Mono-Cyclopentadienyl Titanium and Rare-Earth Metal Catalysts for Syndiospecific Polymerization of Styrene and Its Derivatives
Abstract
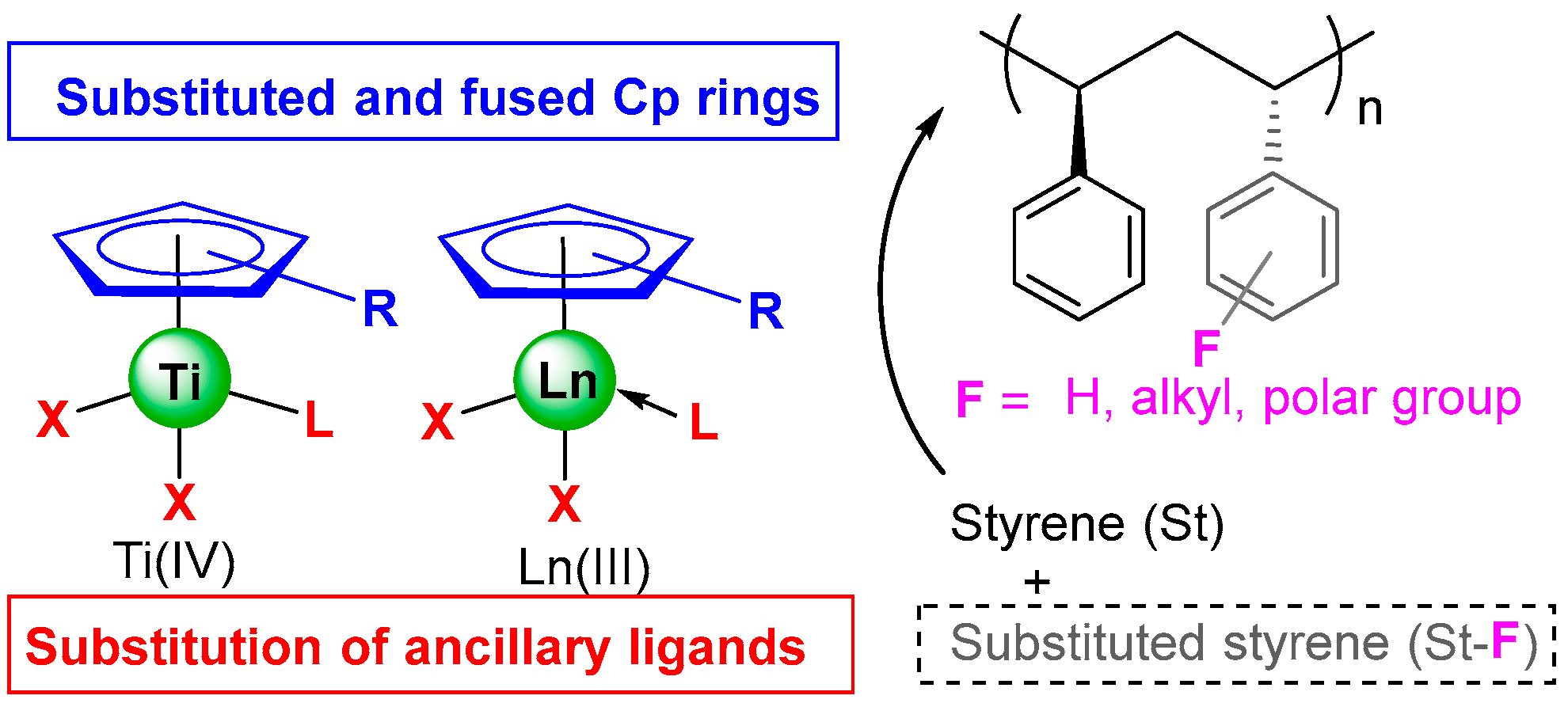
1. Introduction
2. Mono-Cyclopentadienyl Ti Catalysts
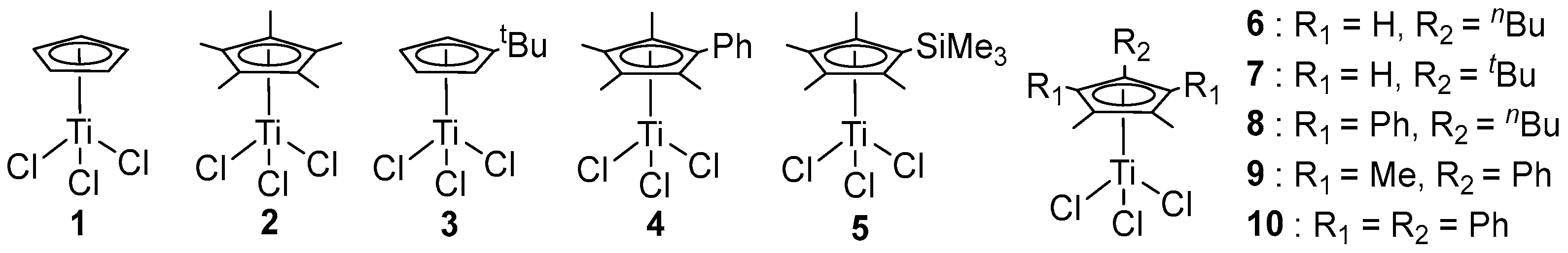
2.1. Substituted Cyclopentadienyl Ti Complexes
| Entry | Complex | Activity a | Mw ×104 g/mol | ĐM | Tm (°C) | Ref. |
|---|---|---|---|---|---|---|
| 1 b | 3 | 670 | 4.3 | 2.2 | 272 | [39] |
| 2 c | 4 | 4131 | 3.0 | 1.9 | 268 | [42] |
| 3 c | 5 | 158 | - | - | 269 | [40] |
| 4 b | 6 | 2800 | 3.1 | 2.2 | 264 | [41] |
| 5 b | 7 | 2100 | 2.1 | 2.0 | 265 | [41] |
| 6 b | 8 | 2900 | 3.7 | 2.1 | 269 | [41] |
| 7 b | 9 | 3900 | 2.0 | 2.0 | 262 | [41] |
| 8 b | 10 | 1700 | 1.0 | 1.7 | 255 | [41] |
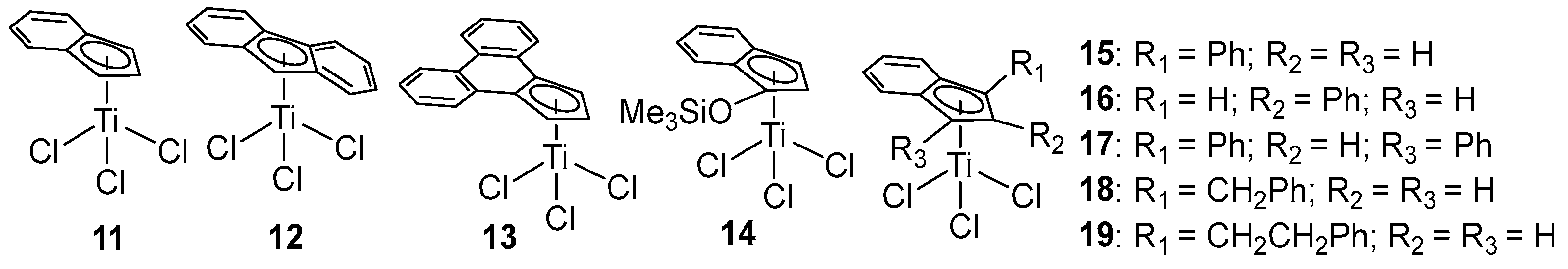
2.2. Fused Cyclopentadienyl Ti Complexes
| Entry | Complex | Activity a | Mw ×104 g/mol | ĐM | Tm (°C) | Ref. |
|---|---|---|---|---|---|---|
| 1 b | 11 | 400 | 10 | 2.0 | 268 | [43] |
| 2 c | 13 | 3600 | 2.9 | - | - | [45] |
| 3 d | 14 | 24 | - | - | - | [46] |
| 4 c | 15 | 3388 | 42.4 | - | 261 | [47] |
| 5 c | 16 | 2288 | 32.3 | - | 262 | [47] |
| 6 c | 17 | 1584 | 49.6 | - | 262 | [47] |
| 7 c | 18 | 88 | 32.3 | - | 268 | [47] |
| 8 c | 19 | 484 | 40.1 | - | 268 | [47] |
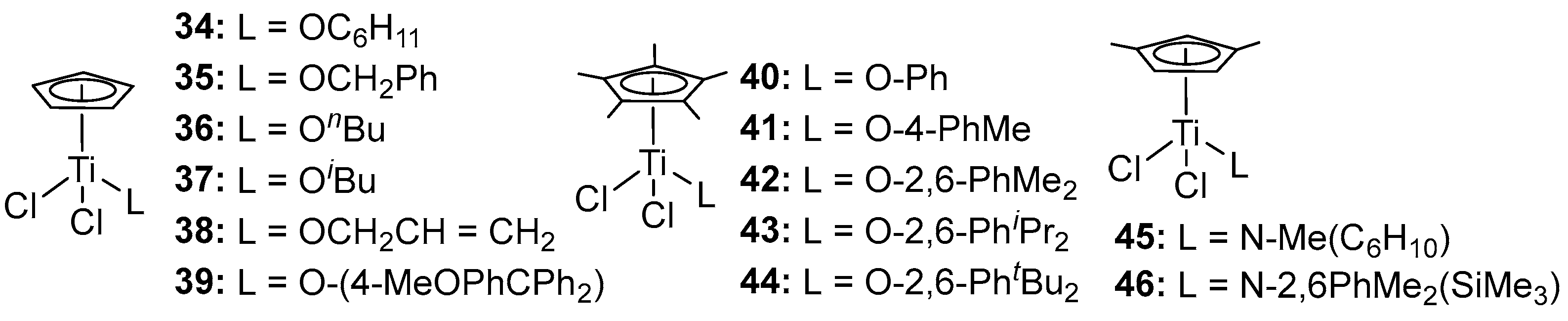
2.3. Substitution of the Ancillary Ligands
| Entry | Complex | Activity a | Mw ×104 g/mol | ĐM | Tm (°C) | Ref. |
|---|---|---|---|---|---|---|
| 1 b | 20 | 3000 | 10 | 2.0 | 265 | [37] |
| 2 c | 21 | 268 | 12 | - | - | [48] |
| 3 b | 22 | 690 | 66 | 2.0 | 275 | [37] |
| 4 d | 23 | 510 | - | - | - | [52] |
| 5 e | 24 | 1200 | 68 | 2.4 | 270 | [53] |
| 6 f | 26 | 59,200 | 18 | 2.1 | 269 | [58] |
| 7 f | 27 | 77,300 | 33 | 1.9 | 272 | [58] |
| 8 f | 28 | 81,200 | 36 | 2.0 | 273 | [58] |
| 9 f | 29 | 75,700 | 36 | 2.2 | 273 | [58] |
| 10 g | 30 | 2060 | 44 | - | 272 | [61] |
| 11 g | 31 | 1980 | 36 | - | 270 | [61] |
| 12 g | 32 | 1860 | 39 | - | 272 | [47] |
| 13 g | 33 | 1540 | 15 | - | 270 | [47] |
