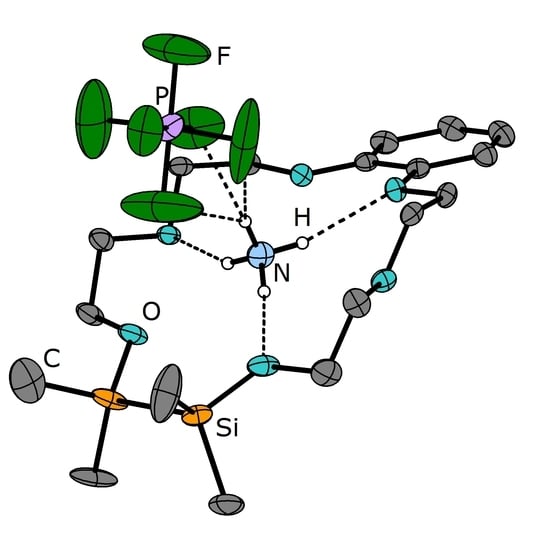
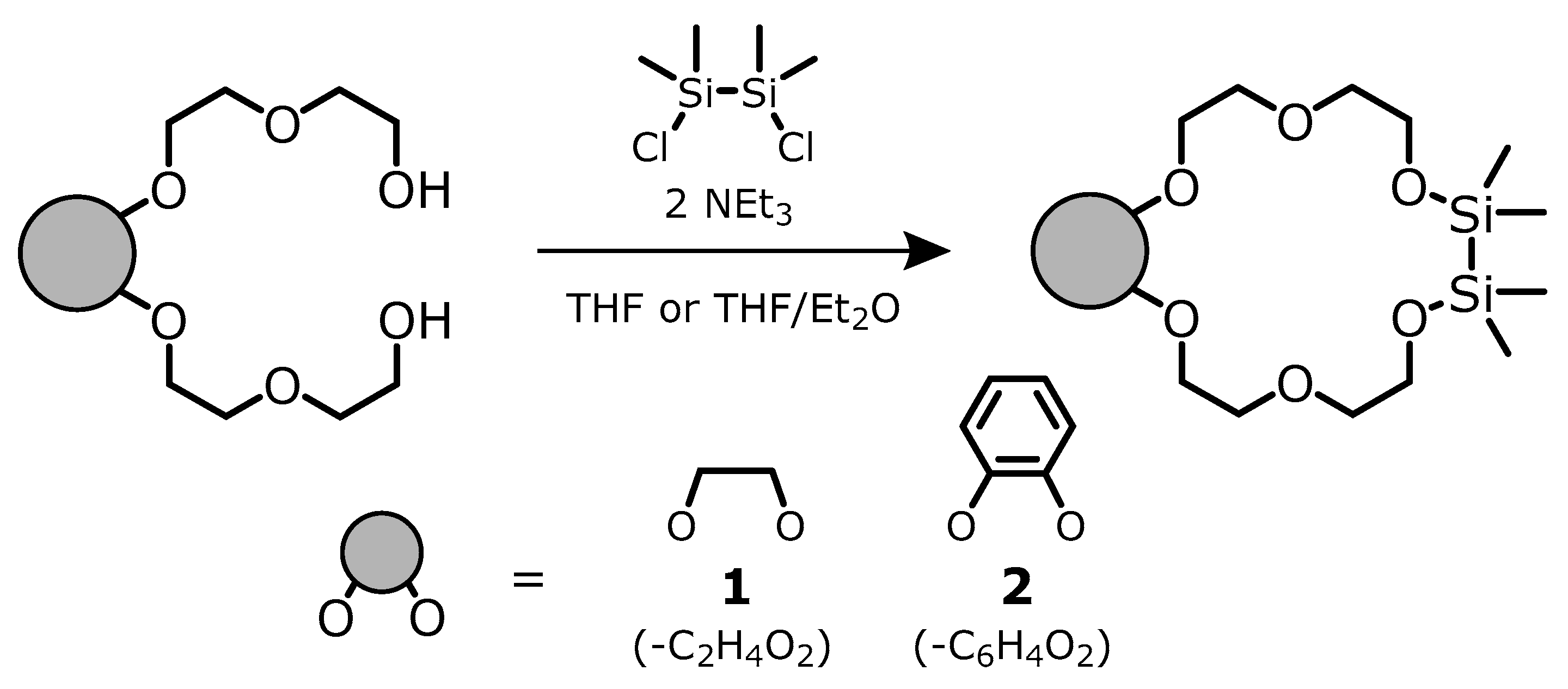
Hybrid Disila-Crown Ethers as Hosts for Ammonium Cations: The O–Si–Si–O Linkage as an Acceptor for Hydrogen Bonding
Abstract
1. Introduction
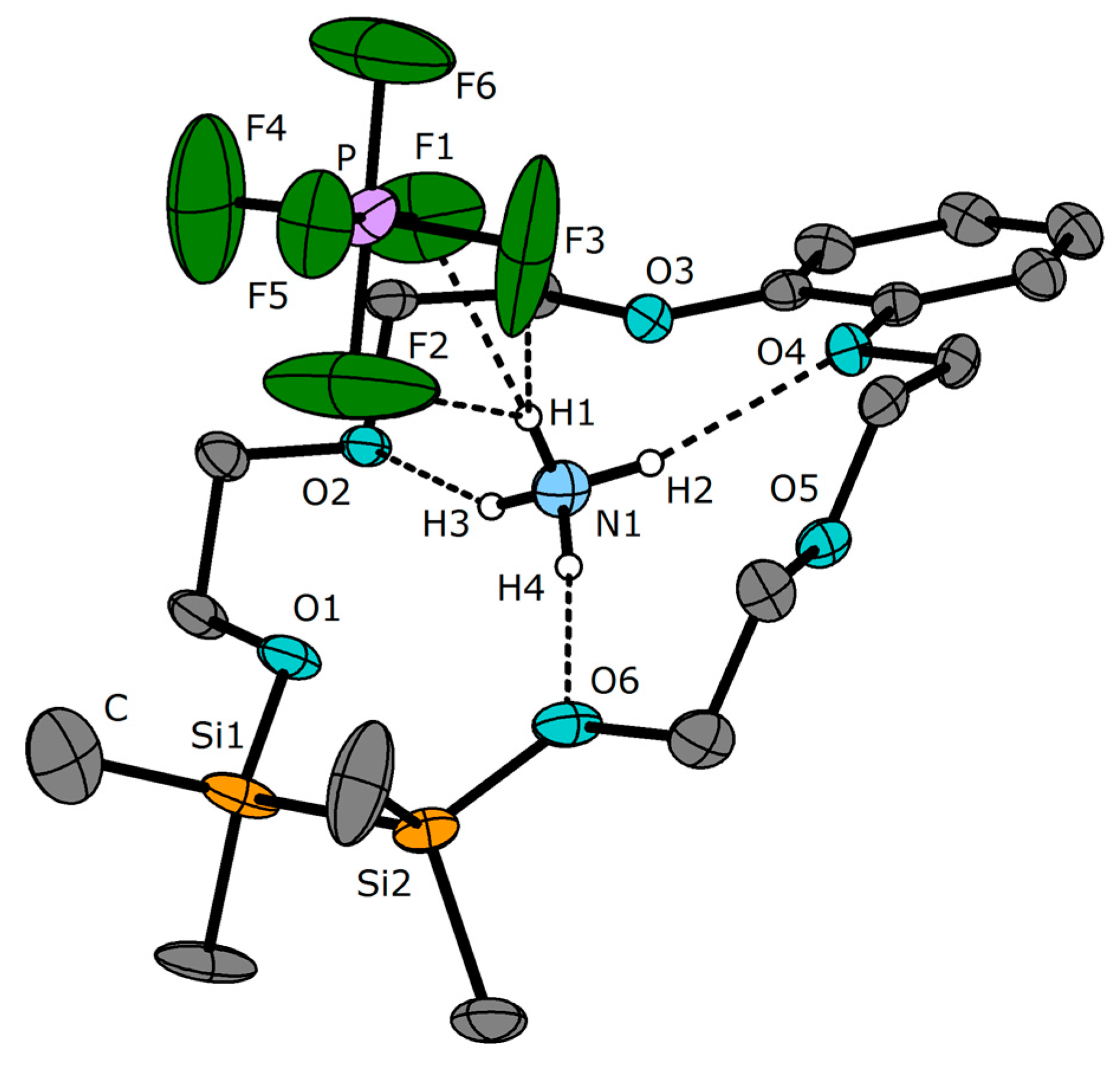
2. Results
3. Materials and Methods
3.1. Laboratory Procedures and Techniques
3.2. Crystal Structures
3.3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weinhold, F.; West, R. The nature of the silicon–oxygen bond. Organometallics 2011, 30, 5815–5824. [Google Scholar] [CrossRef]
- Weinhold, F.; West, R. Hyperconjugative Interactions in Permethylated Siloxanes and Ethers: The Nature of the SiO Bond. J. Am. Chem. Soc. 2013, 135, 5762–5767. [Google Scholar] [CrossRef] [PubMed]
- Ritch, J.S.; Chivers, T. Siliciumanaloga von Kronenethern und Cryptanden: Ein neues Kapitel in der Wirt-Gast-Chemie? Angew. Chem. 2007, 119, 4694–4697. [Google Scholar] [CrossRef]
- Shambayati, S.; Blake, J.F.; Wierschke, S.G.; Jorgensen, W.L.; Schreiber, S.L. Structure and Basicity of Silyl Ethers: A Crystallographic and ab Initio Inquiry into the Nature of Silicon–Oxygen Interactions. J. Am. Chem. Soc. 1990, 112, 697–703. [Google Scholar] [CrossRef]
- Cypryk, M.; Apeloig, Y. Ab Initio Study of Silyloxonium Ions. Organometallics 1997, 16, 5938–5949. [Google Scholar] [CrossRef]
- Gillespie, R.J.; Robinson, E. Models of molecular geometry. Chem. Soc. Rev. 2005, 34, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Passmore, J.; Rautiainen, J.M. On The Lower Lewis Basicity of Siloxanes Compared to Ethers. Eur. J. Inorg. Chem. 2012, 2012, 6002–6010. [Google Scholar] [CrossRef]
- Cameron, T.S.; Decken, A.; Krossing, I.; Passmore, J.; Rautiainen, J.M.; Wang, X.; Zeng, X. Reactions of a Cyclodimethylsiloxane (Me2SiO)6 with Silver Salts of Weakly Coordinating Anions; Crystal Structures of [Ag(Me2SiO)6][Al] ([Al] = [FAl{OC(CF3)3}3], [Al{OC(CF3)3}4]) and Their Comparison with [Ag(18-Crown-6)]2[SbF6]2. Inorg. Chem. 2013, 52, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, M.; Inoue, Y.; Kanzaki, T.; Hakushi, T. Ring-contracted Crown Ethers: 14-Crown-5, 17-Crown-6, and Their Sila-analogues. Drastic Decrease in Cation-binding Ability. Bull. Chem. Soc. Jpn. 1984, 57, 887–888. [Google Scholar] [CrossRef]
