Zinc(II) as a Versatile Template for Efficient Dipolar and Octupolar Second-Order Nonlinear Optical Molecular Materials §
Abstract
1. Introduction
2. Principles of Second-Order Nonlinear Optics
3. Dipolar Complexes
3.1. Monodentate Nitrogen Ligands: Stilbazoles
3.2. Bidentate Nitrogen Ligands: Bipyridines, Phenanthrolines, and Diazafluorens
3.3. Tridentate Nitrogen Ligands: Terpyridines
3.4. Schiff-Bases
4. Octupolar Complexes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prasad, N.P.; Williams, D.J. Introduction to Nonlinear Optical Effects in molecules and Polymers; John Wiley: New York, NY, USA, 1991. [Google Scholar]
- Zyss, J. Molecular Nonlinear Optics: Materials, Physics and Devices; Academic Press: Boston, MA, USA, 1994. [Google Scholar]
- Roundhill, D.M.; Fackler, J.P., Jr. Optoelectronic Properties of Inorganic Compounds; Plenum Press: New York, NY, USA, 1999. [Google Scholar]
- Nalwa, H.S. Organometallic materials for nonlinear optics. Appl. Organomet. Chem. 1991, 5, 349–377. [Google Scholar] [CrossRef]
- Calabrese, J.C.; Cheng, L.-T.; Green, J.C.; Marder, S.R.; Tam, W. Molecular second-order optical nonlinearities of metallocenes. J. Am. Chem. Soc. 1991, 113, 7227–7232. [Google Scholar] [CrossRef]
- Long, N.J. Organometallic Compounds for Nonlinear Optics-The Search for En-light-enment! Angew. Chem. Int. Ed. Engl. 1995, 34, 21–38. [Google Scholar] [CrossRef]
- Whittal, I.R.; McDonagh, A.M.; Humphrey, M.G. Organometallic complexes in nonlinear optics I: Second-order nonlinearities. Adv. Organomet. Chem. 1998, 42, 291–362. [Google Scholar]
- Heck, J.; Dabek, S.; Meyer-Friedrichsen, T.; Wong, H. Mono- and dinuclear sesquifulvalene complexes, organometallic materials with large nonlinear optical properties. Coord. Chem. Rev. 1999, 190–192, 1217–1254. [Google Scholar] [CrossRef]
- Le Bozec, H.; Renouard, T. Dipolar and Non-Dipolar Pyridine and Bipyridine Metal Complexes for Nonlinear Optics. Eur. J. Inorg. Chem. 2000, 2, 229–239. [Google Scholar] [CrossRef]
- Powell, C.E.; Humphrey, M.G. Nonlinear optical properties of transition metal acetylides and their derivatives. Coord. Chem. Rev. 2004, 248, 725–756. [Google Scholar] [CrossRef]
- Di Bella, S. Second-order nonlinear optical properties of transition metal complexes. Chem. Soc. Rev. 2001, 30, 355–366. [Google Scholar] [CrossRef]
- Coe, B.J. Nonlinear Optical Properties of Metal Complexes. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Pergamon: Oxford, UK, 2004; Volume 9, pp. 621–687. [Google Scholar]
- Coe, B.J.; Curati, N.R.M. Metal complexes for molecular electronics and photonics. Comments Inorg. Chem. 2004, 25, 147–184. [Google Scholar] [CrossRef]
- Maury, O.; Le Bozec, H. Molecular Engineering of Octupolar NLO Molecules and Materials Based on Bipyridyl Metal Complexes. Acc. Chem. Res. 2005, 38, 691–704. [Google Scholar] [CrossRef]
- Cariati, E.; Pizzotti, M.; Roberto, D.; Tessore, F.; Ugo, R. Coordination and organometallic compounds and inorganic–organic hybrid cristalline materials for second-order non-linear optics. Coord. Chem. Rev. 2006, 250, 1210–1233. [Google Scholar] [CrossRef]