| 14 h | 34 | 1139 | - | - | 258 | [62] |
| 15 h | 35 | 261 | - | - | 256 | [62] |
| 16 h | 36 | 83 | - | - | 259 | [62] |
| 17 h | 37 | 456 | - | - | 259 | [62] |
| 18 h | 38 | 70 | - | - | 258 | [62] |
| 19 i | 40 | 6390 | 48 | 2.1 | - | [63] |
| 20 i | 41 | 6020 | 24 | 2.3 | - | [63] |
| 21 i | 42 | 12,400 | 21 | 2.1 | - | [63] |
| 22 i | 43 | 4290 | 32 | 2.2 | - | [63] |
| 23 i | 44 | 5690 | 23 | 2.2 | - | [63] |
| 24 i | 45 | 5680 | 5 | 2.2 | - | [64] |
| 25 i | 46 | 4890 | 6 | 2.8 | - | [64] |
3. Mono-Cyclopentadienyl Rare-Earth Metal Complexes
3.1. Substituted Mono-Cyclopentadienyl Ln Complexes
3.2. Fused Mono-Cyclopentadienyl Ln Complexes
3.3. Bridged Mono-Cyclopentadienyl Ln Complexes
3.4. Dinuclear Cyclopentadienyl Ln Complexes
4. Conclusions and Outlook
- (1)
- The cooperative action of steric/electronic modifications on the Cp ring and ancillary ligand is well known in the metal-catalyzed polymerization of styrene. How do we use the combination of steric/electronic modifications on the Cp and ancillary ligand moieties to achieve positive effects on styrene polymerization? We believe that exploiting this synergy more deliberately through advanced ligand design and computational modeling holds immense promise. In addition, AI-assisted catalyst design would provide a promising future.
- (2)
- Mono-cyclopentadienyl titanium catalysts typically possess a Ti(IV) state, necessitating reduction to the active Ti(III) species through the use of an excess of alkylaluminum compounds. However, the requirement for large quantities of alkylaluminum and MAO reagents raises safety and cost concerns in practical industrial applications. Therefore, developing novel activation strategies that minimize or eliminate the need for large quantities of alkylaluminum and MAO cocatalysts represents a critical research priority. Additionally, due to the high electrophilicity of titanium, these catalysts demonstrate significantly low polymerization activity toward polar styrenic monomers.
