Transformative Si8R8 Siliconoids
Abstract
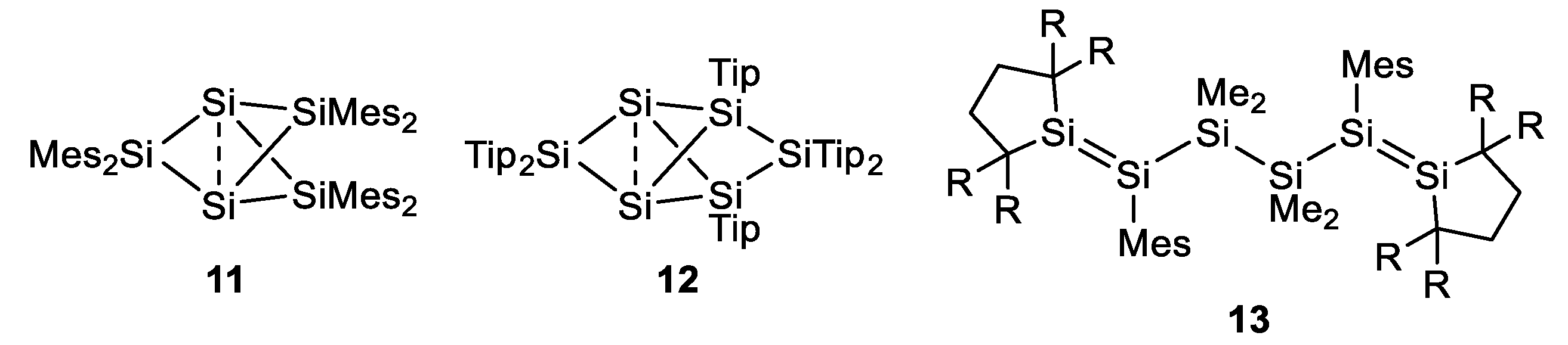
1. Introduction
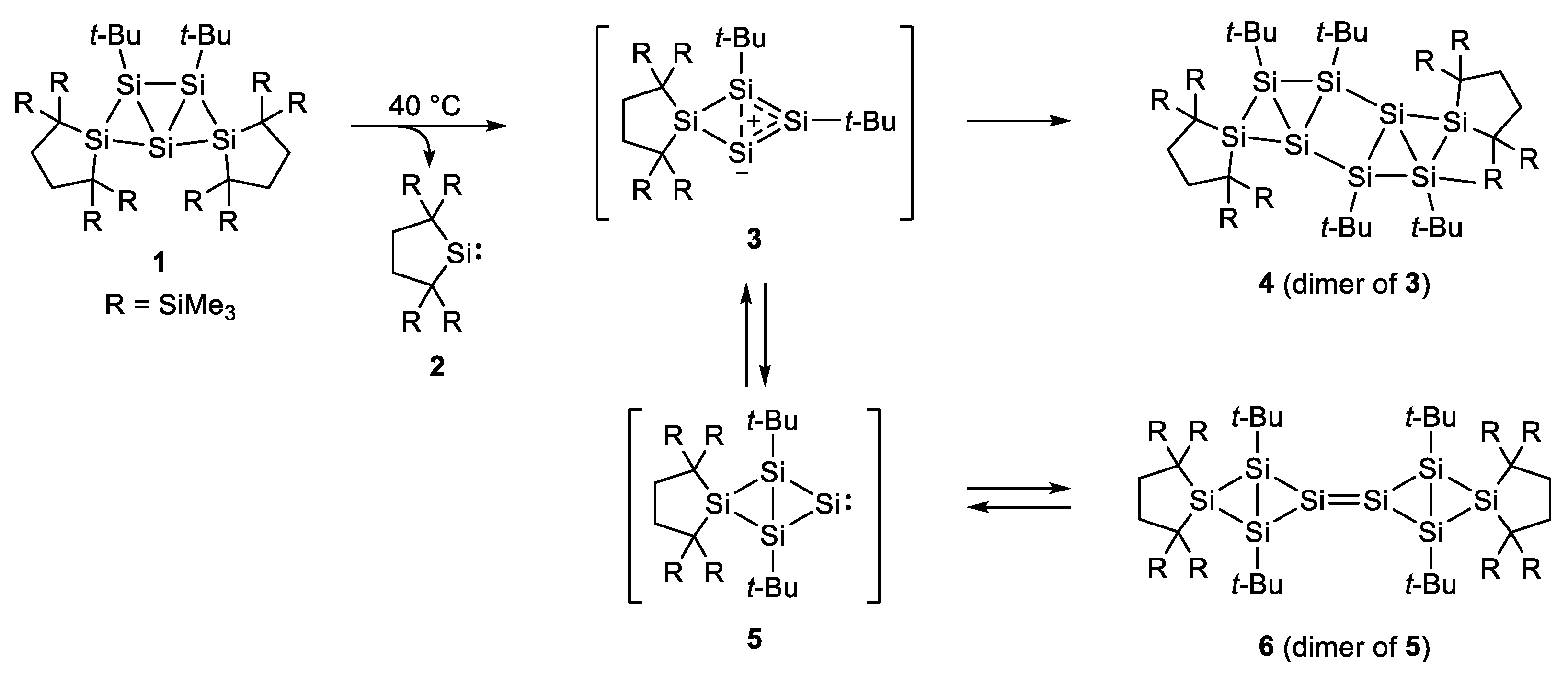
2. Results
2.1. Thermal Reactions of 4
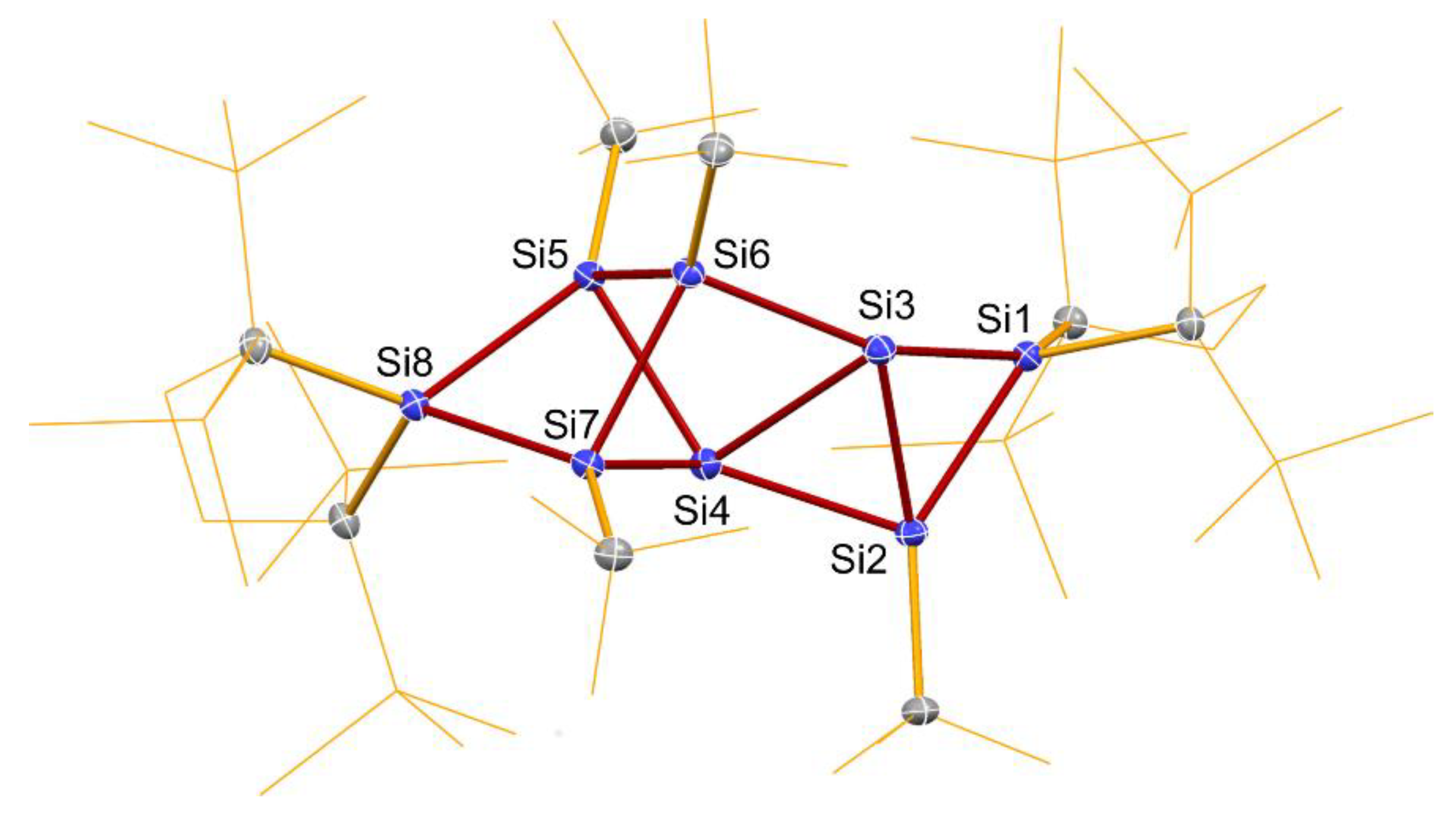
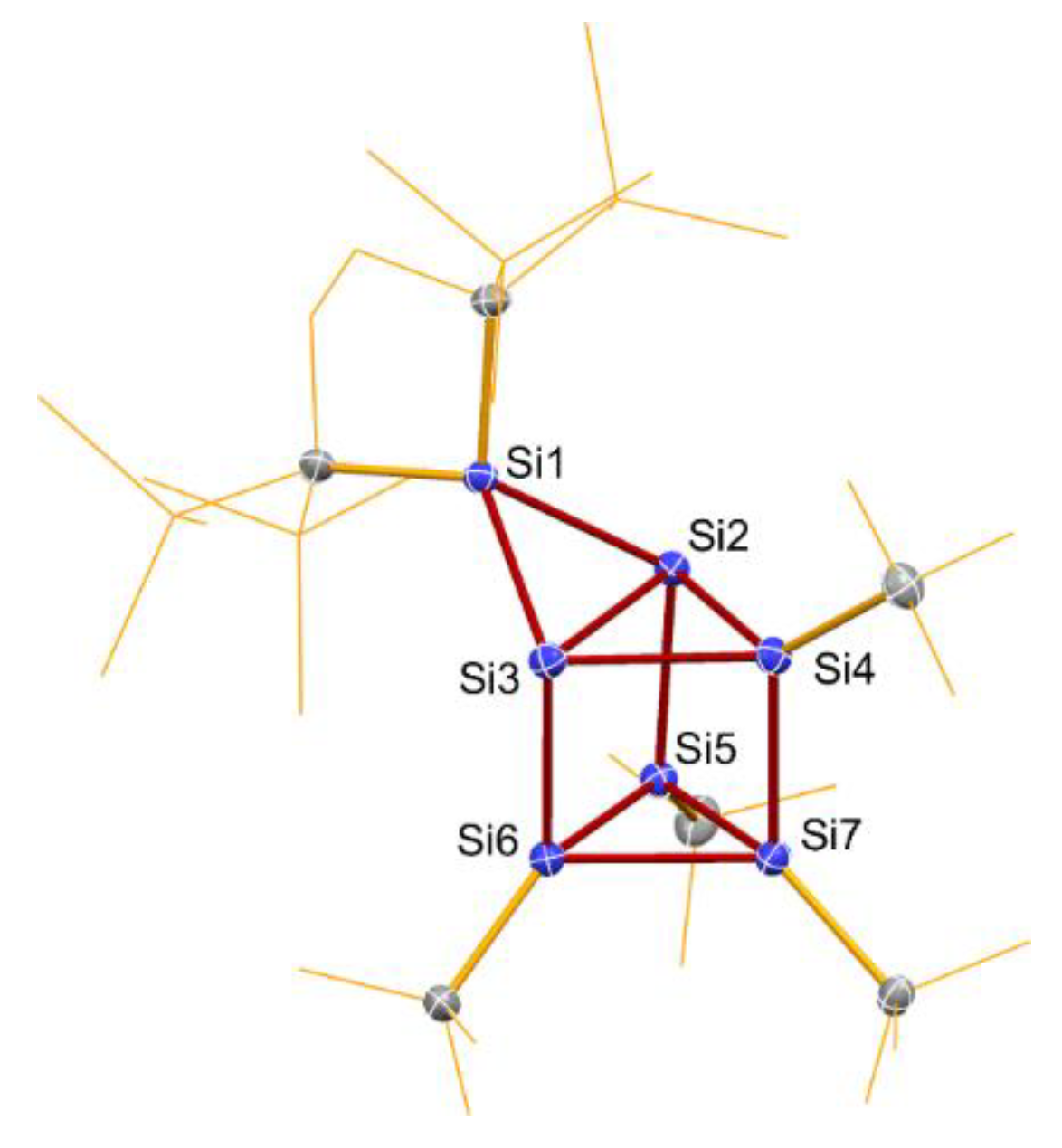
2.2. Molecular Structure of 7
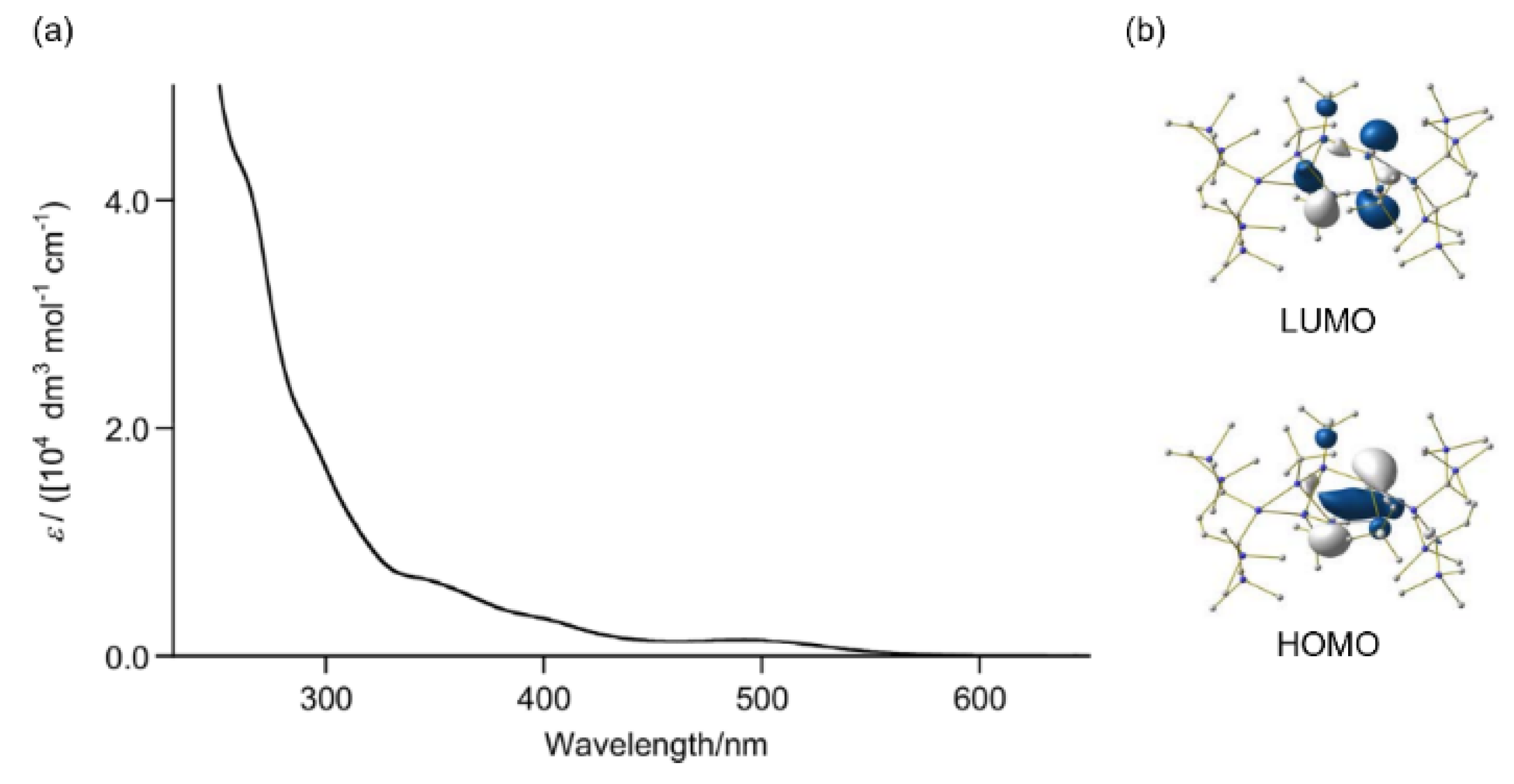
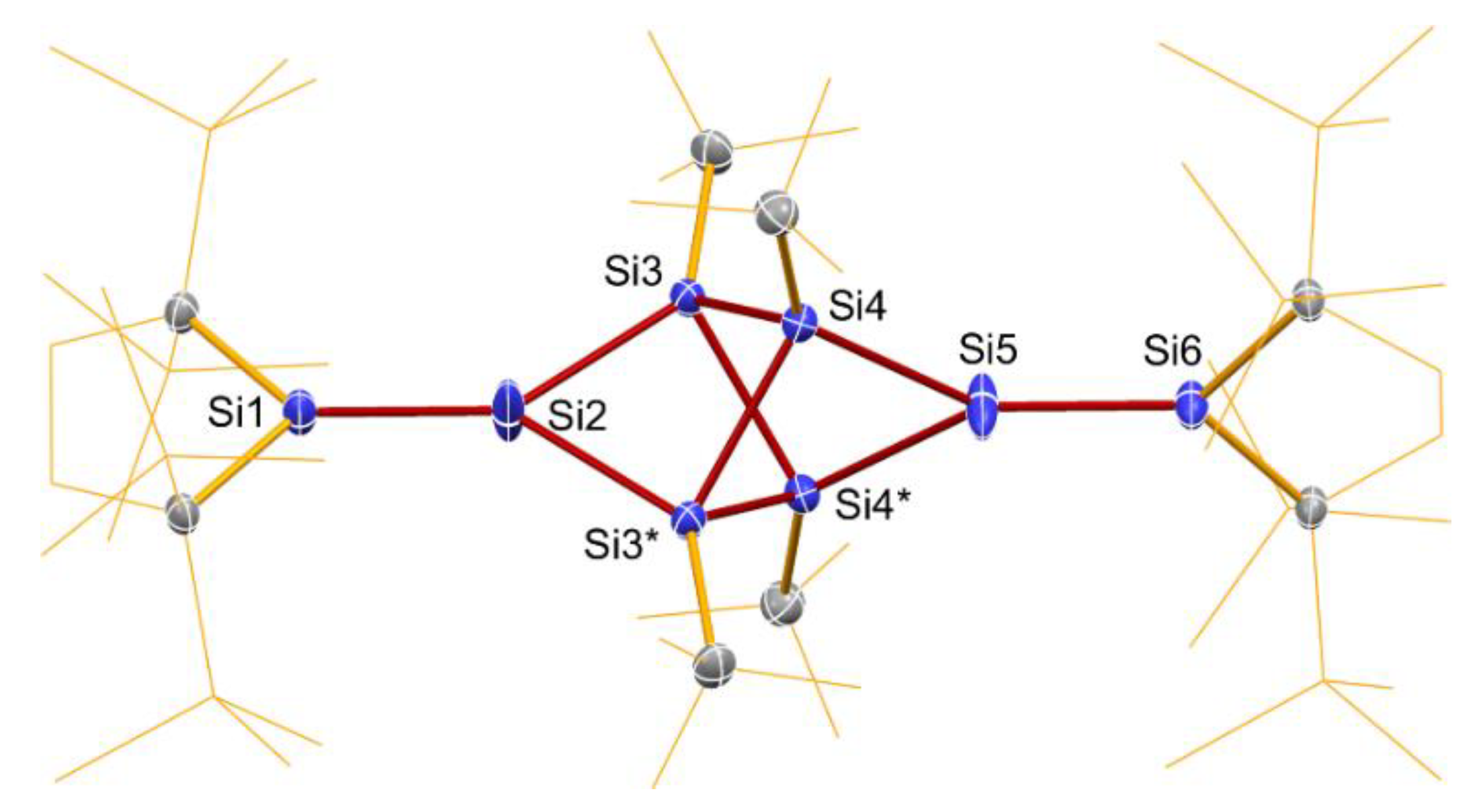
2.3. Molecular Structure of 8
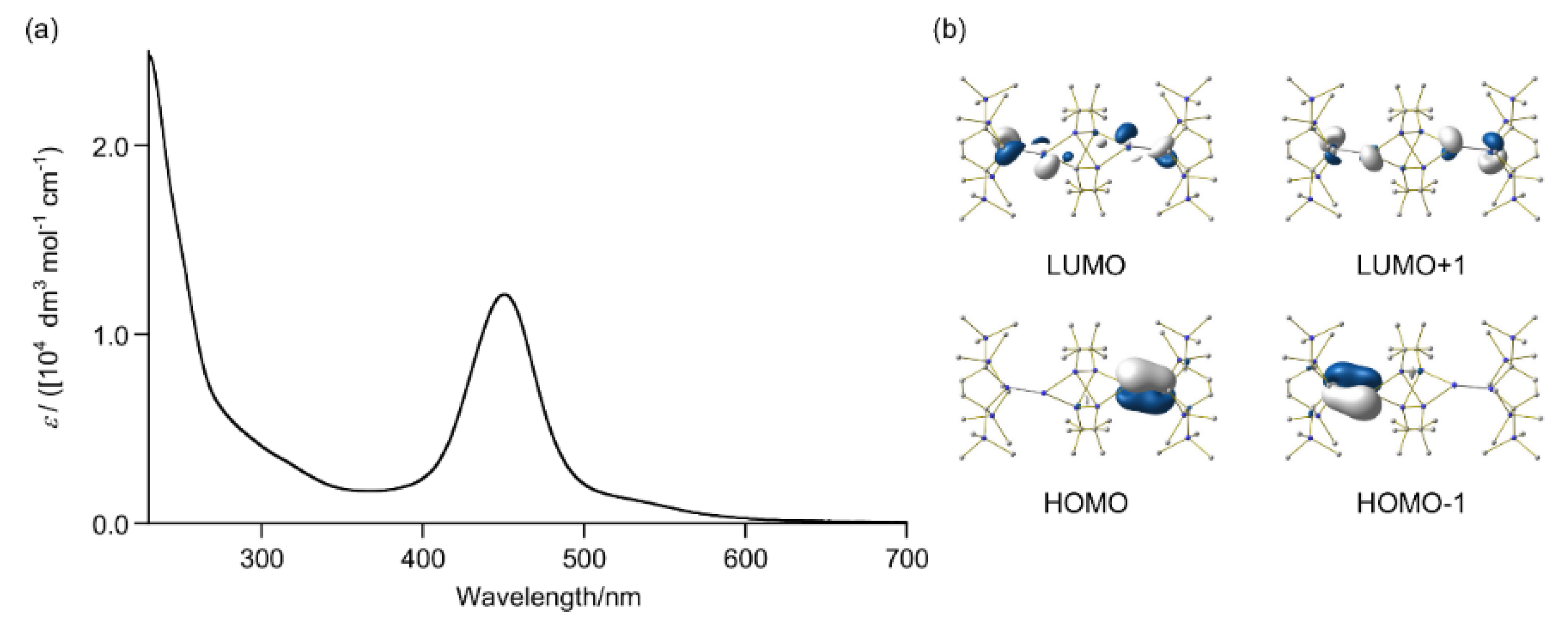
2.4. Molecular Structure of 9
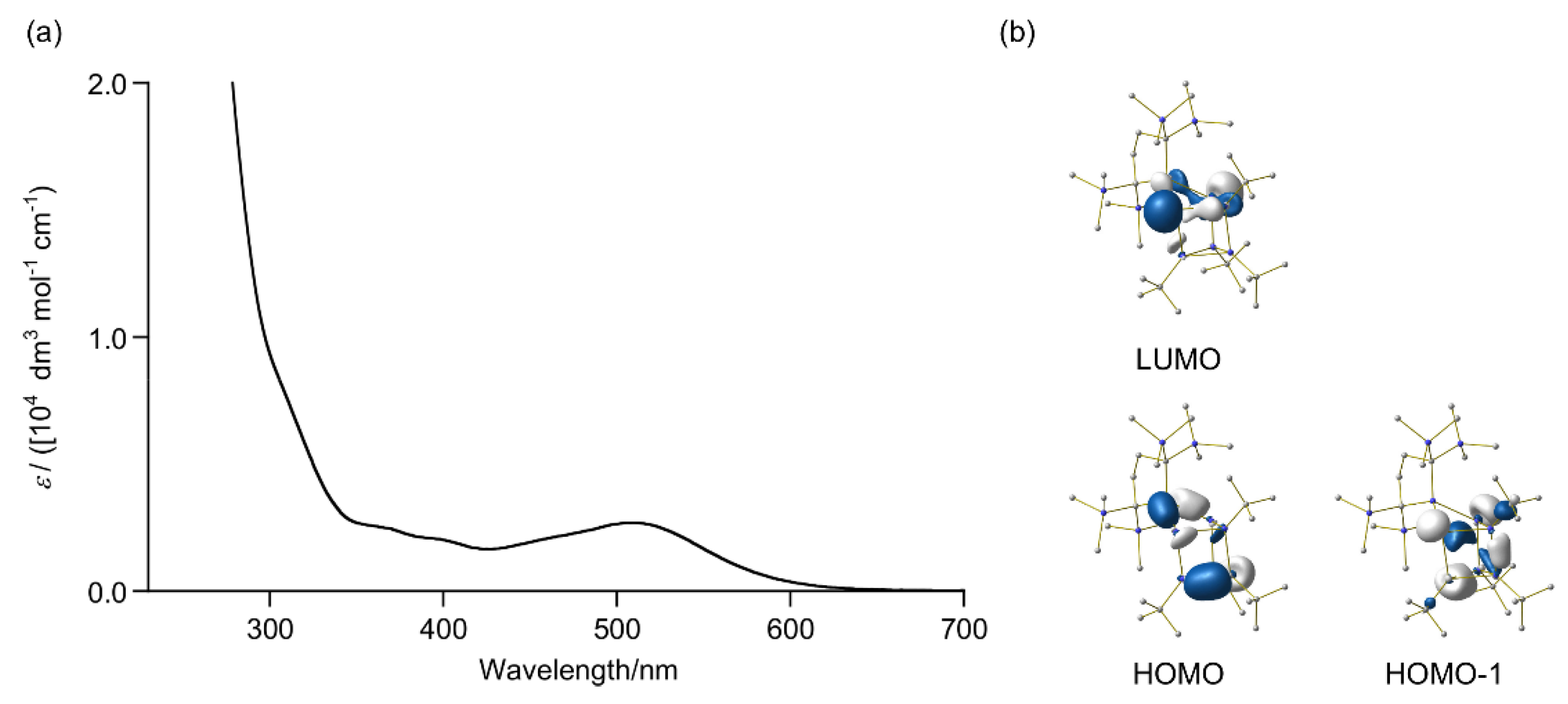
2.5. Theoretical Study
3. Materials and Methods
3.1. General Procedures
3.2. Materials
3.3. Thermolysis of 4
3.4. Isolation of 7
3.5. Isolation of 8 and 9
3.6. X-ray Analysis
3.7. Theoretical Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hengge, E.; Janoschek, R. Homocyclic silanes. Chem. Rev. 1995, 95, 1495–1526. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Sakurai, H. Cage and cluster compounds of silicon, germanium, and tin. Adv. Organomet. Chem. 1995, 37, 1–38. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Nagase, S. Polyhedral silicon compounds. In The Chemistry of Organic Silicon Compounds; Rappoport, Z., Apeloig, Y., Eds.; Wiley: Chichester, UK, 1998; Volume 2, pp. 119–152. [Google Scholar]
- Hengge, E.; Stüger, H. Recent advances in the chemistry of cyclopolysilanes. In The Chemistry of Organic Silicon Compounds; Rappoport, Z., Apeloig, Y., Eds.; Wiley: Chichester, UK, 1998; Volume 2, pp. 2177–2216. [Google Scholar]
