A Recycling Hydrogen Supply System of NaBH4 Based on a Facile Regeneration Process: A Review
Abstract
1. Introduction
2. NaBH4 Regeneration via the Reaction between Metal or Other Hydrides and NaBO2
3. NaBH4 Regeneration via using NaBO2∙xH2O as Raw Materials
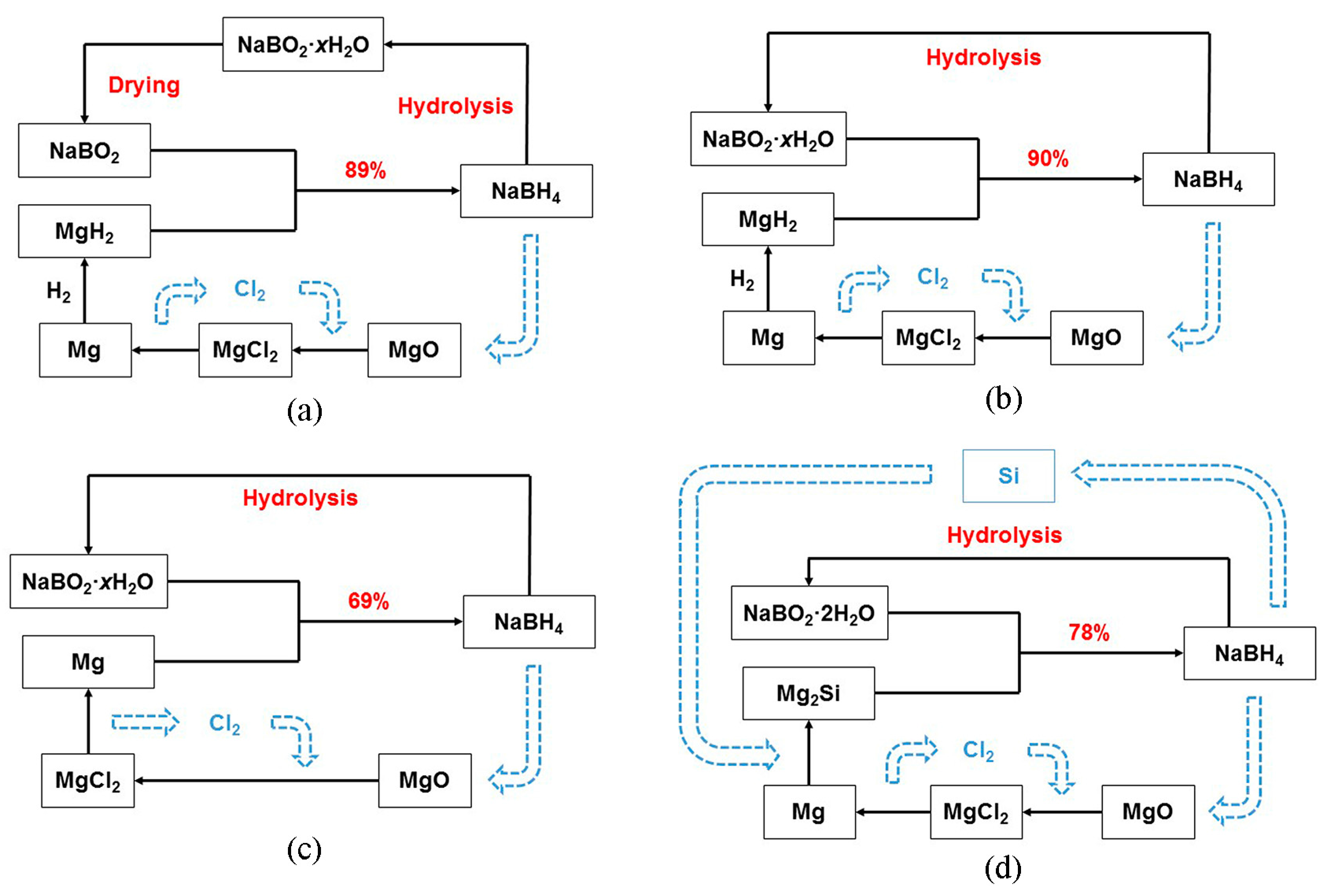
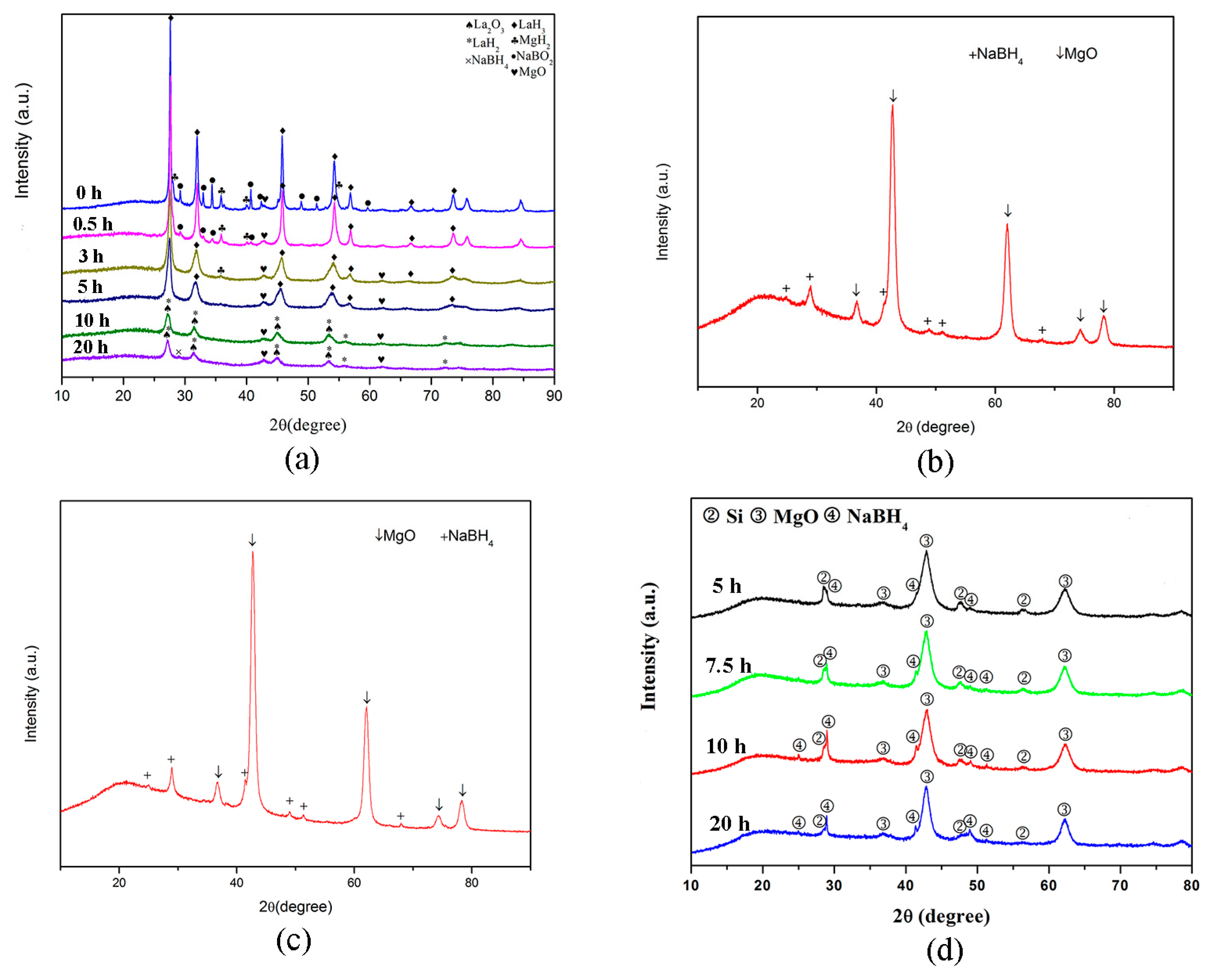
3.1. NaBH4 Regeneration via the Reaction between MgH2 and NaBO2∙xH2O
3.2. NaBH4 Regeneration via the Reaction between Mg and NaBO2∙xH2O
3.3. NaBH4 Regeneration via the Reaction between Mg2Si and NaBO2∙2H2O
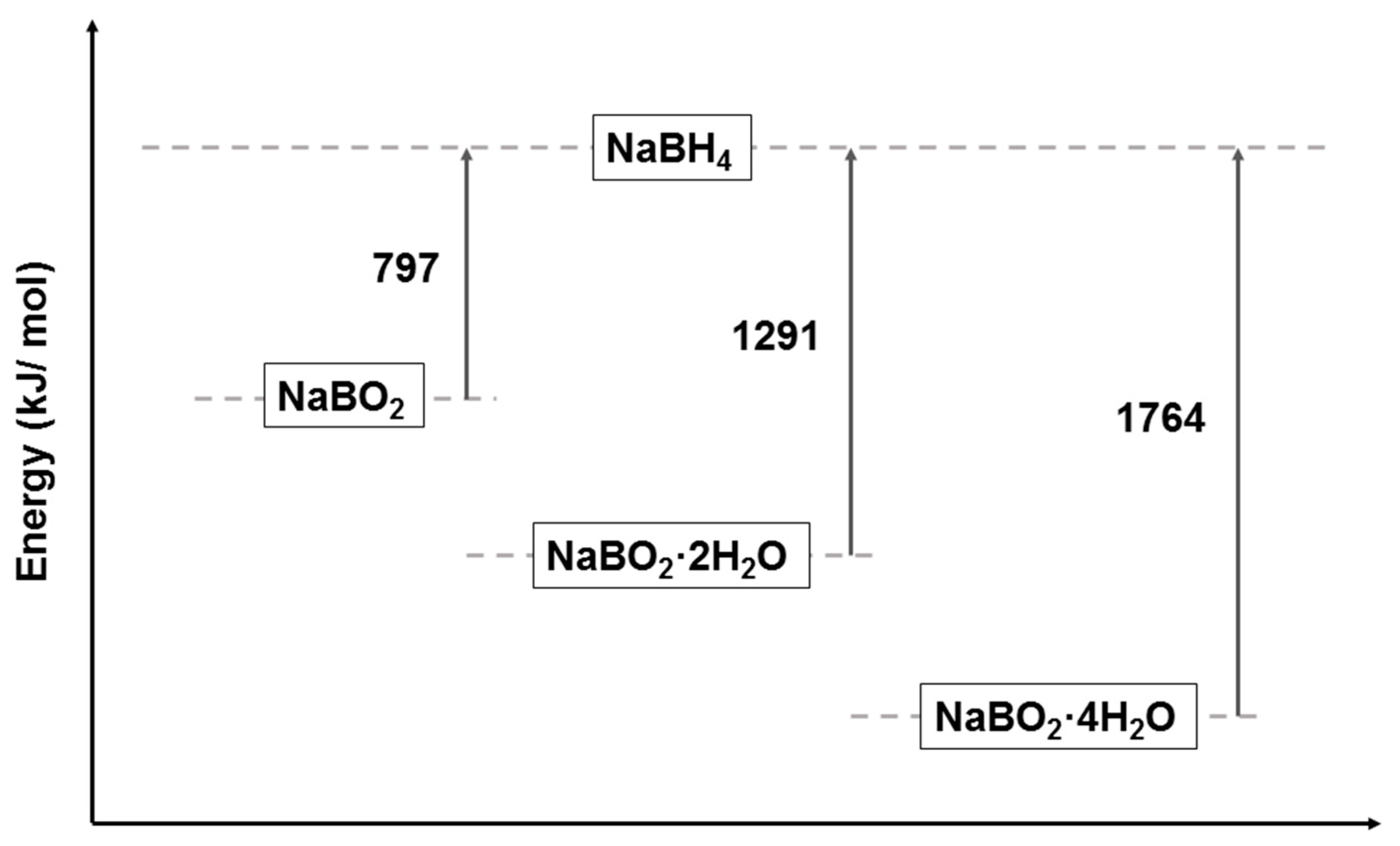
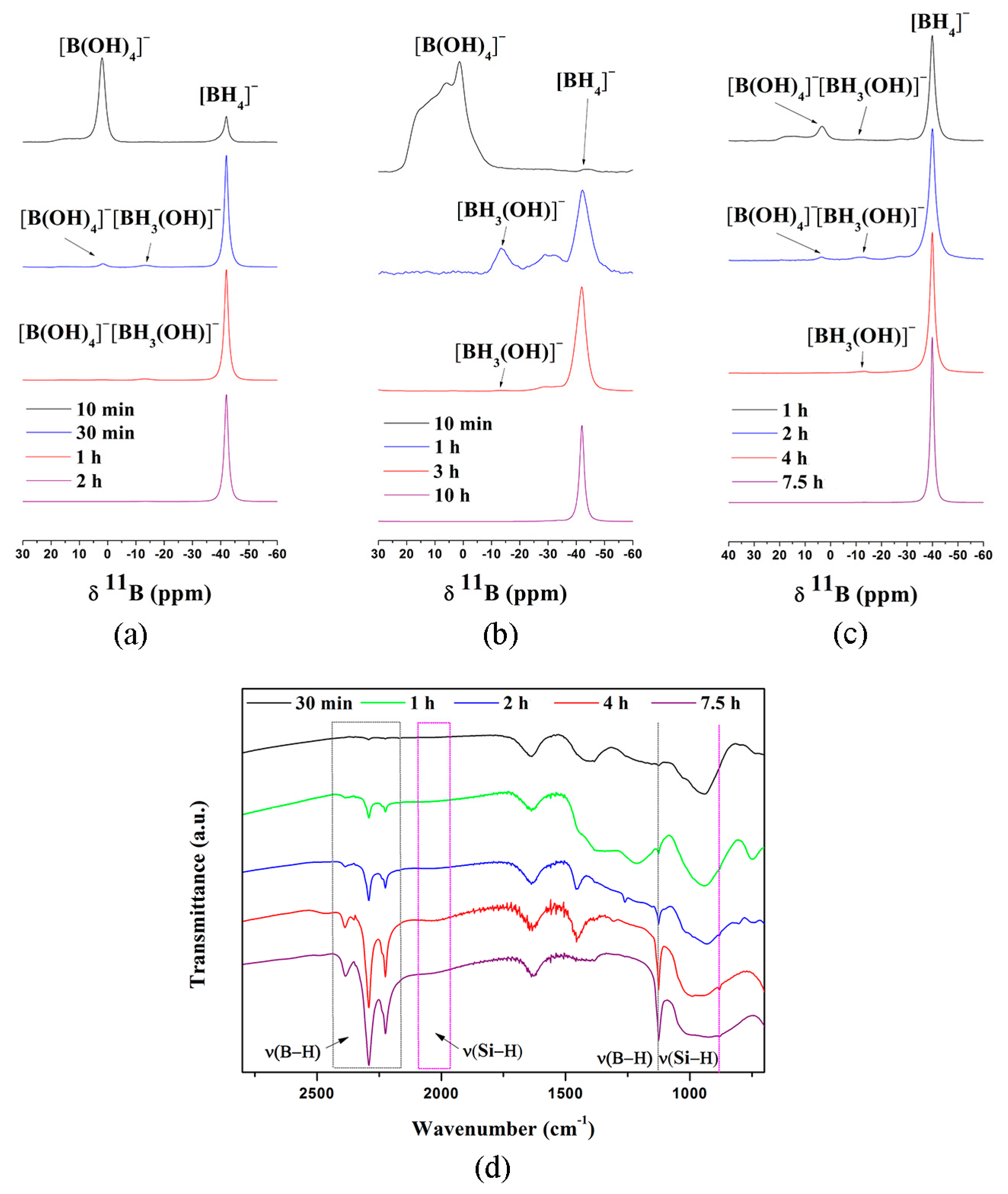
4. Mechanism of NaBH4 Regeneration Using NaBO2∙xH2O as Raw Materials
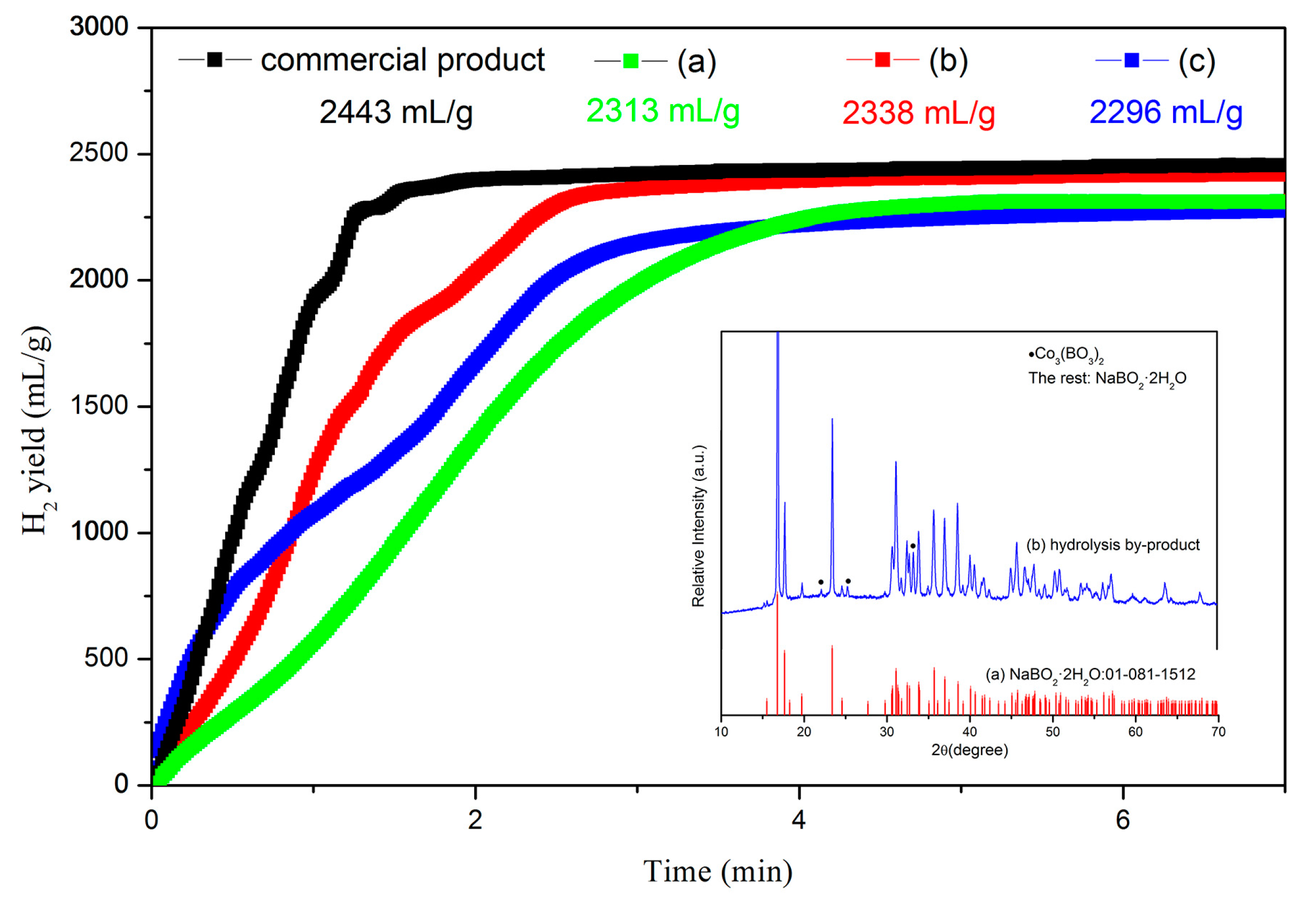
5. Hydrolysis Property of Regenerated NaBH4 Using NaBO2∙xH2O as Raw Materials
6. Summary and Perspective
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Wei, Z.; Guo, Q.; Tian, H. Kinetics of sodium borohydride hydrolysis catalyzed via carbon nanosheets supported Zr/Co. J. Power Sources 2013, 231, 190–196. [Google Scholar] [CrossRef]
- Awad, A.S.; El-Asmar, E.; Tayeh, T.; Mauvy, F.; Nakhl, M.; Zakhour, M.; Bobet, J.L. Effect of carbons (G and CFs), TM (Ni, Fe and Al) and oxides (Nb2O5 and V2O5) on hydrogen generation from ball milled Mg-based hydrolysis reaction for fuel cell. Energy 2016, 95, 175–186. [Google Scholar] [CrossRef]
- Brack, P.; Dann, S.E.; Wijayantha, K.G.U. Heterogeneous and homogenous catalysts for hydrogen generation by hydrolysis of aqueous sodium borohydride (NaBH4) solutions. Energy Sci. Eng. 2015, 3, 174–188. [Google Scholar] [CrossRef]
- Luo, Y.-C.; Liu, Y.-H.; Hung, Y.; Liu, X.-Y.; Mou, C.-Y. Mesoporous silica supported cobalt catalysts for hydrogen generation in hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2013, 38, 7280–7290. [Google Scholar] [CrossRef]
- Ding, Q.; Meng, F.; English, C.R.; Cabán-Acevedo, M.; Shearer, M.J.; Liang, D.; Daniel, A.S.; Hamers, R.J.; Jin, S. Efficient photoelectrochemical hydrogen generation using heterostructures of Si and chemically exfoliated metallic MoS2. J. Am. Chem. Soc. 2014, 136, 8504–8507. [Google Scholar] [CrossRef] [PubMed]
- Ladomenou, K.; Natali, M.; Iengo, E.; Charalampidis, G.; Scandola, F.; Coutsolelos, A.G. Photochemical hydrogen generation with porphyrin-based systems. Coord. Chem. Rev. 2015, 304, 38–54. [Google Scholar] [CrossRef]