- (3)
- Pure sPS is inherently brittle; its current industrial applications typically depend on the incorporation of glass fibers to enhance performance. However, copolymerizing styrene with alkyl-substituted styrenes or specific olefin monomers presents a promising route to modify the polymer chain structure and improve impact resistance. Importantly, the direct incorporation of functional polar comonomers facilitated by advanced rare-earth catalysts could simultaneously impart desired functionality and potentially modulate mechanical properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shrivastava, A. 2—Polymerization. In Introduction to Plastics Engineering; Shrivastava, A., Ed.; William Andrew Publishing: New York, NY, USA, 2018; pp. 17–48. [Google Scholar]
- Men, H.; Yin, C.; Shi, Y.; Liu, X.; Fang, H.; Han, X.; Liu, J. Quantification of Acrylonitrile Butadiene Styrene Odor Intensity Based on a Novel Odor Assessment System With a Sensor Array. IEEE Access 2020, 8, 33237–33249. [Google Scholar] [CrossRef]
- Vaughan, A.S.; Bassett, D.C. 12—Crystallization and Morphology. In Comprehensive Polymer Science and Supplements; Allen, G., Bevington, J.C., Eds.; Pergamon: Amsterdam, The Netherlands, 1989; pp. 415–457. [Google Scholar]
- Van Krevelen, D.W. Chapter 2—Typology of Polymers. In Properties of Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 7–47. [Google Scholar]
- Lutz, J.F. Sequence-Controlled Polymerizations: The next Holy Grail in Polymer Science? Polym. Chem. 2010, 1, 55–62. [Google Scholar] [CrossRef]
- Stanford, M.J.; Dove, A.P. Stereocontrolled Ring-Opening Polymerisation of Lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Gómez-García, D.; Domínguez-Rodríguez, A.; Todd, R.I. Ultra-fast and Energy-efficient Sintering of Ceramics by Electric Current Concentration. Sci. Rep. 2015, 5, 8513. [Google Scholar] [CrossRef]
- Nakamura, Y.; Adachi, M.; Kato, Y.; Fujii, S.; Sasaki, M.; Urahama, Y.; Sakurai, S. Effects of Polystyrene Block Content on Morphology and Adhesion Property of Polystyrene Block Copolymer. J. Adhes. Sci. Technol. 2011, 25, 869–881. [Google Scholar] [CrossRef]
- Dörr, J.M.; Scheidelaar, S.; Koorengevel, M.C.; Dominguez, J.J.; Schäfer, M.; van Walree, C.A.; Killian, J.A. The Styrene–maleic Acid Copolymer: A Versatile Tool in Membrane Research. Eur. Biophys. J. 2016, 45, 3–21. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P. Frontiers of Mechanistic Studies of Coordination Polymerization and Oligomerization of α-olefins. Coord. Chem. Rev. 2012, 256, 2994–3007. [Google Scholar] [CrossRef]
- Woo, E.M.; Sun, Y.S.; Yang, C.P. Polymorphism, Thermal Behavior, and Crystal Stability in Syndiotactic Polystyrene vs. its Miscible Blends. Prog. Polym. Sci. 2001, 26, 945–983. [Google Scholar] [CrossRef]
- Laur, E.; Kirillov, E.; Carpentier, J.-F. Engineering of Syndiotactic and Isotactic Polystyrene-Based Copolymers via Stereoselective Catalytic Polymerization. Molecules 2017, 22, 594. [Google Scholar] [CrossRef]
- Liu, J.W.; Mackay, M.E.; Duxbury, P.M. Molecular Dynamics Simulation of Intramolecular Cross-Linking of BCB/Styrene Copolymers. Macromolecules 2009, 42, 8534–8542. [Google Scholar] [CrossRef]
- Annunziata, L.; Monasse, B.; Rizzo, P.; Guerra, G.; Duc, M.; Carpentier, J.-F. On the Crystallization Behavior of Syndiotactic-b-atactic Polystyrene Stereodiblock Copolymers, Atactic/Syndiotactic Polystyrene Blends, and aPS/sPS Blends Modified with sPS-b-aPS. Mater. Chem. Phys. 2013, 141, 891–902. [Google Scholar] [CrossRef]
- Danusso, F.; Sianesi, D. 95—Stereospecific PolymerIzation of Styrene. Note IV: The Influence of the Catalytic Ratio Al(C2H5)3/TiCl4. In Stereoregular Polymers and Stereospecific Polymerizations; Natta, G., Danusso, F., Eds.; Pergamon: Oxford, UK, 1967; p. 561. [Google Scholar]