- Inoue, Y.; Ouchi, M.; Hakushi, T. Molecular Design of Crown Ethers. 3. Extraction of Alkaline Earth and Heavy Metal Picrates with 14- to 17-Crown-5 and 17- to 22-Crown-6. Bull. Chem. Soc. Jpn. 1985, 58, 525–530. [Google Scholar] [CrossRef]
- Reuter, K.; Buchner, M.R.; Thiele, G.; von Hänisch, C. Stable Alkali-Metal Complexes of Hybrid Disila-Crown Ethers. Inorg. Chem. 2016, 55, 4441–4447. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Thiele, G.; Hafner, T.; Uhlig, F.; von Hänisch, C. Synthesis and coordination ability of a partially silicon based crown ether. Chem. Commun. 2016, 52, 13265–13268. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Rudel, S.S.; Buchner, M.R.; Kraus, F.; von Hänisch, C. Crown ether complexes of alkali metal chlorides from SO2. Chem. Eur. J. 2017, 23, 9607–9617. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Dankert, F.; Donsbach, C.; von Hänisch, C. Structural Study of Mismatched Disila-Crown Ether Complexes. Inorganics 2017, 5, 11. [Google Scholar] [CrossRef]
- Dankert, F.; Reuter, K.; Donsbach, C.; von Hänisch, C. A structural study of alkaline earth metal complexes with hybrid disila-crown ethers. Dalton Trans. 2017, 46, 8727–8735. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Whatley, L.S.; Lake, K.J. Hydrogen Bonding Studies. V. The Relative Basicities of Ethers, Alkoxysilanes and Siloxanes and the Nature of the Silicon–Oxygen Bond. J. Am. Chem. Soc. 1961, 83, 761–764. [Google Scholar] [CrossRef]
- West, R.; Wilson, L.S.; Powell, D.L. Basicity of siloxanes, alkoxysilanes and ethers toward hydrogen bonding. J. Organomet. Chem. 1979, 178, 5–9. [Google Scholar] [CrossRef]
- Popowski, E.; Schulz, J.; Feist, K.; Kelling, H.; Jancke, H. Basizität und 29Si-NMR-spektroskopische Untersuchungen von Ethoxysiloxanen. Z. Anorg. Allg. Chem. 1988, 558, 206–216. [Google Scholar] [CrossRef]
- Yilgör, E.; Burgaz, E.; Yurtsever, E.; Yilgör, I. Comparison of hydrogen bonding in polydimethylsiloxane and polyether based urethane and urea copolymers. Polymer 2000, 41, 849–857. [Google Scholar] [CrossRef]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Grabowsky, S.; Beckmann, J.; Luger, P. The Nature of Hydrogen Bonding Involving the Siloxane Group. Aust. J. Chem. 2012, 65, 785–795. [Google Scholar] [CrossRef]