- Marschner, C. Oligosilanes. In Structure and Bonding 156, Functional Molecular Silicon Compounds I. Regular Oxidation States; Scheschkewitz, D., Ed.; Springer: New York, NY, USA, 2014; Volume 156, pp. 163–228. [Google Scholar]
- Lyon, J.T.; Gruene, P.; Fielicke, A.; Meijer, G.; Janssens, E.; Claes, P.; Lievens, P. Structures of silicon cluster cations in the gas phase. J. Am. Chem. Soc. 2009, 131, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Peppernick, S.J.; Gunaratne, K.D.D.; Castleman, A.W. The relative abundances of silicon hydride clusters, SinHx− (n = 8–12 and 0 ≤ x ≤ 25), investigated with high-resolution time-of-flight mass spectrometry. Int. J. Mass. Spectr. 2010, 290, 65–71. [Google Scholar] [CrossRef]
- Haertelt, M.; Lyon, J.T.; Claes, P.; de Haeck, J.; Lievens, P.; Fielicke, A. Gas-phase structures of neutral silicon clusters. J. Chem. Phys. 2012, 136, 064301. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.J.; Rzepa, H.S.; Scheschkewitz, D. Ring currents in the dismutational aromatic Si6R6. Angew. Chem. Int. Ed. 2010, 49, 10006–10009. [Google Scholar] [CrossRef] [PubMed]
- Abersfelder, K.; Russell, A.; Rzepa, H.S.; White, A.J.P.; Haycock, P.R.; Scheschkewitz, D. Contraction and expansion of the silicon scaffold of stable Si6R6 isomers. J. Am. Chem. Soc. 2012, 134, 16008–16016. [Google Scholar] [CrossRef] [PubMed]
- Scheschkewitz, D. A molecular silicon cluster with a “naked” vertex atom. Angew. Chem. Int. Ed. 2005, 44, 2954–2956. [Google Scholar] [CrossRef] [PubMed]
