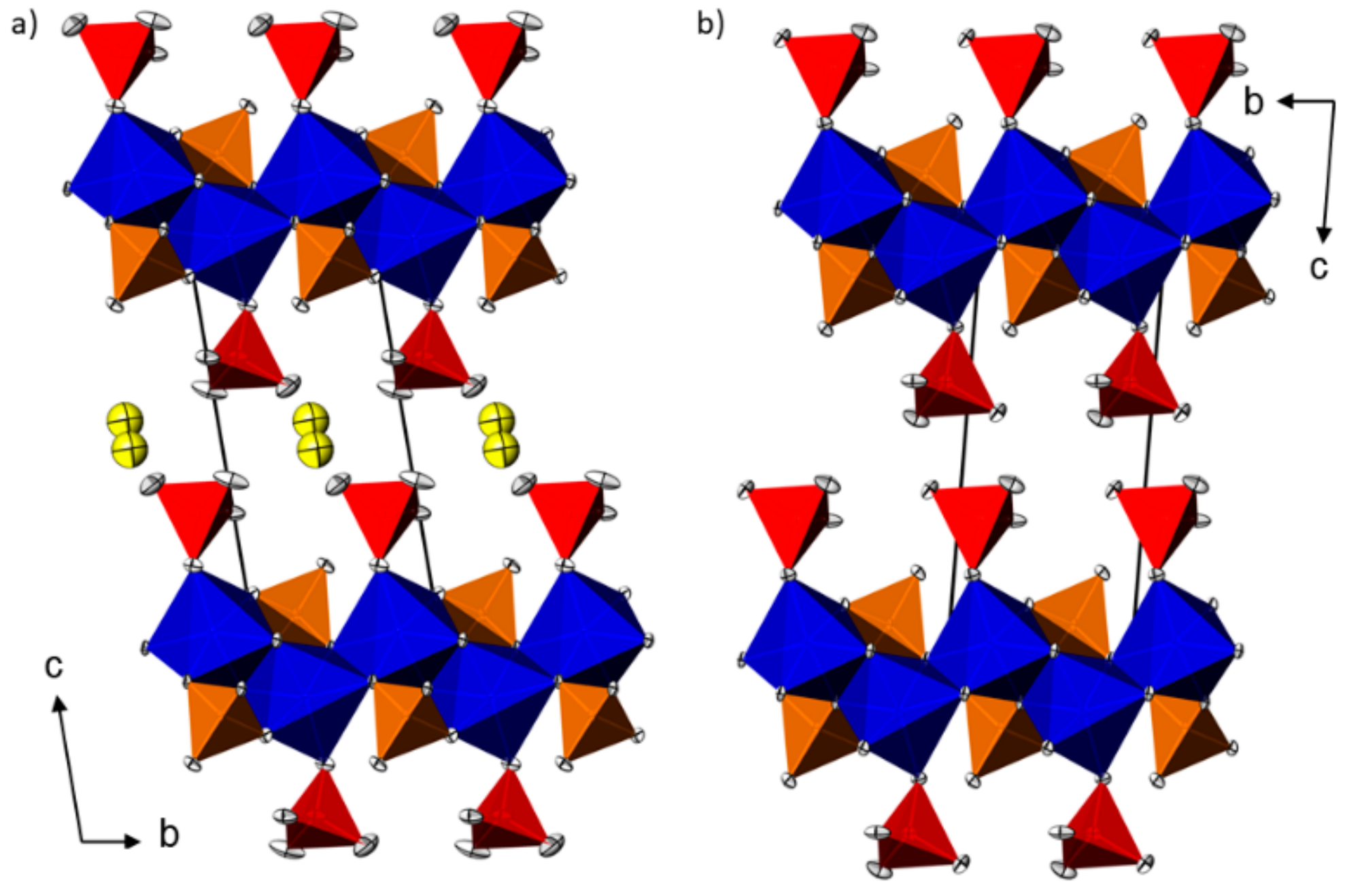
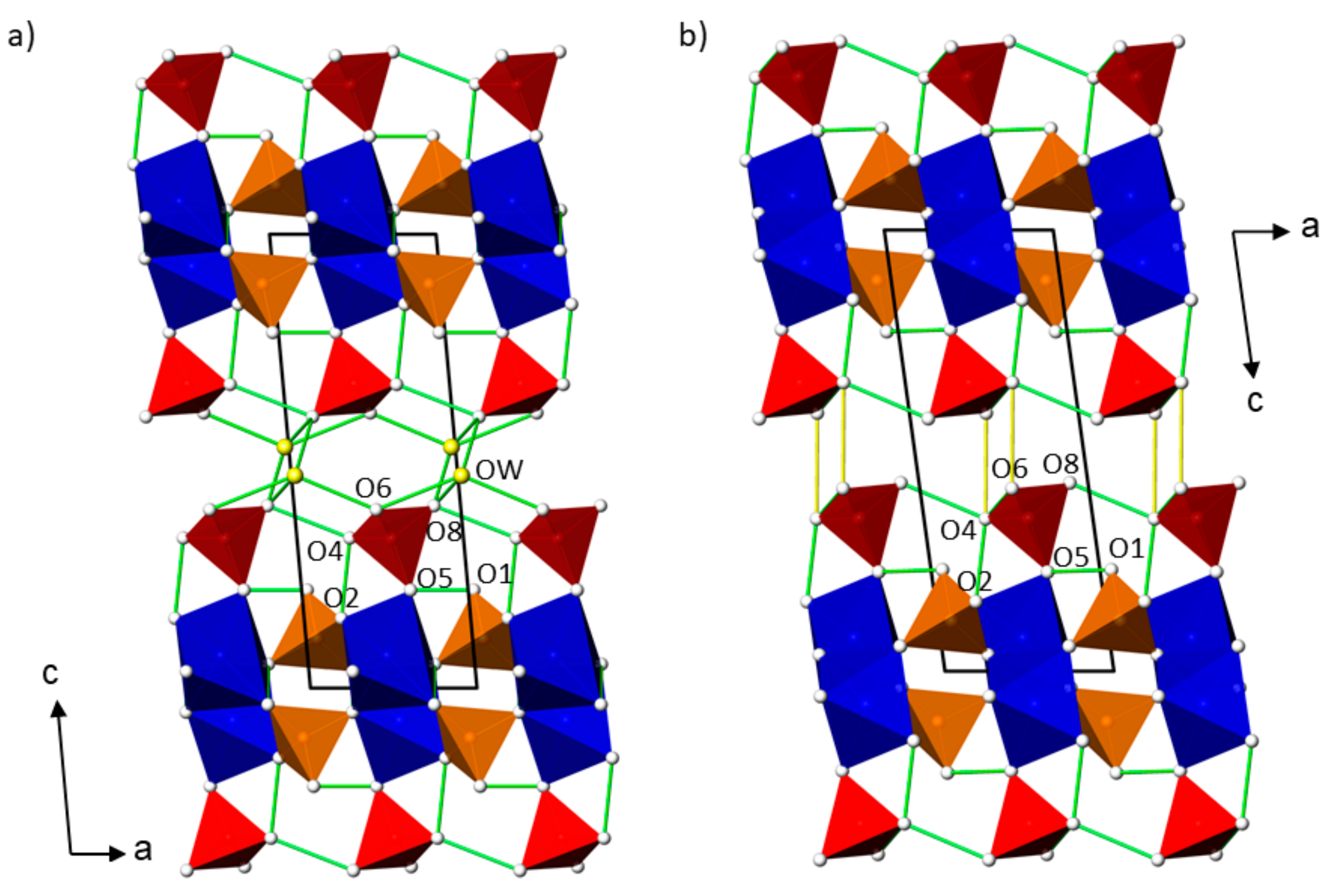
| Mn(HAsO4)(H3AsO4)(H2O)0.5 | Cd(HAsO4)(H3AsO4)(H2O)0.5 | Zn(HAsO4)(H3AsO4) | ||||||||||||
| Mn1 | – | O3 | 2.149(2) | Cd1 | – | O3 | 2.242(3) | Zn1 | – | O3 | 2.031(3) | |||
| – | O5 | 2.162(3) | – | O5 | 2.253(4) | – | O7 | 2.048(3) | ||||||
| – | O7 | 2.186(3) | – | O7 | 2.273(3) | – | O2 | 2.123(4) | ||||||
| – | O7 | 2.202(3) | – | O7 | 2.307(3) | – | O5 | 2.127(4) | ||||||
| – | O2 | 2.229(3) | – | O2 | 2.307(3) | – | O7 | 2.180(4) | ||||||
| – | O3 | 2.229(3) | – | O3 | 2.314(3) | – | O3 | 2.204(4) | ||||||
| av. | 2.19 | av. | 2.28 | av. | 2.12 | |||||||||
| As1 | – | O7 | 1.666(3) | As1 | – | O7 | 1.663(3) | As1 | – | O7 | 1.669(3) | |||
| – | O3 | 1.673(3) | – | O3 | 1.672(3) | – | O3 | 1.673(3) | ||||||
| – | O2 | 1.698(3) | – | O2 | 1.696(3) | – | O2 | 1.690(4) | ||||||
| – | O1 | 1.708(3) | – | O1 | 1.714(4) | – | O1 | 1.710(4) | ||||||
| av. | 1.686 | av. | 1.686 | av. | 1.689 | |||||||||
| As2 | – | O5 | 1.654(3) | As2 | – | O5 | 1.649(4) | As2 | – | O5 | 1.668(4) | |||
| – | O4 | 1.666(3) | – | O4 | 1.670(4) | – | O4 | 1.673(4) | ||||||
| – | O6 | 1.692(5) | – | O6 | 1.686(7) | – | O8 | 1.687(4) | ||||||
| – | O8 | 1.705(5) | – | O8 | 1.702(6) | – | O6 | 1.698(5) | ||||||
| av. | 1.680 | av. | 1.677 | av. | 1.682 | |||||||||
| BVS | ||||||||||||||
| Mn1 2.03, As1 5.01, As2 5.11, O1 1.18, O2 1.52, O3 1.98, O4 1.33, O5 1.73, O6 1.23, O7 2.00, O8 1.19. | Cd1 2.16, As1 5.01, As2 5.15, O1 1.16, O2 1.55, O3 2.03, O4 1.31, O5 1.78, O6 1.25, O7 2.04, O8 1.20. | Zn1 1.98, As1 5.02, As2 5.07, O1 1.17, O2 1.56, O3 1.97 O4 1.30, O5 1.64, O6 1.21, O7 1.99, O8 1.25. | ||||||||||||
| Mg(H2AsO4)2(H3AsO4)2 | Mn(H2AsO4)2(H3AsO4)2 | Co(H2AsO4)2(H3AsO4)2 | ||||||||||||