- Huang, M.; Ouyang, L.; Wang, H.; Liu, J.; Zhu, M. Hydrogen generation by hydrolysis of MgH2 and enhanced kinetics performance of ammonium chloride introducing. Int. J. Hydrog. Energy 2015, 40, 6145–6150. [Google Scholar] [CrossRef]
- Bulut, A.; Yurderi, M.; Ertas, İ.E.; Celebi, M.; Kaya, M.; Zahmakiran, M. Carbon dispersed copper-cobalt alloy nanoparticles: A cost-effective heterogeneous catalyst with exceptional performance in the hydrolytic dehydrogenation of ammonia-borane. Appl. Catal. B 2016, 180, 121–129. [Google Scholar] [CrossRef]
- Dudoladov, A.O.; Buryakovskaya, O.A.; Vlaskin, M.S.; Zhuk, A.Z.; Shkolnikov, E.I. Generation of hydrogen by aluminium oxidation in aquaeous solutions at low temperatures. Int. J. Hydrog. Energy 2016, 41, 2230–2237. [Google Scholar] [CrossRef]
- Huynh, K.; Napolitano, K.; Wang, R.; Jessop, P.G.; Davis, B.R. Indirect hydrolysis of sodium borohydride: Isolation and crystallographic characterization of methanolysis and hydrolysis by-products. Int. J. Hydrog. Energy 2013, 38, 5775–5782. [Google Scholar] [CrossRef]
- Jiang, J.; Materna, K.L.; Hedström, S.; Yang, K.R.; Crabtree, R.H.; Batista, V.S.; Brudvig, G.W. Antimony Complexes for Electrocatalysis: Activity of a Main-Group Element in Proton Reduction. Angew. Chem. Int. Ed. 2017, 56, 9111–9115. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Kanoglu, M. Thermodynamic evaluation of geothermal energy powered hydrogen production by PEM water electrolysis. Energy 2014, 69, 592–602. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Amaral, L.; Santos, D.M.F.; Šljukić, B.; Sequeira, C.A.C.; Macciò, D.; Saccone, A. Enhancement of hydrogen evolution in alkaline water electrolysis by using nickel-rare earth alloys. Int. J. Hydrog. Energy 2015, 40, 4295–4302. [Google Scholar] [CrossRef]
- Kibria, M.G.; Qiao, R.; Yang, W.; Boukahil, I.; Kong, X.; Chowdhury, F.A.; Trudeau, M.L.; Ji, W.; Guo, H.; Himpsel, F.J.; et al. Atomic-Scale Origin of Long-Term Stability and High Performance of p-GaN Nanowire Arrays for Photocatalytic Overall Pure Water Splitting. Adv. Mater. 2016, 28, 8388–8397. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, S.; Kuang, Y.; Akiyama, S.; Hisatomi, T.; Nakabayashi, M.; Shibata, N.; Katayama, M.; Minegishi, T.; Domen, K. Visible light-driven Z-scheme water splitting using oxysulfide H2 evolution photocatalysts. J. Phys. Chem. Lett. 2016, 7, 3892–3896. [Google Scholar] [CrossRef] [PubMed]