- Coe, B.J. Switchable Nonlinear Optical Metallochromophores with Pyridinium Electron Acceptor Groups. Acc. Chem. Res. 2006, 39, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.J. Ruthenium Complexes as Versatile Chromophores with Large, Switchable Hyperpolarizabilities. In Non-Linear Optical Properties of Matter; Papadopoulos, M.G., Sadlej, A.J., Leszczynski, J., Eds.; Springer: New York, NY, USA, 2006; pp. 571–608. [Google Scholar]
- Morrall, J.P.; Dalton, G.T.; Humphrey, M.G.; Samoc, M. Organotransition Metal Complexes for Nonlinear Optics. Adv. Organomet. Chem. 2007, 55, 61–136. [Google Scholar]
- Di Bella, S.; Dragonetti, C.; Pizzotti, M.; Roberto, D.; Tessore, F.; Ugo, R. Coordination and organometallic complexes as second-order nonlinear optical materials. In Molecular Organometallic Material for Optics; Bozec, H., Guerchais, V., Eds.; Springer: Heidelberg, Germany, 2010; pp. 1–55. [Google Scholar]
- Guerchais, V.; Boixel, J.; Le Bozec, H. Linear and Nonlinear Optical Molecular Switches Based on Photochromic Metal Complexes. In Photon-Working Switches; Yokoyama, Y., Nakatani, K., Eds.; Springer: Tokyo, Japan, 2017; pp. 363–384. [Google Scholar]
- Maury, O.; Viau, L.; Sénéchal, K.; Corre, B.; Guégan, J.P.; Renouard, T.; Ledoux, I.; Zyss, J.; Le Bozec, H. Synthesis, Linear, and Quadratic-Nonlinear Optical Properties of Octupolar D3 and D2d Bipyridyl Metal Complexes. Chem. Eur. J. 2004, 10, 4454–4466. [Google Scholar] [CrossRef]
- Righetto, S.; Rondena, S.; Locatelli, D.; Roberto, D.; Tessore, F.; Ugo, R.; Quici, S.; Roma, S.; Korystov, D.; Srdanov, V. An investigation on the two-photon absorption activity of various terpyridines and related homoleptic and heteroleptic cationic Zn(II) complexes. J. Mater. Chem. 2006, 16, 1439–1444. [Google Scholar] [CrossRef]
- Mazzucato, S.; Fortunati, I.; Scolaro, S.; Zerbetto, M.; Ferrante, C.; Signorini, R.; Pedron, D.; Bozio, R.; Locatelli, D.; Righetto, S.; et al. Two-photon absorption of Zn(II) octupolar molecules. Phys. Chem. Chem. Phys. 2007, 9, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Dragonetti, C.; Balordi, M.; Colombo, A.; Roberto, D.; Ugo, R.; Fortunati, I.; Garbin, E.; Ferrante, C.; Bozio, R.; Abbotto, A.; et al. Two-photon absorption properties of Zn(II) complexes: Unexpected large TPA cross section of dipolar [ZnY2(4,4’-bis(para-di-n-butylaminostyryl)-2,2’-bipyridine)] (Y = Cl, CF3CO2). Chem. Phys. Lett. 2009, 475, 245–249. [Google Scholar] [CrossRef]
- Grisanti, L.; Sissa, C.; Terenziani, F.; Painelli, A.; Roberto, D.; Tessore, F.; Ugo, R.; Quici, S.; Fortunati, I.; Garbin, E.; et al. Enhancing the efficiency of two-photon absorption by metal coordination. Phys. Chem. Chem. Phys. 2009, 11, 9450–9457. [Google Scholar] [CrossRef] [PubMed]