- Frolov, Y.L.; Voronkov, M.G.; Strashnikova, N.V.; Shergina, N.I. Hydrogen bonding involving the siloxane group. J. Mol. Struct. 1992, 270, 205–215. [Google Scholar] [CrossRef]
- Grabowsky, S.; Hesse, M.F.; Paulmann, C.; Luger, P.; Beckmann, J. How to Make the Ionic Si–O Bond More Covalent and the Si–O–Si Linkage a Better Acceptor for Hydrogen Bonding. Inorg. Chem. 2009, 48, 4384–4393. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.P.; Dumas, P.; Moras, D. The H3O+ Cation: Molecular Structure of an Oxonium-Macrocyclic Polyether Complex. J. Am. Chem. Soc. 1982, 104, 4540–4543. [Google Scholar] [CrossRef]
- Atwood, J.L.; Bott, S.G.; Means, C.M.; Coleman, A.W.; Zhang, H.; May, M.T. Synthesis of salts of the hydrogen dichloride anion in aromatic solvents. 2. Syntheses and crystal structures of [K∙18-crown-6][Cl–H–Cl], [Mg∙18-crown-6][Cl–H–Cl]2, [H3O∙18-crown-6][Cl–H–Cl], and the Related [H3O∙18-crown-6][Br–H–Br]. Inorg. Chem. 1990, 29, 467–470. [Google Scholar] [CrossRef]
- Atwood, J.L.; Bott, S.G.; Robinson, K.D.; Bishop, E.J.; May, M.T. Preparation and X-ray structure of [H3O+ ·18-crown-6]-[(H5O2+)(Cl−)2], a compound containing both H3O+ and H5O2+ crystallized from aromatic solution. J. Crystallogr. Spectrosc. Res. 1991, 21, 459–462. [Google Scholar] [CrossRef]
- Atwood, J.L.; Junk, P.C.; May, M.T.; Robinson, K.D. Synthesis and X-ray structure of [H3O+ · 18-crown-6] [Br–Br–Br−]; a compound containing both H3O+ and a linear and symmetrical Br3− ion crystallized from aromatic solution. J. Chem. Crystallogr. 1994, 24, 243–245. [Google Scholar] [CrossRef]
- Saleh, M.I.; Kusrini, E.; Fun, H.-K.; Teh, J.B.-J. Dicyclohexano-18-crown-6 hydroxonium tribromide. Acta Cryst. 2007, E63, o3790–o3791. [Google Scholar] [CrossRef]
- Kloo, L.; Svensson, P.H.; Taylor, M.J. Investigations of the polyiodides H3O·Ix (x = 3, 5 or 7) as dibenzo-18-crown-6 complexes. J. Chem. Soc. Dalton Trans. 2000, 1061–1065. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, X.; Luo, Q.; Duan, X.; Pei, C. Cocrystals of ammonium perchlorate with a series of crown ethers: Preparation, structures, and properties. CrystEngComm 2016, 18, 8487–8496. [Google Scholar] [CrossRef]