- Cazzaniga, L.; Cohen, R.E. Anionic Synthesis of Isotactic Polystyrene. Macromolecules 1989, 22, 4125–4128. [Google Scholar] [CrossRef]
- Ishihara, N.; Seimiya, T.; Kuramoto, M.; Uoi, M. Crystalline Syndiotactic Polystyrene. Macromolecules 1986, 19, 2464–2465. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Sun, Z.; Zhang, Q.; Wei, P.; Mu, X.; Zhou, H.; Li, C.; Ma, S.; He, D. Superparamagnetic Enhancement of Thermoelectric Performance. Nature 2017, 549, 247–251. [Google Scholar] [CrossRef]
- Owen, M.M.; Okechukwu, A.E.; Zafir, R.A.; Akil, H.M. Recent Advances on Improving the Mechanical and Thermal Properties of Kenaf Fibers/Engineering Thermoplastic Composites using Novel Coating Techniques: A Review. Compos. Interfaces 2023, 30, 849–875. [Google Scholar] [CrossRef]
- Li, L.; Han, L.; Hu, H.; Zhang, R. A Review on Polymers and Their Composites for Flexible Electronics. Mater. Adv. 2023, 4, 726–746. [Google Scholar] [CrossRef]
- Zhou, W.L.; Shen, Z.G.; Chen, D.Q.; Tu, J.J.; Lu, W.K. Novel Engineering Plastics-Syndiotactic Polystyrene (sPS). Rare Met. Mater. Eng. 2001, 30, 374–378. [Google Scholar]
- Rueping, M.; Nachtsheim, B.J.; Scheidt, T. Efficient Metal-Catalyzed Hydroarylation of Styrenes. Org. Lett. 2006, 8, 3717–3719. [Google Scholar] [CrossRef]
- Schellenberg, J.; Leder, H.J. Syndiotactic Polystyrene: Process and Applications. Adv. Polym. Technol. 2006, 25, 141–151. [Google Scholar] [CrossRef]
- Ishihara, N.; Kuramoto, M.; Uoi, M. Stereospecific Polymerization of Styrene Giving the Syndiotactic Polymer. Macromolecules 1988, 21, 3356–3360. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Cui, D.; Liu, X. Precisely Controlled Polymerization of Styrene and Conjugated Dienes by Group 3 Single-Site Catalysts. ChemCatChem 2018, 10, 42–61. [Google Scholar] [CrossRef]
- Arndt, S.; Okuda, J. Cationic Alkyl Complexes of the Rare-earth metals: Synthesis, Structure, and Reactivity. Adv. Synth. Catal. 2005, 347, 339–354. [Google Scholar] [CrossRef]
- Zinck, P.; Bonnet, F.; Mortreux, A.; Visseaux, M. Functionalization of Syndiotactic Polystyrene. Prog. Polym. Sci. 2009, 34, 369–392. [Google Scholar] [CrossRef]
- Okuda, J. Molecular Olefin Polymerization Catalysts: From Metallocenes to Half-sandwich Complexes with Functionalized Cyclopentadienyl ligands. J. Organomet. Chem. 2023, 1000, 122833. [Google Scholar] [CrossRef]
- Gillis, D.J.; Tudoret, M.J.; Baird, M.C. Novel Arene Complexes of Titanium(IV), Zirconium(IV), and Hafnium(IV). J. Am. Chem. Soc. 1993, 115, 2543–2545. [Google Scholar] [CrossRef]
- Longo, P.; Proto, A.; Oliva, L. Zirconium Catalysts for the Syndiotactic Polymerization of Styrene. Macromol. Rapid Commun. 1994, 15, 151–154. [Google Scholar] [CrossRef]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative Chain Transfer Polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Fayzan Shakir, H.M. Stereospecific Polymerization Techniques. In Advanced Functional Polymers: Synthesis to Applications; Shaker, K., Hafeez, A., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 3–21. [Google Scholar]
- Nomura, K.; Izawa, I.; Yi, J.; Nakatani, N.; Aoki, H.; Harakawa, H.; Ina, T.; Mitsudome, T.; Tomotsu, N.; Yamazoe, S. Solution XAS Analysis for Exploring Active Species in Syndiospecific Styrene Polymerization and 1-Hexene Polymerization Using Half-Titanocene–MAO Catalysts: Significant Changes in the Oxidation State in the Presence of Styrene. Organometallics 2019, 38, 4497–4507. [Google Scholar] [CrossRef]
- Tomotsu, N.; Ishihara, N. Novel Catalysts for Syndiospecific Polymerization of Styrene. Catal. Surv. Asia 1997, 1, 89–110. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Liu, X.; Pollick, D.J.; Brungard, N.L.; Chang, M. Ziegler-Natta Catalysts for Propylene Polymerization: Chemistry of Reactions Leading to the Formation of Active Centers. J. Mol. Catal. A Chem. 2008, 287, 45–52. [Google Scholar] [CrossRef]