| Mg1 | – | O7 | 2.0680(13) | 2× | Mn1 | – | O7 | 2.1629(9) | 2× | Co1 | – | O7 | 2.050(3) | 2× |
| – | O5 | 2.0853(14) | 2× | – | O5 | 2.1707(9) | 2× | – | O5 | 2.146(3) | 2× | |||
| – | O3 | 2.1205(14) | 2× | – | O3 | 2.2120(11) | 2× | – | O3 | 2.146(4) | 2× | |||
| av. | 2.09 | av. | 2.18 | av. | 2.11 | |||||||||
| As1 | – | O3 | 1.6484(14) | As1 | – | O3 | 1.6458(10) | As1 | – | O3 | 1.650(3) | |||
| – | O1 | 1.6852(14) | – | O1 | 1.6831(10) | – | O1 | 1.687(3) | ||||||
| – | O4 | 1.6983(16) | – | O4 | 1.6983(11) | – | O4 | 1.696(3) | ||||||
| – | O2 | 1.7096(15) | – | O2 | 1.7037(12) | – | O2 | 1.711(4) | ||||||
| av. | 1.685 | av. | 1.683 | av. | 1.686 | |||||||||
| As2 | – | O5 | 1.6562(15) | As2 | – | O5 | 1.6552(9) | As2 | – | O5 | 1.663(3) | |||
| – | O7 | 1.6579(14) | – | O7 | 1.6561(9) | – | O7 | 1.665(3) | ||||||
| – | O6 | 1.7119(14) | – | O6 | 1.7076(11) | – | O6 | 1.709(4) | ||||||
| – | O8 | 1.7161(15) | – | O8 | 1.7131(11) | – | O8 | 1.717(4) | ||||||
| av. | 1.686 | av. | 1.683 | av. | 1.689 | |||||||||
| Ni(H2AsO4)2(H3AsO4)2 | ||||||||||||||
| Ni1 | – | O7 | 2.037(3) | 2× | ||||||||||
| – | O5 | 2.097(3) | 2× | |||||||||||
| – | O3 | 2.117(3) | 2× | |||||||||||
| av. | 2.08 | |||||||||||||
| As1 | – | O3 | 1.644(3) | |||||||||||
| – | O1 | 1.694(3) | ||||||||||||
| – | O4 | 1.696(3) | ||||||||||||
| – | O2 | 1.712(3) | ||||||||||||
| av. | 1.689 | |||||||||||||
| As2 | – | O5 | 1.656(3) | |||||||||||
| – | O7 | 1.664(3) | ||||||||||||
| – | O6 | 1.713(3) | ||||||||||||
| – | O8 | 1.713(3) | ||||||||||||
| av. | 1.687 | |||||||||||||
| D | H | A | D–H | H⋯A | D⋯A | D–H⋯A |
|---|---|---|---|---|---|---|
| Mg(H2AsO4)2(H3AsO4)2 | ||||||
| O2 | H1 | O8 | 0.890(10) | 1.918(18) | 2.769(2) | 160(4) |