- Annoni, E.; Pizzotti, M.; Ugo, R.; Quici, S.; Morotti, T.; Bruschi, M.; Mussini, P. Synthesis, Electronic characterization and Significant Second Order Non Linear Optical Responses of meso Tetraphenylporphyrins and their Zn(II) Complexes Carrying a Push or Pull Group in β Pyrrolic Position. Eur. J. Inorg. Chem. 2005, 3857–3874. [Google Scholar] [CrossRef]
- Morotti, T.; Pizzotti, M.; Ugo, R.; Quici, S.; Bruschi, M.; Mussini, P.; Righetto, S. Electronic Characterisation and Significant Second Order NLO response of 10,20-Diphenylporphyrins and their Zn(II) complexes Substituted in the meso position with π-Delocalised Linkers Carrying Push or Pull Groups. Eur. J. Inorg. Chem. 2006, 1743–1757. [Google Scholar] [CrossRef]
- Tessore, F.; Orbelli Biroli, A.; Di Carlo, G.; Pizzotti, M. Porphyrins for Second Order Nonlinear Optics (NLO): An Intriguing History. Inorganics 2018, 6, 81. [Google Scholar] [CrossRef]
- Oudar, J.L.; Chemla, D.S. Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 1977, 66, 2664–2668. [Google Scholar] [CrossRef]
- Oudar, J.L. Optical nonlinearities of conjugated molecule. Stilbene derivatives and highly polar aromatic compounds. J. Chem. Phys. 1977, 67, 446–457. [Google Scholar] [CrossRef]
- Oudar, J.L.; Le Person, H. Second-order polarizabilities of some aromatic molecules. Opt. Commun. 1975, 15, 258–262. [Google Scholar] [CrossRef]
- Di Bella, S. On the determination of the molecular static first hyperpolarisability: How reliable are literature data? New J. Chem. 2002, 26, 495–497. [Google Scholar] [CrossRef]
- Roberto, D.; Ugo, R.; Bruni, S.; Cariati, E.; Cariati, F.; Fantucci, P.C.; Invernizzi, I.; Quici, S.; Ledoux, I.; Zyss, J. Quadratic Hyperpolarizability Enhancement of para-Substituted Pyridines upon Coordination to Organometallic Moieties: The Ambivalent Donor or Acceptor Role of the Metal. Organometallics 2000, 19, 1775–1788. [Google Scholar] [CrossRef]
- Ledoux, I.; Zyss, J. Influence of the molecular environment in solution measurements of the Second-order optical susceptibility for urea and derivatives. J. Chem. Phys. 1982, 73, 203–213. [Google Scholar] [CrossRef]
- Maker, P.D. Spectral broadening of elastic second-harmonic light scattering in liquids. Phys. Rev. 1970, 1, 923–951. [Google Scholar] [CrossRef]
- Clays, K.; Pearson, A. Hyper-Rayleigh Scattering in Solution. Phys. Rev. Lett. 1991, 66, 2980–2983. [Google Scholar] [CrossRef]
- Zyss, J.; Ledoux, I. Nonlinear optics in multipolar media: Theory and experiments. Chem. Rev. 1994, 94, 77–105. [Google Scholar] [CrossRef]
- Roberto, D.; Ugo, R.; Tessore, F.; Lucenti, E.; Quici, S.; Vezza, S.; Fantucci, P.C.; Invernizzi, I.; Bruni, S.; Ledoux-Rak, I.; et al. Effect of the Coordination to M(II) Metal Centers (M = Zn, Cd, Pt) on the Quadratic Hyperpolarizability of Various Substituted 5-X-1,10-phenanthrolines (X = Donor Group) and of trans-4-(Dimethylamino)-4′-stilbazole. Organometallics 2002, 21, 161–170. [Google Scholar] [CrossRef]
