Improvement in the Electrochemical Lithium Storage Performance of MgH2
Abstract
:1. Introduction
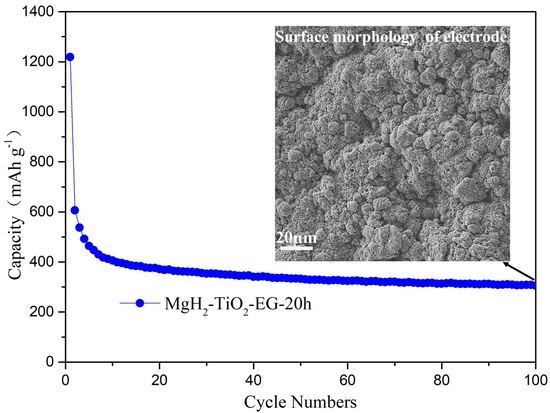
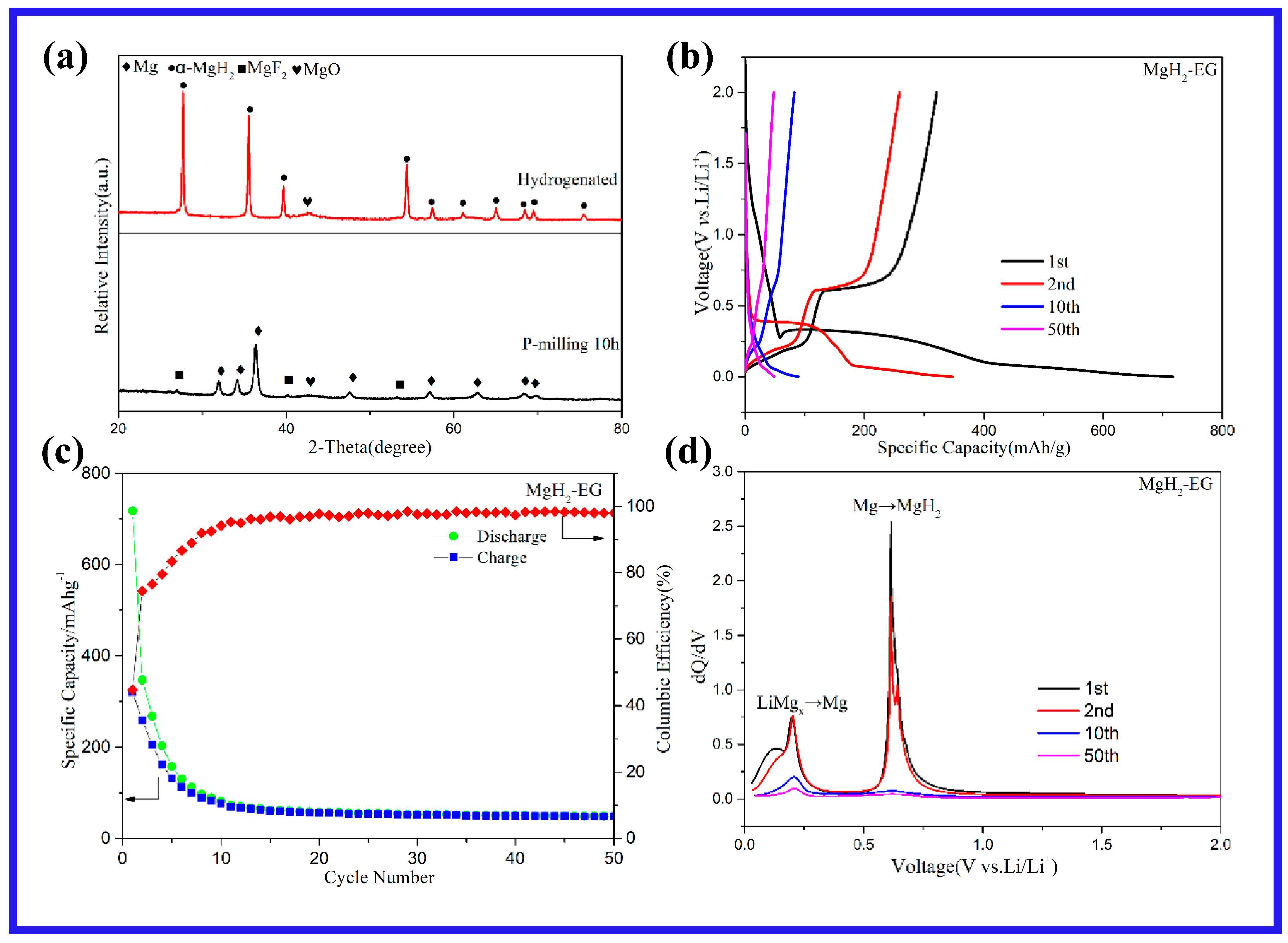
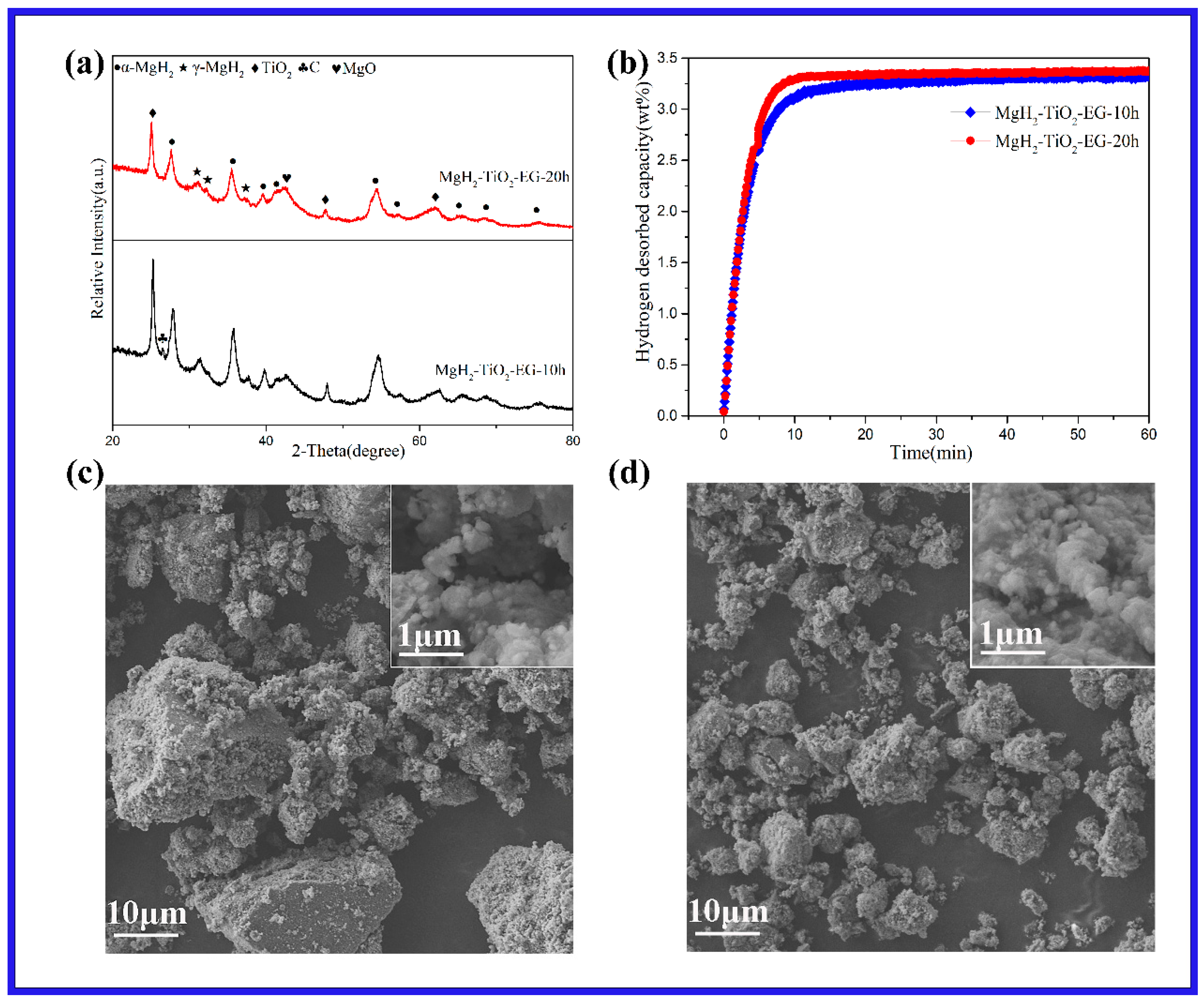
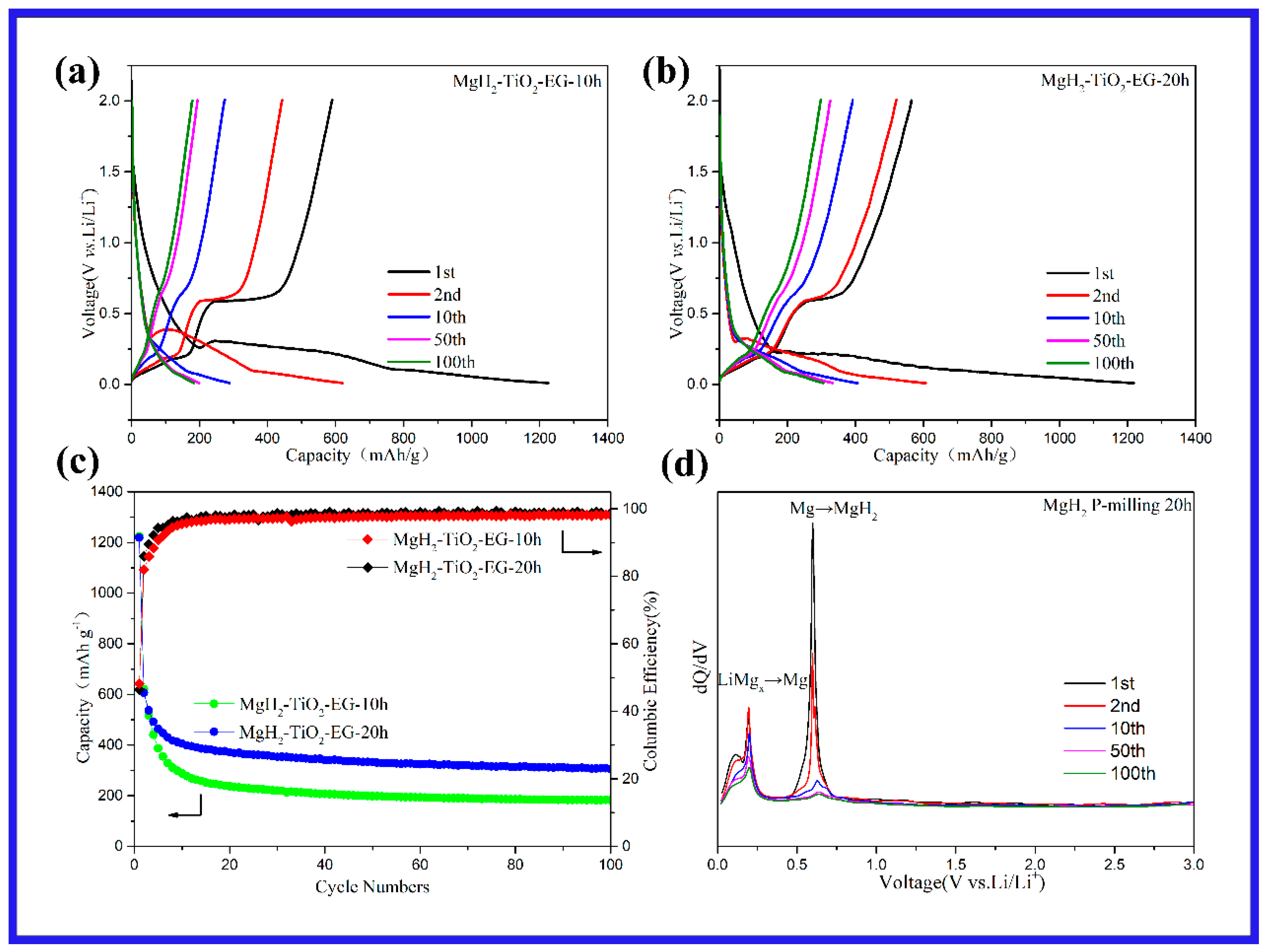
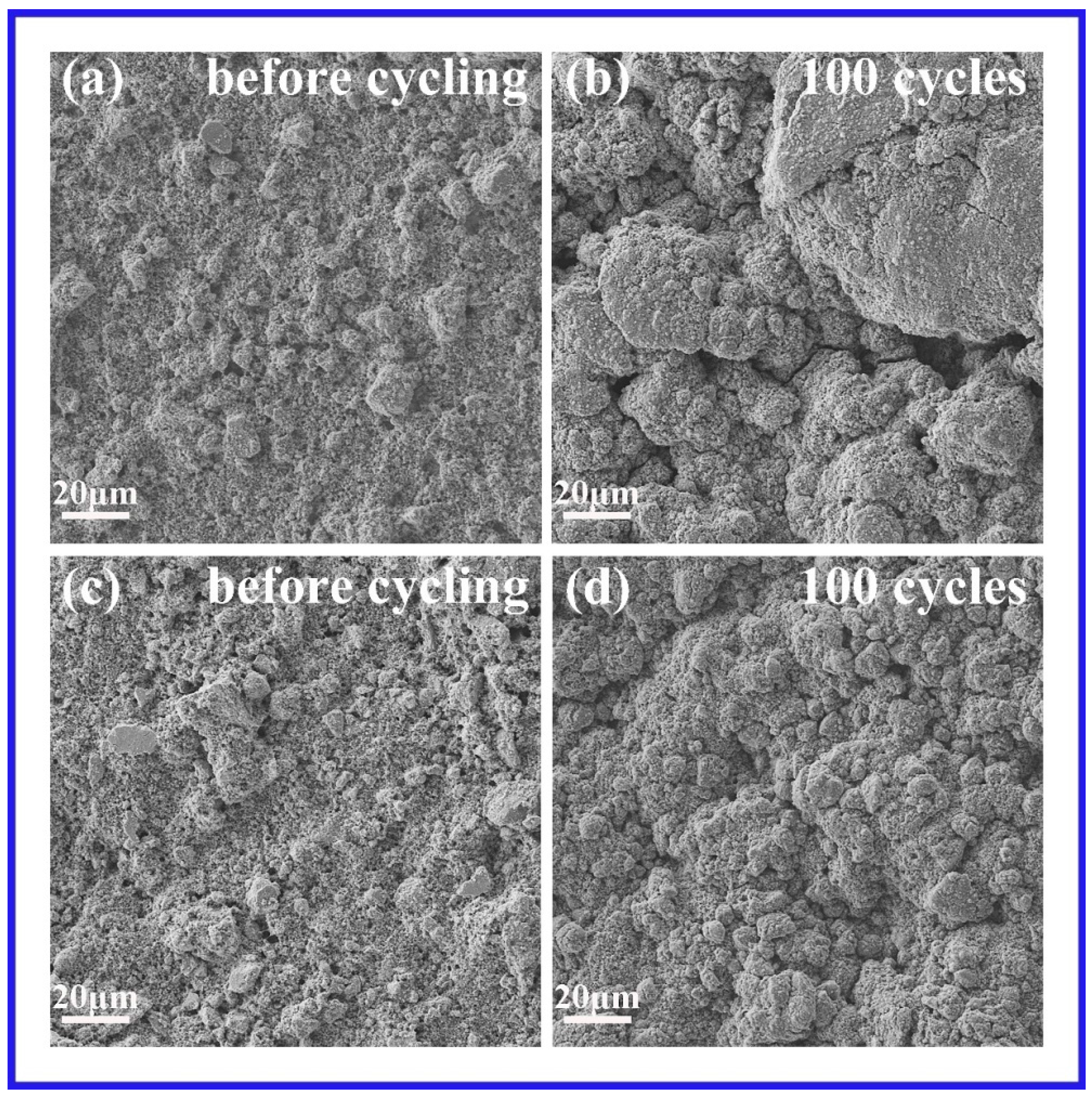
2. Results and Discussion
3. Experimental
3.1. Materials Preparation
3.2. Material Characterization
3.3. Electrochemical Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aguey-Zinsou, K.F.; Ares-Fernandez, J.R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.J.; Cai, W.T.; Ouyang, L.Z.; Zhu, M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—A review of recent progress. J. Alloys Compd. 2016, 658, 280–300. [Google Scholar] [CrossRef]
- Mohtadi, R.; Orimo, S.I. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2017, 2, 16091–16106. [Google Scholar] [CrossRef]
- Muthukumar, P.; Groll, M. Metal hydride based heating and cooling systems: A review. Int. J. Hydrogen Energy 2010, 35, 8816–8829. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef] [PubMed]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.