Abstract
Crystals of LiCs2[N(CN)2]3 were obtained from the reaction of stoichiometric amounts of aqueous solutions of LiCl and CsBr with Ag[N(CN)2]. X-ray single-crystal structure analysis showed that LiCs2[N(CN)2]3 crystallizes isotypically to NaCs2[N(CN)2]3 and adopts the hexagonal space group P63/m (No. 176), with a = 6.8480(8), c = 14.1665(17) Å, and Z = 2. The IR and Raman spectra of the title compound exhibit modes typical for the dicyanamide anion.
1. Introduction
Nitrogen-based materials are interesting not only for research purposes but also for industrial applications. Whether used as fertilizers, high-performance steel coatings, or lithium-ion battery materials [1,2], the research on nitrogen compounds has advanced and recently focused on complex nitrogen-containing compounds opposed to simple nitrides [3]. One interesting inorganic moiety is the boomerang-shaped dicyanamide anion [N(CN)2]− which is often dubbed as [dca]−. This [dca] species has been found to allow for a rich diversity of compounds, simply due to its chemical stability as well as its ability to coordinate metal ions through terminal and/or bridging nitrogen atoms. Whenever coordination with all three nitrogen atoms of the [dca] anion occurs, ferromagnetic and antiferromagnetic transition-metal compounds with a rutile-like structure result [4]. On the other hand, [dca]− also forms one-dimensional (1D)- and two-dimensional (2D)-structures, which makes this anion interesting for crystal engineering [5]. Moreover, some dicyanamides show promise as water-oxidation catalysts [6], whilst Li[dca] has been suggested as a potential lithium-ion battery material [7]. In total, there is an enormous variety of pseudo-binary dicyanamide compounds available with examples known for ammonium [8], alkali metals [9,10,11,12], alkaline-earth metals [13], transition metals [4,5,6,14,15,16,17,18,19], and rare-earth metals [20,21,22]. Additionally, a number of pseudo-ternary dicyanamides has also been reported with KCs[dca]2 [23], LiK[dca]2 [24], LiRb[dca]2 [24], NaRb2[dca]3∙H2O [23], and NaCs2[dca]3 [25]. We here report the synthesis and single-crystal structure determination of the new pseudo-ternary compound LiCs2[dca]3 according to its IR and Raman spectra.
2. Results and Discussion
2.1. Structural Description and Discussion
LiCs2[dca]3 crystallizes isotypically to NaCs2[dca]3 [25] in the hexagonal space group P63/m (No. 176) with a = 6.8480(4), c = 14.1665(17) Å, and Z = 2. The lattice parameters of LiCs2[dca]3 are smaller than those of NaCs2[dca]3 (a = 7.0001(4), c = 14.4929(7) Å) due to the smaller cationic size of lithium compared to sodium. The boomerang-shaped dicyanamide anion exhibits bond lengths and angles consistent with those given in the literature: the bond length of d(C–N1) = 1.16 Å of the terminal C–N pairs indicates a triple bond, while the central C–N pairing with d(C–N2) = 1.31 Å is found to be in the expected distance range of a C–N single bond. The angles of the [dca] anion are also typically found for such a moiety with ∡(N1–C–N2) = 172.1° and ∡(C–N1–C) = 119.8° (Figure 1, Table 1).
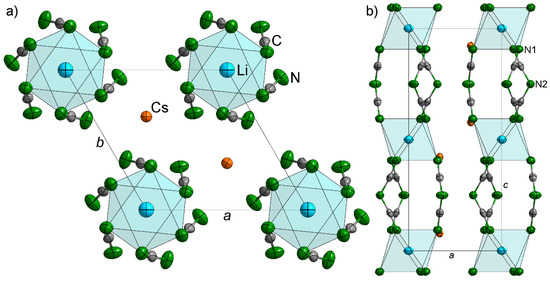
Figure 1.
Crystal structure of LiCs2[dca]3 with (a) viewed along the c axis and (b) along the b axis. The thermal ellipsoids correspond to 90% probability using the refined ADPs.

Table 1.
Selected bond lengths (Å) and angles (°) of LiCs2[dca]3 in comparison to NaCs2[dca]3.