- Senthil, V.; Badapanda, T.; Chithambararaj, A.; Chandra Bose, A.; Panigrahi, S. Impedance spectroscopy and photocatalysis water splitting for hydrogen production with cerium modified SrBi2Ta2O9 ferroelectrics. Int. J. Hydrog. Energy 2016, 41, 22856–22865. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Ren, J.; Wang, S.; Wang, R.; Fu, G.; Hu, Y. Passivation of defect states in anatase TiO2 hollow spheres with Mg doping: Realizing efficient photocatalytic overall water splitting. Appl. Catal. B 2017, 202, 127–133. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Principi, G.; Agresti, F.; Maddalena, A.; Lo Russo, S. The problem of solid state hydrogen storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Lim, K.L.; Kazemian, H.; Yaakob, Z.; Daud, W.R.W. Solid-state Materials and Methods for Hydrogen Storage: A Critical Review. Chem. Eng. Technol. 2010, 33, 213–226. [Google Scholar] [CrossRef]
- Khafidz, N.Z.A.K.; Yaakob, Z.; Lim, K.L.; Timmiati, S.N. The kinetics of lightweight solid-state hydrogen storage materials: A review. Int. J. Hydrog. Energy 2016, 41, 13131–13151. [Google Scholar] [CrossRef]
- Amendola, S.C.; Sharp-Goldman, S.L.; Janjua, M.S.; Kelly, M.T.; Petillo, P.J.; Binder, M. An ultrasafe hydrogen generator: Aqueous, alkaline borohydride solutions and Ru catalyst. J. Power Sources 2000, 85, 186–189. [Google Scholar] [CrossRef]
- Cao, N.; Hu, K.; Luo, W.; Cheng, G. RuCu nanoparticles supported on graphene: A highly efficient catalyst for hydrolysis of ammonia borane. J. Alloys Compd. 2014, 590, 241–246. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Dong, H.W.; Peng, C.H.; Sun, L.X.; Zhu, M. A new type of Mg-based metal hydride with promising hydrogen storage properties. Int. J. Hydrog. Energy 2007, 32, 3929–3935. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.Z.; Cao, Z.J.; Wang, H.; Liu, J.W.; Sun, D.L.; Zhang, Q.A.; Zhu, M. Enhanced dehydriding thermodynamics and kinetics in Mg(In)–MgF2 composite directly synthesized by plasma milling. J. Alloys Compd. 2014, 586, 113–117. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Huang, J.M.; Fang, C.J.; Zhang, Q.A.; Sun, D.L.; Zhu, M. The controllable hydrolysis rate for LaMg12 hydride. Int. J. Hydrog. Energy 2012, 37, 12358–12364. [Google Scholar] [CrossRef]
- Ouyang, L.; Ma, M.; Huang, M.; Duan, R.; Wang, H.; Sun, L.; Zhu, M. Enhanced Hydrogen Generation Properties of MgH2-Based Hydrides by Breaking the Magnesium Hydroxide Passivation Layer. Energies 2015, 8, 4237–4252. [Google Scholar] [CrossRef]