- Feinberg, H.; Columbus, I.; Cohen, S.; Rabinovitz, M.; Shoham, G.; Selig, H. Crystallographic evidence for different modes of interaction of BF3/BF4− species with water molecules and 18-crown-6. Polyhedron 1993, 12, 2913–2919. [Google Scholar] [CrossRef]
- Dapporto, P.; Paoli, P.; Matijašić, I.; Tuśek-Božić, L. Crystal structures of complexes of ammonium and potassium hexafluorophosphate with dibenzo-18-crown-6. Molecular mechanics studies on the uncomplexed macrocycle. Inorg. Chim. Acta 1996, 252, 383–389. [Google Scholar] [CrossRef]
- Ponomarova, V.V.; Rusanova, J.A.; Rusanov, E.B.; Domasevitch, K.V. Unusual centrosymmetric structure of [M(18-crown-6)]+ (M = Rb, Cs and NH4) complexes stabilized in an environment of hexachloridoantimonate(V) anions. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Hurd, D.T. On the Mechanism of the Acid-catalyzed Rearrangement of Siloxane Linkages in Organopolysiloxanes. J. Am. Chem. Soc. 1955, 77, 2998–3001. [Google Scholar] [CrossRef]
- Cypryk, M.; Apeloig, Y. Mechanism of the Acid-Catalyzed Si–O Bond Cleavage in Siloxanes and Siloxanols. A Theoretical Study. Organometallics 2002, 21, 2165–2175. [Google Scholar] [CrossRef]
- Wu, D.H.; Wu, Q.Q. Ammonium hexafluoridophosphate-18-crown-6 (1/1). Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Shephard, D.S.; Zhou, W.; Maschmeyer, T.; Matters, J.M.; Roper, C.L.; Parsons, S.; Johnson, B.F.G.; Duer, M.J. Ortsspezifische Derivatisierung von MCM-41: Molekulare Erkennung und Lokalisierung funktioneller Gruppen in mesoporösen Materialien durch hochauflösende Transmissionselektronenmikroskopie. Angew. Chem. 1998, 110, 2847–2851. [Google Scholar] [CrossRef]
- Kociok-Köhn, G.; Molloy, K.C.; Price, G.J.; Smith, D.R.G. Structural characterisation of trimethylsilyl-protected DNA bases. Supramol. Chem. 2008, 20, 697–707. [Google Scholar] [CrossRef][Green Version]