-W. Hydrogen—A sustainable energy carrier. Prog. Natl. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Crivello, J.C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: development and optimization. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.J. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Natl. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 85. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, C.; Shen, S.; Zou, J.; Mao, S.S.; Yao, X. Combination of nanosizing and interfacial effect: Future perspective for designing Mg-based nanomaterials for hydrogen storage. Renew. Sustain. Energy Rev. 2015, 44, 289–303. [Google Scholar] [CrossRef]
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601. [Google Scholar] [CrossRef]
- Oumellal, Y.; Rougier, A.; Nazri, G.A.; Tarascon, J.M.; Aymard, L. Metal hydrides for lithium-ion batteries. Nat. Mater. 2008, 7, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, W.; Oumellal, Y.; Bonnet, J.P.; Zhang, J.; Cuevas, F.; Latroche, M.; Bobet, J.L.; Aymard, L. Carboxymethylcellulose and carboxymethylcellulose-formate as binders in MgH2–carbon composites negative electrode for lithium-ion batteries. J. Power Sources 2011, 196, 2854–2857. [Google Scholar] [CrossRef]
- Oumellal, Y.; Zlotea, C.; Bastide, S.; Cachet-Vivier, C.; Leonel, E.; Sengmany, S.; Leroy, E.; Aymard, L.; Bonnet, J.P.; Latroche, M. Bottom-up preparation of MgH2 nanoparticles with enhanced cycle life stability during electrochemical conversion in Li-ion batteries. Nanoscale 2014, 6, 14459–14466. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Aymard, L.; Bonnet, J.P. MgH2–TiH2 mixture as an anode for lithium-ion batteries: Synergic enhancement of the conversion electrode electrochemical performance. J. Mater. Chem. A 2015, 3, 15091–15096. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Gigli, G.; Paolone, A.; Reale, P.; Doublet, M.L.; Brutti, S. Origin of the Voltage Hysteresis of MgH2 Electrodes in Lithium Batteries. J. Phys. Chem. C 2015, 119, 17044–17052. [Google Scholar] [CrossRef]
- Li, D.X.; Zhang, T.R.; Yang, S.Q.; Tao, Z.L.; Chen, J. Ab initio investigation of structures, electronic and thermodynamic properties for Li–Mg–H ternary system. J. Alloys Compd. 