- Schellenberg, J. Recent Transition Metal Catalysts for Syndiotactic Polystyrene. Prog. Polym. Sci. 2009, 34, 688–718. [Google Scholar] [CrossRef]
- Kaminsky, W.; Lenk, S.; Scholz, V.; Roesky, H.W.; Herzog, A. Fluorinated Half-Sandwich Complexes as Catalysts in Syndiospecific Styrene Polymerization. Macromolecules 1997, 30, 7647–7650. [Google Scholar] [CrossRef]
- Nakatani, H.; Nitta, K.-h.; Takata, T.; Soga, K. Polymerization of 4-n-alkylstyrenes with Typical Ziegler-Natta and Metallocene Catalysts. Polym. Bull. 1997, 38, 43–48. [Google Scholar] [CrossRef]
- Duncalf, D.J.; Wade, H.J.; Waterson, C.; Derrick, P.J.; Haddleton, D.M.; McCamley, A. Synthesis and Mechanism of Formation of Syndiotactic Polystyrene Using a (tert-Butylcyclopentadienyl)titanium Complex. Macromolecules 1996, 29, 6399–6403. [Google Scholar] [CrossRef]
- Pinkas, J.; Lyčka, A.; Šindelář, P.; Gyepes, R.; Varga, V.; Kubišta, J.; Horáček, M.; Mach, K. Effects of Substituents in Cyclopentadienyltitanium Trichlorides on Electronic Absorption and 47,49Ti NMR Spectra and Styrene Polymerization Activated by Methylalumoxane. J. Mol. Catal. A Chem. 2006, 257, 14–25. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.W.; Seo, H.; Lee, I.S.; Chung, Y.K. Synthesis of Titanium Trichloride Complexes of 1,2,3-trisubstituted Cyclopentadienyls and Their Use in Styrene Polymerization. J. Organomet. Chem. 2001, 627, 233–238. [Google Scholar] [CrossRef]
- Schellenberg, J.; Tomotsu, N. Syndiotactic Polystyrene Catalysts and Polymerization. Prog. Polym. Sci. 2002, 27, 1925–1982. [Google Scholar] [CrossRef]
- Ready, T.E.; Gurge, R.; Chien, J.C.W.; Rausch, M.D. Oxidation States of Active Species for Syndiotactic-Specific Polymerization of Styrene. Organometallics 1998, 17, 5236–5239. [Google Scholar] [CrossRef]
- Knjazhanski, S.Y.; Cadenas, G.; García, M.; Pérez, C.M.; Nifant’ev, I.E.; Kashulin, I.A.; Ivchenko, P.V.; Lyssenko, K.A. (Fluorenyl)titanium Triisopropoxide and Bis(fluorenyl)titanium Diisopropoxide: A Facile Synthesis, Molecular Structure, and Catalytic Activity in Styrene Polymerization. Organometallics 2002, 21, 3094–3099. [Google Scholar] [CrossRef]
- Schneider, N.; Prosenc, M.-H.; Brintzinger, H.-H. Cyclopenta Phenanthrene Titanium Trichloride Derivatives: Syntheses, Crystal Structure and Properties as Catalysts for Styrene Polymerization. J. Organomet. Chem. 1997, 545–546, 291–295. [Google Scholar] [CrossRef]
- Tian, G.; Xu, S.; Zhang, Y.; Wang, B.; Zhou, X. Siloxy-substituted Tetramethylcyclopentadienyl and Indenyl Trichlorotitanium Complexes for Syndiospecific Polymerization of Styrene. J. Organomet. Chem. 1998, 558, 231–233. [Google Scholar] [CrossRef]
- Foster, P.; Chien, J.C.W.; Rausch, M.D. Highly Stable Catalysts for the Stereospecific Polymerization of Styrene. Organometallics 1996, 15, 2404–2409. [Google Scholar] [CrossRef]
- Kucht, A.; Kucht, H.; Barry, S.; Chien, J.C.W.; Rausch, M.D. New Syndiospecific Catalysts for Styrene Polymerization. Organometallics 1993, 12, 3075–3078. [Google Scholar] [CrossRef]
- Kucht, H.; Kucht, A.; Rausch, M.D.; Chien, J.C.W. (η5-Pentamethylcyclopentadienyl)trimethyltitanium as a Precursor for the Syndiospecific Polymerization of Styrene. Appl. Organomet. Chem. 1994, 8, 393–396. [Google Scholar] [CrossRef]
- Newman, T.H.; Borodychuk, K.K.; Schellenberg, J. Syndiotactic Vinylidene Aromatic Polymer Production, used in Moulding Involves Using Catalyst Comprising Gp=IV Metal Complex, Activating Cocatalyst and Hydrocarbylsilane Adjuvant. Patent WO9742234-A1, 13 November 1997. [Google Scholar]
- Erben, M.; Merna, J.; Hylský, O.; Kredatusová, J.; Lyčka, A.; Dostál, L.; Padělková, Z.; Novotný, M. Synthesis, Characterization and Styrene Polymerization Behavior of Alkoxysilyl-substituted Monocyclopentadienyltitanium(IV) Complexes. J. Organomet. Chem. 2013, 725, 5–10. [Google Scholar] [CrossRef]