- Tessore, F.; Roberto, D.; Ugo, R.; Mussini, P.; Quici, S.; Ledoux-Rak, I.; Zyss, J. Large, Concentration-Dependent Enhancement of the Quadratic Hyperpolarizability of [Zn(CH3CO2)2(L)2] in CHCl3 on Substitution of Acetate by Triflate. Angew. Chem. Int. Ed. 2003, 42, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Tessore, F.; Locatelli, D.; Righetto, S.; Roberto, D.; Ugo, R.; Mussini, P. An Investigation on the Role of the Nature of Sulfonate Ancillary Ligands on the Strength and Concentration Dependence of the Second-Order NLO Responses in CHCl3 of Zn(II) Complexes with 4,4′-trans-NC5H4CH=CHC6H4NMe2 and 4,4′-trans,trans-NC5H4(CH=CH)2C6H4NMe2. Inorg. Chem. 2005, 44, 2437–2442. [Google Scholar] [PubMed]
- Lucenti, E.; Cariati, E.; Dragonetti, C.; Manassero, L.; Tessore, F. Effect of the Coordination to the “Os3(CO)11” Cluster Core on the Quadratic Hyperpolarizability of trans-4-(4′-X-styryl)pyridines (X = NMe2, t-Bu, CF3) and trans,trans-4-(4′-NMe2-phenyl-1,3-butadienyl)pyridine. Organometallics 2004, 23, 687–692. [Google Scholar]
- Bourgault, M.; Mountassir, C.; Le Bozec, H.; Ledoux, I.; Pucetti, G.; Zyss, J. Synthesis and second-order nonlinear optical properties of new bipyridyl metal complexes. J. Chem. Soc. Chem. Commun. 1993, 1623–1624. [Google Scholar] [CrossRef]
- Bourgault, M.; Baum, K.; Le Bozec, H.; Pucetti, G.; Ledoux, I.; Zyss, J. Synthesis and molecular hyperpolarisabilities of donor–acceptor bipyridyl metal complexes (M = Re, Zn, Hg). New J. Chem. 1998, 517–522. [Google Scholar] [CrossRef]
- Hilton, A.; Renouard, T.; Maury, O.; Le Bozec, H.; Ledoux, I.; Zyss, J. New bipyridyl ligands bearing azo- and imino-linked chromophores. Synthesis and nonlinear optical studies of related dipolar zinc complexes. Chem. Commun. 1999, 2521–2522. [Google Scholar] [CrossRef]
- Todescato, F.; Fortunati, I.; Carlotto, S.; Ferrante, C.; Grisanti, L.; Sissa, C.; Painelli, A.; Colombo, A.; Dragonetti, C.; Roberto, D. Dimers of polar chromophores in solution: Role of excitonic interactions in one- and two-photon absorption properties. Phys. Chem. Chem. Phys. 2011, 13, 11099–11109. [Google Scholar] [CrossRef] [PubMed]
- Aubert, V.; Guerchais, V.; Ishow, E.; Hoang-Thy, K.; Ledoux, I.; Nakatani, K.; Le Bozec, H. Efficient Photoswitching of the Nonlinear Optical Properties of Dipolar Photochromic Zinc(II) Complexes. Angew. Chem. Int. Ed. 2008, 47, 577–580. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Valore, A.; Benincori, T.; Colombo, F.; Sannicolò, F. Novel highly conjugated push-pull 4,5-diazafluoren-9-ylidene based efficient NLO chromophores as a springboard for coordination complexes with large second-order NLO properties. J. Mat. Chem. 2012, 22, 19761–19766. [Google Scholar] [CrossRef]
- Das, S.; Jana, A.; Ramanathan, V.; Chakraborty, T.; Ghosh, S.; Das, P.K.; Bharadwaj, P.K. Design and synthesis of 1,10-phenanthroline based Zn(II) complexes bearing 1D push–pull NLO-phores for tunable quadratic nonlinear optical properties. J. Organomet. Chem. 2006, 691, 2512–2516. [Google Scholar] [CrossRef]