2011, 509, 8228–8234. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Gigli, G.; Paolone, A.; Vitucci, F.; Brutti, S. Incorporation of Lithium by MgH2: An Ab Initio Study. J. Phys. Chem. C 2013, 117, 22467–22477. [Google Scholar] [CrossRef]
- Brutti, S.; Mulas, G.; Piciollo, E.; Panero, S.; Reale, P. Magnesium hydride as a high capacity negative electrode for lithium ion batteries. J. Mater. Chem. 2012, 22, 14531–14537. [Google Scholar] [CrossRef]
- Ikeda, S.; Ichikawa, T.; Kawahito, K.; Hirabayashi, K.; Miyaoka, H.; Kojima, Y. Anode properties of magnesium hydride catalyzed with niobium oxide for an all solid-state lithium-ion battery. Chem. Commun. 2013, 49, 7174–7176. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Ichikawa, T.; Goshome, K.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y. Anode properties of Al2O3-added MgH2 for all-solid-state lithium-ion batteries. J. Solid State Electrochem. 2015, 19, 3639–3644. [Google Scholar] [CrossRef]
- Brutti, S.; Meggiolaro, D.; Paolone, A.; Reale, P. Magnesium hydride as negative electrode active material in lithium cells: A review. Mater. Today Energy 2017, 3, 53–59. [Google Scholar] [CrossRef]