- Varin, R.A.; Jang, M.; Czujko, T.; Wronski, Z.S. The effect of ball milling under hydrogen and argon on the desorption properties of MgH2 covered with a layer of Mg(OH)2. J. Alloys Compd. 2010, 493, L29–L32. [Google Scholar] [CrossRef]
- Kadri, A.; Jia, Y.; Chen, Z.; Yao, X. Catalytically Enhanced Hydrogen Sorption in Mg-MgH2 by Coupling Vanadium-Based Catalyst and Carbon Nanotubes. Materials 2015, 8, 3491–3507. [Google Scholar] [CrossRef]
- Hiraki, T.; Hiroi, S.; Akashi, T.; Okinaka, N.; Akiyama, T. Chemical equilibrium analysis for hydrolysis of magnesium hydride to generate hydrogen. Int. J. Hydrog. Energy 2012, 37, 12114–12119. [Google Scholar] [CrossRef]
- Tayeh, T.; Awad, A.S.; Nakhl, M.; Zakhour, M.; Silvain, J.F.; Bobet, J.L. Production of hydrogen from magnesium hydrides hydrolysis. Int. J. Hydrog. Energy 2014, 39, 3109–3117. [Google Scholar] [CrossRef]
- Fernandes, R.; Patel, N.; Paris, A.; Calliari, L.; Miotello, A. Improved H2 production rate by hydrolysis of Ammonia Borane using quaternary alloy catalysts. Int. J. Hydrog. Energy 2013, 38, 3313–3322. [Google Scholar] [CrossRef]
- Kantürk Figen, A.; Coşkuner, B. A novel perspective for hydrogen generation from ammonia borane (NH3BH3) with Co–B catalysts: “Ultrasonic Hydrolysis”. Int. J. Hydrog. Energy 2013, 38, 2824–2835. [Google Scholar] [CrossRef]
- Rakap, M. PVP-stabilized Ru–Rh nanoparticles as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. J. Alloys Compd. 2015, 649, 1025–1030. [Google Scholar] [CrossRef]
- Yang, X.J.; Li, L.L.; Sang, W.L.; Zhao, J.L.; Wang, X.X.; Yu, C.; Zhang, X.H.; Tang, C.C. Boron nitride supported Ni nanoparticles as catalysts for hydrogen generation from hydrolysis of ammonia borane. J. Alloys Compd. 2017, 693, 642–649. [Google Scholar] [CrossRef]
- Durak, H.; Gulcan, M.; Zahmakiran, M.; Ozkar, S.; Kaya, M. Hydroxyapatite-nanosphere supported ruthenium(0) nanoparticle catalyst for hydrogen generation from ammonia-borane solution: Kinetic studies for nanoparticle formation and hydrogen evolution. Rsc. Adv. 2014, 4, 28947–28955. [Google Scholar] [CrossRef]
- Garralda, M.A.; Mendicute-Fierro, C.; Rodriguez-Dieguez, A.; Seco, J.M.; Ubide, C.; Zumeta, I. Efficient hydridoirida-beta-diketone-catalyzed hydrolysis of ammonia- or amine-boranes for hydrogen generation in air. Dalton Trans. 2013, 42, 11652–11660. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M. Catalytic and photo hydrolysis of ammonia borane complex using Pd-doped Co nanofibers. Appl. Catal. A 2013, 451, 21–27. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today 2011, 170, 56–63. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Chen, X.; Shore, S.G. Ammonia borane, past as prolog. J. Organomet. Chem. 2014, 751, 60–66. [Google Scholar] [CrossRef]