- Roberto, D.; Tessore, F.; Ugo, R.; Bruni, S.; Manfredi, A.; Quici, S. Terpyridine Zn(II), Ru(III) and Ir(III) complexes as new asymmetric chromophores for nonlinear optics: First evidence for a shift from positive to negative value of the quadratic hyperpolarizability of a ligand carrying an electron donor substituent upon coordination to different metal centres. Chem. Commun. 2002, 846–847. [Google Scholar]
- Tessore, F.; Roberto, D.; Ugo, R.; Pizzotti, M.; Quici, S.; Cavazzini, M.; Bruni, S.; De Angelis, F. Terpyridine Zn(II), Ru(III), and Ir(III) Complexes: The Relevant Role of the Nature of the Metal Ion and of the Ancillary Ligands on the Second-Order Nonlinear Response of Terpyridines Carrying Electron Donor or Electron Acceptor Groups. Inorg. Chem. 2005, 44, 8967–8978. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, D.; Quici, S.; Righetto, S.; Roberto, D.; Tessore, F.; Ashwell, G.J.; Amiri, M. Second-harmonic generation from monolayer Langmuir-Blodgett films of various push–pull pyridine and terpyridine metal complexes. Prog. Solid State Chem. 2005, 33, 223–232. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragalà, I.; Ledoux, I.; Diaz-Garcia, M.A.; Marks, T.J. Synthesis, Characterization, Optical Spectroscopic, Electronic Structure, and Second-Order Nonlinear Optical (NLO) Properties of a Novel Class of Donor−Acceptor Bis(salicylaldiminato)nickel(II) Schiff Base NLO Chromophores. J. Am. Chem. Soc. 1997, 119, 9550–9557. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragalà, I.; Ledoux, I.; Zyss, J. Dipolar Donor‒Acceptor-Substituted Schiff Base Complexes with Large Off-Diagonal Second-Order Nonlinear Optical Tensor Components. Chem. Eur. J. 2001, 7, 3738–3743. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragalà, I.; Guerri, A.; Dapporto, P.; Nakatani, K. Synthesis, crystal structure, and second-order nonlinear optical properties of [N,N′-bis(1H-pyrrol-2-ylmethylene)-1,2-benzenediaminato]nickel(II) Schiff base complexes. Inorg. Chim. Acta 2004, 357, 1161–1167. [Google Scholar] [CrossRef]
- Costes, J.P.; Lamère, J.F.; Lepetit, C.; Lacroix, P.G.; Dahan, F.; Kakatani, K. Synthesis, Crystal Structures, and Nonlinear Optical (NLO) Properties of New Schiff-Base Nickel(II) Complexes. Toward a New Type of Molecular Switch? Inorg. Chem. 2005, 44, 1973–1982. [Google Scholar] [CrossRef]
- Rigamonti, L.; Demartin, F.; Forni, A.; Righetto, S.; Pasini, A. Copper(II) Complexes of salen Analogues with Two Differently Substituted (Push–Pull) Salicylaldehyde Moieties. A Study on the Modulation of Electronic Asymmetry and Nonlinear Optical Properties. Inorg. Chem. 2006, 45, 10976–10989. [Google Scholar] [CrossRef]
- Trujillo, A.; Fuentealba, M.; Carrillo, D.; Manzur, C.; Ledoux-Rak, I.; Hamon, J.-R.; Saillard, J.-Y. Synthesis, Spectral, Structural, Second-Order Nonlinear Optical Properties and Theoretical Studies On New Organometallic Donor–Acceptor Substituted Nickel(II) and Copper(II) Unsymmetrical Schiff-Base Complexes. Inorg. Chem. 2010, 49, 2750–2764. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragalà, I. Synthesis and second-order nonlinear optical properties of bis(salicylaldiminato)M(II) metalloorganic materials. Synth. Met. 2000, 115, 191–196. [Google Scholar] [CrossRef]