- Xu, G.; Chung, T.C. Synthesis of Syndiotactic Polystyrene Derivatives Containing Amino Groups. Macromolecules 2000, 33, 5803–5809. [Google Scholar] [CrossRef]
- Grassi, A.; Pellecchia, C.; Oliva, L.; Laschi, F. A Combined NMR and Electron Spin Resonance Investigation of The (C5(CH3)5)Ti(CH2C6H5)3/B(C6F5)3 Catalytic System Active in the Syndiospecific Styrene Polymerization. Macromol. Chem. Phys. 1995, 196, 1093–1100. [Google Scholar] [CrossRef]
- Newman, T.H.; Malanga, M.T. Syndiotactic Polystyrene Polymerization Results Using a Titanium(III) Complex, Cp*Ti(OMe)2 and Implications to the Mechanism of Polymerization. J. Macromol. Sci. Part A 1997, 34, 1921–1927. [Google Scholar] [CrossRef]
- Dong, J.Y.; Manias, E.; Chung, T. Functionalized Syndiotactic Polystyrene Polymers Prepared by the Combination of Metallocene Catalyst and Borane Comonomer. Macromolecules 2002, 35, 3439–3447. [Google Scholar] [CrossRef]
- Zhu, F.M.; Wang, Q.F.; Fang, Y.T.; Lin, S.A. Synthesis of Syndiotactic Polystyrene with Novel Titanocene Catalysts Activated by Modified Methylaluminoxane. Chin. Chem. Lett. 1999, 10, 171–174. [Google Scholar]
- Huang, Q.; Sheng, Y.; Yang, W. Synthesis, Structure, and properties of syndiotactic polystyrene catalyzed by Cp*Ti(OBz)3/MAO/TIBA. J. Appl. Polym. Sci. 2007, 103, 501–505. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, L.; Lin, S.; Wu, Q.; Zhu, F.; Shiyan; Fu, Z.; Yang, W. Syndiospecific polymerization of styrene catalyzed by half-titanocene catalysts. Polymer 2006, 47, 767–773. [Google Scholar] [CrossRef]
- Shen, Z.G.; Zhao, W.W.; Li, H.M.; Zhu, F.M.; Lin, S.A. In-situ preparation of sPS/Montmorillonite nanocomposites with monetitanocene catalytic system. Acta Polym. Sin. 2004, 1, 50–53. [Google Scholar]
- Zhu, F.M.; Lin, S.A. Metallocene Catalysts for Syndiospecific Polymerization of Styrene. Chem. Res. Chin. Univ. 1997, 18, 2065–2069. [Google Scholar]
- Shen, Z.G.; Zhou, W.L.; Zhu, F.M.; Lin, S.A. New highly active catalysts Cp*Ti(O-C6H4-X)3 for bulk syndiospecific polymerization of styrene. Acta Polym. Sin. 2004, 1, 45–49. [Google Scholar]
- Liu, J.; Ma, H.; Huang, J.; Qian, Y. Syndiotactic Polymerization of Styrene with CpTiCl2(OR)/MAO System. Eur. Polym. J. 2000, 36, 2055–2058. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J.; Padmanabhan, S.; Kitiyanan, B. Nonbridged Half-metallocenes Containing Anionic Ancillary Donor Ligands: New Promising Candidates as Catalysts for Precise Olefin Polymerization. J. Mol. Catal. A Chem. 2007, 267, 1–29. [Google Scholar] [CrossRef]
- Byun, D.J.; Fudo, A.; Tanaka, A.; Fujiki, M.; Nomura, K. Effect of Cyclopentadienyl and Anionic Ancillary Ligand in Syndiospecific Styrene Polymerization Catalyzed by Nonbridged Half-Titanocenes Containing Aryloxo, Amide, and Anilide Ligands: Cocatalyst Systems. Macromolecules 2004, 37, 5520–5530. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, Y.; Tezuka, H.; Xie, P.; Tardif, O.; Koizumi, T.-a.; Yamazaki, H.; Wakatsuki, Y. C5Me5/ER-Ligated Samarium(II) Complexes with the Neutral “C5Me5M” Ligand (ER = OAr, SAr, NRR‘, or PHAr; M = K or Na): A Unique Catalytic System for Polymerization and Block-Copolymerization of Styrene and Ethylene. J. Am. Chem. Soc. 2000, 122, 10533–10543. [Google Scholar] [CrossRef]
- Kirillov, E.; Lehmann, C.W.; Razavi, A.; Carpentier, J.-F. Highly Syndiospecific Polymerization of Styrene Catalyzed by Allyl Lanthanide Complexes. J. Am. Chem. Soc. 2004, 126, 12240–12241. [Google Scholar] [CrossRef]
- Lin, F.; Wang, X.; Pan, Y.; Wang, M.; Liu, B.; Luo, Y.; Cui, D. Nature of the Entire Range of Rare Earth Metal-Based Cationic Catalysts for Highly Active and Syndioselective Styrene Polymerization. ACS Catal. 2016, 6, 176–185. [Google Scholar] [CrossRef]
- Pan, Y.; Rong, W.; Jian, Z.; Cui, D. Ligands Dominate Highly Syndioselective Polymerization of Styrene by Using Constrained-geometry-configuration Rare-earth Metal Precursors. Macromolecules 2012, 45, 1248–1253. [Google Scholar] [CrossRef]