- Abersfelder, K.; White, A.J.P.; Rzepa, H.S.; Scheschkewitz, D. A tricyclic aromatic isomer of hexasilabenzene. Science 2010, 327, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Abersfelder, K.; White, A.J.P.; Berger, R.J.F.; Rzepa, H.S.; Scheschkewitz, D. A stable derivative of the global minimum on the Si6H6 potential energy surface. Angew. Chem., Int. Ed. 2011, 50, 7936–7939. [Google Scholar] [CrossRef] [PubMed]
- Willmes, P.; Leszczynska, K.; Heider, Y.; Abersfelder, K.; Zimmer, M.; Huch, V.; Scheschkewitz, D. Isolation and versatile derivatization of an unsaturated anionic silicon cluster (siliconoid). Angew. Chem. Int. Ed. 2016, 55, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Huch, V.; Mayer, P.; Vasisht, S.K.; Veith, M.; Wiberg, N. Si8(SitBu3)6: A hitherto unknown cluster structure in silicon chemistry. Angew. Chem. Int. Ed. 2005, 44, 7884–7887. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Vasisht, S.K.; Fischer, G.; Mayer, P. A reactive Si4 cage: K(SitBu3)3Si4. J. Organomet. Chem. 2010, 695, 667–672. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Vasisht, S.K.; Mayer, P. Spirocycle (SitBu3)6Si9Cl2: The first of its kind among group 14 elements. Eur. J. Inorg. Chem. 2010, 2010, 3256–3260. [Google Scholar] [CrossRef]