- Lacroix, P.G. Second-Order Optical Nonlinearities in Coordination Chemistry: The Case of Bis(salicylaldiminato)metal Schiff Base Complexes. Eur. J. Inorg. Chem. 2001, 339–348. [Google Scholar] [CrossRef]
- Nayar, C.R.; Ravikumar, R. Second order nonlinearities of Schiff bases derived from salicylaldehyde and their metal complexes. J. Coord. Chem. 2014, 67, 1–16. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Lacroix, P.G.; Di Bella, S.; Ledoux, I. Synthesis and Second-Order Nonlinear Optical Properties of New Copper(II), Nickel(II), and Zinc(II) Schiff-Base Complexes. Toward a Role of Inorganic Chromophores for Second Harmonic Generation. Chem. Mater. 1996, 8, 541–545. [Google Scholar] [CrossRef]
- Di Bella, S.; Oliveri, I.P.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D. An unprecedented switching of the second-order nonlinear optical response in aggregate bis(salicylaldiminato)zinc(II) Schiff-base complexes. Dalton Trans. 2012, 41, 7013–7016. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Oliveri, I.P.; Consiglio, G.; Failla, S.; Di Bella, S. On the Lewis acidic character of bis(salicylaldiminato)zinc(II) Schiff-base complexes: A computational and experimental investigation on a series of compounds varying the bridging diamine. Dalton Trans. 2017, 46, 4571–4581. [Google Scholar] [CrossRef]
- Consiglio, G.; Failla, S.; Finocchiaro, P.; Oliveri, I.P.; Purrello, R.; Di Bella, S. Supramolecular Aggregation/Deaggregation in Amphiphilic Dipolar Schiff-Base Zinc(II) Complexes. Inorg. Chem. 2010, 49, 5134–5142. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Di Bella, S. Lewis basicity of relevant monoanions in a non-protogenic organic solvent using a zinc(II) Schiff-base complex as reference Lewis acid. Dalton Trans. 2017, 46, 11608–11614. [Google Scholar] [CrossRef]
- Gradinaru, J.; Forni, A.; Druta, V.; Tessore, F.; Zecchin, S.; Quici, S.; Garbalau, N. Structural, Spectral, Electric-Field-Induced Second Harmonic, and Theoretical Study of Ni(II), Cu(II), Zn(II), and VO(II) Complexes with [N2O2] Unsymmetrical Schiff Bases of S-Methylisothiosemicarbazide Derivatives. Inorg. Chem. 2007, 46, 884–895. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Failla, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Di Bella, S. Synthesis, characterization, optical absorption/fluorescence spectroscopy, and second-order nonlinear optical properties of aggregate molecular architectures of unsymmetrical Schiff-base zinc(II) complexes. Dalton Trans. 2014, 43, 2168–2175. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragalà, I.; Ratner, M.A.; Marks, T.J. Chromophore Environmental Effects in Saltlike Nonlinear Optical Materials. A Computational Study of Architecture/Anion Second-Order Response Relationships in High-β Stilbazolium Self-Assembled Films. Chem. Mater. 1995, 7, 400–404. [Google Scholar] [CrossRef]