- Ha, H.-J.; Choi, C.-J.; Ahn, Y.-G.; Yun, H.; Dong, Y.; Lee, W.K. Cycloaddition of Lewis Acid-Induced N-Methyleneanilines as Azadienes to 1,2-Bistrimethylsilyloxycyclobutene and Oxidative Ring Expansion to 1,2,4,5-Tetrahydro-1-benzazocine-3,6-diones. J. Org. Chem. 2000, 65, 8384–8386. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Baranowska, K.; Chojnacki, J.; Wojnowski, W. Cubane-like structure of a silanethiol—Primary amine assembly—A novel, unusual hydrogen bond pattern. Chem. Commun. 2004, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, A.P.; Makarova, N.N.; Astapova, T.V. X-ray diffraction investigation of trans-2,8-dihydroxy-2,8-diphenyl-4,4′,6,6′,10,10′, 12,12′-octamethylcyclohexasiloxane and trans-2,8-dihydroxy-2,4,4′,6,6′,8,10,10′, 12,12′-decamethyl-5-carbahexacyclosiloxane. Crystallogr. Rep. 2002, 47, 798–804. [Google Scholar] [CrossRef]
- Spielberger, A.; Gspaltl, P.; Siegl, H.; Hengge, E.; Gruber, K. Syntheses, structures and properties of dihydroxypermethylcyclosilanes and permethyloxahexasilanorbornanes. J. Organomet. Chem. 1995, 499, 241–246. [Google Scholar] [CrossRef]
- Driver, T.G.; Franz, A.K.; Woerpel, K.A. Diastereoselective Silacyclopropanations of Functionalized Chiral Alkenes. J. Am. Chem. Soc. 2002, 124, 6524–6525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Myers, A.G.; Dragovich, P.S. A Reaction Cascade Leading to 1,6-didehydro[10]annulene → 1,5-dehydronaphthalene Cyclization Initiated by Thiol Addition. J. Am. Chem. Soc. 1993, 115, 7021–7022. [Google Scholar] [CrossRef]
- Veith, M.; Rammo, A. Synthese und Struktur eines neuartigen molekularen λ5-Organospirosilikats und dessen dynamisches Verhalten in Lösung. J. Organomet. Chem. 1996, 521, 429–433. [Google Scholar] [CrossRef]
- Heyns, A.M.; van Schalkwyk, G.J. A study of the infrared and Raman spectra of ammonium hexafluorophosphate NH4PF6 over a wide range of temperatures. Spectrochim. Acta Part A Mol. Spectrosc. 1973, 29, 1163–1175. [Google Scholar] [CrossRef]
- Willcott, M.R. MestRe Nova. J. Am. Chem. Soc. 2009, 131, 13180. [Google Scholar] [CrossRef]
- OPUS; Version 7.2; Bruker Opt. GmbH: Ettlingen, Germany, 2012.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Diamond; Version 4.4.1; Crystal and Molecular Structure Visualization; Putz, H. & Brandenburg, K. GbR: Bonn, Germany, 2017.