Li+ is octahedrally coordinated by six terminal nitrogen atoms of six different [dca] moieties with d(Li–N1) = 2.28 Å. This distance is in good agreement with Li–N distances for the octahedrally coordinated lithium cation in Li[dca] (2.22–2.29 Å) [9]. These octahedra are connected with each other by sharing three [N(CN)2]− and they are bonded by the terminal nitrogen atoms. In this way columns are formed parallel to the crystallographic c axis. These columns are packed hexagonally, forming channels hosting the cesium cations (Figure 1).
NaCs2[dca]3 was reported to incorporate coordination spheres around cesium with twelve nitrogen atoms from nine [dca]-groups and with two different distances [25]. A closer look at the structure of NaCs2[dca]3 reveals that there are four different d(Cs–N) (Table 1). The calculated valence bond sum (VBS [26]) confirms that all twelve nitrogen atoms are part of the coordination sphere for Cs+ in NaCs2[dca]3. This is not the case for the title compound. Calculations via VBS analysis reveals that the coordination sphere of Cs+ contains nine nitrogen atoms of nine different dicyanamides with three different distances (Figure 2, Table 1), generating a tri-capped trigonal prism according to IUPAC nomenclature. Two cesium atoms share six of these groups. Half of these anions coordinate the Cs+ via their bridging nitrogen with d(Cs–N2 = 3.52 Å); for the other half, it was found that six nitrogen atoms coordinate terminally to the cesium cation with d(Cs–N1 = 3.25 Å). The coordination sphere is completed by three terminal nitrogen atoms in the layers below or above the Cs+ with d(Cs–N1 = 3.23 Å). These distances are in good agreement with Cs–N distances in Cs[dca] (3.26–3.62 Å with CN = 10 and 3.15–3.31 Å with CN = 8) [12].
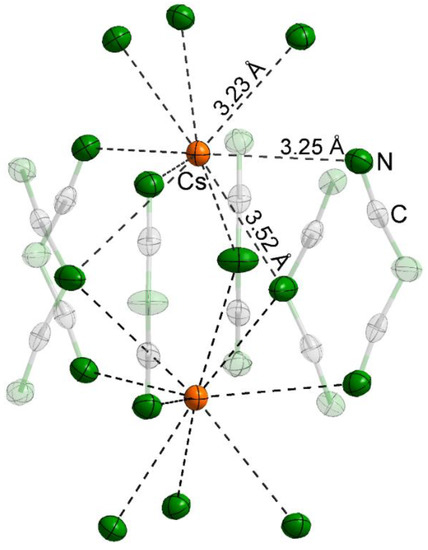
Figure 2.
Coordination sphere of Cs in LiCs2[dca]3. Atoms not involved have been greyed out. The thermal ellipsoids correspond to 90% probability using the refined ADPs.
2.2. Vibrational Spectra
The frequencies obtained from the IR and Raman spectra of the title compound confirm the presence of the [dca] moiety and agree very well to the IR/Raman data of the isostructural NaCs2[dca]3 [25] (Figure 3, Table 2). The IR spectrum was measured under atmospheric conditions, therefore it shows the presence of water due the hygroscopic nature of the title compound.
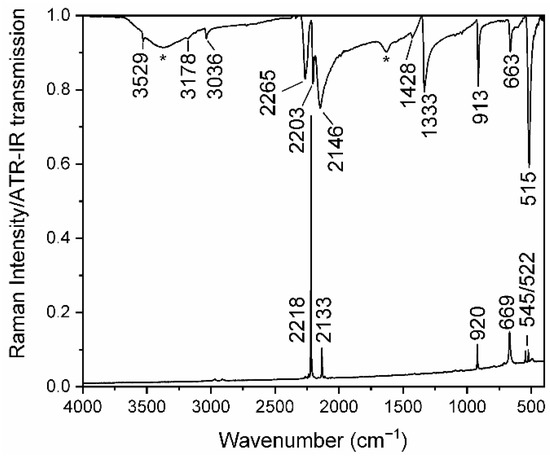
Figure 3.
IR and Raman spectra of LiCs2[dca]3. The asterisks mark vibrations of water.

Table 2.