| O1 | H2 | O7 | 0.898(10) | 1.620(11) | 2.517(2) | 174(4) |
| O4 | H3 | O5 | 0.895(10) | 1.758(14) | 2.634(2) | 167(4) |
| O6 | H4 | O1 | 0.884(10) | 1.96(2) | 2.760(2) | 149(4) |
| O8 | H5 | O3 | 0.893(10) | 1.686(11) | 2.577(2) | 174(4) |
| Mn(H2AsO4)2(H3AsO4)2 | ||||||
| O2 | H1 | O8 | 0.890(10) | 1.921(11) | 2.8018(16) | 170(3) |
| O1 | H2 | O7 | 0.898(10) | 1.654(13) | 2.5358(14) | 167(4) |
| O4 | H3 | O5 | 0.895(10) | 1.759(12) | 2.6452(15) | 170(3) |
| O6 | H4 | O1 | 0.884(10) | 1.932(12) | 2.7968(14) | 166(3) |
| O8 | H5 | O3 | 0.893(10) | 1.723(13) | 2.6007(14) | 167(4) |
| Co(H2AsO4)2(H3AsO4)2 | ||||||
| O2 | H1 | O8 | 0.898(10) | 1.94(3) | 2.812(5) | 163(8) |
| O1 | H2 | O7 | 0.901(10) | 1.69(3) | 2.564(5) | 162(8) |
| O4 | H3 | O5 | 0.898(10) | 1.75(3) | 2.616(5) | 162(7) |
| O6 | H4 | O1 | 0.896(10) | 1.94(3) | 2.799(4) | 159(7) |
| O8 | H5 | O3 | 0.899(10) | 1.71(2) | 2.592(5) | 167(8) |
| Ni(H2AsO4)2(H3AsO4)2 | ||||||
| O2 | H1 | O8 | 0.897(10) | 1.92(3) | 2.762(4) | 155(6) |
| O1 | H2 | O7 | 0.898(10) | 1.629(17) | 2.516(4) | 169(6) |
| O4 | H3 | O5 | 0.899(10) | 1.75(3) | 2.610(4) | 159(6) |
| O6 | H4 | O1 | 0.897(10) | 1.90(2) | 2.755(4) | 159(6) |
| O8 | H5 | O3 | 0.900(10) | 1.658(14) | 2.553(4) | 172(7) |
| Mn(HAsO4)(H3AsO4)(H2O)0.5 | ||||||
| O1 | O5 | 2.635(4) | ||||
| O4 | O2 | 2.458(5) | ||||
| O8 | O4 | 2.633(6) | ||||
| O6 | OW | 2.61(2) | ||||
| O8 | OW | 2.64(2) | ||||
| OW | O8 | 2.90(2) | ||||
| OW | O6 | 2.92(2) | ||||
| Cd(HAsO4)(H3AsO4)(H2O)0.5 | ||||||
| O1 | O5 | 2.625(5) | ||||
| O4 | O2 | 2.470(5) | ||||
| O8 | O4 | 2.670(7) | ||||
| O6 | OW | 2.75(4) | ||||
| O8 | OW | 2.60(4) | ||||
| OW | O6 | 2.84(4) | ||||
| OW | O8 | 2.94(4) | ||||
| Zn(HAsO4)(H3AsO4) | ||||||
| O1 | O5 | 2.610(6) | ||||
| O4 | O2 | 2.429(6) | ||||