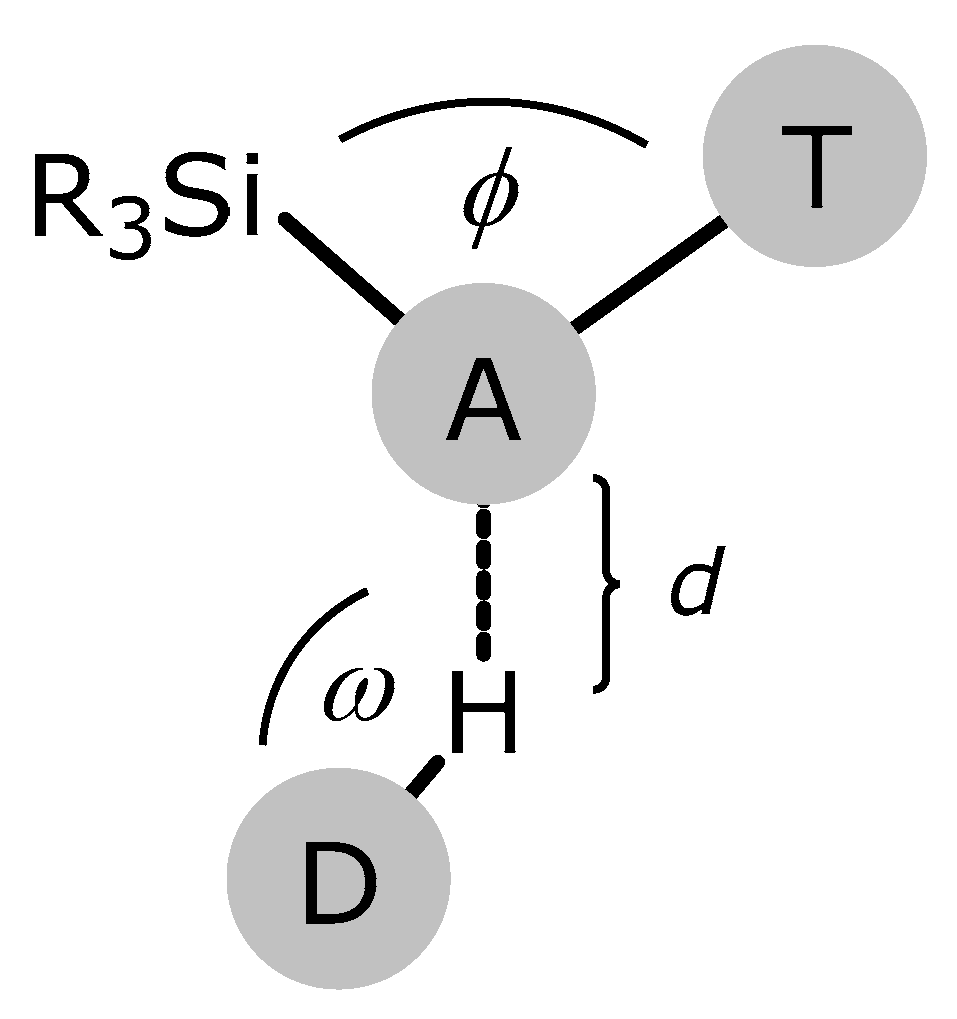
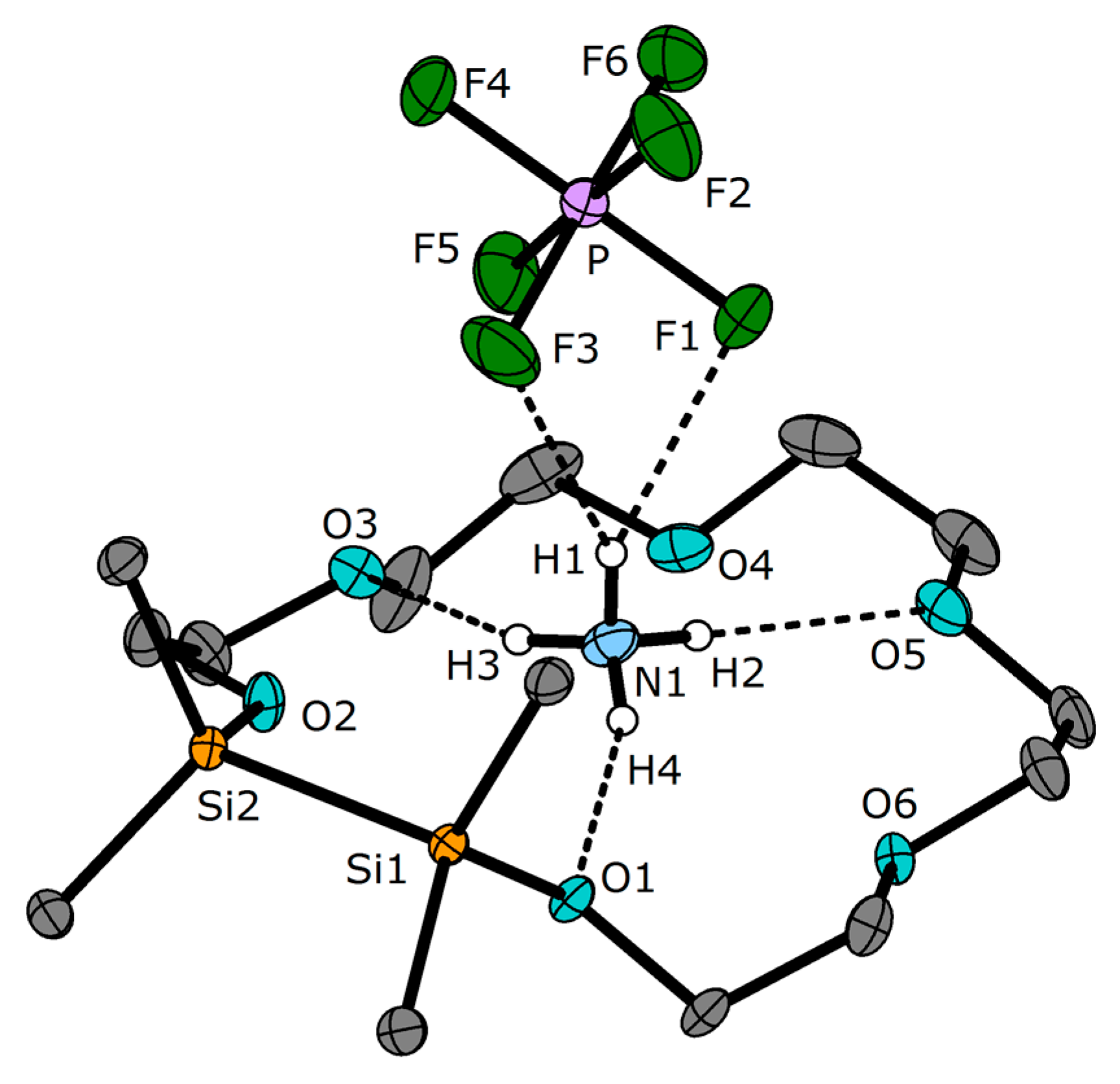
| Compound or CSD Refcode 1 | D–H 2 | A | d [pm] | T 3 | ω [°] | ϕ [°] | Ref. 4 |
|---|---|---|---|---|---|---|---|
| 3 | N–H2 | O5 | 206 | CE | 160 | 113 | * |
| N–H3 | O3 | 203 | CE | 156 | 108 | * | |
| N–H4 | O1 | 205 | C | 159 | 122 | * | |
| 4 | N–H2 | O4 | 214 | CE | 165 | 117 | * |
| N–H3 | O2 | 209 | CE | 151 | 110 | * | |
| N–H4 | O6 | 200 | C | 172 | 118 | * | |
| VONMOB | N–H | O | 226 | C | 174 | 128 | [38] |
| MEQFOD | N–H | O | 247 | C | 141 | 131 | [39] |
| ITUBUI | N–H | O | 198–210 | C | 157–160 | 130–132 | [40] |
| MOLYUO | O–H | O | 192 | Si | 167 | 116 | [23] |
| TAKFOB | O–H | O | 257–260 | Si | 148–152 | 139–148 | [41] |
| ZEMXAQ | O–H | O | 199 | Si | 156 | 116 | [42] |
| EGEKAC | O–H | O | 199–200 | C | 155–161 | 114–115 | [43] |
| PERWIS | O–H | O | 199 | C | 166 | 123 | [44] |
| REXXAT | O–H | O | 199 | C | 173 | 111 | [45] |
| 3 | 4∙DCM | |
|---|---|---|
| Empirical formula | C14H36F6NO6PSi2 | C19H38Cl2F6NO6PSi2 |