- Aymard, L.; Oumellal, Y.; Bonnet, J.P. Metal hydrides: An innovative and challenging conversion reaction anode for lithium-ion batteries. Beilstein J. Nanotechnol. 2015, 6, 1821–1839. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Cuevas, F.; Latroche, M. Metal hydrides used as negative electrode materials for Li-ion batteries. Appl. Phys. Mater. 2016, 122, 135. [Google Scholar] [CrossRef]
- Liu, H.; Hu, R.Z.; Zeng, M.Q.; Liu, J.W.; Zhu, M. Enhancing the performance of Sn–C nanocomposite as lithium ion anode by discharge plasma assisted milling. J. Mater. Chem. 2012, 22, 8022–8028. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Gao, M.; Pan, H.; Liu, Y. A Novel synthesis of MgS and its application as electrode material for lithium-ion batteries. J. Alloys Compd. 2014, 603, 158–166. [Google Scholar] [CrossRef]
- Wang, Y.K.; Yang, L.C.; Hu, R.Z.; Sun, W.; Liu, J.W.; Ouyang, L.Z.; Yuan, B.; Wang, H.H.; Zhu, M. A stable and high-capacity anode for lithium-ion battery: Fe2O3 wrapped by few layered graphene. J. Power Sources 2015, 288, 314–319. [Google Scholar] [CrossRef]