| O8 | O4 | 2.666(6) | ||||
| O6 | O4 | 2.962(7) | ||||
| O6 | O4 | 3.071(7) | ||||
| Compound | Mn(HAsO4)-(H3AsO4)(H2O)0.5 | Cd(HAsO4)(H3AsO4)-(H2O)0.5 | Zn(HAsO4)-(H3AsO4) | Mg(H2AsO4)2-(H3AsO4)2 | Mn(H2AsO4)2-(H3AsO4)2 | Co(H2AsO4)2-(H3AsO4)2 | Ni(H2AsO4)2-(H3AsO4)2 | |
|---|---|---|---|---|---|---|---|---|
| MR | 345.82 | 403.28 | 347.24 | 590.07 | 620.70 | 624.69 | 624.47 | |
| Temp./°C | 23 | 23 | 23 | –173 | 23 | 23 | 23 | |
| Radiation; λ/Å | — Mo Kα; 0.71073 — | |||||||
| Diffractometer | APEXII CCD | SMART CCD | APEXII CCD | APEXII CCD | APEXII CCD | APEXII CCD | APEXII CCD | |
| Crystal size/mm3 | 0.48 × 0.18 × 0.02 | 0.20 × 0.15 × 0.02 | 0.12 × 0.12 × 0.01 | 0.10 × 0.06 × 0.01 | 0.12 × 0.09 × 0.02 | 0.12 × 0.06 × 0.01 | 0.09 × 0.06 × 0.04 | |
| Crystal color; form | light-pink; plate | colorless; plate | colorless; fragment | colorless; plate | light-pink; plate | violet; plate | yellow; plate | |
| Space group | P1 | P1 | P1 | P1 | P1 | P1 | P1 | |
| Formula units, Z | 2 | 2 | 2 | 1 | 1 | 1 | 1 | |
| a/Å | 4.9750(10) | 5.0188(9) | 4.9187(3) | 5.4558(3) | 5.5602(2) | 5.495(3) | 5.4297(7) | |
| b/Å | 5.4747(11) | 5.6180(10) | 5.2357(3) | 7.3180(4) | 7.4100(3) | 7.394(4) | 7.3308(9) | |
| c/Å | 13.603(3) | 13.734(2) | 12.8459(8) | 8.3382(5) | 8.4276(4) | 8.330(5) | 8.2795(10) | |
| α/° | 98.86(3) | 99.254(3) | 83.987(3) | 100.231(2) | 100.110(2) | 100.604(15) | 100.356(5) | |
| β/° | 93.63(3) | 93.756(3) | 81.286(3) | 98.614(2) | 98.578(2) | 97.550(14) | 98.088(5) | |
| γ/° | 99.09(3) | 98.845(3) | 80.117(3) | 93.022(2) | 92.744(2) | 92.858(12) | 92.982(5) | |
| V/Å3 | 360.02(13) | 376.02(12) | 321.09(3) | 322.84(3) | 337.03(2) | 328.8(3) | 319.95(7) | |
| μ/mm−1 | 10.966 | 11.648 | 14.054 | 10.399 | 10.808 | 11.379 | 11.869 | |