| Formula weight [g·mol−1] | 515.59 | 648.55 |
| Crystal colour, shape | colourless block | colourless rod |
| Crystal size [mm] | 0.487 × 0.432 × 0.346 | 0.076 × 0.106 × 0.522 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21 | Cc |
| Formula units | 2 | 4 |
| Temperature [K] | 100(2) | 100(2) |
| Unit cell dimensions [Å, °] | a = 10.4542(5) | a = 14.9582(7) |
| b = 12.3869(6) | b = 12.6454(6) | |
| c = 10.6140(5) | c = 16.3325(8) | |
| β = 117.912(1) | β = 104.950(2) | |
| Cell volume [Å3] | 1214.57(10) | 2984.8(2) |
| ρ calc [g/cm3] | 1.410 | 1.443 |
| μ [mm−1] | 0.286 | 0.422 |
| 2θ range [°] | 4.342 to 50.568 | 4.280 to 53.570 |
| Reflections measured | 34532 | 68974 |
| Independent reflections | 4419 | 6355 |
| R1 (I > 2σ(I)) | 0.0203 | 0.0535 |
| wR2 (all data) | 0.0529 | 0.1329 |
| GooF | 1.076 | 1.035 |
| Largest diff. peak/hole [e∙Å−3] | 0.24/−0.27 | 0.51/−0.44 |