- Evans, C.; Luneau, D. New Schiff base zinc(II) complexes exhibiting second harmonic generation. J. Chem. Soc. Dalton Trans. 2002, 83–86. [Google Scholar] [CrossRef]
- Sénéchal, K.; Maury, O.; Le Bozec, H.; Ledoux, I.; Zyss, J. Zinc(II) as a Versatile Template for the Design of Dipolar and Octupolar NLO-phores. J. Am. Chem. Soc. 2002, 124, 4560–4561. [Google Scholar] [CrossRef]
- Akdas-Kilig, H.; Malval, J.-P.; Morlet-Savary, F.; Singh, A.; Toupet, L.; Ledoux-Rak, I.; Zyss, J.; Le Bozec, H. The synthesis of tetrahedral bipyridyl metallo-octupoles with large second- and third-order nonlinear optical properties. Dyes Pigment. 2011, 92, 681–688. [Google Scholar] [CrossRef]
- Coe, B.J.; Foxon, S.P.; Helliwell, M.; Rusanova, D.; Brunschwig, B.S.; Clays, K.; Depotter, G.; Nyk, M.; Samoc, M.; Wawrzynczyk, D.; et al. Heptametallic, Octupolar Nonlinear Optical Chromophores with Six Ferrocenyl Substituents. Chem. Eur. J. 2013, 19, 6613–6629. [Google Scholar] [CrossRef]
- Fiorini, C.; Charra, F.; Nunzi, J.-M.; Samuel, I.D.W.; Zyss, J. Light-induced second-harmonic generation in an octupolar dye. Opt. Lett. 1995, 20, 2469–2471. [Google Scholar] [CrossRef]
- Viau, L.; Bidault, S.; Maury, O.; Brasselet, S.; Ledoux, I.; Zyss, J.; Ishow, E.; Nakatani, K.; Le Bozec, H. All-Optical Orientation of Photoisomerizable Octupolar Zinc(II) Complexes in Polymer Films. J. Am. Chem. Soc. 2004, 126, 8386–8387. [Google Scholar] [CrossRef]
- Bidault, S.; Viau, L.; Maury, O.; Brasselet, S.; Zyss, J.; Ishow, E.; Nakatani, K.; Le Bozec, H. Optically Tunable Nonlinearities in Polymers Based on Photoisomerizable Metal-Based Coordination Complexes. Adv. Funct. Mater. 2006, 16, 2252–2262. [Google Scholar] [CrossRef]
- Ordronneau, L.; Aubert, V.; Guerchais, V.; Boucekkine, A.; Le Bozec, H.; Singh, A.; Ledoux, I.; Jacquemin, D. The First Hexadithienylethene-Substituted Tris(bipyridine)metal Complexes as Quadratic NLO Photoswitches: Combined Experimental and DFT Studies. Chem. Eur. J. 2013, 19, 5845–5849. [Google Scholar] [CrossRef]
- Sanhueza, L.; Cortés-Arriagada, D.; Ledoux-Rak, I.; Crivelli, I.; Loeb, B. Nonlinear optical response of octupolar Zn(II) complexes incorporating highly aromatic polypyridinic ligands: Insights into the role of the metal center. Synth. Met. 2017, 234, 9–17. [Google Scholar] [CrossRef]
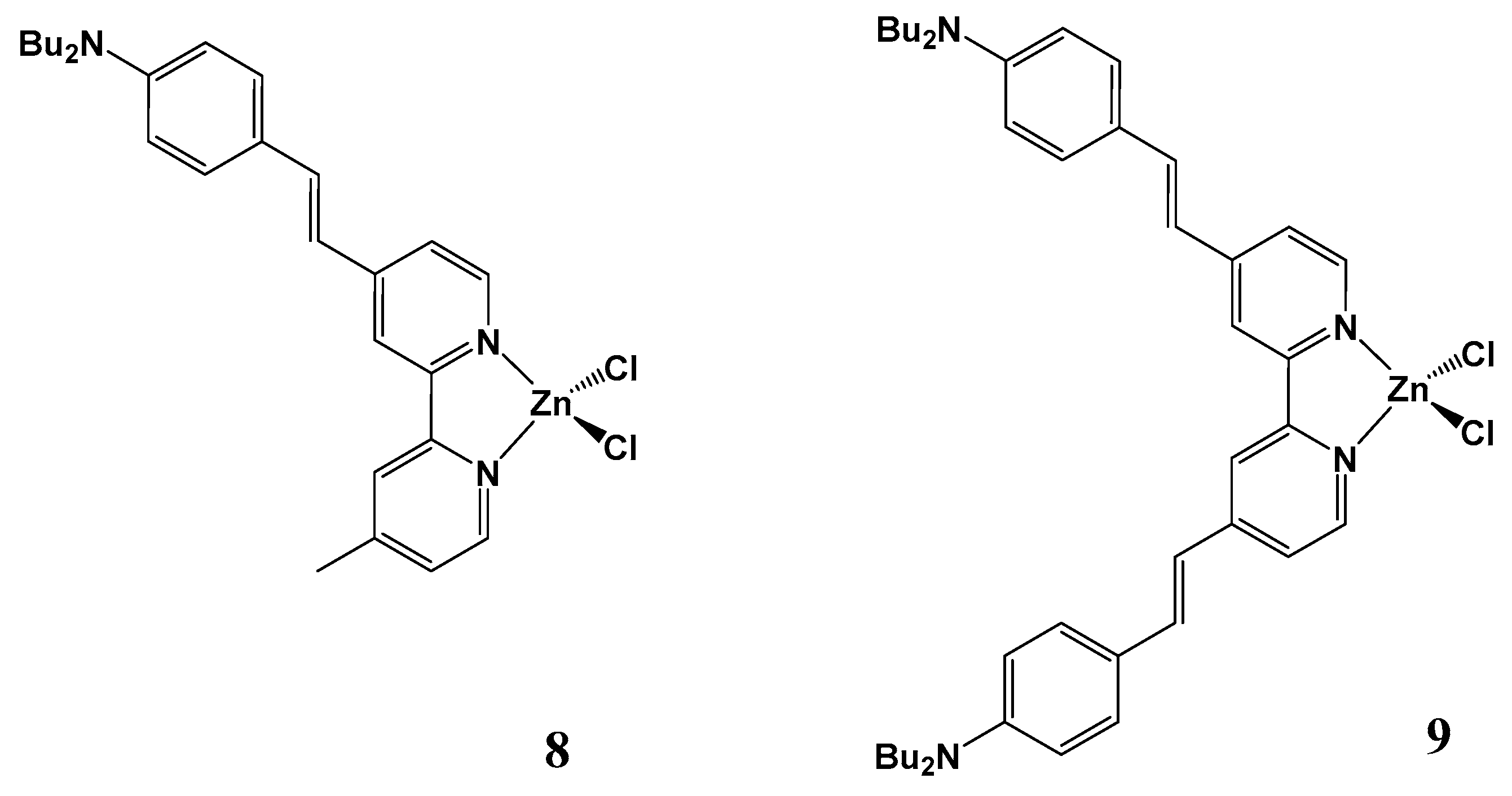
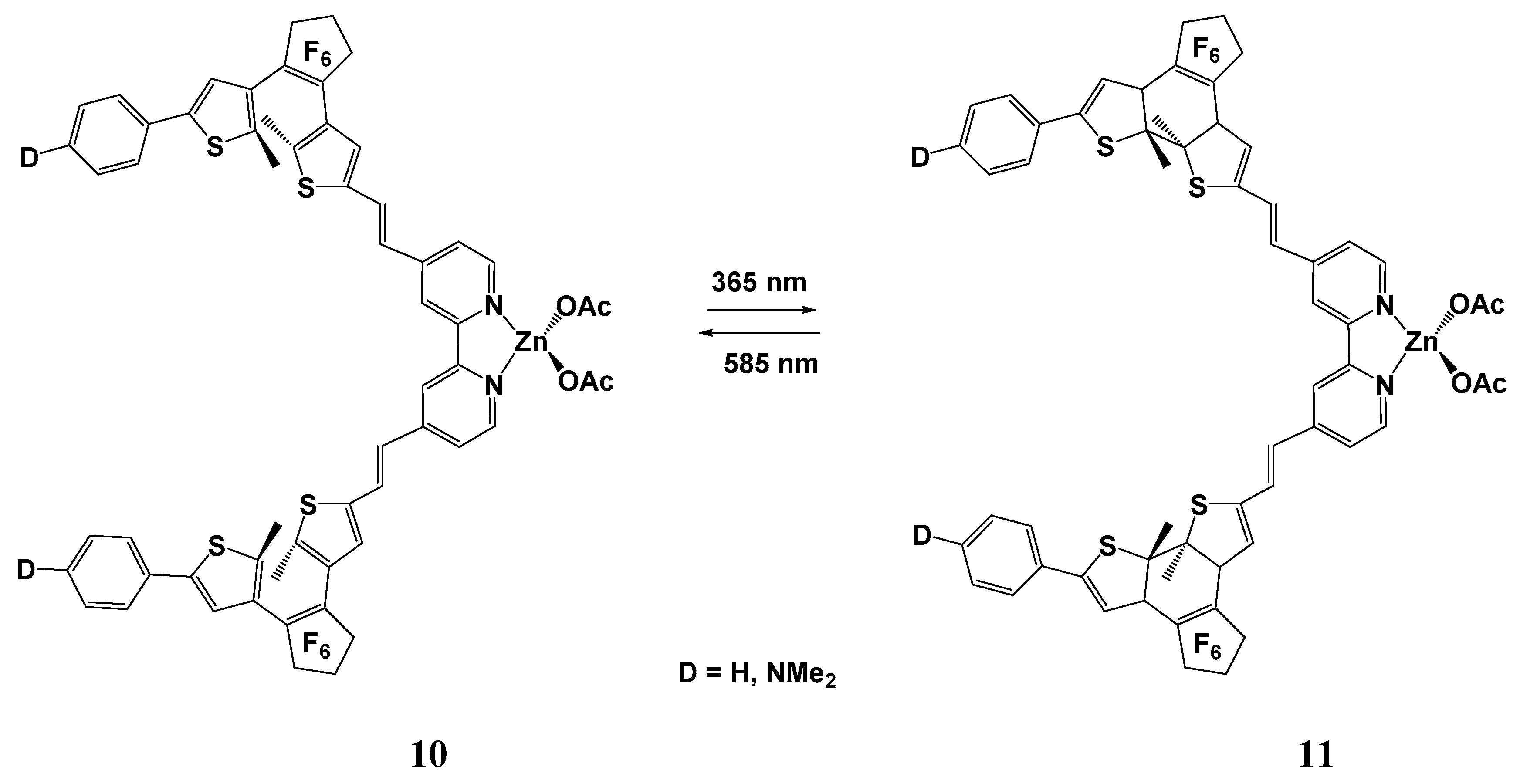
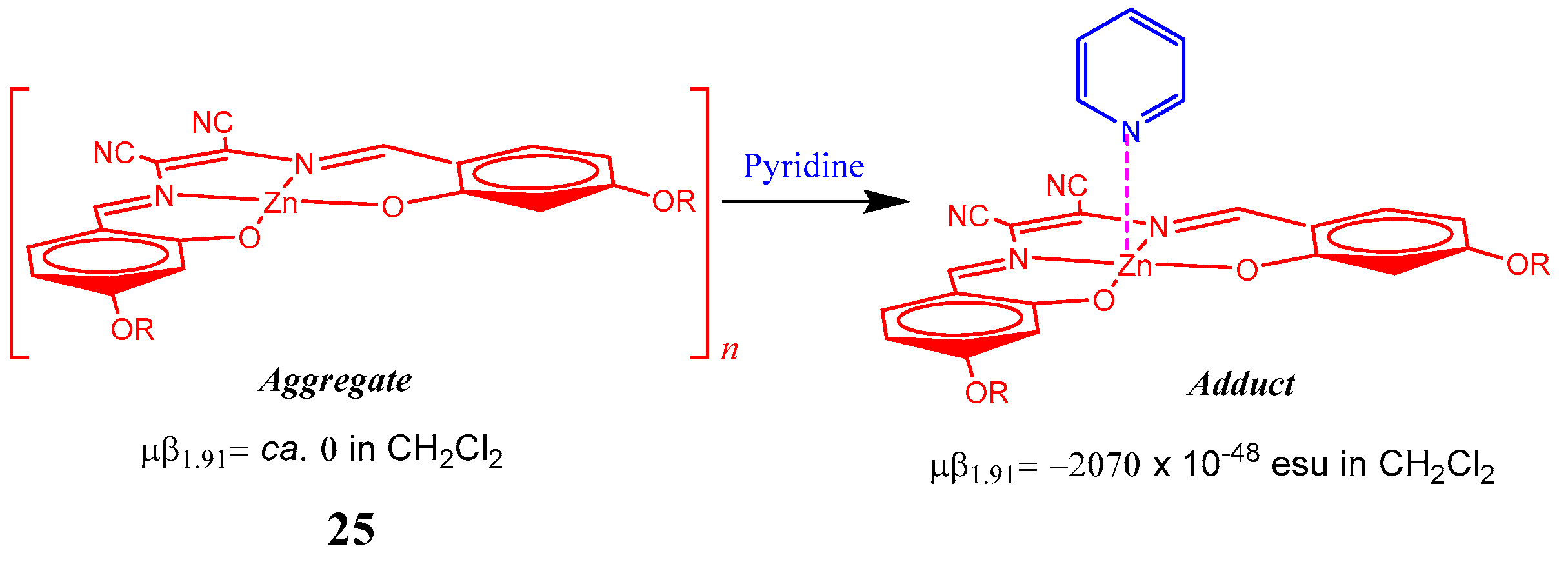
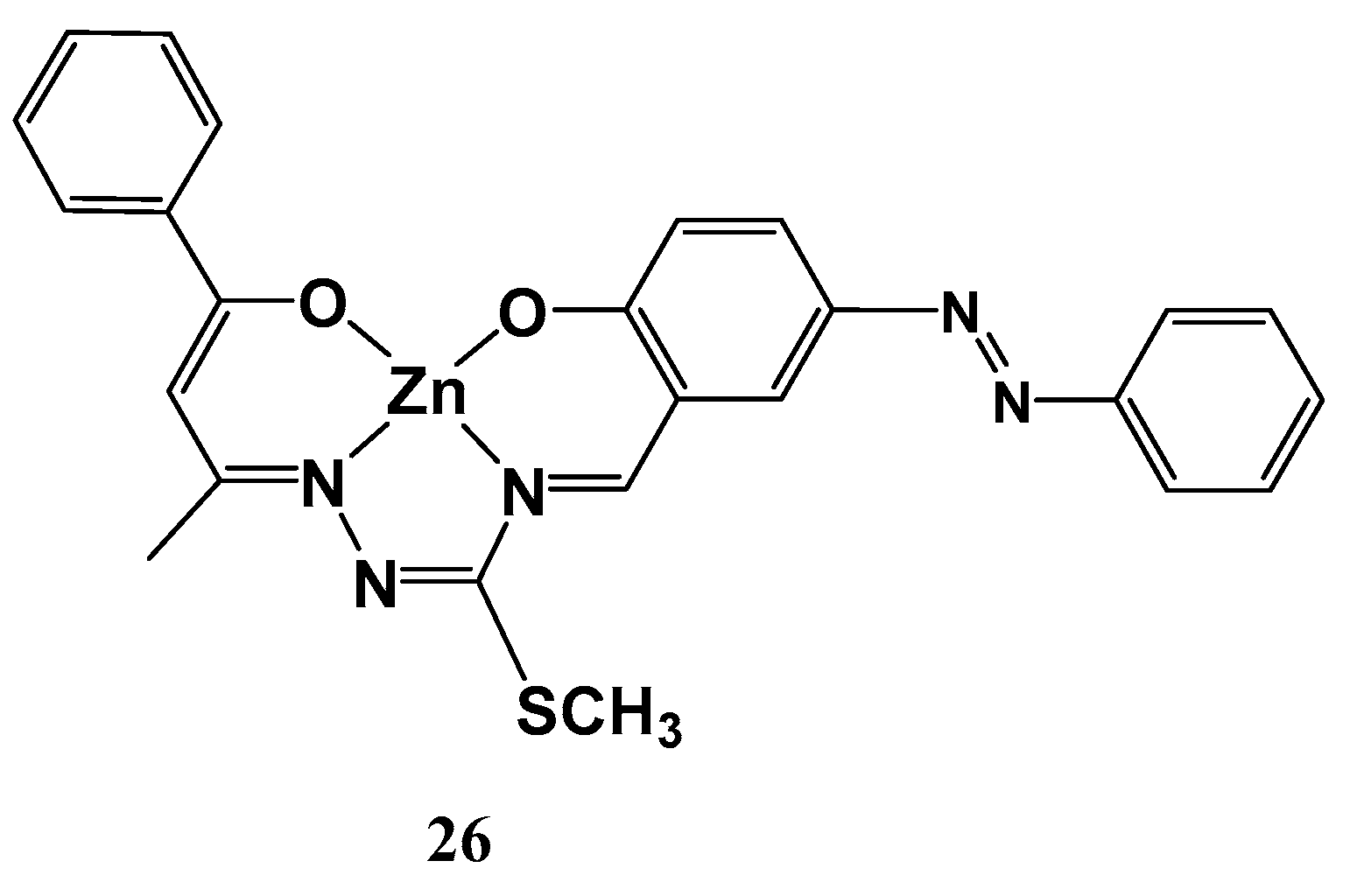



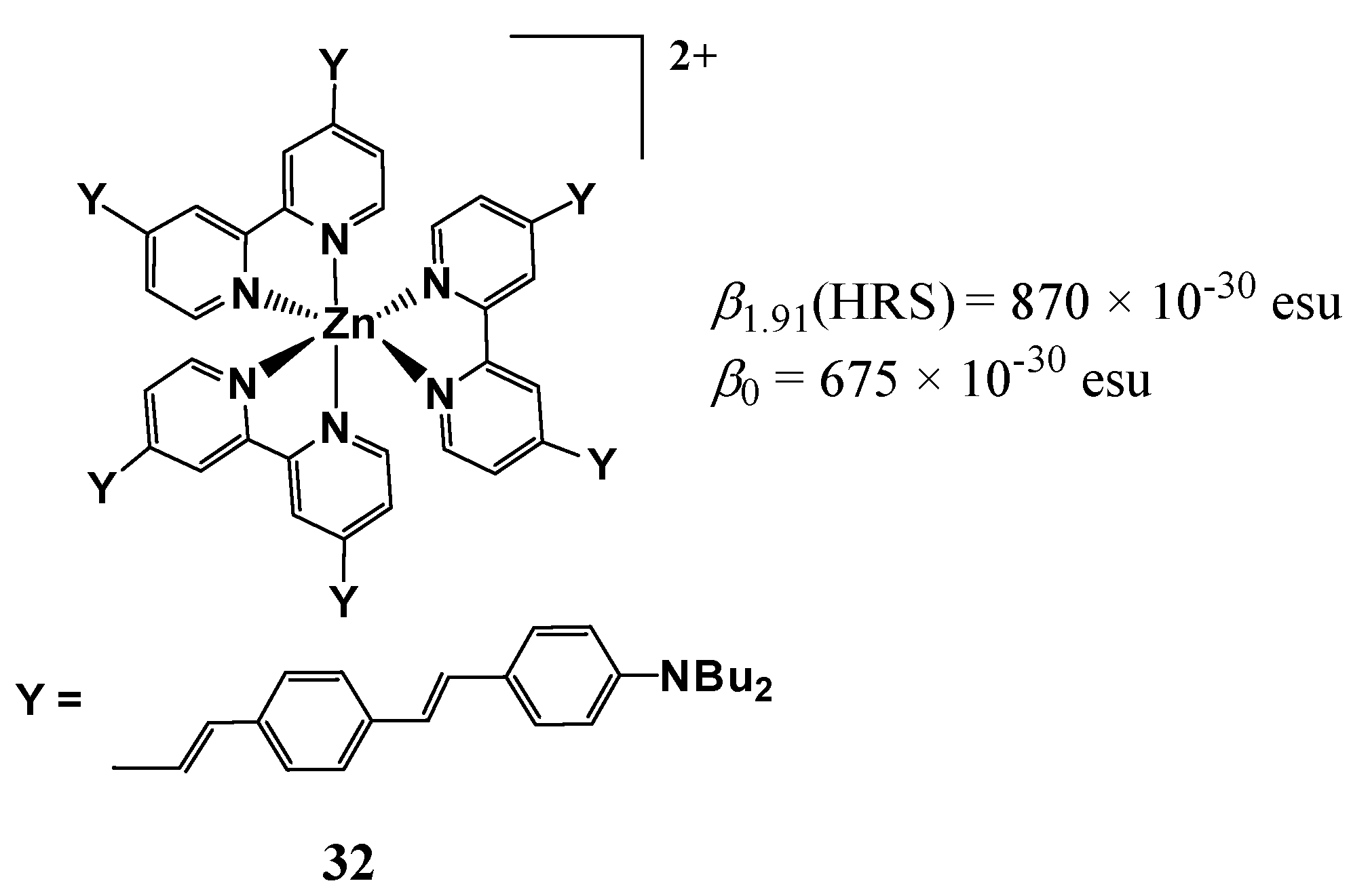
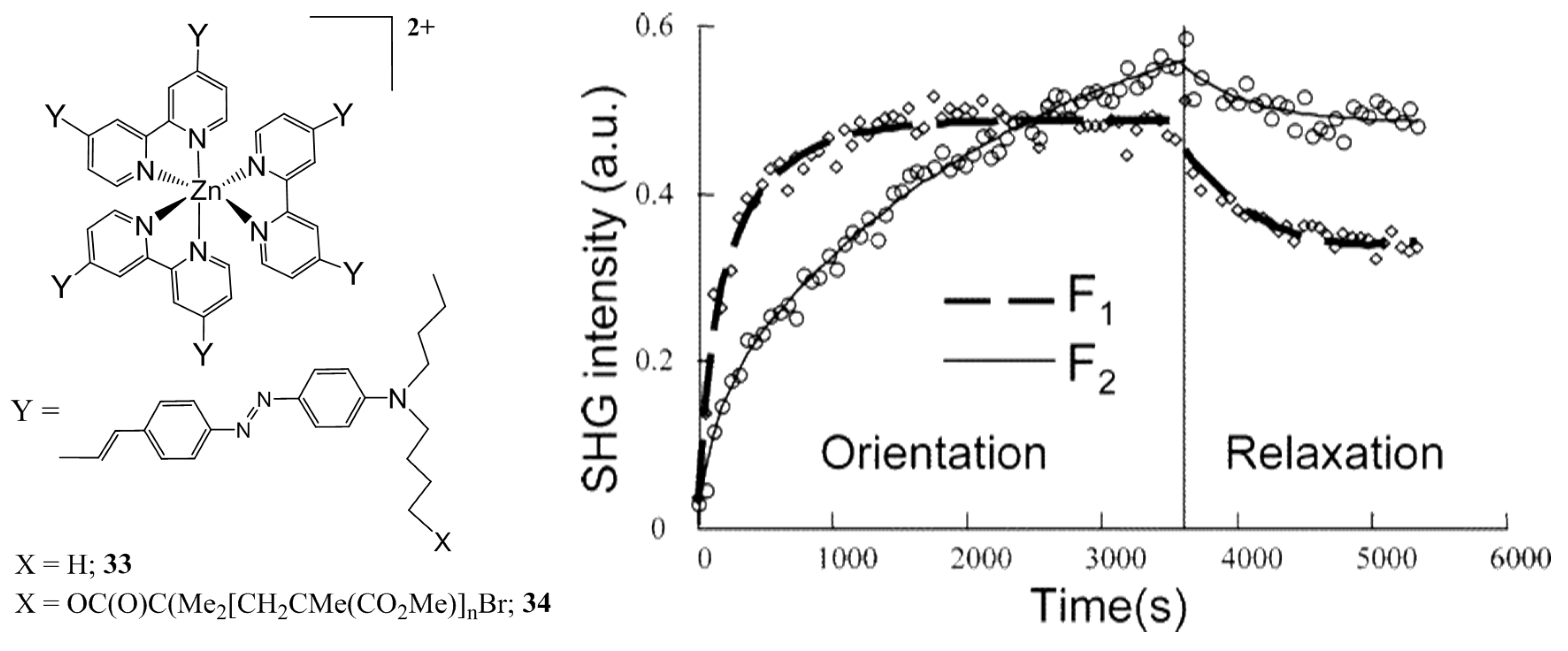
| Molecule | Y (Complex) | λmax (nm) | µβ1.91(EFISH) (10−48 esu) a | μ (10−18 esu) | β1.91(EFISH) (10−30 esu) |
|---|---|---|---|---|---|
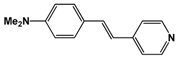 | 374 | 136 | 3.9 | 35 | |
 | CH3CO2 (1) | 376 | 316 | 8.0 | 39 |
| CF3CO2 (2) | 420 | 512 | 10.5 | 49 | |
| CF3SO3 (3) | 490 | 2715 | 16.7 | 163 | |
| CH3SO3 (4) | 475 | 450 | 15.5 | 29 | |