- Summerscales, O.T.; Gordon, J.C. Regeneration of ammonia borane from spent fuel materials. Dalton Trans. 2013, 42, 10075–10084. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.D.; Davis, B.L.; Bhattacharyya, K.X.; Ellis, B.D.; Gordon, J.C.; Power, P.P. Recycle of tin thiolate compounds relevant to ammonia-borane regeneration. Chem. Commun. 2010, 46, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, C.; Fang, Y.; Zhu, F.; Liu, H.; Ge, H. Hydrogen generation mechanism of spontaneous hydrolysis: A sight from ab initio calculation. Int. J. Hydrog. Energy 2016, 41, 22668–22676. [Google Scholar] [CrossRef]
- Retnamma, R.; Novais, A.Q.; Rangel, C.M. Kinetics of hydrolysis of sodium borohydride for hydrogen production in fuel cell applications: A review. Int. J. Hydrog. Energy 2011, 36, 9772–9790. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, H.; Liu, J.W.; Sun, D.L.; Fang, F.; Zhang, Q.A.; Ouyang, L.Z.; Zhu, M. Enhanced hydrolysis properties and energy efficiency of MgH2-base hydrides. J. Alloys Compd. 2016, 680, 419–426. [Google Scholar] [CrossRef]
- Nunes, H.X.; Ferreira, M.J.F.; Rangel, C.M.; Pinto, A.M.F.R. Hydrogen generation and storage by aqueous sodium borohydride (NaBH4) hydrolysis for small portable fuel cells (H2 – PEMFC). Int. J. Hydrog. Energy 2016, 41, 15426–15432. [Google Scholar] [CrossRef]
- Chinnappan, A.; Puguan, J.M.C.; Chung, W.-J.; Kim, H. Hydrogen generation from the hydrolysis of sodium borohydride using chemically modified multiwalled carbon nanotubes with pyridinium based ionic liquid and decorated with highly dispersed Mn nanoparticles. J. Power Sources 2015, 293, 429–436. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Efficient hydrogen production from NaBH4 hydrolysis catalyzed by a magnetic cobalt/carbon composite derived from a zeolitic imidazolate framework. Chem. Eng. J. 2016, 296, 243–251. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt in NaBH4 hydrolysis. Phys. Chem. Chem. Phys. 2010, 12, 14651–14665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, R.; Niu, W.; Wu, Y.; Gou, X. Fast hydrogen generation from NaBH4 hydrolysis catalyzed by carbon aerogels supported cobalt nanoparticles. Int. J. Hydrog. Energy 2013, 38, 10864–10870. [Google Scholar] [CrossRef]