| Flack parameter | 0.004(14) | 0.048(16) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dankert, F.; Reuter, K.; Donsbach, C.; Von Hänisch, C. Hybrid Disila-Crown Ethers as Hosts for Ammonium Cations: The O–Si–Si–O Linkage as an Acceptor for Hydrogen Bonding. Inorganics 2018, 6, 15. https://doi.org/10.3390/inorganics6010015
Dankert F, Reuter K, Donsbach C, Von Hänisch C. Hybrid Disila-Crown Ethers as Hosts for Ammonium Cations: The O–Si–Si–O Linkage as an Acceptor for Hydrogen Bonding. Inorganics. 2018; 6(1):15. https://doi.org/10.3390/inorganics6010015
Chicago/Turabian StyleDankert, Fabian, Kirsten Reuter, Carsten Donsbach, and Carsten Von Hänisch. 2018. "Hybrid Disila-Crown Ethers as Hosts for Ammonium Cations: The O–Si–Si–O Linkage as an Acceptor for Hydrogen Bonding" Inorganics 6, no. 1: 15. https://doi.org/10.3390/inorganics6010015
APA StyleDankert, F., Reuter, K., Donsbach, C., & Von Hänisch, C. (2018). Hybrid Disila-Crown Ethers as Hosts for Ammonium Cations: The O–Si–Si–O Linkage as an Acceptor for Hydrogen Bonding. Inorganics, 6(1), 15. https://doi.org/10.3390/inorganics6010015