| X-ray Dens./g·cm−3 | 3.190 | 3.562 | 3.592 | 3.035 | 3.058 | 3.155 | 3.241 | |
| Range θmin–θmax | 3.87–30.00 | 3.02–31.06 | 3.96–30.98 | 3.79–41.89 | 2.80–41.52 | 2.81–31.00 | 2.53–36.71 | |
| Range | h | −6→6 | −7→7 | −7→6 | −10→10 | −10→10 | −7→7 | −9→8 |
| k | −7→3 | −7→8 | −7→7 | −13→13 | −13→14 | −10→10 | −12→12 | |
| l | −19→19 | −19→19 | −18→18 | −15→15 | −15→15 | −12→11 | 0→13 | |
| Meas. refl. | 4677 | 4430 | 7650 | 19986 | 17841 | 4582 | 3000 | |
| Indep. refl. | 2087 | 2326 | 2011 | 4436 | 4843 | 2057 | 3000 | |
| Obs.refl. [I > 2σ(I)] | 1831 | 1996 | 1600 | 3262 | 3767 | 1568 | 2566 | |
| Ri | 0.0287 | 0. 0287 | 0.0461 | 0.0656 | 0.0285 | 0.0364 | - | |
| Abs. corr. | SADABS | SADABS | SADABS | SADABS | SADABS | SADABS | TWINABS | |
| Trans. coef. Tmin; Tmax | 0.526; 0.748 | 0. 443; 0.663 | 0.480; 0.747 | 0.531; 0.748 | 0.479; 0.748 | 0.592; 0.747 | 0.257; 0.439 | |
| Number of parameters | 104 | 104 | 100 | 113 | 113 | 113 | 114 | |
| R[F2 > 2σ(F2)] | 0.0332 | 0.0350 | 0.0380 | 0.0353 | 0.0264 | 0.0380 | 0.0387 | |
| wR2(F2 all) | 0.1053 | 0.0950 | 0.0994 | 0.0712 | 0.0538 | 0.0850 | 0.0880 | |
| Goof | 1.1324 | 1.041 | 1.032 | 1.019 | 1.010 | 1.037 | 1.082 | |
| CSD number | 1951017 | 1951013 | 1951019 | 1951015 | 1951016 | 1951014 | 1951018 | |
| Zn(H2AsO4)2(H3AsO4)2 versus M(H2AsO4)2(H3AsO4)2 | ||||||
| M1 | Mg | Mn | (Co,P) b | (Co,As) | Ni | Cu c |
| Atom, atomic displacement |u|/Å | ||||||
| Zn1/M1 | 0 | 0 | 0 | 0 | 0 | 0 |
| As1/As1(P1) | 0.0111 | 0.0289 | 0.0746 | 0.0077 | 0.0200 | 0.3198 |
| As2/As2(P2) | 0.0176 | 0.0434 | 0.0852 | 0.0041 | 0.0122 | 0.1569 |
| O1 | 0.0242 | 0.0737 | 0.1646 | 0.0134 | 0.0394 | 0.2641 |
| O2 | 0.0241 | 0.0305 | 0.1641 | 0.0165 | 0.0285 | 0.3981 |
| O3 | 0.0295 | 0.0500 | 0.1157 | 0.0272 | 0.0508 | 0.6942 |