- Nied, D.; Koppe, R.; Klopper, W.; Schnockel, H.; Breher, F. Synthesis of a pentasilapropellane. Exploring the nature of a stretched silicon–silicon bond in a nonclassical molecule. J. Am. Chem. Soc. 2010, 132, 10264–10265. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Otsuka, K.; Toma, Y.; Kyushin, S. An organosilicon cluster with an octasilacuneane core: A missing silicon cage motif. Angew. Chem. Int. Ed. 2013, 52, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, A.; Iizuka, C.; Otsuka, K.; Kyushin, S. Cyclopentasilane-fused hexasilabenzvalene. J. Am. Chem. Soc. 2013, 135, 16340–16343. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, A.; Kamiyama, J.; Kyushin, S. Tetrasilane-bridged bicyclo[4.1.0]heptasil-1-ene. J. Am. Chem. Soc. 2014, 136, 12896–12898. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Akasaka, N.; Ishida, S. A heavy analogue of the smallest bridgehead alkene stabilized by a base. Nat. Commun. 2014, 5, 5353. [Google Scholar] [CrossRef] [PubMed]
- Hamers, R.J.; Köhler, U.K.; Demuth, J.E. Nucleation and growth of epitaxial silicon on Si(001) and Si(111) surfaces by scanning tunneling microscopy. Ultramicroscopy 1989, 31, 10–19. [Google Scholar] [CrossRef]
- Swihart, M.T.; Girshick, S.L. Thermochemistry and kinetics of silicon hydride cluster formation during thermal decomposition of silane. J. Phys. Chem. B 1999, 103, 64–76. [Google Scholar] [CrossRef]
- Swihart, M.T.; Girshick, S.L. Ab initio structures and energetics of selected hydrogenated silicon clusters containing six to ten silicon atoms. Chem. Phys. Lett. 1999, 307, 527–532. [Google Scholar] [CrossRef]