- Luo, Y.; Baldamus, J.; Hou, Z. Scandium Half-Metallocene-Catalyzed Syndiospecific Styrene Polymerization and Styrene−Ethylene Copolymerization: Unprecedented Incorporation of Syndiotactic Styrene−Styrene Sequences in Styrene−Ethylene Copolymers. J. Am. Chem. Soc. 2004, 126, 13910–13911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, Y.; Zhang, K.; Cai, Z.-Y.; Li, S.-H.; Cui, D.-M. DMAO-activated Rare-earth Metal Catalysts for Styrene and Its Derivative Polymerization. Chin. J. Polym. Sci. 2021, 39, 1185–1190. [Google Scholar] [CrossRef]
- Peng, D.-Q.; Yan, X.-W.; Zhang, S.-W.; Li, X.-F. Syndiotactic Polymerization of Styrene and Copolymerization with Ethylene Catalyzed by Chiral Half-sandwich Rare-earth Metal Dialkyl Complexes. Chin. J. Polym. Sci. 2018, 36, 222–230. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, C.; Wu, C.; Zhao, Z.; Cui, D. Additive-Triggered Chain Transfer to a Solvent in Coordination Polymerization. Macromolecules 2020, 53, 1205–1211. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Tong, X.; Zhang, H.; Chen, Y.; Liu, Y.; Liu, H.; Wang, X.; Nishiura, M.; He, H.; et al. Aluminum Effects in the Syndiospecific Copolymerization of Styrene with Ethylene by Cationic Fluorenyl Scandium Alkyl Catalysts. Organometallics 2013, 32, 1445–1458. [Google Scholar]
- Wang, Q.; Zhang, Z.; Jiang, Y.; Li, S.; Cui, D. Synthesis of Ethylene–Styrene Multiblock Copolymers Possessing High Strength and Toughness Using Binuclear Scandium Catalysts. Macromolecules 2025, 58, 2609–2618. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Z.Y.; Pan, Y.P.; Dou, Y.L.; Li, S.H.; Cui, D.M. Substituent Effects of Pyridyl-methylene Cyclopentadienyl Rare-earth Metal Complexes on Styrene Polymerization. Chin. J. Polym. Sci. 2019, 37, 570–577. [Google Scholar] [CrossRef]
- Huang, Z.; Li, A.; Chen, J.; Luo, Y. Syndiospecific Copolymerization of Styrene with para-Methoxystyrene Catalyzed by Functionalized Fluorenyl-Ligated Rare-Earth Metal Complexes. Inorg. Chem. 2023, 62, 4322–4329. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nishiura, M.; Mori, K.; Mashiko, T.; Hou, Z. Cationic Scandium Aminobenzyl Complexes. Synthesis, Structure and Unprecedented Catalysis of Copolymerization of 1-hexene and Dicyclopentadiene. Chem. Commun. 2007, 40, 4137–4139. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Guo, F.; Li, Y.; Hou, Z. Synthesis of Amino-containing Syndiotactic Polystyrene as Efficient Polymer Support for Palladium Nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 5–9. [Google Scholar] [CrossRef]
- Shi, X.; Nishiura, M.; Hou, Z. Simultaneous Chain-Growth and Step-Growth Polymerization of Methoxystyrenes by Rare-Earth Catalysts. Angew. Chem. Int. Ed. 2016, 55, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, M.; Chai, Y.; Wan, X.; Cui, D. Self-Activated Coordination Polymerization of Alkoxystyrenes by a Yttrium Precursor: Stereocontrol and Mechanism. ACS Catal. 2019, 9, 2618–2625. [Google Scholar] [CrossRef]
- Liu, B.; Wu, C.; Cui, D. Sequence Controlled Ethylene/styrene Copolymerization Catalyzed by Scandium Complexes. Polym. Chem. 2018, 10, 235–243. [Google Scholar]
- Jian, Z.; Cui, D.; Hou, Z. Rare-Earth-Metal–Hydrocarbyl Complexes Bearing Linked Cyclopentadienyl or Fluorenyl Ligands: Synthesis, Catalyzed Styrene Polymerization, and Structure–Reactivity Relationship. Chem. Eur. J. 2012, 18, 2674–2684. [Google Scholar] [CrossRef]
- Liu, D.; Wang, R.; Wang, M.; Wu, C.; Wang, Z.; Yao, C.; Liu, B.; Wan, X.; Cui, D. Syndioselective Coordination Polymerization of Unmasked Polar Methoxystyrenes using a Pyridenylmethylene Fluorenyl Yttrium Precursor. Chem. Commun. 2015, 51, 4685–4688. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, D.; Cui, D. Statistically Syndioselective Coordination (Co)polymerization of 4-Methylthiostyrene. Macromolecules 2016, 49, 781–787. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Liu, D.; Liu, B.; Cui, D. Highly Syndioselective Coordination (Co)Polymerization of Para-Chlorostyrene. Macromolecules 2020, 53, 8333–8339. [Google Scholar] [CrossRef]