| O4 | 0.0253 | 0.0353 | 0.0889 | 0.0206 | 0.0184 | 0.2552 |
| O5 | 0.0386 | 0.0497 | 0.1497 | 0.0185 | 0.0129 | 0.0974 |
| O6 | 0.0230 | 0.0384 | 0.0669 | 0.0100 | 0.0337 | 0.2862 |
| O7 | 0.0061 | 0.0807 | 0.1612 | 0.0051 | 0.0266 | 0.3203 |
| O8 | 0.0295 | 0.0165 | 0.1616 | 0.0224 | 0.0308 | 0.3185 |
| degree of lattice distortion (S) | 0.0049 | 0.0078 | 0.0176 | 0.0030 | 0.0046 | 0.0243 |
| arithmetic mean (dav) | 0.0218 | 0.0426 | 0.1174 | 0.0139 | 0.0260 | 0.2963 |
| measure of similarity (Δ) | 0.014 | 0.033 | 0.037 | 0.017 | 0.011 | 0.112 |
| Mn(HAsO4)(H3AsO4)(H2O)0.5versus Cd(HAsO4)(H3AsO4)(H2O)0.5 | ||||||
| Atom, atomic displacement |u|/Å | ||||||
| Mn1/Cd1 | 0.0576 | |||||
| As1 | 0.0523 | |||||
| As2 | 0.0816 | |||||
| O1 | 0.0540 | |||||
| O2 | 0.0835 | |||||
| O3 | 0.0492 | |||||
| O4 | 0.0645 | |||||
| O5 | 0.1206 | |||||
| O6 | 0.1899 | |||||
| O7 | 0.0747 | |||||
| O8 | 0.0497 | |||||
| OW | 0.1435 | |||||
| degree of lattice distortion (S) | 0.0095 | |||||
| arithmetic mean (dav) | 0.0851 | |||||
| measure of similarity (Δ) | 0.037 | |||||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weil, M. Arsenates of Divalent Metals Comprising Arsenic Acid—An Update. Inorganics 2019, 7, 122. https://doi.org/10.3390/inorganics7100122
Weil M. Arsenates of Divalent Metals Comprising Arsenic Acid—An Update. Inorganics. 2019; 7(10):122. https://doi.org/10.3390/inorganics7100122
Chicago/Turabian StyleWeil, Matthias. 2019. "Arsenates of Divalent Metals Comprising Arsenic Acid—An Update" Inorganics 7, no. 10: 122. https://doi.org/10.3390/inorganics7100122
APA StyleWeil, M. (2019). Arsenates of Divalent Metals Comprising Arsenic Acid—An Update. Inorganics, 7(10), 122. https://doi.org/10.3390/inorganics7100122