- Li, C.P.; Li, X.J.; Yang, J.C. Silicon hydride clusters Si5Hn (n = 3–12) and their anions: Structures, thermochemistry, and electron affinities. J. Phys. Chem. A 2006, 110, 12026–12034. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Effect of hydrogen on ground state properties of silicon clusters (SinHm; n = 11–15, m = 0–4): A density functional based tight binding study. J. Phys. Condens. Matt. 2008, 20, 045226. [Google Scholar] [CrossRef]
- Adamczyk, A.J.; Broadbelt, L.J. Thermochemical property estimation of hydrogenated silicon clusters. J. Phys. Chem. A 2011, 115, 8969–8982. [Google Scholar] [CrossRef] [PubMed]
- Gapurenko, O.A.; Minyaev, R.M.; Minkin, V.I. Silicon analogues of pyramidane: A quantum-chemical study. Mendeleev Commun. 2012, 22, 8–10. [Google Scholar] [CrossRef]
- Thingna, J.; Prasad, R.; Auluck, S. Photo-absorption spectra of small hydrogenated silicon clusters using the time-dependent density functional theory. J. Phys. Chem. Solids 2011, 72, 1096–1100. [Google Scholar] [CrossRef]
- Kaftory, M.; Kapon, M.; Botoshansky, M. The structural chemistry of organosilicon compounds. In The Chemistry of Organic Silicon Compounds; Rappoport, Z., Apeloig, Y., Eds.; Wiley: Chichester, UK, 1998; Volume 2, pp. 181–266. [Google Scholar]
- Okazaki, R.; West, R. Chemistry of stable disilenes. Adv. Organomet. Chem. 1996, 39, 231–273. [Google Scholar] [CrossRef]
- Kira, M.; Iwamoto, T. Progress in the chemistry of stable disilenes. Adv. Organomet. Chem. 2006, 54, 73–148. [Google Scholar] [CrossRef]