| p-CH3C6H4SO3 (5) | 476 | 428 | 16.0 | 27 | |
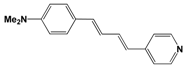 | 396 | 310 | 4.5 | 69 | |
 | CH3CO2 (6) | 406 | 680 | 6.7 | 101 |
| CF3SO3 (7) | 519 | 3840 | 14.7 | 261 |
| Molecule | R (Complex) | λmax (nm) | µβ1.34(EFISH) (10−48 esu) a | µ (10−18 esu) | β1.34(EFISH) (10−30 esu) |
|---|---|---|---|---|---|
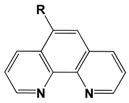 | OMe | 272 | 16 | 4.0 | 4 |
| NMe2 | 328 | 27 | 3.8 | 7.2 | |
| trans- CH=CHC6H4-4′-NMe2 | 371 | 201 | 4.9 | 41 | |
| trans,trans- (CH=CH)2C6H4-4′-NMe2 | 399 | 368 | 4.9 | 75 | |
 | OMe (12) | 284 | 99 | 7.6 | 13 |
| NMe2 (13) | 344 | 254 | 7.7 | 33 | |
| trans- CH=CHC6H4-4′-NMe2 (14) | 419 | 616 | 8.0 | 77 | |
| trans,trans- (CH=CH)2C6H4-4′-NMe2 (15) | 432 | 862 | 7.7 | 112 |
| Molecule | Y (Complex) | λmax (nm) | Concentration (10−4 M) | µβ1.91(EFISH) (10−48 esu) |
|---|---|---|---|---|
 | 485 | 10 | 998 | |
 | CH3CO2 (16) | 490 | 10 | 1230 |
| CF3CO2 (17) | 548 | 10 | 1900 | |
| CF3SO3 (18) | 560 | 10 | 2230 | |
| 5 | 3170 | |||
| 1 | 5750 | |||
| 0.5 | 12,000 | |||
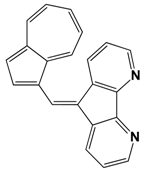 | 439 | 10 | 760 | |
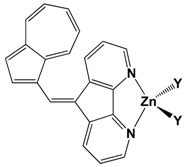 | CF3CO2 (19) | 447 | 10 | 1320 |
| CF3SO3 (20) | 486 | 10 | 1640 | |
| 0.5 | 3570 |
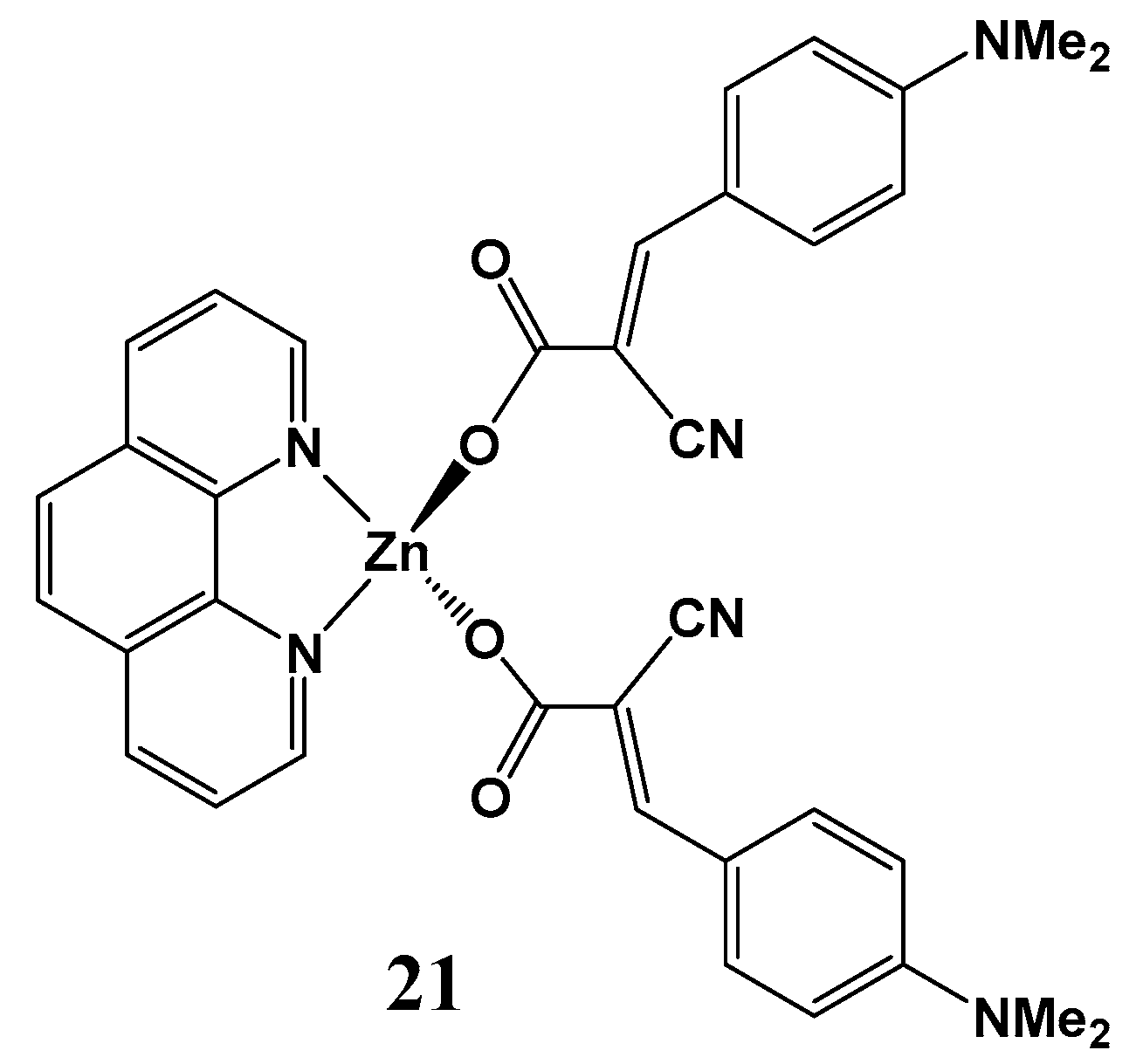
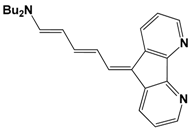
| Molecule | R (Complex) | λmax (nm) | µβ1.34(EFISH) (10−48 esu) a | µ (10−18 esu) | β1.34(EFISH) (10−30 esu) |
|---|---|---|---|---|---|
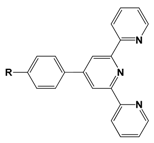 | NBu2 | 360 | 46 | 2.1 | 22 |
| trans- CH=CHC6H4-p-NBu2 | 395 | 187 | 3.6 | 52 | |
| trans,trans- (CH=CH)2C6H4-p-NMe2 | 399 | 370 | 3.9 | 95 | |
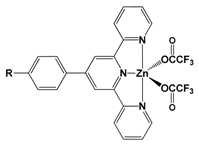 | NBu2 (22) | 427 | 880 | 10 | 88 |
| trans- CH=CHC6H4-p-NBu2 (23) | 454 | 1502 | 8.3 | 181 | |
| trans,trans- (CH=CH)2C6H4-p-NMe2 (24) | 444 | 1219 | 8.9 | 137 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bella, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D. Zinc(II) as a Versatile Template for Efficient Dipolar and Octupolar Second-Order Nonlinear Optical Molecular Materials §. Inorganics 2018, 6, 133. https://doi.org/10.3390/inorganics6040133
Di Bella S, Colombo A, Dragonetti C, Righetto S, Roberto D. Zinc(II) as a Versatile Template for Efficient Dipolar and Octupolar Second-Order Nonlinear Optical Molecular Materials §. Inorganics. 2018; 6(4):133. https://doi.org/10.3390/inorganics6040133
Chicago/Turabian StyleDi Bella, Santo, Alessia Colombo, Claudia Dragonetti, Stefania Righetto, and Dominique Roberto. 2018. "Zinc(II) as a Versatile Template for Efficient Dipolar and Octupolar Second-Order Nonlinear Optical Molecular Materials §" Inorganics 6, no. 4: 133. https://doi.org/10.3390/inorganics6040133
APA StyleDi Bella, S., Colombo, A., Dragonetti, C., Righetto, S., & Roberto, D. (2018). Zinc(II) as a Versatile Template for Efficient Dipolar and Octupolar Second-Order Nonlinear Optical Molecular Materials §. Inorganics, 6(4), 133. https://doi.org/10.3390/inorganics6040133