- Wang, M.C.; Ouyang, L.Z.; Liu, J.W.; Wang, H.; Zhu, M. Hydrogen generation from sodium borohydride hydrolysis accelerated by zinc chloride without catalyst: A kinetic study. J. Alloys Compd. 2017, 717, 48–54. [Google Scholar] [CrossRef]
- Program, U.S.D.o.E.H. Go/No-Go Recommendation for Sodium Borohydride for On-Board Vehicular Hydrogen Storage. Available online: http://www.hydrogen.energy.gov/pdfs/42220.pdf (accessed on 1 July 2017).
- Demirci, U.B.; Akdim, O.; Miele, P. Ten-year efforts and a no-go recommendation for sodium borohydride for on-board automotive hydrogen storage. Int. J. Hydrog. Energy 2009, 34, 2638–2645. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E. The Preparation of Sodium Borohydride by the High Temperature Reaction of Sodium Hydride with Borate Esters1. J. Am. Chem. Soc. 1953, 75, 205–209. [Google Scholar] [CrossRef]
- Johnson, W.C.; Isenberg, S. Hydrogen Compounds of Silicon. I. The Preparation of Mono- and Disilane. J. Am. Chem. Soc. 1935, 57, 1349–1353. [Google Scholar] [CrossRef]
- Li, Z.P.; Morigazaki, N.; Liu, B.H.; Suda, S. Preparation of sodium borohydride by the reaction of MgH2 with dehydrated borax through ball milling at room temperature. J. Alloys Compd. 2003, 349, 232–236. [Google Scholar] [CrossRef]
- Çakanyildirim, Ç.; Gürü, M. The Production of NaBH4 from Its Elements by Mechano-chemical Reaction and Usage in Hydrogen Recycle. Energy Sources Part A 2011, 33, 1912–1920. [Google Scholar] [CrossRef]
- Bilen, M.; Gürü, M.; Çakanyıldırım, Ç. Role of NaCl in NaBH4 production and its hydrolysis. Energ Convers. Manag. 2013, 72, 134–140. [Google Scholar] [CrossRef]
- Marrero-Alfonso, E.Y.; Gray, J.R.; Davis, T.A.; Matthews, M.A. Minimizing water utilization in hydrolysis of sodium borohydride: The role of sodium metaborate hydrates. Int. J. Hydrog. Energy 2007, 32, 4723–4730. [Google Scholar] [CrossRef]
- Ved, A.S.; Miley, G.H.; Seetaraman, T.S. Recycling Sodium Metaborate to Sodium Borohydride Using Wind-Solar Energy System for Direct Borohydride Fuel Cell. In Proceedings of the ASME 2010 8th International Fuel Cell Science, Engineering and Technology, Brooklyn, NY, USA, 14–16 June 2010; pp. 139–141. [Google Scholar]
- Sanli, A.E.; Kayacan, İ.; Uysal, B.Z.; Aksu, M.L. Recovery of borohydride from metaborate solution using a silver catalyst for application of direct rechargable borohydride/peroxide fuel cells. J. Power Sources 2010, 195, 2604–2607. [Google Scholar] [CrossRef]