- Kabe, Y.; Kuroda, M.; Honda, Y.; Yamashita, O.; Kawase, T.; Masamune, S. Reductive oligomerization of 1,2-di-tert-butyl-1,1,2,2-tetrachlorodisilane: The tricyclo[2.2.0.02,5]hexasilane and tetracyclo[3.3.0.02,7.03,6]octasilane systems. Angew. Chem. Int. Ed. Engl. 1988, 27, 1725–1727. [Google Scholar] [CrossRef]
- Iwamoto, T.; Uchiyama, K.; Kabuto, C.; Kira, M. Synthesis of tricyclo[3.1.0.02,4]hexasilane and its photochemical isomerization to tricyclo[2.2.0.02,5]hexasilane. Chem. Lett. 2007, 36, 368–369. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhang, J.; Song, H.; Cui, C. Isolation of R6Si6 dianion: A bridged tricyclic isomer of dianionic hexasilabenzene. J. Am. Chem. Soc. 2018, 140, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Yatabe, T.; Kabuto, C.; Sakurai, H. The missing hexasilaprismane: Synthesis, x-ray analysis and photochemical reactions. J. Am. Chem. Soc. 1993, 115, 5853–5854. [Google Scholar] [CrossRef]
- Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C. The first isolable dialkylsilylene. J. Am. Chem. Soc. 1999, 121, 9722–9723. [Google Scholar] [CrossRef]
- Tang, M.; Lu, W.; Wang, C.Z.; Ho, K.M. Search for most stable structure of Si8H8 cluster. Chem. Phys. Lett. 2003, 377, 413–418. [Google Scholar] [CrossRef]
- Matsumoto, H.; Higuchi, K.; Hoshino, Y.; Koike, H.; Naoi, Y.; Nagai, Y. The first octasilacubane system: Synthesis of octakis-(t-butyldimethylsilyl)pentacyclo[4.2.0.02,5.03,8.04,7]octasilane. J. Chem. Soc. Chem. Commun. 1988, 1083–1084. [Google Scholar] [CrossRef]