IR and Raman data of LiCs2[dca]3 in comparison to NaCs2[dca]3. All numbers are given in cm−1.
3. Materials and Methods
3.1. Synthesis
All synthetic steps involving AgNO3 or Ag[dca] were carried out under exclusion of light. Ag[dca] was synthesized by mixing aqueous solutions of Na[dca] (974.45 mg, 10.95 mmol in 10 mL) and AgNO3 (1965.26 mg, 11.57 mmol in 10 mL). After 1 h of stirring, the white Ag[dca] (1827.96 mg, 10.51 mmol, yield = 96%) was filtered off, washed with water, and dried under vacuum.
LiCs2[dca]3 was obtained by adding stoichiometric amounts of CsBr (382.89 mg, 1.80 mmol) and LiCl (38.31 mg, 0.90 mmol) to an aqueous Ag[dca] (472.06 mg, 2.71 mmol, 5 mL H2O) suspension. The suspension was stirred for 12 h. The solid silver halides were decanted off and LiCs2[dca]3 (314.37 mg, 0.722 mmol, yield = 79.9% compared to Ag[dca]) was obtained after water evaporation. Colorless, transparent crystals suitable for X-ray diffraction were selected and measured.
3.2. Single-Crystal Diffraction
Suitable single crystals were mounted on glass fibers. Intensity data were collected with a Bruker SMART APEX CCD detector (Bruker AXS GmbH, Karlsruhe, Germany) equipped with an Incoatec microsource (Mo-Kα1 radiation, λ = 0.71073 Å, multilayer optics). Temperature control was achieved using an Oxford Cryostream 700 (Oxford Cryosystems Ltd, Oxford, United Kingdom) at 100 K. Collected data were integrated with SAINT+ [27] and multi-scan absorption corrections were applied with SADABS [28]. The structure was solved by charge-flipping methods (Superflip [29]) and refined on F2 as implemented in Jana2006 [30]. More crystallographic details can be found in Table 3, Table 4 and Table 5 and in the supplementary materials. Additional details concerning the structure determination are available in CIF format and have been deposited under the CCDC entry number 1866007. Copies of the data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk)

Table 3.
Crystallographic data of LiCs2[dca]3.

Table 4.
Atomic coordinates and equivalent isotropic displacement parameters Ueq (Å2) of LiCs2[dca]3.

Table 5.
Anisotropic displacement parameters Uij (Å2) of LiCs2[dca]3.
3.3. Infrared and Raman Spectra
For the recording of the IR spectrum, an ALPHA II FT-IR-spectrometer (Bruker Optik GmbH, Ettlingen, Germany) equipped with an ATR Platinum Diamond measuring cell was used. All measurements were performed within the range of 4000 to 400 cm−1.
Raman-spectroscopic investigations were performed on a microscope laser Raman spectrometer (Jobin Yvon, Unterhaching, Germany, 4 mW, equipped with a HeNe laser with an excitation line at λ = 632.82 nm, 50× magnification, 2 × 40 s accumulation time). The Raman spectrum was recorded on a crystal sealed in a thin-walled glass capillary.
4. Conclusion
The compound LiCs2[dca]3 was synthesized, its crystal structure determined, and its IR and Raman spectra were reported. The acquired data of the vibrational spectra as well as the structural results are similar to the data of the previously reported alkali metal dicyanamides NaCs2[dca]3, Li[dca] and Cs[dca], although the smaller Li+ changes the coordination of Cs+.
Supplementary Materials
The following are available online at http://www.mdpi.com/2304-6740/6/4/108/s1: CIF and CIFchecked files.
Author Contributions
M.M. and O.R. conceived and designed the experiment; M.M. performed the synthesis, SXRD, and ATR-IR experiment; results were discussed with the assistance of O.R. and R.D.; and M.M. wrote the paper with the assistance of O.R. and R.D.
Funding
This research received no external funding.