- McLafferty, J.; Colominas, S.; Macdonald, D.D. Attempts to cathodically reduce boron oxides to borohydride in aqueous solution. Electrochim. Acta 2010, 56, 108–114. [Google Scholar] [CrossRef]
- Kojima, Y.; Haga, T. Recycling process of sodium metaborate to sodium borohydride. Int. J. Hydrog. Energy 2003, 28, 989–993. [Google Scholar] [CrossRef]
- Hsueh, C.-L.; Liu, C.-H.; Chen, B.-H.; Chen, C.-Y.; Kuo, Y.-C.; Hwang, K.-J.; Ku, J.-R. Regeneration of spent-NaBH4 back to NaBH4 by using high-energy ball milling. Int. J. Hydrog. Energy 2009, 34, 1717–1725. [Google Scholar] [CrossRef]
- Kong, L.; Cui, X.; Jin, H.; Wu, J.; Du, H.; Xiong, T. Mechanochemical Synthesis of Sodium Borohydride by Recycling Sodium Metaborate. Energy Fuels 2009, 23, 5049–5054. [Google Scholar] [CrossRef]
- Çakanyıldırım, Ç.; Gürü, M. Processing of NaBH4 from NaBO2 with MgH2 by ball milling and usage as hydrogen carrier. Renew. Energy 2010, 35, 1895–1899. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Zhong, H.; Li, Z.M.; Cao, Z.J.; Wang, H.; Liu, J.W.; Zhu, X.K.; Zhu, M. Low-cost method for sodium borohydride regeneration and the energy efficiency of its hydrolysis and regeneration process. J. Power Sources 2014, 269, 768–772. [Google Scholar] [CrossRef]
- Lang, C.; Jia, Y.; Liu, J.; Wang, H.; Ouyang, L.; Zhu, M.; Yao, X. NaBH4 regeneration from NaBO2 by high-energy ball milling and its plausible mechanism. Int. J. Hydrog. Energy 2017, 42, 13127–13135. [Google Scholar] [CrossRef]
- Liu, B. Kinetic characteristics of sodium borohydride formation when sodium meta-borate reacts with magnesium and hydrogen. Int. J. Hydrog. Energy 2008, 33, 1323–1328. [Google Scholar] [CrossRef]
- Eom, K.; Cho, E.; Kim, M.; Oh, S.; Nam, S.-W.; Kwon, H. Thermochemical production of sodium borohydride from sodium metaborate in a scaled-up reactor. Int. J. Hydrog. Energy 2013, 38, 2804–2809. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Zhu, J.K.; Morigasaki, N.; Suda, S. Sodium Borohydride Synthesis by Reaction of Na2O Contained Sodium Borate with Al and Hydrogen. Energy Fuels 2007, 21, 1707–1711. [Google Scholar] [CrossRef]
- Beaird, A.M.; Li, P.; Marsh, H.S.; Al-Saidi, W.A.; Johnson, J.K.; Matthews, M.A.; Williams, C.T. Thermal Dehydration and Vibrational Spectra of Hydrated Sodium Metaborates. Ind. Eng. Chem. Res. 2011, 50, 7746–7752. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Zhu, J.K. Sodium borohydride formation when Mg reacts with hydrous sodium borates under hydrogen. J. Alloys Compd. 2009, 476, L16–L20. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, L.Z.; Liu, J.W.; Yao, X.D.; Wang, H.; Liu, Z.W.; Zhu, M. Hydrolysis and regeneration of sodium borohydride (NaBH4)—A combination of hydrogen production and storage. J. Power Sources 2017, 359, 400–407. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, S.; Fang, F.; Chen, G.; Sang, G.; Sun, D. Synthesis of NaBH4 based on a solid-state reaction under Ar atmosphere. J. Alloys Compd. 2009, 484, 352–355. [Google Scholar] [CrossRef]
- Ouyang, L.; Chen, W.; Liu, J.; Felderhoff, M.; Wang, H.; Zhu, M. Enhancing the Regeneration Process of Consumed NaBH4 for Hydrogen Storage. Adv. Energy Mater. 2017, 7, 1700299. [Google Scholar] [CrossRef]
- Zhong, H.; Ouyang, L.Z.; Ye, J.S.; Liu, J.W.; Wang, H.; Yao, X.D.; Zhu, M. An one-step approach towards hydrogen production and storage through regeneration of NaBH4. Energy Storage Mater. 2017, 7, 222–228. [Google Scholar] [CrossRef]
- Andrieux, J.; Demirci, U.B.; Hannauer, J.; Gervais, C.; Goutaudier, C.; Miele, P. Spontaneous hydrolysis of sodium borohydride in harsh conditions. Int. J. Hydrog. Energy 2011, 36, 224–233. [Google Scholar] [CrossRef]
- Chong, M.; Karkamkar, A.; Autrey, T.; Orimo, S.-I.; Jalisatgi, S.; Jensen, C.M. Reversible dehydrogenation of magnesium borohydride to magnesium triborane in the solid state under moderate conditions. Chem. Commun. 2011, 47, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.-B.; Ma, G.-L.; Kang, X.-D.; Wang, P. Hydrogen generation from coupling reactions of sodium borohydride and aluminum powder with aqueous solution of cobalt chloride. Catal. Today 2011, 170, 50–55. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, L.; Zhong, H.; Li, H.-W.; Zhu, M. A Recycling Hydrogen Supply System of NaBH4 Based on a Facile Regeneration Process: A Review. Inorganics 2018, 6, 10. https://doi.org/10.3390/inorganics6010010
Ouyang L, Zhong H, Li H-W, Zhu M. A Recycling Hydrogen Supply System of NaBH4 Based on a Facile Regeneration Process: A Review. Inorganics. 2018; 6(1):10. https://doi.org/10.3390/inorganics6010010
Chicago/Turabian StyleOuyang, Liuzhang, Hao Zhong, Hai-Wen Li, and Min Zhu. 2018. "A Recycling Hydrogen Supply System of NaBH4 Based on a Facile Regeneration Process: A Review" Inorganics 6, no. 1: 10. https://doi.org/10.3390/inorganics6010010
APA StyleOuyang, L., Zhong, H., Li, H.-W., & Zhu, M. (2018). A Recycling Hydrogen Supply System of NaBH4 Based on a Facile Regeneration Process: A Review. Inorganics, 6(1), 10. https://doi.org/10.3390/inorganics6010010