- Furukawa, K.; Fujino, M.; Matsumoto, N. Cubic silicon cluster. Appl. Phys. Lett. 1992, 60, 2744–2745. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Yatabe, T.; Kamatani, H.; Kabuto, C.; Sakurai, H. Preparation, characterization, and crystal structures of octasilacubanes and octagermacubanes. J. Am. Chem. Soc. 1992, 114, 6260–6262. [Google Scholar] [CrossRef]
- Matsumoto, H.; Higuchi, K.; Kyushin, S.; Goto, M. Octakis(1,1,2-trimethylpropyl)octasilacubane: Synthesis, molecular structure, and unusual properties. Angew. Chem. Int. Ed. Engl. 1992, 31, 1354–1356. [Google Scholar] [CrossRef]
- Furukawa, K.; Fujino, M.; Matsumoto, N. Superlattice structure of octa-tert-butylpentacyclo[4.2.0.02,5.03,8.04,7]octasilane found by reinvestigation of x-ray structure analysis. J. Organomet. Chem. 1996, 515, 37–41. [Google Scholar] [CrossRef]
- Unno, M.; Matsumoto, T.; Mochizuki, K.; Higuchi, K.; Goto, M.; Matsumoto, H. Structure and oxidation of octakis(tert-butyldimethylsilyl)octasilacubane. J. Organomet. Chem. 2003, 685, 156–161. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Empirical Absorption Correction Program; Institute for Inorganic Chemistry: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014/7; Program for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Maeda, S.; Harabuchi, Y.; Osada, Y.; Taketsugu, T.; Morokuma, K.; Ohno, K. Available online: http://grrm.chem.tohoku.ac.jp/GRRM/ (accessed on 1 October 2018).