Acknowledgments
It is a pleasure to thank Armin Schulz (MPI-FKF, Stuttgart) for having measured the Raman spectrum.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sougrati, M.T.; Darwiche, A.; Liu, X.; Mahmoud, A.; Hermann, R.P.; Jouen, S.; Monconduit, L.; Dronskowski, R.; Stievano, L. Transition-Metal Carbodiimides as Molecular Negative Electrode Materials for Lithium- and Sodium-Ion Batteries with Excellent Cycling Properties. Angew. Chem. Int. Ed. 2016, 55, 5090–5095. [Google Scholar] [CrossRef] [PubMed]
- Sougrati, M.T.; Arayamparambil, J.J.; Liu, X.; Mann, M.; Slabon, A.; Stievano, L.; Dronskowski, R. Carbodiimides as energy materials: Which directions for a reasonable future? Dalton Trans. 2018, 47, 10827–10832. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; Görne, A.L.; Dronskowski, R. Itinerant nitrides and salt-like guanidinates—The diversity of solid-state nitrogen chemistry. Prog. Solid State Chem. 2018, 51, 1–18. [Google Scholar] [CrossRef]
- Manson, J.L.; Kmety, C.R.; Huang, Q.-z.; Lynn, J.W.; Bendele, G.M.; Pagola, S.; Stephens, P.W.; Liable-Sands, L.M.; Rheingold, A.L.; Epstein, A.J.; et al. Structure and Magnetic Ordering of MII[N(CN)2]2 (M = Co, Ni). Chem. Mater. 1998, 10, 2552–2560. [Google Scholar] [CrossRef]
- Raebiger, J.W.; Manson, J.L.; Sommer, R.D.; Geiser, U.; Rheingold, A.L.; Miller, J.S. 1-D and 2-D Homoleptic Dicyanamide Structures, [Ph4P]2{CoII[N(CN)2]4} and [Ph4P]{M[N(CN)2]3} (M = Mn, Co). Inorg. Chem. 2001, 40, 2578–2581. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.V.K.; Basaran, A.T.; Ülker, E.; Mishra, R.; Karadas, F. Metal Dicyanamides as Efficient and Robust Water-Oxidation Catalysts. ChemCatChem 2017, 9, 300–307. [Google Scholar] [CrossRef]
- Yoon, H.; Lane, G.H.; Shekibi, Y.; Howlett, P.C.; Forsyth, M.; Best, A.S.; MacFarlane, D.R. Lithium electrochemistry and cycling behaviour of ionic liquids using cyano based anions. Energy Environ. Sci. 2013, 6, 979–986. [Google Scholar] [CrossRef]
- Jürgens, B.; Höppe, H.A.; Irran, E.; Schnick, W. Transformation of Ammonium Dicyanamide into Dicyandiamide in the Solid. Inorg. Chem. 2002, 41, 4849–4851. [Google Scholar] [CrossRef] [PubMed]
- Reckeweg, O.; DiSalvo, F.J.; Schulz, A.; Blaschkowski, B.; Jagiella, S.; Schleid, T. Synthesis, Crystal Structure, and Vibrational Spectra of the Anhydrous Lithium Dicyanamide Li[N(CN)2]. Z. Anorg. Allg. Chem. 2014, 640, 851–855. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Schneider, J.; Schnick, W. Trimerization of NaC2N3 to Na3C6N9 in the Solid: Ab Initio Crystal Structure Determination of Two Polymorphs of NaC2N3 and of Na3C6N9 from X-ray Powder Diffractometry. Inorg. Chem. 2000, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Irran, E.; Jürgens, B.; Schnick, W. Trimerization of alkali dicyanamides M[N(CN)2] and formation of tricyanomelaminates M3[C6N9] (M = K, Rb) in the melt: Crystal structure determination of three polymorphs of K[N(CN)2], two of Rb[N(CN)2], and one of K3[C6N9] and Rb3[C6N9] from X-ray powder diffractometry. Chem. Eur. J. 2001, 7, 5372–5381. [Google Scholar] [CrossRef] [PubMed]