- Sun, W.; Hu, R.Z.; Liu, H.; Zeng, M.Q.; Yang, L.C.; Wang, H.H.; Zhu, M. Embedding nano-silicon in graphene nanosheets by plasma assisted milling for high capacity anode materials in lithium ion batteries. J. Power Sources 2014, 268, 610–618. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Wang, H.; Ouyang, L.; Liu, J.; Zhu, M. Improvement in the Electrochemical Lithium Storage Performance of MgH2. Inorganics 2018, 6, 2. https://doi.org/10.3390/inorganics6010002
Yang S, Wang H, Ouyang L, Liu J, Zhu M. Improvement in the Electrochemical Lithium Storage Performance of MgH2. Inorganics. 2018; 6(1):2. https://doi.org/10.3390/inorganics6010002
Chicago/Turabian StyleYang, Shuo, Hui Wang, Liuzhang Ouyang, Jiangwen Liu, and Min Zhu. 2018. "Improvement in the Electrochemical Lithium Storage Performance of MgH2" Inorganics 6, no. 1: 2. https://doi.org/10.3390/inorganics6010002
APA StyleYang, S., Wang, H., Ouyang, L., Liu, J., & Zhu, M. (2018). Improvement in the Electrochemical Lithium Storage Performance of MgH2. Inorganics, 6(1), 2. https://doi.org/10.3390/inorganics6010002