- Maeda, S.; Ohno, K.; Morokuma, K. Systematic exploration of the mechanism of chemical reactions: The global reaction route mapping (GRRM) strategy using the ADDF and AFIR methods. Phys. Chem. Chem. Phys. 2013, 15, 3683–3701. [Google Scholar] [CrossRef] [PubMed]

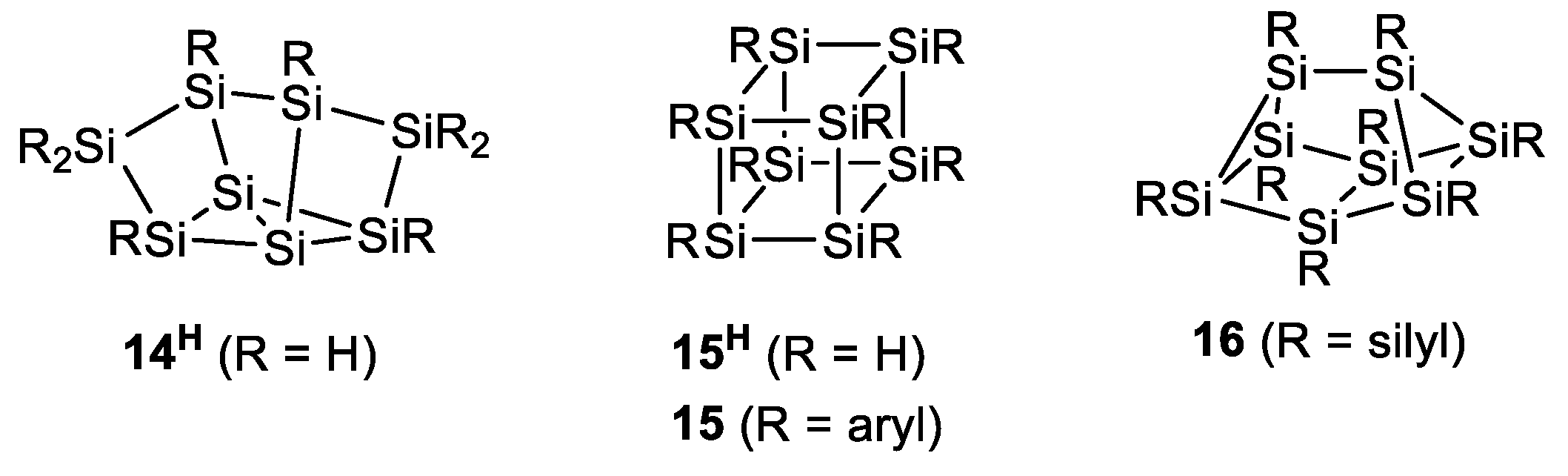
| Compound | ΔG(298.15 K) [kJ/mol] | ΔG(348.15 K) [kJ/mol] |
|---|---|---|
| 4opt | 0.0 | 0.0 |
| 6opt | +16.2 | +14.6 |
| 7opt | +14.2 | +14.6 |
| 8opt | −71.8 | −77.5 |
| 9opt + 2opt | +14.9 | −0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akasaka, N.; Ishida, S.; Iwamoto, T. Transformative Si8R8 Siliconoids. Inorganics 2018, 6, 107. https://doi.org/10.3390/inorganics6040107
Akasaka N, Ishida S, Iwamoto T. Transformative Si8R8 Siliconoids. Inorganics. 2018; 6(4):107. https://doi.org/10.3390/inorganics6040107
Chicago/Turabian StyleAkasaka, Naohiko, Shintaro Ishida, and Takeaki Iwamoto. 2018. "Transformative Si8R8 Siliconoids" Inorganics 6, no. 4: 107. https://doi.org/10.3390/inorganics6040107
APA StyleAkasaka, N., Ishida, S., & Iwamoto, T. (2018). Transformative Si8R8 Siliconoids. Inorganics, 6(4), 107. https://doi.org/10.3390/inorganics6040107