- Chung, T.C.; Rhubright, D. Synthesis of Functionalized Polypropylene. Macromolecules 1991, 24, 970–972. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, Y.; Cai, Z.; Liu, D.; Li, S.; Cui, D. Syndioselective Coordination (Co)Polymerization of Alkyne-Substituted Styrenes Using Rare-Earth Metal Catalysts. Macromolecules 2020, 53, 5895–5902. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Zhao, Z.; Cui, D. Chemo- and Stereoselective Polymerization of Polar Divinyl Monomers by Rare-Earth Complexes. Macromolecules 2021, 54, 3181–3190. [Google Scholar] [CrossRef]




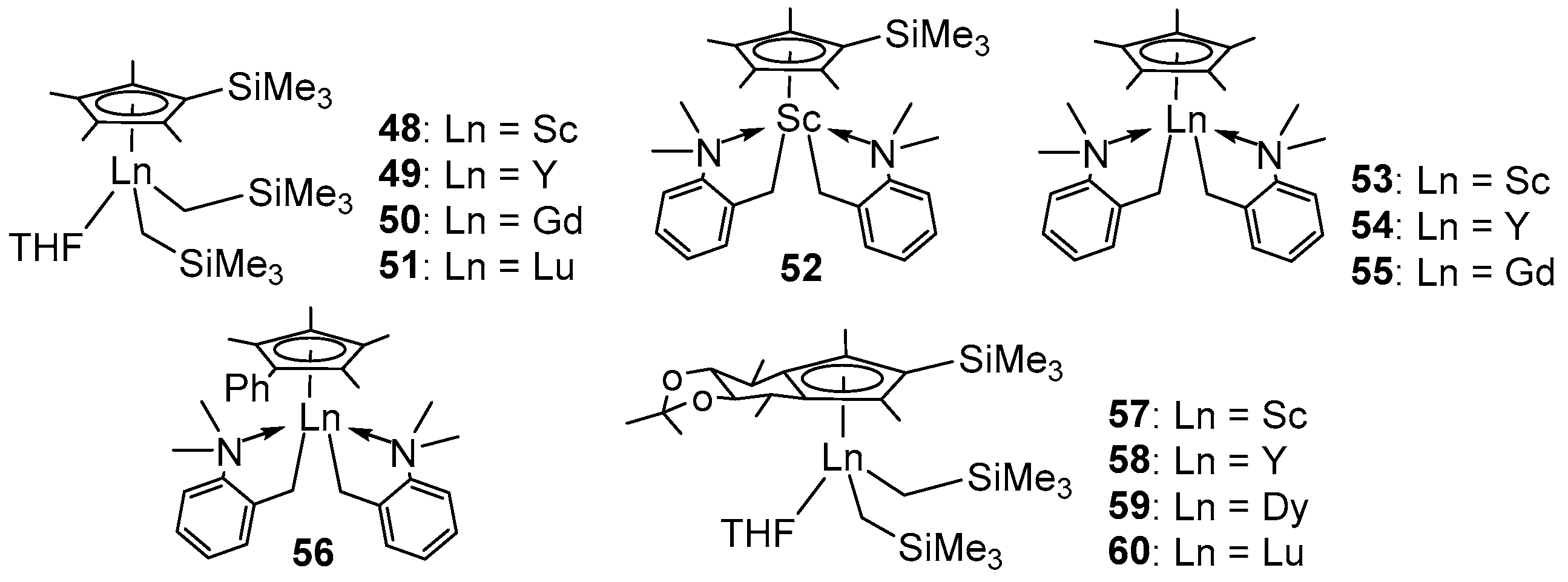
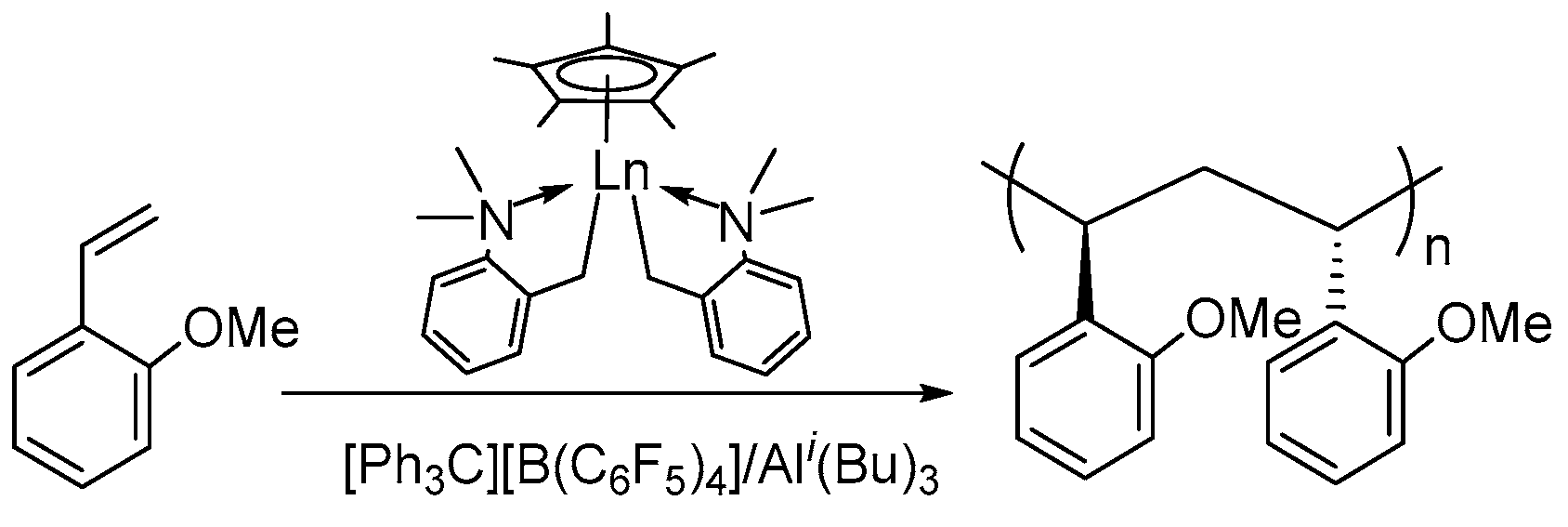


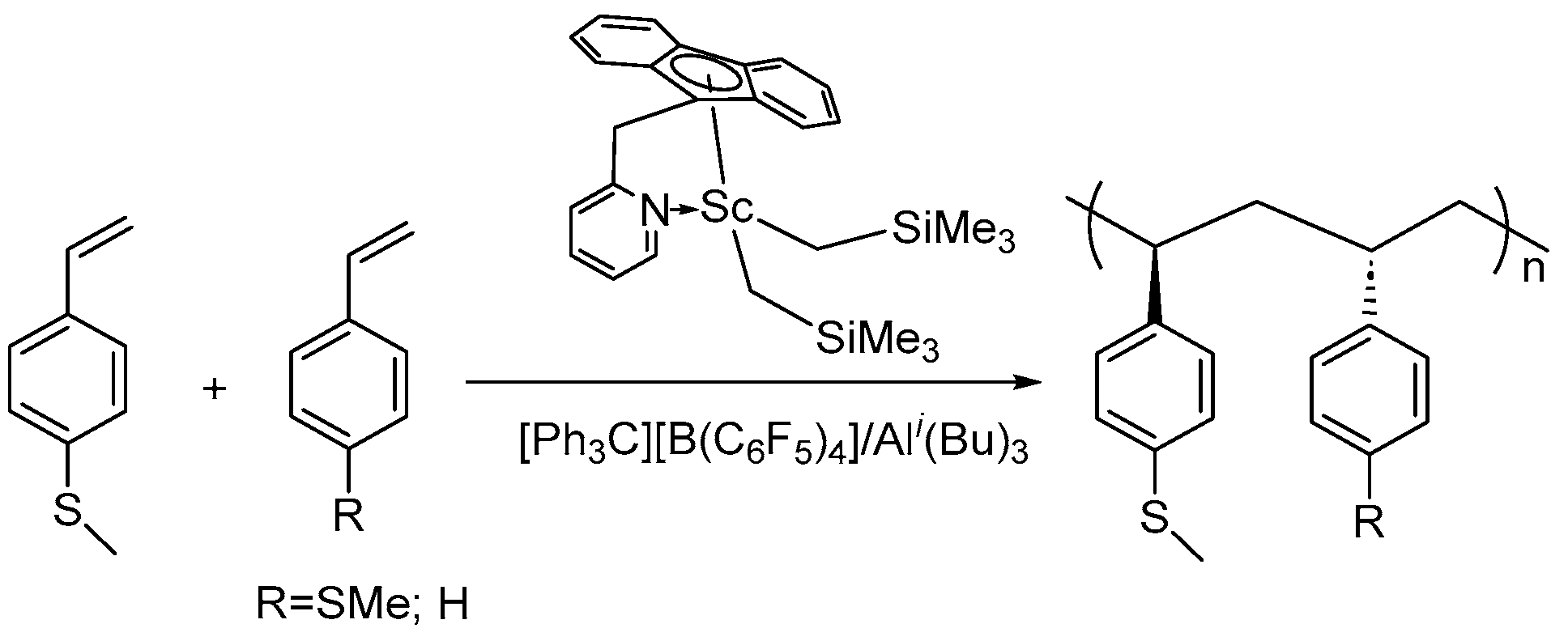

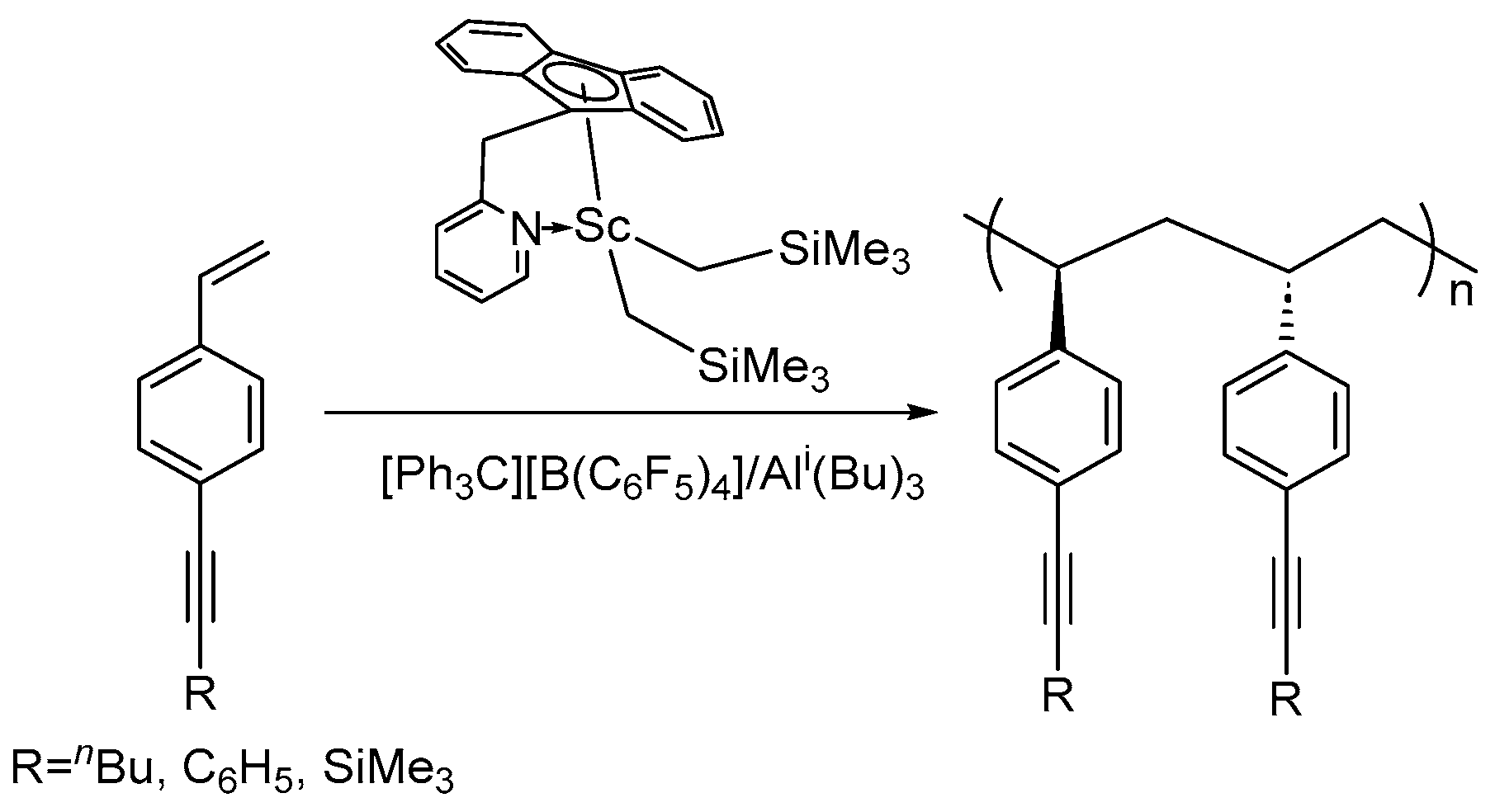



| Entry | Complex | [St]/[Ln] | t (min) | Activity a | Mn ×104 g/mol | ĐM | Tm (°C) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 b | 48 | 2500 | 1 | 13,618 | 37.9 | 1.4 | 273 | [69] |
| 2 b | 49 | 100 | 30 | 13 | 1.1 | 1.4 | 269 | [69] |
| 3 b | 50 | 100 | 30 | 15 | 0.9 | 1.4 | 269 | [69] |
| 4 b | 51 | 100 | 30 | 6 | 0.5 | 1.4 | 268 | [69] |
| 5 c | 56 | 500 | 120 | 1 | 3.2 | 2.3 | 272 | [70] |
| 6 d | 57 | 1500 | 3 | 3126 | 0.6 | 5.7 | 266 | [71] |
| 7 d | 58 | 500 | 90 | 0.7 | - | - | 266 | [71] |
| 8 e | 59 | 500 | 720 | 0.8 | - | - | 267 | [72] |
| 9 d | 60 | 500 | 90 | 0.3 | - | - | 268 | [71] |
| 10 f | 63 | 2500 | 60 | 208 | 43 | 3.8 | 268 | [73] |
| 11 f | 64 | 5500 | 1 | ≥34,370 | 12 | 4.6 | 267 | [73] |
| 12 f | 65 | 5500 | 1 | ≥34,370 | 67 | 2.3 | 272 | [73] |
| 13 f | 66 | 5500 | 1 | ≥34,370 | 64 | 4.1 | 274 | [73] |
| 14 g | 67 | 500 | 2 | 1360 | 37 | 1.9 | 268 | [74] |
| 15 h | 68 | 5000 | 2 | 15,600 | 48.5 | 2.4 | 270 | [68] |
| 16 h | 69 | 5000 | 2 | 15,600 | 49.5 | 2.6 | 270 | [68] |
| 17 h | 70 | 5000 | 2 | 15,600 | 48.7 | 2.4 | 270 | [68] |
| 18 h | 72 | 1000 | 240 | 15 | 3.0 | 1.4 | 228 | [68] |
| 19 h | 73 | 1000 | 240 | 17 | 3.6 | 1.6 | 234 | [68] |
| 20 h | 74 | 1000 | 180 | 143 | 3.5 | 2.1 | 269 | [75] |
| 21 f | 77 | 2500 | 300 | 13 | 77 | 1.8, 1.4 | 265 | [73] |
| 22 f | 78 | 2500 | 300 | 7 | 122 | 2.0, 1.6 | 267 | [73] |
| 23 h | 82 | 1000 | 1 | 6240 | 3.5 | 1.9 | 270 | [76] |
| 24 h | 83 | 500 | 5 | 474 | 2.2 | 1.5 | 270 | [76] |
| 25 g | 84 | 500 | 2 | 240 | 44 | 2.1 | 269 | [74] |
| 26 g | 85 | 500 | 5 | 180 | 78 | 2.0 | 269 | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Bai, M.; Wang, W.; Zheng, H.; Feng, C.; Gu, J.; Mao, G.; Gao, H. Mono-Cyclopentadienyl Titanium and Rare-Earth Metal Catalysts for Syndiospecific Polymerization of Styrene and Its Derivatives. Inorganics 2025, 13, 274. https://doi.org/10.3390/inorganics13080274
Wang J, Bai M, Wang W, Zheng H, Feng C, Gu J, Mao G, Gao H. Mono-Cyclopentadienyl Titanium and Rare-Earth Metal Catalysts for Syndiospecific Polymerization of Styrene and Its Derivatives. Inorganics. 2025; 13(8):274. https://doi.org/10.3390/inorganics13080274
Chicago/Turabian StyleWang, Junsong, Mingming Bai, Wenyan Wang, Handou Zheng, Chunyu Feng, Jiayue Gu, Guoliang Mao, and Haiyang Gao. 2025. "Mono-Cyclopentadienyl Titanium and Rare-Earth Metal Catalysts for Syndiospecific Polymerization of Styrene and Its Derivatives" Inorganics 13, no. 8: 274. https://doi.org/10.3390/inorganics13080274
APA StyleWang, J., Bai, M., Wang, W., Zheng, H., Feng, C., Gu, J., Mao, G., & Gao, H. (2025). Mono-Cyclopentadienyl Titanium and Rare-Earth Metal Catalysts for Syndiospecific Polymerization of Styrene and Its Derivatives. Inorganics, 13(8), 274. https://doi.org/10.3390/inorganics13080274