- Starynowicz, P. Structure of caesium dicyanamide. Acta Crystallogr. Sect. C 1991, 47, 2198–2199. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Schnick, W. Syntheses, Vibrational Spectroscopy, and Crystal Structure Determination from X-Ray Powder Diffraction Data of Alkaline Earth Dicyanamides M[N(CN)2]2 with M = Mg, Ca, Sr, and Ba. J. Solid State Chem. 2001, 157, 241–249. [Google Scholar] [CrossRef]
- Manson, J.L.; Kmety, C.R.; Epstein, A.J.; Miller, J.S. Spontaneous Magnetization in the M[N(CN)2]2 (M = Cr, Mn) Weak Ferromagnets. Inorg. Chem. 1999, 38, 2552–2553. [Google Scholar] [CrossRef]
- Hodgson, S.A.; Hunt, S.J.; Sørensen, T.J.; Thompson, A.L.; Reynolds, E.M.; Faulkner, S.; Goodwin, A.L. Anomalous Thermal Expansion and Luminescence Thermochromism in Silver(I) Dicyanamide. Eur. J. Inorg. Chem. 2016, 2016, 4378–4381. [Google Scholar] [CrossRef]
- Reckeweg, O.; Schulz, A.; Schneck, C.; Lissner, F.; Schleid, T. Syntheses, single-crystal structures, vibrational spectra and DSC/TG analyses of orthorhombic and trigonal Ag[N(CN)2]. Z. Naturforsch. B 2016, 71, 827–834. [Google Scholar] [CrossRef]
- Jürgens, B.; Irran, E.; Höppe, H.A.; Schnick, W. Phase Transition of a Dicyanamide with Rutile-like Structure: Syntheses and Crystal Structures of α- and β-Cd[N(CN)2]2. Z. Anorg. Allg. Chem. 2004, 630, 219–223. [Google Scholar] [CrossRef]
- Reckeweg, O.; Dinnebier, R.E.; Schulz, A.; Blaschkowski, B.; Schneck, C.; Schleid, T. About the air- and water-stable copper(I) dicyanamide: Synthesis, crystal structure, vibrational spectra and DSC/TG analysis of Cu[N(CN)2]. Z. Naturforsch, B 2017, 72, 159–165. [Google Scholar] [CrossRef]
- Manson, J.L.; Lee, D.W.; Rheingold, A.L.; Miller, J.S. Buckled-layered Structure of Zinc Dicyanamide, ZnII[N(CN)2]2. Inorg. Chem. 1998, 37, 5966–5967. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, B.; Irran, E.; Schnick, W. Synthesis and characterization of the rare-earth dicyanamides Ln[N(CN)2]3 with Ln = La, Ce, Pr, Nd, Sm, and Eu. J. Solid State Chem. 2005, 178, 72–78. [Google Scholar] [CrossRef]
- Nag, A.; Schnick, W. Synthesis, Crystal Structure and Thermal Behavior of Gadolinium Dicyanamide Dihydrate Gd[N(CN)2]3·2H2O. Z. Anorg. Allg. Chem. 2006, 632, 609–614. [Google Scholar] [CrossRef]
- Nag, A.; Schmidt, P.J.; Schnick, W. Synthesis and Characterization of Tb[N(CN)2]3·2H2O and Eu[N(CN)2]3·2H2O: Two New Luminescent Rare-Earth Dicyanamides. Chem. Mater. 2006, 18, 5738–5745. [Google Scholar] [CrossRef]
- Reckeweg, O.; Wakabayashi, R.H.; DiSalvo, F.J.; Schulz, A.; Schneck, C.; Schleid, T. About alkali metal dicyanamides: Syntheses, single-crystal structure determination, DSC/TG and vibrational spectra of KCs[N(CN)2]2 and NaRb2[N(CN)2]3·H2O. Z. Naturforsch. B 2015, 70, 365–372. [Google Scholar] [CrossRef]
- Reckeweg, O.; DiSalvo, F.J. Synthesis and single-crystal structure of the pseudo-ternary compounds LiA[N(CN)2]2 (A = K or Rb). Z. Naturforsch. B 2016, 71, 157–160. [Google Scholar] [CrossRef]
- Jürgens, B.; Milius, W.; Morys, P.; Schnick, W. Trimerisierung von Dicyanamid-Ionen C2N3—im Festkörper—Synthesen, Kristallstrukturen und Eigenschaften von NaCs2(C2N3)3 und Na3C6N9·3H2O. Z. Anorg. Allg. Chem. 1998, 624, 91–97. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. Sect. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Bruker AXS Inc. SAINT Version 7.68A; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker AXS Inc. SADABS Version 2004/1; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).